Abstract
Background
Persistent atrial fibrillation may lead to a higher probability of inappropriate shocks in heart failure patients with an implantable cardioverter‐defibrillator (ICD). The aim of this study was to evaluate the impact of His‐Purkinje conduction system pacing combined with atrioventricular node ablation in improving heart function and preventing inappropriate shock therapy in these patients.
Methods and Results
A total of 86 consecutive patients with persistent atrial fibrillation and heart failure who had indications for ICD implantation were enrolled from January 2010 to March 2018. His‐Purkinje conduction system pacing with ICD and atrioventricular node ablation was attempted in 55 patients, and the remaining patients underwent ICD implantation only. Left ventricular (LV) ejection fraction, LV end‐systolic volume, New York Heart Association (NYHA) classification, shock therapies, and drug therapy were assessed during follow‐up. Overall, 31 patients received ICD implantation with optimal drug therapy (group 1). atrioventricular node ablation combined with His‐Purkinje conduction system pacing was successfully achieved in 52 patients (group 2). During follow‐up, patients in group 2 had lower incidence of inappropriate shock (15.6% versus 0%, P<0.01) and adverse events (P=0.011). Meanwhile, improvement in LV ejection fraction and reduction in LV end‐systolic volume were significantly higher in group 2 than in group 1 (15% versus 3%, P<0.001; and 40 versus 2 mL, P<0.01, respectively). NYHA functional class improved in both groups from a baseline 2.57±0.68 to 1.73±0.74 in group 1 and 2.73±0.59 to 1.42±0.53 in group 2 (P<0.01).
Conclusions
His‐Purkinje conduction system pacing combined with atrioventricular node ablation is feasible and safe with a high success rate in persistent atrial fibrillation patients with heart failure and ICD indication. It can significantly reduce the incidence of inappropriate shocks and improve LV function.
Keywords: atrial fibrillation, atrioventricular node ablation, His bundle pacing, inappropriate shock, left bundle branch pacing
Subject Categories: Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator, Heart Failure, Pacemaker, Arrhythmias
Clinical Perspective
What Is New?
This study is the first to evaluate the impact of His‐Purkinje conduction system pacing (HPSP) combined with atrioventricular node (AVN) ablation in persistent atrial fibrillation patients with heart failure and implantable cardioverter‐defibrillator implantation.
HPSP combined with AVN ablation can significantly improve left ventricular function and reduce adverse events (heart failure hospitalization, inappropriate shocks, or death) compared with implantable cardioverter‐defibrillator implantation and drug therapy in patients with persistent atrial fibrillation and heart failure.
HPSP combined with AVN ablation was feasible and safe, with a 94.5% success rate in this study.
What Are the Clinical Implications?
HPSP combined with AVN ablation can be considered in patients with persistent atrial fibrillation and heart failure who do not respond to optimal drug therapy.
HPSP combined with AVN ablation may be an important therapeutic option for persistent atrial fibrillation and heart failure patients with recurrent inappropriate shocks caused by AF despite optimal drug therapy.
Introduction
Implantable cardioverter‐defibrillator (ICD) effectively treats malignant arrhythmias, including persistent ventricular tachycardia (VT), torsades de pointes, and ventricular fibrillation.1 Randomized controlled trials have shown the effectiveness of ICD in primary and secondary prevention of sudden cardiac death.2, 3, 4 However, inappropriate shock (IS) caused by atrial fibrillation (AF), supraventricular tachycardia, noise, or T‐wave oversensing in ICD recipients has a significant impact on their physical and mental well‐being.3, 5, 6
AF is a common arrhythmia in patients with ICD and heart failure (HF). An epidemiological survey in 2010 showed that the number of patients with AF in the world is estimated to be 33.5 million and is increasing at the rate of 5 million per year.7 AF is often associated with HF.8 Current therapeutic options for AF include upstream treatment, anticoagulation therapy, and rate and rhythm control. At present, the main methods of rate and rhythm control in patients with AF include drug therapy, AF ablation, and atrioventricular node ablation (AVN) combined with pacing. Antiarrhythmic drugs are ineffective sometimes and can lead to significant side effects. Catheter ablation is still associated with high recurrence rates of AF. AVN ablation and pacing have been performed for >40 years in patients with AF who are intolerant of or unresponsive to intensive rate and rhythm control therapy. Studies have shown that AVN ablation and permanent pacing can improve cardiac function, quality of life, and clinical symptoms in patients compared with drug therapy alone.9, 10 However, right ventricular apical pacing after AVN ablation may lead to pacing‐induced cardiomyopathy and/or worsening of HF in some patients.11 Compared with right ventricular pacing (RVP), biventricular pacing (BVP) can better maintain cardiac function.12, 13, 14 However, in patients whose QRS duration is <130 ms, BVP may cause dysynchrony of cardiac contraction after AVN ablation.15 Recent studies have found that His‐Purkinje system pacing (HPSP), including His bundle pacing (HBP) and left bundle‐branch pacing, can provide physiologic ventricular activation, avoiding ventricular dysynchrony and improving cardiac function16, 17 along with greater control of rate and rhythm of AF.18, 19, 20, 21 It is effective in patients with narrow QRS and maintains normal His‐Purkinje activation.
In this study, we analyzed the follow‐up data of patients with persistent AF and HF who received ICD implantation in our center to evaluate the outcome, feasibility, and safety of AVN ablation combined with HPSP.
Methods
This study was a single‐center, retrospective, case–control study of persistent AF patients with HF who had indications for ICD implantation and AVN ablation. The study protocol was approved by the institutional review board of the first affiliated hospital of Wenzhou Medical University. All patients completed written informed consent. The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
Eighty‐six consecutive patients who met the inclusion criteria between January 2010 and March 2018 were recruited into the study. The study was carried out in the first affiliated hospital of Wenzhou Medical University. The following inclusion criteria were used: (1) patients met ICD implantation indications as per the 2008 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines1; (2) patients had a narrow QRS duration (≤130 ms, except right bundle‐branch block); (3) patients had symptomatic HF and long‐lasting persistent or permanent AF even though their heart rate was controlled (ventricular rate ≤100 beats/min [bpm] over the 24‐hour recording period) with pharmacologic treatment; (4) patients were at least 18 years old and not pregnant. Patients were excluded if they had (1) severe mitral or aortic valve regurgitation, (2) congenital heart disease requiring cardiac surgery, or (3) severe chronic obstructive pulmonary disease. AVN ablation combined with HPSP was carried out if the patient consented to this strategy.
