Abstract
Background
Data are limited on use patterns of low‐dose aspirin and its role for primary prevention of cardiovascular disease (CVD) in different racial and ethnic groups.
Methods and Results
Overall, 65 231 non‐Hispanic black and white people aged 40 to 79 years with no history of CVD enrolled from 2002 through 2009 in the SCCS (Southern Community Cohort Study). At cohort entry, the simplified Framingham 10‐year CVD risk was calculated, and data related to low‐dose aspirin use and clinical and socioeconomic covariates were collected. Race‐ and ethnicity‐specific adjusted odds ratios for characteristics of low‐dose aspirin users and hazard ratios for ischemic cardiac death according to aspirin use were calculated using multivariate logistic and Cox regression models. Black participants were less likely to take low‐dose aspirin compared with white participants, regardless of CVD risk and covariates (adjusted odds ratio: 0.79; 95% CI, 0.75–0.82). Over a median follow‐up of 11.3 years, low‐dose aspirin use was associated with a trend toward decreased risk of ischemic cardiac death in white participants (adjusted hazard ratio: 0.86; 95% CI, 0.68–1.10), especially in women (adjusted hazard ratio: 0.72; 95% CI, 0.51–1.02), but not in black participants (adjusted hazard ratio: 1.18; 95% CI, 0.98–1.40). Similar trends were observed when the analysis was restricted to high‐risk individuals aged 50 to 69 or 50 to 59 years, ages for which guidelines consider aspirin for CVD primary prevention.
Conclusions
Low‐dose aspirin use for primary prevention of CVD is lower among black than white patients. Its use might be associated with a disparate impact on ischemic cardiac death according to race and ethnicity. Although additional studies are required, these findings provide no evidence of a beneficial effect of aspirin among black patients for CVD primary prevention.
Keywords: aspirin, ethnicity, ischemic heart disease, primary prevention
Subject Categories: Primary Prevention, Race and Ethnicity, Cardiovascular Disease
Clinical Perspective
What Is New?
This study, which included 65 231 adults without known cardiovascular disease who were from the southeastern United States and followed up for 11 years, is one of the first to determine the prevalence of low‐dose aspirin use for primary prevention and its association with incident fatal ischemic heart disease in a predominantly high‐risk, low‐income, non‐Hispanic black and non‐Hispanic white population.
Low‐dose aspirin use for primary prevention of cardiovascular disease was consistently lower among black than white participants, and its use might be associated with a disparate impact on ischemic cardiac death according to race and ethnicity.
What Are the Clinical Implications?
Although additional studies are required, our findings provide no evidence of a beneficial effect of aspirin use among black patients for cardiovascular disease primary prevention.
Introduction
In 2016, the US Preventive Services Task Force (USPSTF) recommended that physicians consider initiating low‐dose aspirin for the primary prevention of cardiovascular disease (CVD) in adults aged 50 to 59 years with a high predicted risk of CVD and without elevated bleeding risk.1 The decision to initiate low‐dose aspirin in high‐risk adults aged 60 to 69 years should be an individual one, according to the USPSTF.1 Nevertheless, researchers recognized a significant evidence gap regarding recommendations for CVD primary prevention in subpopulations.2
Current evidence is insufficient to assess the balance of benefits and harms of initiating aspirin for the primary prevention of CVD in adults aged <50 or ≥70 years. Notably, no data exist on the role of aspirin therapy in different racial and ethnic groups.1 The benefit of aspirin for primary prevention has not been assessed in black Americans because major trials did not include a sufficient sample to perform subgroup analyses by race/ethnicity.3, 4 Similarly, little information is available on the patterns of low‐dose aspirin use for primary prevention of CVD by race/ethnicity.5, 6, 7, 8
The objectives of this study were to analyze the prevalence and patterns of low‐dose aspirin use for the primary prevention of CVD and to study the association between low‐dose aspirin use and CVD incidence by race/ethnicity in the SCCS (Southern Community Cohort Study). The SCCS is an ongoing, large, prospective, cohort study designed to investigate the incidence of various chronic diseases, including differential patterns by race/ethnicity and sex in a low‐income underinsured US population that is underrepresented in previous studies.9
Methods
Study Sample and Data Collection
Between 2002 and 2009, the SCCS enrolled ≈85 000 adults (approximately two thirds black Americans) who were aged 40 to 79 years and residing in 12 states in the southeastern United States. A detailed description of SCCS methods was published previously.9 Briefly, sociodemographic data, lifestyle and anthropometric characteristics, and personal medical history were ascertained at cohort enrollment through standardized computer‐assisted personal interviews for community health center participants (≈86% of participants) and a self‐administered mailed questionnaire for the general population participants (≈14% of participants).
For this study, we used data obtained from the SCCS Baseline Questionnaire (available at https://www.southerncommunitystudy.org/). Participants who reported having a prior myocardial infarction, coronary artery bypass surgery, stroke, and/or transient ischemic attack were excluded. Analyses were restricted to self‐reported black or non‐Hispanic black and non‐Hispanic white SCCS participants because too few participants in other racial/ethnic groups were available for stable statistical analysis.10, 11 SCCS participants provided written informed consent, and protocols were approved by the institutional review boards of Vanderbilt University Medical Center and Meharry Medical College. A data access request was submitted via the SCCS Online Request System, and the research proposal was approved under the request identifier 219. Data and methods used in the analysis are available from the corresponding author on reasonable request and approval by the SCCS Data and Biospecimen Use Committee.
Definition of Low‐Dose Aspirin Use and Other Variables of Interest
Information regarding low‐dose aspirin use was obtained at enrollment. Participants were asked if they used low‐dose aspirin regularly in the previous year to prevent heart disease or stroke. Low‐dose aspirin was defined as baby aspirin, half tablets of aspirin, or low‐dose aspirin itself. Regular use was defined as taking low‐dose aspirin ≥2 days/week for ≥1 month. Self‐reported medical history of peptic ulcer or concomitant use of over‐the‐counter NSAIDs was also collected.
Year of SCCS enrollment was classified in the following categories: 2002–2003, 2004–2005, 2006–2007, and 2008–2009. Participant age at enrollment (baseline interview) was classified in the following categories: 40 to 49, 50 to 59, 60 to 69, and 70 to 79 years. Annual household income was reported in categories of <$15 000, $15 000 to $24 999, $25 000 to $49 999, $50 000 to $99 999, and $100 000 or more, with the 2 highest categories combined owing to small numbers. Educational attainment was classified as less than high school (<12 years), high school completed (12 years; completed high school or General Educational Development), or higher than high school (some education beyond completion of high school including vocational school, some college or junior college, and college graduate or beyond). Health insurance coverage was classified as none or coverage by any type of health insurance including Medicaid, Medicare, private or employer insurance, military insurance, and “other” types of insurance.
Calculation of Predicted CVD Risk
The 10‐year CVD risk for each participant at enrollment was calculated by using the simplified Framingham risk equation, which includes age, diabetes mellitus, smoking, treated and untreated systolic blood pressure (SBP), and body mass index (as replacement for lipid levels), and standardized assignment of points for each component.12 As described previously, 10‐year CVD risk was stratified into 3 mutually exclusive categories: low risk (<6%), intermediate risk (6–9.9%), and high risk (≥10%).6, 13 Actual information for calculating the SBP component of the score was available only for a proportion of SCCS participants assessed at community health centers (n=9568, ≈15%). Missing SBP values were imputed using the normal values as presented in the 2008 generalized CVD calculator study and as done previously by others.12, 14 Consequently, values of 125 and 135 mm Hg of SBP were assigned respectively for those participants without or with a diagnosis of hypertension (defined as high blood pressure reported by a doctor at any time). A sensitivity analysis was performed in the subset of participants with SBP measurements by comparing the assigned overall risk category accounted with actual measured versus imputed blood pressure values. The global agreement and area under the receiver operating characteristic curve between both estimated risks were 87.0% (95% CI, 86.3–87.7%) and 0.86 (95% CI, 0.85–0.87), respectively.
Outcome Follow‐Up
Vital status and cause of death were ascertained by linkage of the cohort with the US Social Security Administration's Death Master File and the National Death Index, respectively. Death due to ischemic heart disease was defined as International Classification of Diseases, Tenth Revision (ICD‐10) codes I20–I25. Follow‐up of vital status was extended through December 31, 2016. The follow‐up duration was defined as the number of months between a participant's date of enrollment and date of death, date lost to follow‐up, or December 31, 2016, whichever occurred first.
