Abstract
Background
Genetic variation in catechol‐O‐methyltransferase (COMT), a key enzyme in estrogen and catecholamine metabolism, has plausible physiological links to cardiovascular disease (CVD) and its risk factors. In WHS (Women's Health Study), COMT variants rs4818 and rs4680 were associated with a lower risk of CVD among women receiving placebo but not aspirin, suggesting a possible role of COMT in thrombosis.
Methods and Results
To evaluate potential pathways linking COMT with CVD, and COMT effect modification of aspirin in prevention, we examined COMT association with CVD risk and subclinical measures, coronary artery calcium, and carotid intima‐media thickness in MESA (Multi‐Ethnic Study of Atherosclerosis). In 65 957 person‐years of follow‐up, during which 498 events occurred, COMT rs4818 was associated with lower CVD risk (hazard ratio, 0.85; 95% CI, 0.74–0.97 [P=0.02]). This association remained virtually unchanged after adjusting for common CVD risk factors. Fibrinogen was the only risk factor associated with rs4818 (β, −3.65; SE, 1.35 mg/dL [P=0.007]). Results were directionally similar but not significant for rs4680. Adjusted hazard ratios for COMT rs4818 CVD association were 0.79 (95% CI, 0.65–0.95; P=0.02) among individuals who used aspirin <3 days per week and 0.89 (95% CI, 0.71–1.13; P=0.34) among more frequent users (P interaction=0.39). Neither intima‐media thickness nor coronary artery calcium was associated with COMT.
Conclusions
In a multiethnic prospective cohort of men and women, the COMT rs4818G allele was associated with lower CVD risk and lower fibrinogen levels but not with radiographic measures of subclinical atherosclerosis. These results suggest a plausible role of COMT in the latter stages of CVD.
Keywords: aspirin, cardiovascular disease risk factors, catecholamine, catecholaminergic polymorphic ventricular tachycardia, catechol‐O‐methyltransferase
Subject Categories: Cardiovascular Disease; Epidemiology; Risk Factors; Race and Ethnicity; Genetic, Association Studies
Clinical Perspective
What Is New?
A common coding variant in the catechol‐O‐methyltransferase locus (rs4818) was associated with lower rates of incident cardiovascular disease in MESA (Multi‐Ethnic Study of Atherosclerosis) cohort.
Two variants (rs4818 and rs4680) linked closely in white populations exhibited much less linkage in other populations, enabling separate analyses that were generally stronger for the former variant.
What Are the Clinical Implications?
The catechol‐O‐methyltransferase variant was also associated with lower levels of fibrinogen, but not with radiographic measures of subclinical atherosclerosis, suggesting that the underlying mechanism of action likely impacts the latter stages of cardiovascular disease.
Introduction
Genetic variation in catechol‐O‐methyltransferase (COMT), a key enzyme in catecholamine metabolism, has plausible physiological links to both cardiovascular disease (CVD) and its risk factors.1 COMT metabolizes catechol estrogen and the catecholamines epinephrine, norepinephrine, and dopamine by catalyzing the transfer of a methyl group from S‐adenosyl methionine onto the catechol moieties.2 COMT links to estrogen regulation are made more complex by the presence of estrogen receptor response elements in the COMT promoter region,3 and the downregulatory effects of estradiol on COMT.4 These estradiol effects are thought to contribute to sexual variation in COMT effects, with lower activity in women compared with men.5, 6 COMT is expressed in platelets,7 and the highest levels are expressed in the liver and adrenal glands.8 COMT's roles in reducing the toxic effects of catechol estrogen exposure and catecholamine flux are important in maintaining cardiovascular and renal function. Hence, rs4818 and rs4680, genetic single nucleotide polymorphisms (SNPs) in COMT, have been associated with CVD,1, 9 hypertension,1, 10, 11, 12, 13 type 2 diabetes mellitus,14, 15 and preeclampsia.16, 17 The rs4680 variant encodes a functional G (Val) to A (Met) substitution that results in a 3‐ to 4‐fold reduction in COMT enzyme activity. Both rs4680 and rs4818 disrupt stability of COMT mRNA and have effects related to COMT mRNA secondary structure and gene expression.18 We previously demonstrated that both of these COMT variants were associated with rates of CVD in women of European ancestry1 in the genetics cohort of the WHS (Women's Health Study),19 the WGHS (Women's Genome Health Study),20 and men and women in CARDIoGRAM (Coronary Artery Disease Genome‐Wide Replication and Meta‐Analysis).21 In the WGHS and large international consortia, COMT was also associated with several CVD risk factors including systolic blood pressure (BP),1 triglycerides, and glycated hemoglobin (HbA1c) levels.14, 15 We also observed a significant COMT‐aspirin interaction, such that the statistically significant 33% CVD protection associated with the G alleles of rs4818 and rs4680 was attenuated with randomized assignment to aspirin (P interaction<0.001), suggesting that benefit may result from antiplatelet activity that is superseded by aspirin. However, the WGHS comprised a white population of women aged 45 years or older, limiting the generalizability of the WGHS findings. Further, the WGHS included few physiological measures with which to evaluate potential pathophysiologic pathways of CVD. To address more fully the cardiovascular effects of the COMT locus on rates of clinical and subclinical CVD and the potential interaction with aspirin, we studied these factors in MESA (Multi‐Ethnic Study of Atherosclerosis), an observational study of individuals of European, African, Asian, and Hispanic ancestry free of CVD at baseline.