Procedural Details
His bundle pacing
Previous studies have described the HBP procedure in detail.19, 21 Briefly, the delivery sheath (model C304 or C315; Medtronic) was inserted via the left axillary vein into the His bundle region in the atrioventricular septum. The Medtronic 3830 lead (SelectSecure; Medtronic) was then advanced through the sheath for unipolar mapping and pacing. When the pacing lead could not achieve HBP, a multielectrode mapping catheter was used to identify the His bundle potential, and then the SelectSecure lead was used for HBP. The SelectSecure pacing lead was fixed when pacing parameters were acceptable.
Left bundle‐branch pacing
The procedural steps for delivering left bundle‐branch pacing (LBBP) were recently reported in several studies.22, 23, 24 We attempted LBBP in patients with predefined inadequate HBP parameters, which remained consistent throughout the study. HBP was not accepted if the His capture threshold was >2 V at 0.5 ms or if there was a rise in His capture threshold of >1 V following AVN ablation. The Medtronic 3830 lead (SelectSecure) was used for mapping supported by a C315 His delivery catheter (Medtronic), and the lead connected to an electrophysiology recording system (GE CardioLab EP Recording System 2000) during mapping. The fluoroscopic location of the HBP lead was set as a marker for the implantation of the LBBP lead. The LBB lead was positioned ≈1 cm distal to the HBP site in the direction of the right ventricular apex. During intrinsic rhythm or LBB block correction by selective HBP, the LBB potential can be recorded. The paced QRS morphology usually showed a “w” shape with a notch at the nadir of the QRS in lead V1 at the initial site in the right ventricular septum before fixation. LBBP was achieved by screwing the lead deeply into the interventricular septum; the paced QRS showed a right bundle‐branch block morphology. The criteria for successful LBBP are described in detail in our recent article.23
AVN ablation
The technique for AVN ablation has been described previously.19, 21 We performed AVN ablation at the time of HBP lead implantation. A quadripolar 7‐Fr 4‐mm tip ablation catheter (Therapy Cool Flex, St. Jude Medical Inc or Celsius, Biosense Webster Inc) was used to perform AV node ablation. An 8.5‐Fr nondeflectable sheath (SR0 or SL1; St Jude Medical) or a deflectable sheath (Agilis; Abbott Electrophysiology) was inserted through the femoral vein to the atrioventricular junction region (including the AVN and nearby proximal His bundle). The HBP lead served as a marker, and we aimed to perform ablation at least 8 mm proximal to the His pacing lead tip. Ablation was performed until complete heart block was achieved. The full criteria for successful AVN ablation have been described previously.21
Following AVN ablation, the lower rate for permanent HBP was initially set at 80 bpm; the rate was decreased to 70 bpm at 1 to 3 months after the procedure. Where a backup right or left ventricular (LV) lead was implanted, it was programmed to pace with a long delay so that pacing would occur only if there was loss of capture from the His lead.
Back‐up lead
We implanted a back‐up LV lead (positioned via the coronary sinus) in 31 patients. This allowed us the option of delivering BVP if there was a threshold rise on the His lead. In these patients, the His lead/LBBP lead was connected to the atrial port. We did not have any episodes of significant threshold rise; therefore, later in the study, we changed the protocol so that a back‐up LV lead was not required. In 8 patients we implanted an atrial lead to allow the option of establishing sinus rhythm during follow‐up. A dual‐chamber ICD was implanted in 13 patients, with the His lead connected to the atrial port.
BVP was recommended for AF patients with cardiomyopathy and HF after AVN ablation. In this study, some patients chose RVP as backup pacing given their economic situations.
ICD programming
The ICD was programmed in the following way. For primary‐prevention patients, the ventricular fibrillation zone was set at a heart rate >240 bpm with a duration of 30 intervals, the VT2 zone was programmed as >187 bpm for 30 intervals, and the VT monitor zone was also programmed. For secondary prevention, the ventricular fibrillation zone was set at a heart rate of >240 bpm for 30 intervals. The VT2 zone was set at a heart rate of >187 bpm or 10 to 20 bpm lower than the VT rate for 30 intervals; the VT zone was set to deliver therapy at 10 to 20 bpm lower than the VT rate or as a monitor zone. Supraventricular tachycardia discriminators, far‐field morphology, onset, and stability were turned on. Individual parameters varied slightly according to each patient's characteristics.
Definitions
HPSP was defined as HBP or LBBP. Appropriate ICD shock was defined as therapy delivered for monomorphic or polymorphic VT or for ventricular fibrillation. IS was defined as shock delivered by the device for other reasons, such as arrhythmic causes (eg, AF or flutter with rapid ventricular rates), nonsustained VT, or nonarrhythmic causes (eg, due to ICD lead noise, myopotentials, electromechanical interference, T wave, or other oversensing). A shock episode was defined as an episode during which ≥1 appropriate shocks were delivered until the episode was over; a separate episode triggered by the same type of arrhythmia occurring within 5 minutes of a previous therapy was classified as the same episode.3 Other adverse events were also collected during the follow‐up period, including hospitalization due to HF or death.
Follow‐Up
Baseline and follow‐up data were collected from the time of the first ICD implantation and at every follow‐up visit until March 2019. Postimplantation clinical assessment data including echocardiography, pacing threshold, and sensed R‐wave amplitude were collected at standard time periods (1, 3, and 6 months and annually). ICD interrogation was performed in patients at each follow‐up visit to monitor arrhythmia episodes and therapies. The intracardiac electrograms stored by the device from the arrhythmia episodes and 12‐lead ECGs (if available) were used to distinguish appropriate shocks from ISs. All device interrogations were independently reviewed by an experienced clinician. Only treated arrhythmias were adjudicated. Lead‐related complications including infection, dislodgement, loss of capture, and early battery depletion were also tracked during follow‐up.