Statistical Analysis
We computed means and standard deviations or median and interquartile range for continuous variables and counts and percentages for categorical variables. Chi‐square tests were used to test for unadjusted differences in prevalences of low‐dose aspirin use across categories of participant characteristics within low‐, intermediate‐, and high‐risk CVD groups. Multivariate logistic regression models were used for the adjusted analysis of factors associated with low‐dose aspirin use for CVD primary prevention. Covariates were selected a priori based on previous literature5, 6 or on their potential influence on drug prescription (eg, history of ulcer, concomitant use of NSAIDs).2, 15 Insurance coverage was not included in the models because of known collinearity issues with household income and education.6 Results were reported as odds ratios with 95% CIs. The full adjusted model was subsequently stratified for the study of the prevalence of low‐dose aspirin use in high‐risk participants by race/ethnicity across different categories of covariates of interest.
Ischemic cardiac death incidence rates were calculated by dividing the number of event cases by person‐time of follow‐up and were presented per 1000 person‐years stratified by age, sex, and race/ethnicity. The 95% CIs were calculated using the quadratic approximation to the Poisson log likelihood for the log‐rate parameter.16
For the analysis of the association of low‐dose aspirin use and ischemic cardiac death, the failure distributions during follow‐up of participants with and without low‐dose aspirin treatment were estimated by the Kaplan–Meier method, followed by the Cox proportional hazards regression model. Covariates were selected a priori based on their described association with ischemic heart disease (clinical plausibility) or a potential confounding effect, including 10‐year CVD risk category, age of the participant at enrollment, sex, race/ethnicity, diabetes mellitus status, and annual household income. Subpopulation analyses were performed in high‐risk participants according to the USPSTF 2016 guidelines on low‐dose aspirin use for primary prevention of CVD (grade B recommendation for the 50–59 age group; grade C recommendation for the 60–69 age group). The proportional hazards assumption was confirmed by including time‐dependent covariates and by performing the Schoenfeld residual‐based test of proportional hazards. As a sensitivity analysis, competing‐risk regression models that account for other mortality causes were run and showed similar results (data not shown).
As an additional sensitivity analysis, multiple imputation using chained equations as implemented by the command mi was performed to impute the SBP component of the Framingham risk score. Missing SBP values were filled using a truncated regression imputation method with a restricted range. The range used was the one observed in the participants with actual SBP values (ie, lower limit: 78 mm Hg; upper limit: 244 mm Hg). The following variables were included as auxiliary variables: age, sex, race/ethnicity, body mass index, diabetes mellitus status, hypertension status, antihypertensive medication use, and insurance coverage. Number of imputations was set at 50. A random‐number seed was set to ensure reproducibility of the imputed values. Estimations on the imputed data were run with the mi estimate command using similar multivariate logistic regression and Cox proportional hazards regression models, as described earlier. The estimations adjust coefficients and standard errors for the variability between imputations according to the combination rules by Rubin.17 Estimation to continue was allowed even if the estimation sample varied across imputations. Diagnostic checks of the imputation model were obtained using the vartable and dftable options of the mi estimate command.
All statistical analyses were performed using Stata v15.1 (StataCorp).
Results
General Characteristics of the Population
The flow chart of the study is shown in Figure 1. A total of 65 231 SCCS participants without prior CVD were included in this analysis. Average age at enrollment was 51.5 years (SD: 8.5). The participants were 60.1% female, 70.2% non‐Hispanic black, and 29.8% non‐Hispanic white. Approximately two thirds of the population showed high predicted 10‐year CVD risk at enrollment, whereas ≈20% and ≈13% showed intermediate and low risk, respectively. A detailed description of the sociodemographic and clinical characteristics of the population at enrollment, stratified by 10‐year CVD risk categories, is presented in Table 1.
Figure 1.
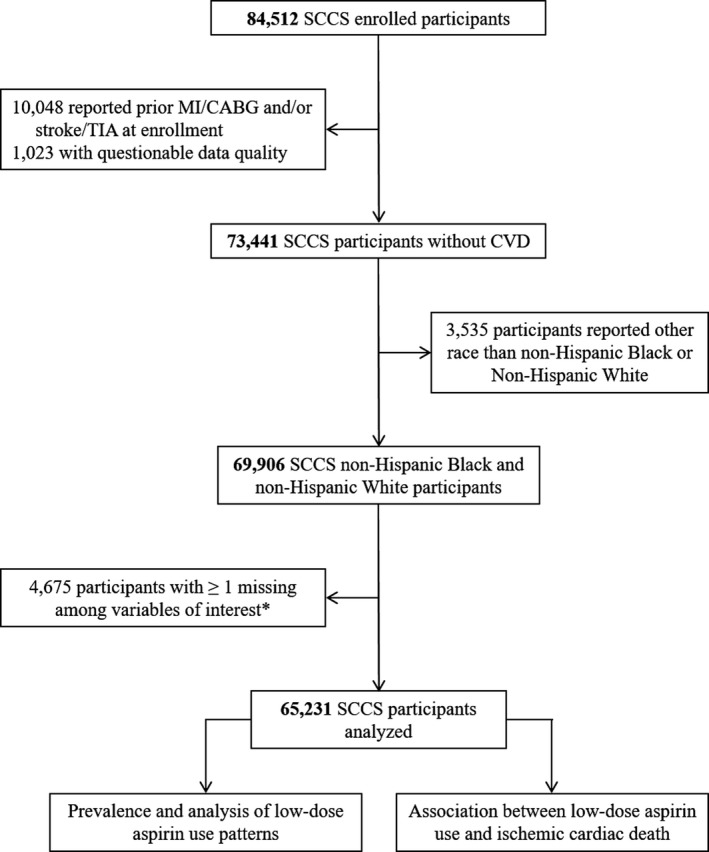
Study flow chart. A total of 65 231 SCCS (Southern Community Cohort Study) participants were included in this study. *Information of interest included vital status and the following variables obtained from the SCCS baseline questionnaire: year of SCCS enrollment, age at enrollment, sex, hypertension status, smoking status, diabetes mellitus status, body mass index, low‐dose aspirin use, race/ethnicity, household income, education, concomitant use of NSAIDs, and medical history of ulcer. CABG indicates coronary artery bypass grafting; CVD, cardiovascular disease; MI, myocardial infarction; TIA, transient ischemic attack.
Table 1.
Clinical and Sociodemographic Characteristics of the SCCS Population by 10‐Year CVD Risk Categories
| Variable | Low Risk (n=8231) | Intermediate Risk (n=12 409) | High Risk (n=44 591) | P Valuea |
|---|---|---|---|---|
| Year of SCCS enrollment | <0.001 | |||
| 2002–2003 | 2215 (26.9) | 3342 (26.9) | 12 905 (28.9) | |
| 2004–2005 | 3320 (40.3) | 4969 (40.0) | 16 189 (36.3) | |
| 2006–2007 | 1768 (21.5) | 2700 (21.8) | 9647 (21.6) | |
| 2008–2009 | 928 (11.3) | 1398 (11.3) | 5850 (13.1) | |
| Age, y | <0.001 | |||
| 40–49 | 7108 (86.4) | 8463 (68.2) | 15 710 (35.2) | |
| 50–59 | 1096 (13.3) | 3352 (27.0) | 17 808 (39.9) | |
| 60–69 | 27 (0.3) | 587 (4.7) | 8476 (19.0) | |
| 70–79 | 0 (0.0) | 7 (0.1) | 2597 (5.8) | |
| Sex | <0.001 | |||
| Female | 7798 (94.7) | 10 181 (82.1) | 21 219 (47.6) | |
| Male | 433 (5.3) | 2228 (18.0) | 23 372 (52.4) | |
| Race/ethnicity | <0.001 | |||
| Black | 5566 (67.6) | 8384 (67.6) | 31 822 (71.4) | |
| White | 2665 (32.4) | 4025 (32.4) | 12 769 (28.6) | |
| Diabetes mellitus | <0.001 | |||
| No | 8202 (99.7) | 12 035 (97.0) | 32 581 (73.1) | |
| Yes | 29 (0.4) | 374 (3.0) | 12 010 (26.9) | |
| Health insurance | <0.001 | |||
| No | 3389 (41.3) | 5503 (44.5) | 18 345 (41.3) | |
| Yes | 4818 (58.7) | 6867 (55.5) | 26 095 (58.7) | |
| Annual household income | <0.001 | |||
| <$15 000 | 3793 (46.1) | 6247 (50.3) | 25 913 (58.1) | |
| $15 000 to <$25 000 | 1898 (23.1) | 2804 (22.6) | 9497 (21.3) | |
| $25 000 to <$50 000 | 1401 (17.0) | 1984 (16.0) | 5741 (12.9) | |
| ≥$50 000 | 1139 (13.8) | 1374 (11.1) | 3440 (7.7) | |
| Education | <0.001 | |||
| Less than high school | 1568 (19.1) | 2747 (22.1) | 14 190 (31.8) | |
| High school completed | 2714 (33.0) | 4324 (34.9) | 15 135 (33.9) | |
| Higher than high school | 3949 (48.0) | 5338 (43.0) | 15 266 (34.2) |
Values are frequencies (percentages). Framingham 10‐year CVD risk scores were stratified into 3 mutually exclusive categories: low risk (<6%), intermediate risk (6–9.9%), and high risk (≥10%). CVD indicates cardiovascular disease; SCCS, Southern Community Cohort Study.