Statistical Analyses
Continuous variables were expressed as mean±SD or median (interquartile range). Independent 2‐sample t tests were performed to compare the differences between the 2 groups, and paired t tests were used to compare the differences between 2 time points within the same group during follow‐up if they were normally distributed. Otherwise, Mann‐Whitney U tests for between‐group comparisons or Wilcoxon signed rank tests for within‐group comparisons were utilized to assess the above‐mentioned differences. The nonadjusted Kaplan–Meier life‐table method was used to graphically show the time to first event and calculate the cumulative event rates for each group and within each group by risk factors. The results were compared using the log‐rank statistic. ANCOVA was used to compare the data (echocardiography, pacing threshold, sensed R‐wave amplitude) that were collected at baseline and subsequent follow‐up time points. The categorical data were described as numbers (percentages), and the χ2 test or Fisher exact test was used to examine the above‐mentioned differences. Data management and analysis were applied with SPSS v23.0 (IBM Corp). All tests were 2‐sided, and P≤0.05 was considered statistically significant.
Results
Patient Characteristics and Implantation Results
Of the 86 enrolled patients, 31 did not consent to AVN ablation and HBP; therefore, they underwent ICD implantation and continued medical therapy for rate control. In total, 55 patients consented to HPSP combined with AVN ablation. The success rate of HPSP combined with AVN ablation was 94.5% (52/55). One patient failed AVN ablation and received a single‐chamber ICD and rate‐control medication. In 2 of the 55 patients, permanent conduction system pacing was not delivered because of high His pacing thresholds; these patients received BVP. Overall, 39 patients received HPSP with implantation of a cardiac resynchronization therapy defibrillator device. A dual‐chamber ICD was implanted in the remaining 13 of 52 patients. Of the 52 patients, HBP was achieved in 44 patients and LBBP was performed in the remaining 8 patients. Group 1 was defined as 31 patients with ICD implantation and drug therapy; group 2 was defined as 52 patients with AVN ablation and permanent HPSP (Figure 1).
Figure 1.
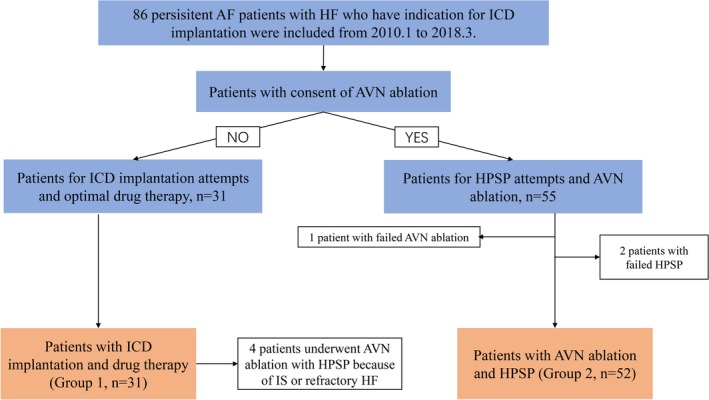
Schematic summary of study and patient flow. AF indicates atrial fibrillation; AVN, atrioventricular node; HF, heart failure; HPSP, His‐Purkinje conduction system pacing; ICD, implantable cardioverter‐defibrillator; IS, inappropriate shock.
The baseline characteristics of groups 1 and 2 are summarized in Table 1. The mean age of the study population was 67.75±9.98 years, and 64 of 86 participants (74.4%) were male. The average baseline ventricular rate was 84.23±12.01 bpm in group 1 and 88.90±16.85 bpm in group 2. Baseline characteristics were similar in the 2 groups (Table 1) except for the following: group 1 had a higher percentage of patients with a secondary prevention indication for ICD implantation compared with group 2 (51.6% versus 19.2%, respectively). In addition the incidence of ischemic cardiomyopathy was higher in group 2, whereas baseline LV ejection fraction (LVEF) was higher in group 1 compared with group 2 (42.77±15.97% versus 35.09±11.65%, P<0.001). The median follow‐up period was 30.5 months.
Table 1.
Baseline Patient Characteristics
| Group 1 (n=31) | Group 2 (n=52) | P Value | |
|---|---|---|---|
| Sex, male | 25 (80.6) | 37 (71.2) | 0.336 |
| Age, y | 68.25±8.43 | 67.60±10.85 | 0.761 |
| Diabetes mellitus | 8 (25.8) | 20 (38.5) | 0.238 |
| Hypertension | 20 (64.5) | 38 (73.1) | 0.411 |
| Single‐chamber ICD | 27 (87.1) | 0 (0) | <0.001 |
| Dual‐chamber ICD | 4 (12.9) | 13 (25.0) | 0.187 |
| CRTD | 0 | 39 (75.0) | <0.001 |
| Primary prevention | 15 (48.4) | 42 (80.8) | <0.001 |
| Secondary prevention | 16 (51.6) | 10 (19.2) | <0.001 |
| VT/VF | 16 (51.6) | 10 (19.2) | <0.001 |
| DCM | 14 (45.2) | 28 (53.8) | 0.444 |
| HCM | 3 (9.7) | 6 (11.5) | 0.792 |
| ICM | 2 (6.1) | 13 (25.0) | 0.026 |
| Average heart rate, beats/min | 84.23±12.01 | 88.90±16.85 | 0.095 |
| QRS duration | 95.81±24.03 | 96.40±17.05 | 0.193 |
| LVEF | 42.77±15.97 | 35.09±11.65 | <0.001 |
| LVEF ≤35% | 17 (54.8) | 42 (80.8) | 0.012 |
| NYHA class | |||
| II | 17 (54.8) | 18 (34.6) | 0.071 |
| III | 11 (35.5) | 29 (55.8) | 0.074 |
| IV | 3 (9.7) | 5 (9.6) | 0.993 |
Data are shown as mean±SD or n (%). CRTD indicates cardiac resynchronization therapy defibrillator; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter‐defibrillator; ICM, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; VF, ventricular fibrillation; VT, ventricular tachycardia.
Incidence of ISs
ICD shocks were documented in 13 patients during follow‐up (9 patients in group 1 and 4 in group 2). Of a total of 40 ICD shock episodes (Table 2), 29 were appropriate and the other 11 were ISs.
Table 2.
Factors Responsible for ICD Shock Episodes
| Shock Type | Total, n | Shock Episodes, n | P Value | |
|---|---|---|---|---|
| Group 1 | Group 2 | |||
| Appropriate shock | 29 | 24 | 5 | 0.153 |
| IS | 11 | 11 | 0 | <0.001 |
| AF | 10 | 10 | 0 | <0.001 |
| Abnormal sensing | 1 | 1 | 0 | 0.192 |
This table analyzes shock episodes from the standpoint of shock rather than by patient; a given patient could receive ≥1 IS (from any or all of the subcategories) and/or ≥1 appropriate shock. AF indicates atrial fibrillation; ICD, implantable cardioverter‐defibrillator; IS, inappropriate shock.
All ISs occurred in group 1 (the non‐AVN ablation group). AF with a rapid ventricular rate represented the most common cause for IS; 3 of 4 patients had 10 IS episodes caused by AF, and abnormal sensing led to 1 IS episode in the remaining patient. Two patients experienced >1 IS episode, and 3 had IS within in 1 year of ICD implantation. After 5 years of follow‐up, patients in group 1 (medical therapy group) had a 15.6% likelihood of having experienced ≥1 IS. No patient in group 2 (AVN ablation group) was observed to suffer from an IS (Figure 2). The incidence of IS was significantly higher in group 1 than in group 2 (15.6% versus 0%, P<0.001).
Figure 2.
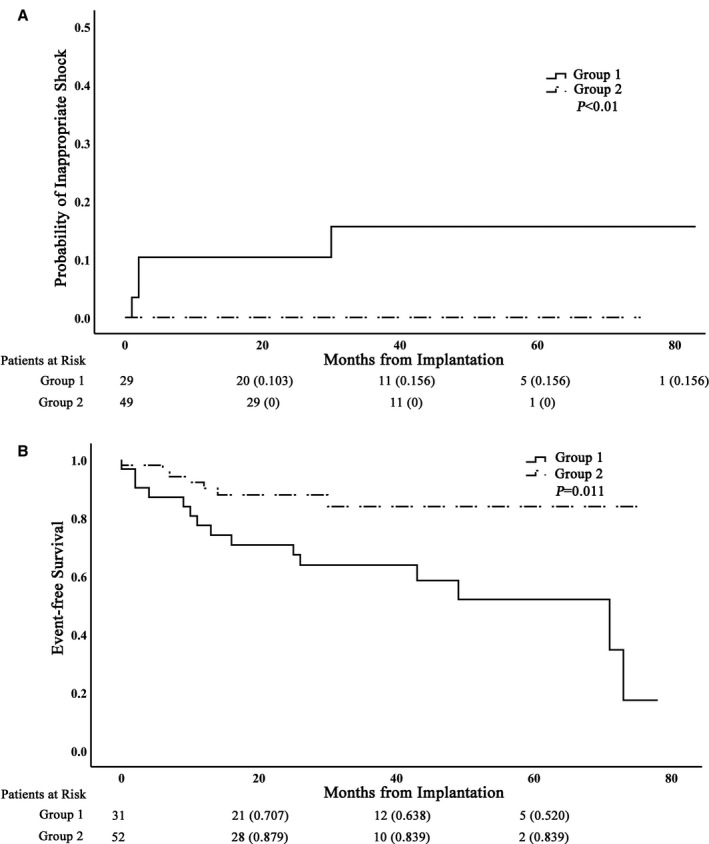
Kaplan–Meier curves showing probability of inappropriate shocks and event‐free survival. A, The cumulative proportion of patients who experienced a first inappropriate therapy is plotted against time. B, Kaplan–Meier survival curves for adverse outcomes (death or heart failure hospitalization). The number of patients at risk at a given time point of follow‐up is indicated below the x‐axis.
We proceeded to perform AVN ablation and upgrade of ICD to HPSP in 2 patients with IS. After ablation, no further IS events were observed during follow‐up in these patients.
Clinical Outcomes of Enrolled Patients
Drug therapy in the 2 groups is summarized in Table 3. No difference in the baseline cardiovascular pharmacotherapy was noted between groups 1 and 2. Change in medication use in group 1 was minimal after ICD implantation. Meanwhile, the number of patients taking amiodarone (25.0% versus 0%, P<0.001) and digoxin (46.2% versus 25.0%, P=0.024) decreased after permanent HPSP with AVN ablation in group 2. There was a significantly greater reduction in amiodarone use in group 2 compared with group 1 (P<0.001).
Table 3.
Utilization of Drugs in the Study Groups
| Group 1 (n=31) | Group 2 (n=52) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | After ICD Implantation | P Value | Baseline | After AVN Ablation | P Value | ||
| β‐Blocker | 26 (83.9) | 20 (64.5) | 0.082 | 42 (80.8) | 40 (76.9) | 0.631 | 0.564 |
| Amiodarone | 13 (41.9) | 9 (29.0) | 0.288 | 13 (25.0) | 0 (0) | <0.001 | <0.001 |
| Digoxin | 19 (61.3) | 13 (41.9) | 0.127 | 24 (46.2) | 13 (25.0) | 0.024 | 0.639 |
| ACEI or ARB | 23 (74.2) | 19 (61.3) | 0.227 | 44 (84.6) | 38 (73.1) | 0.150 | 0.907 |
| Diuretic | 25 (80.6) | 24 (77.4) | 0.755 | 47 (90.4) | 44 (84.6) | 0.374 | 0.943 |
| Calcium channel blocker | 5 (16.1) | 6 (19.4) | 0.740 | 5 (9.6) | 1 (1.9) | 0.093 | 0.129 |
| Statin | 17 (54.8) | 11 (35.5) | 0.126 | 35 (67.3) | 33 (63.5) | 0.680 | 0.409 |
Data are shown as n (%) except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVN, atrioventricular node; ICD, implantable cardioverter‐defibrillator.
In group 2, there was a significant decrease in LV end‐systolic volume (LVESV) and an increase in LVEF compared with baseline over the follow‐up period (Figure 3). The mean LVEF at baseline was 34.80±11.23%, and at last follow‐up, LVEF improved to 49.44±14.90% (P<0.01). Mean LVESV decreased from 122.69±65.18 mL at baseline to 83.68±62.53 mL (P<0.01).
Figure 3.