Crude frequency distributions of categorical variables among categories of CVD risk scores were compared using chi‐square tests.
Prevalence of Low‐Dose Aspirin Use and Impact Across Race and Ethnicity
The average crude prevalence of low‐dose aspirin use for the primary prevention of CVD in the overall SCCS population was 17.1% and increased across predicted 10‐year CVD risk categories: 7.5%, 11.6%, and 20.4% among low‐, intermediate‐, and high‐risk participants, respectively (P<0.001). Among low‐dose aspirin users, the median number of pills consumed was 7 pills/week (first quartile: 7; third quartile: 7). A detailed description of the prevalence of low‐dose aspirin use by 10‐year CVD risk categories according to different sociodemographic and clinical characteristics is presented in Table 2. The prevalence of low‐dose aspirin use initially increased in the participants enrolled from 2002–2003 to 2006–2007 and then stabilized thereafter. Higher prevalence of low‐dose aspirin use was observed among participants who were older, female, white, diabetic, and users of NSAIDs; who reported having some type of health insurance coverage; and who had higher household income or education level. Most of these findings were consistent across all CVD risk categories. In contrast, no consistent differences in the use of low‐dose aspirin were shown in relation to medical history of peptic ulcer.
Table 2.
Prevalence of Low‐Dose Aspirin Use for Primary Prevention of CVD by 10‐Year CVD Risk Categories, According to Different Sociodemographic and Clinical Characteristics, in the SCCS
| Variable | Prevalence of Low‐Dose Aspirin Use, n (%) | |||||
|---|---|---|---|---|---|---|
| Low Risk (n=8231) | P Valuea | Intermediate Risk (n=12 409) | P Valuea | High Risk (n=44 591) | P Valuea | |
| Year of SCCS enrollment | <0.001 | <0.001 | <0.001 | |||
| 2002–2003 | 101 (4.6) | 244 (7.3) | 1784 (13.8) | |||
| 2004–2005 | 285 (8.6) | 638 (12.8) | 3524 (21.8) | |||
| 2006–2007 | 161 (9.1) | 368 (13.6) | 2421 (25.1) | |||
| 2008–2009 | 67 (7.2) | 192 (13.7) | 1358 (23.2) | |||
| Age, y | <0.001 | <0.001 | <0.001 | |||
| 40–49 | 451 (6.3) | 746 (8.8) | 1820 (11.6) | |||
| 50–59 | 156 (14.2) | 540 (16.1) | 3718 (20.9) | |||
| 60–69 | 7 (25.9) | 155 (26.4) | 2611 (30.8) | |||
| 70–79 | ··· | 1 (14.3) | 938 (36.1) | |||
| Sex | 0.001 | <0.001 | <0.001 | |||
| Female | 600 (7.7) | 1296 (12.7) | 5464 (25.8) | |||
| Male | 14 (3.2) | 146 (6.6) | 3623 (15.5) | |||
| Race/ethnicity | <0.001 | <0.001 | <0.001 | |||
| Black | 341 (6.1) | 818 (9.8) | 5665 (17.8) | |||
| White | 273 (10.2) | 624 (15.5) | 3422 (26.8) | |||
| Diabetes mellitus | 0.908 | <0.001 | <0.001 | |||
| No | 612 (7.5) | 1375 (11.4) | 5238 (16.1) | |||
| Yes | 2 (6.9) | 67 (17.9) | 3849 (32.1) | |||
| History of ulcer | 0.004 | 0.364 | 0.002 | |||
| No | 537 (7.2) | 1281 (11.5) | 7882 (20.2) | |||
| Yes | 77 (10.1) | 161 (12.4) | 1205 (22.0) | |||
| Concomitant NSAIDs | <0.001 | <0.001 | <0.001 | |||
| No | 433 (6.6) | 984 (10.1) | 6851 (18.9) | |||
| Yes | 181 (10.9) | 458 (17.4) | 2236 (27.0) | |||
| Health insurance | 0.032 | <0.001 | <0.001 | |||
| No | 228 (6.7) | 516 (9.4) | 2785 (15.2) | |||
| Yes | 385 (8.0) | 918 (13.4) | 6267 (24.0) | |||
| Annual household income | <0.001 | <0.001 | <0.001 | |||
| <$15 000 | 229 (6.0) | 570 (9.1) | 4549 (17.6) | |||
| $15 000 to <$25 000 | 116 (6.1) | 322 (11.5) | 1938 (20.4) | |||
| $25 000 to <$50 000 | 133 (9.5) | 279 (14.1) | 1456 (25.4) | |||
| ≥$50 000 | 136 (11.9) | 271 (19.7) | 1144 (33.3) | |||
| Education | <0.001 | <0.001 | <0.001 | |||
| Less than high school | 90 (5.7) | 232 (8.5) | 2526 (17.8) | |||
| High school completed | 180 (6.6) | 450 (10.4) | 2846 (18.8) | |||
| Higher than high school | 344 (8.7) | 760 (14.2) | 3715 (24.3) | |||
Values are frequencies (percentages). Framingham risk scores were stratified into 3 mutually exclusive categories: low risk (<6%), intermediate risk (6–9.9%), and high risk (≥10%). CVD indicates cardiovascular disease; SCCS, Southern Community Cohort Study.
Crude frequency distributions of categorical variables within each category of CVD risk were compared using chi‐square tests.
These results were confirmed in the multivariate analysis of factors associated with low‐dose aspirin use (Table 3). In the full adjusted model, non‐Hispanic black participants were less likely to use low‐dose aspirin for the primary prevention of CVD compared with white participants (low‐dose aspirin use, adjusted odds ratio: 0.79; 95% CI, 0.75–0.82; P<0.001). Similar results were observed when the analysis was restricted to the high‐risk CVD group (low‐dose aspirin use, adjusted odds ratio; 0.77; 95% CI, 0.73–0.82; P<0.001). Such racial/ethnic differences among high‐risk individuals were more prominent among those who were recruited before 2006, younger than 60 years of age, male, and nondiabetic; who did not report concomitant use of NSAIDs; and who reported lower household income or lower education level (Figure 2). Similar results were observed on multiple imputed data sets (Table 4).
Table 3.
Multivariate Analysis of Factors Associated With Low‐Dose Aspirin Use for Primary Prevention of CVD in the SCCS
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Valuea | OR (95% CI) | P Valuea | OR (95% CI) | P Valuea | |
| Race/ethnicity | ||||||
| White | Ref. | Ref. | Ref. | |||
| Black | 0.61 (0.59–0.64) | <0.001 | 0.73 (0.70–0.76) | <0.001 | 0.79 (0.75–0.82) | <0.001 |
| Year of SCCS enrollment | ||||||
| 2002–2003 | … | … | Ref. | Ref. | ||
| 2004–2005 | … | … | 1.57 (1.48–1.66) | <0.001 | 1.41 (1.33–1.50) | <0.001 |
| 2006–2007 | … | … | 1.87 (1.75–1.99) | <0.001 | 1.77 (1.66–1.89) | <0.001 |
| 2008–2009 | … | … | 1.65 (1.54–1.78) | <0.001 | 1.61 (1.49–1.74) | <0.001 |
| Age, y | ||||||
| 40–49 | … | … | Ref. | Ref. | ||
| 50–59 | … | … | 1.79 (1.70–1.89) | <0.001 | 1.77 (1.67–1.87) | <0.001 |
| 60–69 | … | … | 2.82 (2.64–3.00) | <0.001 | 2.87 (2.69–3.06) | <0.001 |
| 70–79 | … | … | 3.52 (3.21–3.87) | <0.001 | 3.97 (3.60–4.37) | <0.001 |
| Sex | ||||||
| Female | … | … | Ref. | Ref. | ||
| Male | … | … | 0.65 (0.62–0.68) | <0.001 | 0.74 (0.70–0.77) | <0.001 |
| 10‐y CVD risk | ||||||
| Low risk | … | … | Ref. | Ref. | ||
| Intermediate risk | … | … | 1.46 (1.32–1.62) | <0.001 | 1.46 (1.31–1.61) | <0.001 |
| High risk | … | … | 2.49 (2.27–2.73) | <0.001 | 1.96 (1.78–2.16) | <0.001 |
| Diabetes mellitus | ||||||
| No | … | … | … | … | Ref. | |
| Yes | … | … | … | … | 2.45 (2.33–2.58) | <0.001 |
| History of ulcer | ||||||
| No | … | … | … | … | Ref. | |
| Yes | … | … | … | … | 1.06 (1.00–1.14) | 0.060 |
| Concomitant NSAIDs | ||||||
| No | … | … | … | … | Ref. | |
| Yes | … | … | … | … | 1.53 (1.45–1.61) | <0.001 |
| Annual household income | ||||||
| <$15 000 | … | … | … | … | Ref. | |
| $15 000 to <$25 000 | … | … | … | … | 1.18 (1.11–1.25) | <0.001 |
| $25 000 to <$50 000 | … | … | … | … | 1.41 (1.32–1.51) | <0.001 |
| ≥$50 000 | … | … | … | … | 1.85 (1.72–2.00) | <0.001 |
| Education | ||||||
| Less than high school | … | … | … | … | Ref. | |
| High school completed | … | … | … | … | 1.13 (1.07–1.20) | <0.001 |
| Higher than high school | … | … | … | … | 1.25 (1.18–1.33) | <0.001 |
Model 1 includes race/ethnicity. Model 2 includes model 1 plus year of SCCS enrollment, age at enrollment, sex, and Framingham 10‐year CVD risk category. Model 3 includes model 2 plus diabetes mellitus status, history of ulcer, concomitant use of NSAIDs, annual household income, and education level. CVD indicates cardiovascular disease; OR, odds ratio; Ref., referent; SCCS, Southern Community Cohort Study.