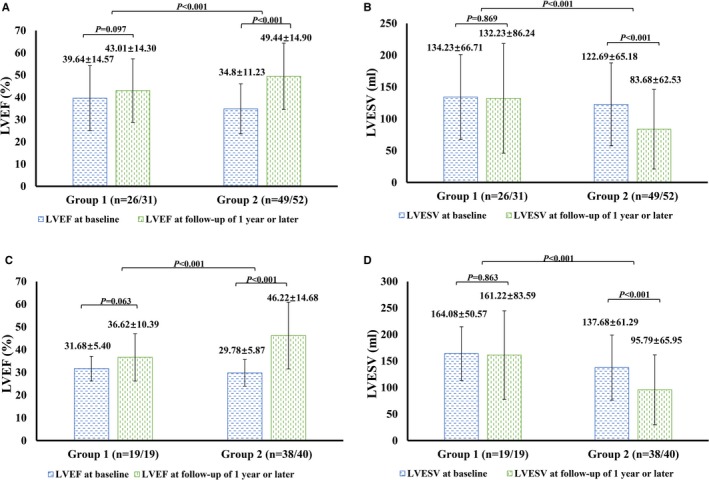
Paired LVEF/LVESV at baseline and during follow‐up. A and B, LVEF (A) and LVESV (B) of all patients in groups 1 and 2 at baseline and during follow‐up. LVEF (C) and LVESV (D) of patients with baseline ejection fraction ≤40% in groups 1 and 2 at baseline and during follow‐up. LVEF indicates left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume.
In group 1, there was a trend toward improvement in echocardiographic parameters during follow‐up, but this did not reach statistical significance (LVEF: 39.64±14.57% versus 43.01±14.30%, P=0.097; LVESV: 134.23±66.71 versus 122.69±65.18 mL, P=0.869). Group 2 had a greater increase in the LVEF compared with baseline than group 1 (P<0.01 versus group 1); however, the reduction in LVESV in group 2 was greater than in group 1 (P<0.01).
In patients with baseline LVEF ≤40%, similar improvement in LVEF and LVESV was observed in both groups (Figure 3C and 3D). Even in the 14 patients with ischemic cardiomyopathy in group 2, significant improvement in LVEF (34.15±5.96% versus 42.16±12.14%, P<0.01) was observed during follow‐up.
Pacing Parameters During HPSP and Follow‐Up
The electrical parameters recorded from implanted devices are summarized in chronological order in Figure 4. The capture threshold of HPSP increased slightly at 3 to 6 months of follow‐up and remained stable at 1‐year follow‐up. The mean His bundle capture threshold at 1 year was 1.26±0.73V/0.5 ms, and the mean left bundle capture threshold was 0.79±0.189V/0.5 ms. Furthermore, the sensed R‐wave amplitude remained stable during the study period (Figure 4B). There were no major complications during device implantation.
Figure 4.
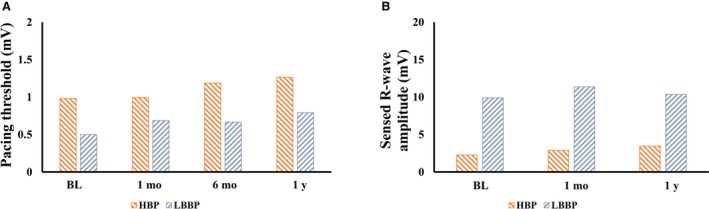
Electrical parameters of His‐Purkinje conduction system pacing at implant (BL) and during the follow‐up period (1 mo, 6 mo, and 1 y). A, Pacing threshold. B, Sensed R‐wave amplitude. BL indicates baseline; HBP, His bundle pacing; LBBP, left bundle‐branch pacing.
HF Hospitalizations or Death
During follow‐up, 8 patients in group 1 (25.8%) died. Two deaths were due to uremia and multiple organ failure; the cause of death in the remaining 6 patients was unknown. There were 6 deaths in group 2 (9.7%); 1 patient experienced sudden cardiac death, 2 patients died from multiple organ failure, 2 patients died from cerebral hemorrhage, and the remaining patients died from respiratory failure. No safety concerns were identified with the AVN ablation and pacing group. Fifteen patients had ≥1 episode of HF‐related hospitalization during follow‐up (9 patients in group 1, and 6 patients in group 2). The incidence of adverse events (HF hospitalization or death) was higher in group 1 compared with group 2 (P=0.01; Figure 2B).
Discussion
This study had several major findings. First, AVN ablation plus HPSP was feasible and safe in a high percentage of patients with persistent AF, HF, and an ICD indication. Second, there was a significant decrease in the use of digoxin and amiodarone in patients with AVN ablation. Third, AVN ablation combined with HPSP was associated with a significant reduction in ISs and the cumulative risk for death or HF hospitalization compared with the medical therapy group.
AVN Ablation and HPSP
Despite underlying cardiomyopathy and HF with an indication for ICD, HPSP plus AVN ablation was safe and feasible in 52 of 55 (94.5%) patients attempted. The pacing parameters remained stable during follow‐up. Prior studies18, 19, 25 have shown the feasibility of AVN ablation and HBP in patients with preserved and reduced LV function in 66% to 95% of patients. In this study, despite significant LV dysfunction, dilated atria, HF, and an indication for ICD, AVN ablation and HPSP was feasible in a high percentage of patients. In our earlier study,19 HBP was feasible in 80.8% (42 of 52) of patients but was unsuccessful in 20% of patients because of high His capture thresholds. In the current study, with the introduction of LBBP, we were able to achieve a 94.5% success rate for HPSP in this population with advanced heart disease.
Change in Cardiovascular Pharmacotherapy
In this study, the use of amiodarone and digoxin for rate control decreased significantly during follow‐up in group 2. Amiodarone, which is widely used in the management of ventricular and atrial tachyarrhythmias, is associated with significant side effects including hepatotoxicity, pulmonary fibrosis, and deterioration of cardiac function.26 Adelstein et al26 reported that in patients with sustained VT and LBB block upgraded from conventional ICDs to cardiac resynchronization therapy defibrillators, concomitant use of amiodarone was associated with less QRS narrowing, less LVEF improvement, and greater risk of death. The significant decrease in the use of amiodarone in group 2 was due to improved rate and rhythm control after AVN. AVN ablation combined with HPSP can improve rate regularization, cardiac synchronization, and cardiac function, which may further reduce the probability of malignant arrhythmias. This improvement in cardiac function may have also contributed to the significant decrease in the use of cardiovascular drugs in group 2.
Among the 4 patients in group 1 who experienced ISs, there was no significant difference in their medication before and after ICD implantation. Consequently, medication change after device implantation does not appear to have been a factor in the ISs in these patients.
Clinical and Echocardiographic Outcomes
Previous studies have shown that AVN ablation combined with permanent pacing benefits patients with AF and low ejection fraction.18, 19 Huang et al19 reported that LV function improved in patients with AF and HF following AVN ablation and that this was accompanied by a mean reduction in LV end‐diastolic diameter compared with baseline. A meta‐analysis10 including 21 studies found that AVN ablation and permanent pacing may improve exercise duration, ventricular function, quality of life, and symptoms compared with medical therapy alone.
RVP and BVP are commonly used in conjunction with AVN ablation. RVP after ablation is not ideal because it produces ventricular synchrony, which is known to be harmful in patients with preexisting LV impairment and may cause RVP‐induced cardiomyopathy.11 BVP is a better alternative to RVP but prolongs ventricular activation time in patients with a narrow QRS duration,15 which may explain why BVP is harmful when it is delivered to patients with a narrow QRS duration and LV impairment.27 Consequently, even BVP may not be the optimal pacing method for patients with HF for whom AVN ablation is being considered.
HBP and LBBP have recently developed rapidly as an alternative pacing option with the potential advantage that it recruits the intrinsic conduction system and thus can deliver physiologic ventricular activation. Previous observational studies have reported the feasibility, efficacy, and safety of these pacing techniques.16, 17, 28, 29 However, no previous studies have investigated the effect of AVN ablation combined with conduction system pacing in patients with HF and an ICD indication.
In our study, 52 patients received combined AVN ablation and conduction system pacing. We observed significant improvements in clinical outcomes compared with the medical therapy group. This included fewer HF hospitalizations, reduced medication use, improved New York Heart Association (NYHA) classification, and improved echocardiographic measurements. LVEF and LVESV were observed after a median follow‐up period of 24 months. One case example is shown in Figure 5.
Figure 5.
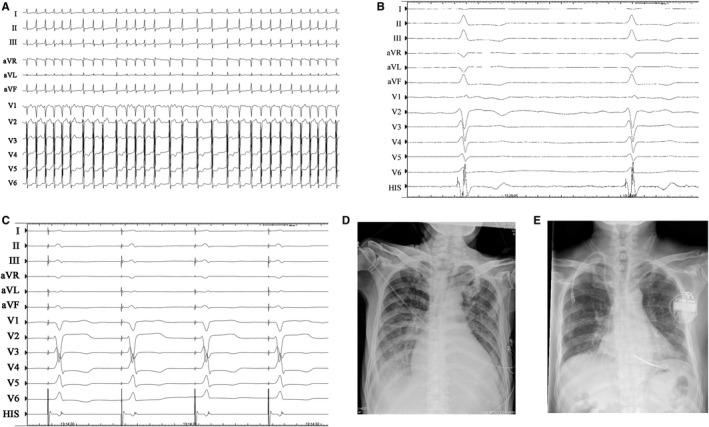
Case example of atrioventricular node (AVN) ablation combined with His bundle pacing (HBP) in a patient with persistent atrial fibrillation with heart failure and cardiac resynchronization therapy defibrillator implantation. A, Native ECG. B, Electrogram after ablation. C, His bundle pacing. Radiograph before (D) and 2 years after (E) AVN ablation and HBP.
We also observed a lower incidence of HF hospitalization or death in the AVN ablation and HPSP group during the median follow‐up of 30.5 months. Our findings are encouraging and suggest that AVN ablation combined with HPSP may be beneficial in patients with AF, HF, and an indication for ICD. Possible mechanisms for these improvements include better ventricular rate control, reduced medication use, and fewer ISs. However, our study was nonrandomized, and thus these findings need to be confirmed in randomized controlled trials.
Inappropriate Shocks
In this study, we observed significantly fewer ISs in the AVN ablation group compared with the medical therapy group. The incidence of ISs was relatively high in our medical therapy group: after a mean follow‐up duration of 5 years, the probability of IS was 15.6%. This is higher than the IS rate observed in the MADIT‐II (Multicenter Automatic Defibrillator Implantation Trial II),3 PAIN‐FREE (Pacing Fast VT Reduces Shock ThErapies),30 and SCD‐HEFT (Sudden Cardiac Death in Heart Failure Trial)31 trials. The percentages of patients with AF in these trials were much lower than in our population; this difference is likely to have contributed to the higher IS rate in our study. The longer follow‐up period and smaller sample size may also have contributed.
AF is a major cause of IS in patients with ICD.3 Consequently, rate and/or rhythm control of AF is crucial for reducing IS. Radiofrequency ablation has been reported to reduce the incidence of IS in patients with AF,32 but despite ablation, there is still a high probability of AF recurrence. A meta‐analysis33 revealed that only 54.1% of paroxysmal AF patients and 41.8% of persistent AF patients maintained sinus rhythm during long‐term follow‐up after radiofrequency ablation for AF. Therefore, having a mechanism for ensuring adequate ventricular rate control remains important even if AF ablation is performed.
AVN ablation combined with permanent pacing is considered an important management option for patients with recurrent AF with HF.18, 19 To the best of our knowledge, the present study is the first to report the impact of AVN ablation combined with physiologic pacing on IS in ICD recipients.
Our study suggests that AVN ablation combined with physiologic pacing is a highly effective method for preventing inappropriate therapies related to rapidly conducted AF in patients with HF and recurrent AF. None of the patients in the AVN ablation group received inappropriate therapies. In addition, 2 patients in group 1 who experienced ISs due to rapidly conducted AF subsequently underwent AVN ablation. Following AVN ablation, there were no further instances of inappropriate therapies for these patients (Figure 6).
Figure 6.