P value from Wald test compared with ref.
Figure 2.
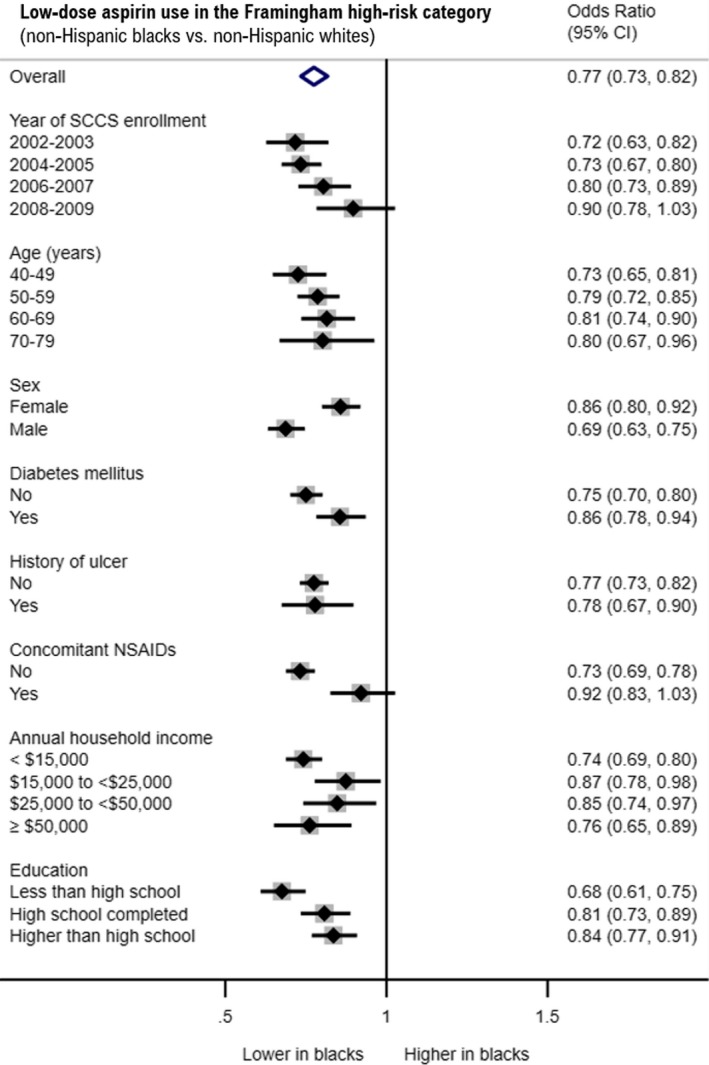
Impact of race/ethnicity on low‐dose aspirin use in the high‐risk category. Forest plot summarizing adjusted odds ratio (95% CI) of low‐dose aspirin use in black and white participants according to selected variables among the SCCS (Southern Community Cohort Study) participants belonging to the Framingham 10‐year high‐risk category (≥10%) at enrollment. Results are derived from stratified multivariate logistic regression models. Models were adjusted for the following variables: year of SCCS enrollment, age at enrollment, sex, diabetes mellitus status, medical history of ulcer, concomitant use of NSAIDs, annual household income, and education.
Table 4.
Multivariate Analysis of Factors Associated With Low‐Dose Aspirin Use for Primary Prevention of CVD in the SCCS Based on Estimations of Multiple Imputed Data
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Valuea | OR (95% CI) | P Valuea | OR (95% CI) | P Valuea | |
| Race/ethnicity | ||||||
| White | Ref. | Ref. | Ref. | |||
| Black | 0.61 (0.59–0.64) | <0.001 | 0.72 (0.69–0.75) | <0.001 | 0.78 (0.74–0.82) | <0.001 |
| Year of SCCS enrollment | ||||||
| 2002–2003 | … | … | Ref. | Ref. | ||
| 2004–2005 | … | … | 1.56 (1.47–1.65) | <0.001 | 1.41 (1.33–1.50) | <0.001 |
| 2006–2007 | … | … | 1.86 (1.74–1.98) | <0.001 | 1.77 (1.66–1.89) | <0.001 |
| 2008–2009 | … | … | 1.64 (1.53–1.77) | <0.001 | 1.61 (1.49–1.73) | <0.001 |
| Age, y | ||||||
| 40–49 | … | … | Ref. | Ref. | ||
| 50–59 | … | … | 1.87 (1.77–1.97) | <0.001 | 1.83 (1.73–1.93) | <0.001 |
| 60–69 | … | … | 2.96 (2.78–3.16) | <0.001 | 2.98 (2.79–3.19) | <0.001 |
| 70–79 | … | … | 3.70 (3.36–4.07) | <0.001 | 4.11 (3.73–4.53) | <0.001 |
| Sex | ||||||
| Female | … | … | Ref. | Ref. | ||
| Male | … | … | 0.67 (0.64–0.70) | <0.001 | 0.76 (0.72–0.79) | <0.001 |
| 10‐y CVD risk | ||||||
| Low risk | … | … | Ref. | Ref. | ||
| Intermediate risk | … | … | 1.32 (1.18–1.49) | <0.001 | 1.29 (1.15–1.45) | <0.001 |
| High risk | … | … | 2.08 (1.88–2.29) | <0.001 | 1.64 (1.48–1.82) | <0.001 |
| Diabetes mellitus | ||||||
| No | … | … | … | … | Ref. | |
| Yes | … | … | … | … | 2.49 (2.37–2.62) | <0.001 |
| History of ulcer | ||||||
| No | … | … | … | … | Ref. | |
| Yes | … | … | … | … | 1.07 (1.00–1.14) | 0.050 |
| Concomitant NSAIDs | ||||||
| No | … | … | … | … | Ref. | |
| Yes | … | … | … | … | 1.53 (1.46–1.61) | <0.001 |
| Annual household income | ||||||
| <$15 000 | … | … | … | … | Ref. | |
| $15 000 to <$25 000 | … | … | … | … | 1.18 (1.11–1.24) | <0.001 |
| $25 000 to <$50 000 | … | … | … | … | 1.41 (1.32–1.50) | <0.001 |
| ≥$50 000 | … | … | … | … | 1.82 (1.69–1.97) | <0.001 |
| Education | ||||||
| Less than high school | … | … | … | … | Ref. | |
| High school completed | … | … | … | … | 1.13 (1.07–1.20) | <0.001 |
| Higher than high school | … | … | … | … | 1.25 (1.18–1.33) | <0.001 |
Model 1: includes race/ethnicity. Model 2: Model 1+year of SCCS enrollment, age at enrollment, sex, and Framingham 10‐year CVD risk category. Model 3: Model 2+diabetes mellitus status, history of ulcer, concomitant use of non‐steroidal anti‐inflammatory drugs (NSAIDs), annual household income, and education level. CVD indicates cardiovascular disease; OR, odds ratio; Ref., referent; SCCS, Southern Community Cohort Study.
P value from Wald test compared with ref.