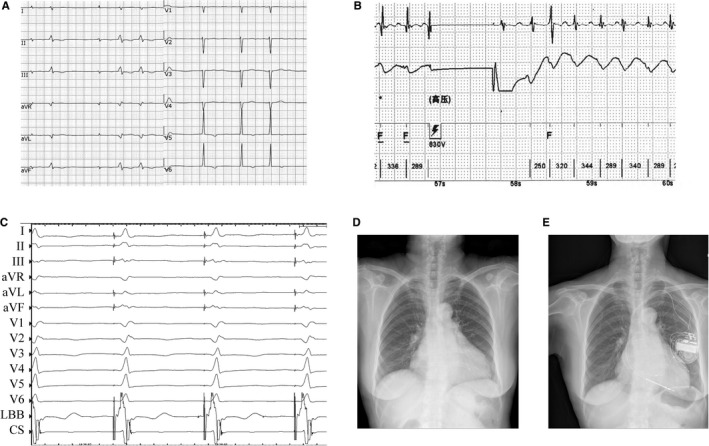
Case example of atrioventricular node (AVN) ablation combined with left bundle‐branch pacing (LBBP) in a persistent atrial fibrillation (AF) patient with heart failure and previous inappropriate implantable cardioverter‐defibrillator shocks. A, Native ECG. B, Inappropriate shock caused by AF. C, LBBP. Radiograph before (D) and 6 months after (E) AVN ablation and LBBP.
Combining conduction system pacing with AVN ablation has the potential to be more technically challenging compared with direct myocardial pacing. It is important that the conduction system is paced distal to the ablation site. We found that this procedure was technically feasible in the majority of patients in our study. Long‐term pacing parameters were satisfactory, and atrioventricular block was maintained during a median follow‐up of 24 months. Our study suggests that this approach is feasible for providing adequate ventricular rate control, preventing ISs, and maintaining physiologic ventricular activation.
Study Limitations
Our study was a nonrandomized single‐center study. Treatment was allocated according to patient choice; therefore, systematic baseline differences between groups may exist that would bias the estimates of treatment effects. Our study demonstrates that HPSP combined with AVN ablation is technically feasible in this population of patients and appears to be safe, with stable pacing parameters during medium‐term follow‐up. We observed reductions in ISs and improvements in LV function, but these findings should be interpreted with caution and need to be confirmed with adequately powered randomized controlled trials. The programmed ICD settings were not uniform in all patients, and this may also have influenced our findings. We observed a reduction in medication use at follow‐up compared with baseline in group 1. The reduction in rate control and HF medication may have contributed to the clinical events observed in this group. The reason for the reduction was intolerance to medication, which is a limitation of drug therapy as a means for obtaining rate control in patients with AF. However, in the 4 patients in group 1 who experienced ISs, we did not observe a decrease in medication use before or after ICD implantation. This suggests that in our study, reduced medication use after ICD implantation was not the driver for inappropriate therapies.
The cause of death could not be verified in all patients in our study. The proportions of primary prevention and secondary prevention indications for ICD therapy were different between the 2 groups, and it is unclear if this had an impact on patient deaths.
Conclusions
Our study suggests that AVN ablation combined with conduction system pacing is a safe and effective method for providing ventricular rate control and maintaining physiologic ventricular activation in patients with persistent AF, LV impairment, and an ICD indication. We found that this procedure was technically feasible in the majority of patients in our study and that pacing parameters remained stable during follow‐up. Following treatment, we observed significant improvements in echocardiographic parameters and NYHA class.
AVN ablation combined with conduction system pacing appears to be a highly effective method of preventing inappropriate therapies caused by rapidly conducted AF while avoiding the potential detrimental effects of nonphysiologic ventricular activation.
Sources of Funding
The study was funded by the Key Research and Development Program of Zhejiang (2019C03012) and the Major Project of the Science and Technology of Wenzhou (ZS2017010)
Disclosures
None.
Acknowledgments
We wish to thank Nadine M. S.M. Ali, National Heart and Lung Institute, Imperial College, London, UK, for help reviewing and revising the article.
(J Am Heart Assoc. 2019;8:e014253 DOI: 10.1161/JAHA.119.014253.)
Contributor Information
Lan Su, Email: 2512057600@qq.com.
Weijian Huang, Email: weijianhuang69@126.com.
References
- 1. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice G, American Association for Thoracic S and Society of Thoracic S . ACC/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. [DOI] [PubMed] [Google Scholar]
- 2. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, Schuger C, Steinberg JS, Higgins SL, Wilber DJ, Klein H, Andrews ML, Hall WJ, Moss AJ, Investigators MI. Inappropriate implantable cardioverter‐defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. [DOI] [PubMed] [Google Scholar]
- 4. Rahmawati A, Chishaki A, Ohkusa T, Sawatari H, Tsuchihashi‐Makaya M, Ohtsuka Y, Nakai M, Miyazono M, Hashiguchi N, Sakurada H, Takemoto M, Mukai Y, Inoue S, Sunagawa K, Chishaki H. Influence of primary and secondary prevention indications on anxiety about the implantable cardioverter‐defibrillator. J Arrhythm. 2016;32:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein RC, Raitt MH, Wilkoff BL, Beckman KJ, Coromilas J, Wyse DG, Friedman PL, Martins JB, Epstein AE, Hallstrom AP, Ledingham RB, Belco KM, Greene HL, Investigators A. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial. J Cardiovasc Electrophysiol. 2003;14:940–948. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ. Background, outcome, and clinical implications of the Multicenter Automatic Defibrillator Implantation Trial (MADIT). Am J Cardiol. 1997;80:28F–32F. [DOI] [PubMed] [Google Scholar]
- 7. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. [DOI] [PubMed] [Google Scholar]
- 9. Marshall HJ, Harris ZI, Griffith MJ, Holder RL, Gammage MD. Prospective randomized study of ablation and pacing versus medical therapy for paroxysmal atrial fibrillation: effects of pacing mode and mode‐switch algorithm. Circulation. 1999;99:1587–1592. [DOI] [PubMed] [Google Scholar]
- 10. Wood MA, Brown‐Mahoney C, Kay GN, Ellenbogen KA. Clinical outcomes after ablation and pacing therapy for atrial fibrillation : a meta‐analysis. Circulation. 2000;101:1138–1144. [DOI] [PubMed] [Google Scholar]