Association Between Low‐Dose Aspirin Use and Fatal Ischemic Heart Disease
After a median follow‐up of 135 months (interquartile range: 110–154 months), there were 11 489 deaths including 1225 deaths due to ischemic heart disease. The overall incidence rate of ischemic cardiac death in the SCCS population was 1.76 per 1000 person‐years (95% CI, 1.66–1.86) and was 20% higher in white than black participants (incidence rate ratio: 1.21; 95% CI, 1.07–1.36). The incidence rate of ischemic cardiac death was consistently higher in white than black participants across most age and sex categories (Table 5).
Table 5.
Incident Cases and Incidence Rates of Ischemic Cardiac Death Among SCCS Participants Stratified by Age, Race/Ethnicity, and Sex
| White | White Women | White Men | Black | Black Women | Black Men | |
|---|---|---|---|---|---|---|
| All ages, n | 19 459 | 12 462 | 6997 | 45 772 | 26 736 | 19 036 |
| Incident cases, n | 395 | 196 | 199 | 830 | 363 | 467 |
| Person‐years | 197 122 | 128 583 | 68 538 | 499 891 | 298 058 | 201 834 |
| Incidence rate | 2.00 (1.82–2.21) | 1.52 (1.33–1.75) | 2.90 (2.53–3.34) | 1.66 (1.55–1.78) | 1.22 (1.10–1.35) | 2.31 (2.11–2.53) |
| 40–49 y, n | 7923 | 4917 | 3006 | 23 358 | 13 168 | 10 190 |
| Incident cases, n | 125 | 56 | 69 | 271 | 101 | 170 |
| Person‐years | 81 886 | 51 909 | 29 976 | 263 697 | 150 200 | 113 497 |
| Incidence rate | 1.53 (1.28–1.82) | 1.08 (0.83–1.40) | 2.30 (1.82–2.91) | 1.03 (0.91–1.16) | 0.67 (0.55–0.82) | 1.50 (1.29–1.74) |
| 50–59 y, n | 6894 | 4491 | 2403 | 15 362 | 8923 | 6439 |
| Incident cases, n | 152 | 71 | 81 | 303 | 125 | 178 |
| Person‐years | 69 804 | 46 481 | 23 323 | 163 993 | 98 605 | 65 388 |
| Incidence rate | 2.18 (1.86–2.55) | 1.53 (1.21–1.93) | 3.47 (2.79–4.32) | 1.85 (1.65–2.07) | 1.27 (1.06–1.51) | 2.72 (2.35–3.15) |
| 60–69 y, n | 3646 | 2396 | 1250 | 5444 | 3518 | 1926 |
| Incident cases, n | 65 | 41 | 24 | 174 | 85 | 89 |
| Person‐years | 36 213 | 23 999 | 12 214 | 56 787 | 38 061 | 18 726 |
| Incidence rate | 1.79 (1.41–2.29) | 1.71 (1.26–2.32) | 1.96 (1.32–2.93) | 3.06 (2.64–3.55) | 2.23 (1.81–2.76) | 4.75 (3.86–5.85) |
| 70–79 y, n | 996 | 658 | 338 | 1608 | 1127 | 481 |
| Incident cases, n | 53 | 28 | 25 | 82 | 52 | 30 |
| Person‐years | 9219 | 6194 | 3025 | 15 415 | 11 192 | 4223 |
| Incidence rate | 5.75 (4.39–7.52) | 4.52 (3.12–6.55) | 8.26 (5.58–12.23) | 5.32 (4.28–6.61) | 4.65 (3.54–6.10) | 7.10 (4.97–10.16) |
Incidence rate is per 1000 person‐years (95% CI). SCCS indicates Southern Community Cohort Study.
In the overall population, low‐dose aspirin use was associated with a trend toward decreased risk of ischemic cardiac death in white participants (adjusted hazard ratio: 0.86; 95% CI, 0.68–1.10), especially in women (adjusted hazard ratio: 0.72; 95% CI, 0.51–1.02), but not in black participants (adjusted hazard ratio: 1.18; 95% CI, 0.98–1.40). Similar results were observed when the analysis was restricted to the high CVD risk group as a whole (Table 6) and to high‐risk individuals aged 50 to 69 or 50 to 59 years (Figure 3). Similar results were observed in multiple imputed data sets (Table 7).
Table 6.
Adjusted Relative Risk of Incident Ischemic Cardiac Death According to Low‐Dose Aspirin Use Among SCCS Participants Stratified by Age, Race/Ethnicity, and Sex
| White | White Women | White Men | Black | Black Women | Black Men | |
|---|---|---|---|---|---|---|
| All risk, all ages | ||||||
| Participants, n | 19 459 | 12 462 | 6997 | 45 772 | 26 736 | 19 036 |
| HR (95% CI) | 0.86 (0.68–1.10) | 0.72 (0.51–1.02) | 1.03 (0.73–1.45) | 1.18 (0.98–1.40) | 1.05 (0.82–1.35) | 1.32 (1.03–1.70) |
| High CVD risk, all ages | ||||||
| Participants, n | 12 769 | 6496 | 6273 | 31 822 | 14 723 | 17 099 |
| HR (95% CI) | 0.82 (0.63–1.06) | 0.71 (0.49–1.03) | 0.94 (0.66–1.34) | 1.17 (0.97–1.40) | 1.02 (0.78–1.33) | 1.33 (1.04–1.71) |
| High CVD risk, 50–69 y | ||||||
| Participants, n | 8253 | 4694 | 3559 | 18 031 | 9889 | 8142 |
| HR (95% CI) | 0.78 (0.57–1.08) | 0.71 (0.45–1.12) | 0.89 (0.56–1.41) | 1.10 (0.88–1.39) | 0.93 (0.67–1.30) | 1.30 (0.95–1.77) |
| High CVD risk, 50–59 y | ||||||
| Participants, n | 5022 | 2713 | 2309 | 12 786 | 6570 | 6216 |
| HR (95% CI) | 0.74 (0.49–1.12) | 0.63 (0.34–1.17) | 0.86 (0.50–1.50) | 1.04 (0.77–1.41) | 0.93 (0.60–1.45) | 1.15 (0.76–1.74) |
Relative risk estimated by HR (95% CI) for fatal ischemic cardiac event among those who used and did not use low‐dose aspirin (reference), obtained from stratified Cox proportional hazards models. All models were adjusted by Framingham 10‐year CVD risk category, age at enrollment, sex, race/ethnicity, diabetes mellitus status, and household income, otherwise were not considered as stratification variables. Results are presented for the overall study population (all risks, all ages) and in high‐risk (≥10% CVD risk) participants by race/ethnicity for any age and according to the US Preventive Services Task Force 2016 recommendations on low‐dose aspirin use for primary prevention of CVD (50–69 and 50–59 years of age). CVD indicates cardiovascular disease; HR, hazard ratio; SCCS, Southern Community Cohort Study.
Figure 3.
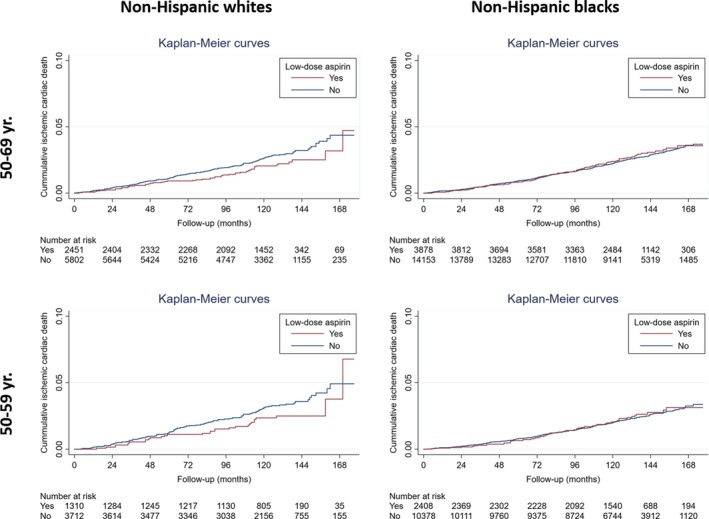
Follow‐up of ischemic cardiac death according to low‐dose aspirin use in high‐risk participants. Race/ethnicity‐stratified Kaplan–Meier curves illustrating cumulative incidence of ischemic cardiac death during follow‐up according to low‐dose aspirin use. Results are presented for participants in the Framingham 10‐year high‐risk category (≥10%) aged 50 to 69 years or 50 to 59 years, for whom the use of low‐dose aspirin may be considered for the primary prevention of CVD according to the US Preventive Services Task Force 2016 recommendations.
Table 7.
Adjusted Relative Risk of Incident Ischemic Cardiac Death According to Low‐Dose Aspirin Use Among SCCS Participants Stratified by Age, Race/Ethnicity, and Sex Based on Estimations of Multiple Imputed Data
| White | White Women | White Men | Black | Black Women | Black Men | |
|---|---|---|---|---|---|---|
| All risk, all ages | ||||||
| Participants, n | 19 459 | 12 462 | 6997 | 45 772 | 26 736 | 19 036 |
| HR (95% CI) | 0.87 (0.68–1.11) | 0.73 (0.51–1.04) | 1.03 (0.73–1.45) | 1.18 (0.99–1.41) | 1.05 (0.82–1.35) | 1.32 (1.03–1.70) |
| High CVD risk, all ages | ||||||
| Participants, n | 12 769 | 6496 | 6273 | 31 822 | 14 723 | 17 099 |
| HR (95% CI) | 0.82 (0.63–1.08) | 0.72 (0.48–1.07) | 0.94 (0.65–1.35) | 1.13 (0.94–1.37) | 0.97 (0.74–1.28) | 1.31 (1.02–1.69) |
| High CVD risk, 50–69 y | ||||||
| Participants, n | 8253 | 4694 | 3559 | 18 031 | 9889 | 8142 |
| HR (95% CI) | 0.78 (0.56–1.10) | 0.72 (0.44–1.16) | 0.88 (0.55–1.40) | 1.11 (0.88–1.40) | 0.94 (0.67–1.33) | 1.28 (0.93–1.76) |
| High CVD risk, 50–59 y | ||||||
| Participants, n | 5022 | 2713 | 2309 | 12 786 | 6570 | 6216 |
| HR (95% CI) | 0.74 (0.48–1.14) | 0.64 (0.33–1.25) | 0.85 (0.48–1.49) | 1.03 (0.75–1.41) | 0.93 (0.59–1.49) | 1.12 (0.74–1.72) |
Relative risk estimated by HR (95% CI) for fatal ischemic cardiac event among those who used and did not use low‐dose aspirin (reference), obtained from stratified Cox proportional hazard models run on multiple imputed data. All models were adjusted by Framingham 10‐year CVD risk category, age at enrollment, sex, race/ethnicity, diabetes mellitus status, and household income, otherwise were not considered as stratification variables. Results are presented for the overall study population (all risk, all ages) and in high‐risk (≥10% CVD risk) participants by race/ethnicity both any age or according to the US Preventive Services Task Force 2016 recommendations on low dose aspirin use for primary prevention of CVD (50–69 and 50–59 years of age). Because estimation samples varied across imputations in high‐risk subgroups, n reflects the number of individuals as in Table 6. CVD indicates cardiovascular disease; HR, hazard ratio; SCCS, Southern Community Cohort Study.
Discussion
This population‐based study examines the prevalence of low‐dose aspirin use and its association with incident fatal ischemic heart disease in a large cohort of non‐Hispanic black and non‐Hispanic white adults without known CVD who were recruited in 2002 through 2009 and followed for an average of 11 years. The main findings were that black participants were less likely than white participants to take low‐dose aspirin for CVD primary prevention, regardless of predicted risk, age, sex, comorbidities, concomitant use of NSAIDs, household income, or education; and low‐dose aspirin use was associated with a trend toward decreased risk of ischemic cardiac death in white participants, especially in women, but not in black participants.
Overall population estimates of self‐reported low‐dose aspirin use for primary CVD prevention obtained from the 2012–2015 National Health Interview Survey ranged from ≈18% for low‐risk individuals to ≈31% for high‐risk individuals.18 Similar estimates were obtained from the National Health and Nutrition Examination Survey 2011–2012.19 The self‐reported rates of low‐dose aspirin use in the SCCS are lower, particularly among black participants (with aspirin use reported by 27% of white and 18% of black participants at high CVD risk; Table 2). This finding may be partially explained by different population characteristics among studies and changes in low‐dose aspirin recommendations over time. Importantly, our study demonstrates that low‐dose aspirin use by black patients is consistently lower compared with white participants across all risk categories examined, even after adjusting for socioeconomic status and other relevant covariates—a detail that could not be investigated in previous smaller studies.5, 6, 7, 8, 20 The reason for these racial and ethnic disparities is unclear but is likely multifactorial in origin.6 Cultural barriers, mistrust of the healthcare establishment, and disparities throughout the continuum of prevention and care, including access to and quality of health care, are plausible factors.21, 22, 23
The large sample of participants allowed us to provide insights into other factors independently associated with low‐dose aspirin use. In the SCCS, the adjusted odds of low‐dose aspirin use were ≈25% lower in male compared with female participants. The Minnesota Heart Survey enrolled a predominantly metropolitan white middle‐ to high‐income population showing consistently lower rates of low‐dose aspirin use for CVD primary prevention in women than in men over time24; however, a recent nationwide survey of US adults did not find differences in use by sex.25 Study setting and population differences, as well as variations in aspirin use ascertainment and statistical modeling, might at least partially explain these conflicting results. We observed that history of peptic ulcer was not associated with reported low‐dose aspirin use, whereas concomitant use of NSAIDs was associated with an increase in use. The latter may be evidence of increasing problems of polypharmacy, especially in elderly patients.26 Interestingly, our data suggest that the use of low‐dose aspirin for the primary prevention of CVD significantly increased from 2002 to 2007 and then stabilized thereafter. A similar temporal evolution was observed across all CVD risk categories and perhaps may be explained by the publication of the USPSTF and American Heart Association (AHA) guidelines on primary prevention in 2002, which encouraged the prescription of low‐dose aspirin for intermediate‐ and high‐risk patients.27, 28
We observed overall higher incidence of ischemic cardiac death in white than black participants, particularly in those aged 40 to 59 years. Available statistics from the United States overall suggest that non‐Hispanic black people have higher ischemic heart disease mortality rates than white people, ranging from 1.3 to 2.8 cases per 1000 person‐years in people aged 45 to 64 years, which might be partially explained by socioeconomic inequalities.29, 30, 31 Within the SCCS, however, socioeconomic status tends to be low among both black and white participants, and we observed lower all‐cause mortality among black versus white participants.10 The reasons for such a lower risk of mortality among black participants in the SCCS are unclear. The influence of confounding from unmeasured resiliency factors in US black communities cannot be excluded. The volunteer participation in the study may be another possibility, including a stronger “healthy volunteer” effect in black than white participants.10 The substantial socioeconomic status overlap between black and white participants in the SCCS and the fact that the SCCS enrolled low‐income rural as well as urban populations that are frequently underrepresented in other cohorts could be another factor responsible of the different racial/ethnic outcome rates observed in other studies.11 Nevertheless, similar lower rates of ischemic heart disease mortality in black participants (compared with white participants) have been also reported in US urban‐centered studies.32 Further research is required to determine the possibility of a racial/ethnic disparity paradox according to particular geographical or socioeconomic characteristics, which would be equally concerning.
Importantly, the potential of differential treatment effects of low‐dose aspirin use by race/ethnicity for primary prevention of CVD has not been well studied. Our data suggest that low‐dose aspirin use is associated with a decreased risk of ischemic cardiac death in white patients, especially in women, but not in black patients. These racial/ethnic and sex differences were consistent when the analysis was restricted to the group with high CVD risk and in high‐risk individuals aged 50 to 69 or 50 to 59 years, for whom the use of low‐dose aspirin may be recommended for the primary prevention of CVD according to USPSTF 2016 guidelines.1 The reasons for this racial/ethnic differential effect are speculative but could include higher rates of inadequate medication adherence,33, 34 reduced response to antiplatelet therapy,35 unrecognized risks of concomitant use of over‐the‐counter drugs diminishing the potential beneficial effect of low‐dose aspirin (eg, NSAIDs),36, 37, 38 and poor control of other risk factors.31, 34 It is unlikely that socioeconomic status plays a major role in this differential effect by race/ethnicity because the participants included in this cohort had minor differences in income levels, which were included in the models for adjustment.
Nevertheless, the role of low‐dose aspirin in the general population without CVD remains controversial. The USPSTF 2016 guidelines narrowed the recommendations to high‐risk individuals aged 50 to 69 years, removed the previous distinction by sex, and downgraded the recommendation from grade A to grade B for the group aged 50 to 59 years and to grade C for the group aged 60 to 69 years. Along the same lines, an overview of systematic reviews concluded that high‐quality evidence supports aspirin for primary CVD prevention.3 In contrast, and as supported by several meta‐analyses and clinical trials suggesting no benefit of low‐dose aspirin and/or a potential increase in severe bleeding risk,39, 40, 41, 42, 43 the “2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice” stated that antiplatelet therapy is not recommended in individuals without CVD.44 Based on recent trials showing no net benefit,45, 46, 47 the 2019 American College of Cardiology and AHA “Guideline on the Primary Prevention of Cardiovascular Disease” recommends that low‐dose aspirin should be used infrequently in the routine primary prevention of CVD.48 Our study suggests that the effect of low‐dose aspirin for the primary prevention of CVD may differ by race/ethnicity, raising the possibility of no benefit among black patients. There are no plausible reasons to think that low‐dose aspirin might be harmful to black patients. Therefore, the trend toward increased risk of ischemic cardiac death shown in particular subgroups of black low‐dose aspirin users in this observational study might be due to residual confounding (ie, black participants on low‐dose aspirin might have been identified as having higher CVD risk in a manner somehow not fully accounted for in the models).