- 11. Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, Frankel DS. Incidence and predictors of right ventricular pacing‐induced cardiomyopathy. Heart Rhythm. 2014;11:1619–1625. [DOI] [PubMed] [Google Scholar]
- 12. Doshi RN, Daoud EG, Fellows C, Turk K, Duran A, Hamdan MH, Pires LA; Group PS . Left ventricular‐based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005;16:1160–5. [DOI] [PubMed] [Google Scholar]
- 13. Orlov MV, Gardin JM, Mara S, Bess RL, Gerald C, William B, Vance P, Horst F, Katerina DM. Biventricular pacing improves cardiac function and prevents further left atrial remodeling in patients with symptomatic atrial fibrillation after atrioventricular node ablation. Am Heart J. 2010;159:264–270. [DOI] [PubMed] [Google Scholar]
- 14. Brignole M, Botto G, Mont L, Iacopino S, De Marchi G, Oddone D, Luzi M, Tolosana JM, Navazio A, Menozzi C. Cardiac resynchronization therapy in patients undergoing atrioventricular junction ablation for permanent atrial fibrillation: a randomized trial. Eur Heart J. 2011;32:2420–2429. [DOI] [PubMed] [Google Scholar]
- 15. Ploux S, Eschalier R, Whinnett ZI, Lumens J, Derval N, Sacher F, Hocini M, Jais P, Dubois R, Ritter P, Haissaguerre M, Wilkoff BL, Francis DP, Bordachar P. Electrical dyssynchrony induced by biventricular pacing: implications for patient selection and therapy improvement. Heart Rhythm. 2015;12:782–791. [DOI] [PubMed] [Google Scholar]
- 16. Shan P, Su L, Chen X, Xu L, Ni X, Huang W. Direct his‐bundle pacing improved left ventricular function and remodelling in a biventricular pacing nonresponder. Can J Cardiol. 2016;32:1577.e1–1577.e4. [DOI] [PubMed] [Google Scholar]
- 17. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Mao G, Vijayaraman P, Ellenbogen KA. Long‐term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart. 2019;105:137–143. [DOI] [PubMed] [Google Scholar]
- 18. Vijayaraman P, Subzposh FA, Naperkowski A. Atrioventricular node ablation and His bundle pacing. Europace. 2017;19:iv10‐iv16. [DOI] [PubMed] [Google Scholar]
- 19. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Ellenbogen KA. Benefits of permanent his bundle pacing combined with atrioventricular node ablation in atrial fibrillation patients with heart failure with both preserved and reduced left ventricular ejection fraction. J Am Heart Assoc. 2017;6:e005309 DOI: 10.1161/JAHA.116.005309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu S, Su L, Wang S, Vijayaraman P, Ellenbogen KA, Huang W. Peri‐left bundle branch pacing in a patient with right ventricular pacing‐induced cardiomyopathy and atrioventricular infra‐Hisian block. Europace. 2019;21:1038. [DOI] [PubMed] [Google Scholar]
- 21. Huang W, Su L, Wu S. Pacing treatment of atrial fibrillation patients with heart failure: his bundle pacing combined with atrioventricular node ablation. Card Electrophysiol Clin. 2018;10:519–535. [DOI] [PubMed] [Google Scholar]
- 22. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Ellenbogen KA. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1736.e1–1736.e3. [DOI] [PubMed] [Google Scholar]
- 23. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner's guide to permanent left bundle branch pacing. Heart Rhythm. 2019. DOI: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 24. Chen X, Wu S, Su L, Su Y, Huang W. The characteristics of the electrocardiogram and the intracardiac electrogram in left bundle branch pacing. J Cardiovasc Electrophysiol. 2019;30:1096–1101. [DOI] [PubMed] [Google Scholar]
- 25. Yarlagadda B, Turagam MK, Dar T, Janagam P, Veerapaneni V, Atkins D, Bommana S, Friedman P, Deshmukh AJ, Doshi R, Reddy VY, Dukkipati SR, Natale A, Lakkireddy D. Safety and feasibility of leadless pacemaker in patients undergoing atrioventricular node ablation for atrial fibrillation. Heart Rhythm. 2018;15:994–1000. [DOI] [PubMed] [Google Scholar]
- 26. Adelstein EC, Althouse AD, Davis L, Schwartzman D, Bazaz R, Jain S, Wang N, Saba S. Amiodarone is associated with adverse outcomes in patients with sustained ventricular arrhythmias upgraded to cardiac resynchronization therapy‐defibrillators. J Cardiovasc Electrophysiol. 2019;30:348–356. [DOI] [PubMed] [Google Scholar]
- 27. Frank R, Abraham WT, Singh JP, Bax JJ, Borer JS, Josep B, Kenneth D, Ian F, John G, Daniel G. Cardiac‐resynchronization therapy in heart failure with a narrow QRS complex. Circulation. 2014;370:578–579. [Google Scholar]
- 28. Shan P, Su L, Zhou X, Wu S, Xu L, Xiao F, Zhou X, Ellenbogen KA, Huang W. Beneficial effects of upgrading to His bundle pacing in chronically paced patients with left ventricular ejection fraction <50. Heart Rhythm. 2018;15:405–412. [DOI] [PubMed] [Google Scholar]
- 29. Su L, Xu L, Wu SJ, Huang WJ. Pacing and sensing optimization of permanent His‐bundle pacing in cardiac resynchronization therapy/implantable cardioverter defibrillators patients: value of integrated bipolar configuration. Europace. 2016;18:1399–1405. [DOI] [PubMed] [Google Scholar]
- 30. Sweeney MO, Wathen MS, Volosin K, Abdalla I, DeGroot PJ, Otterness MF, Stark AJ. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: results from the Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial. Circulation. 2005;111:2898–2905. [DOI] [PubMed] [Google Scholar]
- 31. Mark DB, Nelson CL, Anstrom KJ, Al‐Khatib SM, Tsiatis AA, Cowper PA, Clapp‐Channing NE, Davidson‐Ray L, Poole JE, Johnson G, Anderson J, Lee KL, Bardy GH; SCD‐HeFT Investigators . Cost‐effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT). Circulation. 2006;114:135–142. [DOI] [PubMed] [Google Scholar]
- 32. Mugnai G, Hunuk B, Stroker E, Ruggiero D, Coutino‐Moreno HE, Takarada K, De Regibus V, Choudhury R, Abugattas de Torres JP, Moran D, Iacopino S, Filannino P, Conte G, Sieira J, Poelaert J, Beckers S, Brugada P, de Asmundis C, Chierchia GB. Long‐term outcome of pulmonary vein isolation in patients with paroxysmal atrial fibrillation and Brugada syndrome. Europace. 2018;20:548–554. [DOI] [PubMed] [Google Scholar]
- 33. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts‐Thomson KC, Sanders P. Long‐term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004549 DOI: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]