Our study has several limitations worth noting. Perhaps the greatest is the possibility of confounding by indications that were not completely captured in the covariate adjustments (ie, residual confounding). Another limitation is that we were unable to assess the exact duration of aspirin therapy. For many participants, we had no information on whether and when the individual initiated or stopped low‐dose aspirin use after baseline questionnaire response; this could result in possible misclassification effects of unknown magnitude and direction. Nevertheless, baseline low‐dose aspirin use did not demonstrate a time‐varying effect on mortality in our analysis, meaning that hazard ratios associated with low‐dose aspirin use were constant over time. To account for temporal trends of low‐dose aspirin use, we performed a sensitivity survival analysis including the year at participant enrollment as a covariate, and differences between models were minimal (results not shown). The fact that the study significantly relied on self‐reported data might make the analysis susceptible to recall and misclassification bias; however, these methods are common to many epidemiological studies. Furthermore, a series of independent validation studies demonstrated the reliability of the questionnaire within the SCCS population for many of the collected variables.9 Actual lipid profile was not available, and thus the 10‐year CVD risk based on the pooled cohort equations could not be calculated.13 Nevertheless, the Framingham risk equation has been validated in multiple ethnicities.49 In fact, the Framingham original and offspring cohorts were used, among others, to derive the pooled cohort equations.13 Similarly, a significant proportion of participants in this study did not undergo blood pressure measurements. By imputing normal blood pressure values according to self‐reported hypertension status when they were missing, our calculations may underestimate actual cardiovascular risk.14 Nevertheless, the prevalence of hypertension diagnosis and other clinical and sociodemographic characteristics was similar among individuals with and without actual blood pressure values available (Table 8). Furthermore, the study conclusions were similar when missingness of blood pressure was addressed by several means, including multiple imputation procedures and the replication of the results in the full cohort of participants by considering their predicted CVD risk category based on the number of cardiovascular risk factors (data not shown), as done previously by others.7, 50, 51, 52 The major strengths of our study include the large sample size and the comparison of black and white populations of similar socioeconomic status, allowing examination of low‐dose aspirin use patterns and CVD incidence effects of aspirin by race/ethnicity less confounded by socioeconomic status. The SCCS cohort includes a substantial number of participants from disadvantaged and low‐income populations that have been underrepresented, so our study provides relevant insights not available in most previous studies.
Table 8.
Characteristics of the SCCS Population According to Available Information to Calculate the SBP Component of the Framingham Risk Score
| Variable | Nonmissing SBP (n=9568) | Missing SBP (n=55 663) |
|---|---|---|
| Age, y | ||
| 40–49 | 4457 (46.6) | 26 824 (48.2) |
| 50–59 | 3323 (34.7) | 18 933 (34.0) |
| 60–69 | 1430 (15.0) | 7660 (13.8) |
| 70–79 | 358 (3.7) | 2246 (4.0) |
| Sex | ||
| Female | 6698 (70.0) | 32 500 (58.4) |
| Male | 2870 (30.0) | 23 163 (41.6) |
| Race/ethnicity | ||
| Black | 6494 (67.9) | 39 278 (70.6) |
| White | 3074 (32.1) | 16 385 (29.4) |
| Hypertension | ||
| No | 4797 (50.1) | 26 708 (48.0) |
| Yes | 4771 (49.9) | 28 955 (52.0) |
| Diabetes mellitus | ||
| No | 7237 (75.6) | 45 581 (81.9) |
| Yes | 2331 (24.4) | 10 082 (18.1) |
| Current smoker | ||
| No | 6027 (63.0) | 31 781 (57.1) |
| Yes | 3541 (37.0) | 23 882 (42.9) |
| Overweight/obese | ||
| No | 1949 (20.4) | 15 256 (27.4) |
| Yes | 7619 (79.6) | 40 407 (72.6) |
| Health insurance | ||
| No | 4614 (48.4) | 22 623 (40.8) |
| Yes | 4911 (51.6) | 32 869 (59.2) |
| Annual household income | ||
| <$15 000 | 5727 (59.9) | 30 226 (54.3) |
| $15 000 to <$25 000 | 2309 (24.1) | 11 890 (21.4) |
| $25 000 to <$50 000 | 1168 (12.2) | 7958 (14.3) |
| ≥$50 000 | 364 (3.8) | 5589 (10.0) |
| Education | ||
| Less than high school | 3041 (31.8) | 15 464 (27.8) |
| High school completed | 3480 (36.4) | 18 693 (33.6) |
| Higher than high school | 3047 (31.9) | 21 506 (38.6) |
Values are frequencies (percentages) of participants with (nonmissing SBP) or without (missing SBP) actual information to calculate the SBP component of the Framingham risk score. SBP indicates systolic blood pressure; SCCS, Southern Community Cohort Study.
In conclusion, in this predominantly high‐risk and low‐income biracial/ethnic large cohort, we found that black participants were less likely to take aspirin for primary prevention of CVD and that low‐dose aspirin use was associated with decreased incidence of ischemic cardiac death in white participants, especially in women, but not in black participants. Confounding by indication in these observational data cannot be ruled out, and the findings warrant confirmation in further studies before any clinical recommendation is given.
Sources of Funding
The SCCS (Southern Community Cohort Study) is funded by grants R01 CA092447 and U01 CA202979 from the National Cancer Institute, including American Recovery and Reinvestment Act funding (3R01 CA029447‐08 S1). Data collection was performed by the Survey and Biospecimen Shared Resource, which is supported in part by the Vanderbilt–Ingram Cancer Center (P30 CA68485). This analysis forms part of a project that has received funding from the American Heart Association under grant no. 14SFRN20490315. Fernandez‐Jimenez is a recipient of funding from the European Union Horizon 2020 Research and Innovation Program under the Marie Skłodowska‐Curie grant agreement no. 707642. The Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by the Instituto de Salud Carlos III; the Ministerio de Ciencia, Innovación y Universidades; and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV‐2015‐0505).
Disclosures
None.
Acknowledgments
We thank Emilia Bagiella, director of the Center for Biostatistics at the Icahn School of Medicine at Mount Sinai, for statistical support.
(J Am Heart Assoc. 2019;8:e013404 DOI: 10.1161/JAHA.119.013404.)
This article was handled independently by Cynthia St. Hilaire, PhD, as a guest editor. The editors had no role in the evaluation of the article or in the decision about its acceptance.
References
- 1. Bibbins‐Domingo K; Force USPST . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836–845. [DOI] [PubMed] [Google Scholar]
- 2. Dehmer SP, Maciosek MV, Flottemesch TJ, LaFrance AB, Whitlock EP. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777–786. [DOI] [PubMed] [Google Scholar]
- 3. Karmali KN, Lloyd‐Jones DM, Berendsen MA, Goff DC Jr, Sanghavi DM, Brown NC, Korenovska L, Huffman MD. Drugs for primary prevention of atherosclerotic cardiovascular disease: an overview of systematic reviews. JAMA Cardiol. 2016;1:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guirguis‐Blake JM, Evans CV, Senger CA, O'Connor EA, Whitlock EP. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804–813. [DOI] [PubMed] [Google Scholar]
- 5. Rodondi N, Vittinghoff E, Cornuz J, Butler J, Ding J, Satterfield S, Newman AB, Harris TB, Hulley SB, Bauer DC; Health A and Body Composition Study Research G . Aspirin use for the primary prevention of coronary heart disease in older adults. Am J Med. 2005;118:1288. [DOI] [PubMed] [Google Scholar]
- 6. Sanchez DR, Diez Roux AV, Michos ED, Blumenthal RS, Schreiner PJ, Burke GL, Watson K. Comparison of the racial/ethnic prevalence of regular aspirin use for the primary prevention of coronary heart disease from the Multi‐Ethnic Study of Atherosclerosis. Am J Cardiol. 2011;107:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shahar E, Folsom AR, Romm FJ, Bisgard KM, Metcalf PA, Crum L, McGovern PG, Hutchinson RG, Heiss G. Patterns of aspirin use in middle‐aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1996;131:915–922. [DOI] [PubMed] [Google Scholar]
- 8. Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005;2:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved. 2010;21:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Signorello LB, Cohen SS, Williams DR, Munro HM, Hargreaves MK, Blot WJ. Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104:e98–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akwo EA, Kabagambe EK, Wang TJ, Harrell FE Jr, Blot WJ, Mumma M, Gupta DK, Lipworth L. Heart failure incidence and mortality in the southern community cohort study. Circ Heart Fail. 2017;10:e003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 13. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 14. Ekundayo OJ, Vassar SD, Williams LS, Bravata DM, Cheng EM. Using administrative databases to calculate Framingham scores within a large health care organization. Stroke. 2011;42:1982–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed GW, Abdallah MS, Shao M, Wolski K, Wisniewski L, Yeomans N, Luscher TF, Borer JS, Graham DY, Husni ME, Solomon DH, Libby P, Menon V, Lincoff AM, Nissen SE. Effect of aspirin coadministration on the safety of celecoxib, naproxen, or ibuprofen. J Am Coll Cardiol. 2018;71:1741–1751. [DOI] [PubMed] [Google Scholar]
- 16. StataCorp . Survival Analysis Reference Manual. Release 15. College Station, TX: Stata Press; 2017. [Google Scholar]
- 17. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 18. Stuntz M, Bernstein B. Recent trends in the prevalence of low‐dose aspirin use for primary and secondary prevention of cardiovascular disease in the United States, 2012–2015. Prev Med Rep. 2017;5:183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mainous AG, Tanner RJ, Shorr RI, Limacher MC. Use of aspirin for primary and secondary cardiovascular disease prevention in the United States, 2011–2012. J Am Heart Assoc. 2014;3:e000989 DOI: 10.1161/JAHA.114.000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glasser SP, Cushman M, Prineas R, Kleindorfer D, Prince V, You Z, Howard VJ, Howard G. Does differential prophylactic aspirin use contribute to racial and geographic disparities in stroke and coronary heart disease (CHD)? Prev Med. 2008;47:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mensah GA, Cooper RS, Siega‐Riz AM, Cooper LA, Smith JD, Brown CH, Westfall JM, Ofili EO, Price LN, Arteaga S, Green Parker MC, Nelson CR, Newsome BJ, Redmond N, Roper RA, Beech BM, Brooks JL, Furr‐Holden D, Gebreab SY, Giles WH, James RS, Lewis TT, Mokdad AH, Moore KD, Ravenell JE, Richmond A, Schoenberg NE, Sims M, Singh GK, Sumner AE, Trevino RP, Watson KS, Aviles‐Santa ML, Reis JP, Pratt CA, Engelgau MM, Goff DC Jr, Perez‐Stable EJ. Reducing cardiovascular disparities through community‐engaged implementation research: a National Heart, Lung, and Blood Institute workshop report. Circ Res. 2018;122:213–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- 23. Liao Y, Bang D, Cosgrove S, Dulin R, Harris Z, Taylor A, White S, Yatabe G, Liburd L, Giles W; Division of A, Community Health NCfCDP, Health P, Centers for Disease C and Prevention . Surveillance of health status in minority communities ‐ Racial and Ethnic Approaches to Community Health Across the U.S. (REACH U.S.) risk factor survey, United States, 2009. MMWR Surveill Summ. 2011;60:1–44. [PubMed] [Google Scholar]
- 24. Luepker RV, Steffen LM, Duval S, Zantek ND, Zhou X, Hirsch AT. Population trends in aspirin use for cardiovascular disease prevention 1980–2009: the Minnesota Heart Survey. J Am Heart Assoc. 2015;4:e002320 DOI: 10.1161/JAHA.115.002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams CD, Chan AT, Elman MR, Kristensen AH, Miser WF, Pignone MP, Stafford RS, McGregor JC. Aspirin use among adults in the U.S.: results of a national survey. Am J Prev Med. 2015;48:501–508. [DOI] [PubMed] [Google Scholar]
- 26. Rossello X, Pocock SJ, Julian DG. Long‐term use of cardiovascular drugs: challenges for research and for patient care. J Am Coll Cardiol. 2015;66:1273–1285. [DOI] [PubMed] [Google Scholar]
- 27. Force USPST . Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med. 2002;136:157–160. [DOI] [PubMed] [Google Scholar]
- 28. Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF Jr, Smith SC Jr, Stone NJ, Taubert KA. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. [DOI] [PubMed] [Google Scholar]
- 29. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, Yancy CW; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council . Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 30. Colantonio LD, Gamboa CM, Richman JS, Levitan EB, Soliman EZ, Howard G, Safford MM. Black‐white differences in incident fatal, nonfatal, and total coronary heart disease. Circulation. 2017;136:152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 32. Smilowitz NR, Maduro GA Jr, Lobach IV, Chen Y, Reynolds HR. Adverse trends in ischemic heart disease mortality among young New Yorkers. Particularly young black women. PLoS One. 2016;11:e0149015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu Q, Dillon CF, Eberhardt MS, Wright JD, Burt VL. Preventive aspirin and other antiplatelet medication use among U.S. adults aged >/= 40 years: data from the National Health and Nutrition Examination Survey, 2011–2012. Public Health Rep. 2015;130:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferdinand KC, Senatore FF, Clayton‐Jeter H, Cryer DR, Lewin JC, Nasser SA, Fiuzat M, Califf RM. Improving medication adherence in cardiometabolic disease: practical and regulatory implications. J Am Coll Cardiol. 2017;69:437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waksman R, Maya J, Angiolillo DJ, Carlson GF, Teng R, Caplan RJ, Ferdinand KC. Ticagrelor versus clopidogrel in black patients with stable coronary artery disease: prospective, randomized, open‐label, multiple‐dose, crossover pilot study. Circ Cardiovasc Interv. 2015;8:e002232. [DOI] [PubMed] [Google Scholar]
- 36. Fry RB, Ray MN, Cobaugh DJ, Weissman NW, Kiefe CI, Shewchuk RM, Saag KG, Curtis JR, Allison JJ. Racial/ethnic disparities in patient‐reported nonsteroidal antiinflammatory drug (NSAID) risk awareness, patient‐doctor NSAID risk communication, and NSAID risk behavior. Arthritis Rheum. 2007;57:1539–1545. [DOI] [PubMed] [Google Scholar]
- 37. Nalamachu S, Pergolizzi JV, Raffa RB, Lakkireddy DR, Taylor R Jr. Drug‐drug interaction between NSAIDS and low‐dose aspirin: a focus on cardiovascular and GI toxicity. Expert Opin Drug Saf. 2014;13:903–917. [DOI] [PubMed] [Google Scholar]
- 38. Magnani JW, Mujahid MS, Aronow HD, Cene CW, Dickson VV, Havranek E, Morgenstern LB, Paasche‐Orlow MK, Pollak A, Willey JZ; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council . Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation. 2018;138:e48–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Antithrombotic Trialists C, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ikeda Y, Shimada K, Teramoto T, Uchiyama S, Yamazaki T, Oikawa S, Sugawara M, Ando K, Murata M, Yokoyama K, Ishizuka N. Low‐dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312:2510–2520. [DOI] [PubMed] [Google Scholar]
- 41. Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, Sandercock PA, Fox KA, Lowe GD, Murray GD; Aspirin for Asymptomatic Atherosclerosis Trialists . Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–848. [DOI] [PubMed] [Google Scholar]
- 42. Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators . Low‐dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. [DOI] [PubMed] [Google Scholar]
- 43. Saito Y, Okada S, Ogawa H, Soejima H, Sakuma M, Nakayama M, Doi N, Jinnouchi H, Waki M, Masuda I, Morimoto T; Investigators JT . Low‐dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10‐year follow‐up of a randomized controlled trial. Circulation. 2017;135:659–670. [DOI] [PubMed] [Google Scholar]
- 44. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques‐Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group . 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, Tendera M, Tognoni G, ARRIVE Executive Committee . Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;392:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, Kirpach B, Shah RC, Ives DG, Storey E, Ryan J, Tonkin AM, Newman AB, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Donnan GA, Gibbs P, Johnston CI, Radziszewska B, Grimm R, Murray AM; ASPREE Investigator Group . Effect of aspirin on all‐cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Group ASC , Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 48. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 50. Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, Gibson CM, Pollack CV Jr, Ornato JP, Zalenski RJ, Penney J, Tiefenbrunn AJ, Greenland P; NRMI Investigators . Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306:2120–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daviglus ML, Talavera GA, Aviles‐Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil‐Smoller S, Sorlie PD, Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. National Cholesterol Education Program Expert Panel on Detection National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]