Materials and Methods
Data for the National Heart, Lung, and Blood Institute's SHARe (SNP Health Association Resource), a substudy of the MESA cohort used in this analysis, is publically available through dbGaP (Study Accession: phs000420.v6.p3). The methods and materials used in this analysis are available to any researcher for purposes of reproducing the results or replicating the procedures.
The MESA Cohort
The MESA cohort includes 6814 participants from 6 field centers in the United States: Baltimore, Maryland; Chicago, Illinois; New York, New York; Forsyth County, North Carolina; Los Angeles, California; and St. Paul, Minnesota. Participants self‐identified as white (38%), black (28%), Asian (12%), or Hispanic (22%). Specifics of the MESA design have been previously reported.22 At baseline, participants were free from CVD (as defined by physician‐diagnosed angina, stroke, myocardial infarction, transient ischemic attack, heart failure, or resuscitated cardiac arrest). Since the initiation of the study in 2000, participants have undergone 5 in‐person examinations: baseline (July 17, 2000 to August 29, 2002) and 4 follow‐up examinations, examination 2 (September 9, 2002, to February 7, 2004), examination 3 (March 10, 2004, to September 16, 2005), examination 4 (September 23, 2005, to May 30, 2007), and examination 5 (April 19, 2010, to February 4, 2012). MESA was conducted under institutional review board approval and oversight and with informed consent of participants. The study was performed in accord with the principles of the Declaration of Helsinki. This study was conducted under institutional review board approval and oversight from Partners HealthCare.
Outcome Measures
Our primary cardiovascular outcome was defined as myocardial infarction, stroke, resuscitated cardiac arrest, and death from stroke or coronary heart disease. We prespecified this outcome to be similar to the composite primary CVD outcome in the WGHS, which encompassed myocardial infarction, stroke, or death from CVD. CVD events in MESA were assessed at intervals of 9 to 12 months, between 2000 and 2013, by contacting participants or family members about CVD outpatient diagnoses and procedures, hospitalizations, and deaths. Self‐reports were verified by review of death certificates and medical records for all hospitalizations and selected outpatient cardiovascular diagnoses and procedures. Two MESA physicians independently reviewed and classified events; disagreements were adjudicated by the MESA mortality and morbidity review committee.
Data on CVD risk factors including smoking status, medical history of diabetes mellitus, and hypertension were collected using questionnaires and laboratory evaluation at each examination. Body mass index was calculated as weight/height (kg/m2). BP was determined as the average of the second and third measurements taken after 5 minutes of seated rest. Laboratory measures including HbA1c, fasting glucose, cholesterol, lipoprotein, and triglyceride levels were performed on blood samples following a 12‐hour fast.
Carotid intima‐media thickness (IMT) was assessed at examination 1 by B‐mode ultrasonography of the right and left near and far walls of the internal and common carotid arteries as previously described.23, 24 Maximum IMT from examination 1 was computed as previously described by creating a composite Z score based on the standardized 2 carotid IMT site measurements from the common and internal carotid artery.24 When only one of the measures was available, that one was used. Coronary artery calcium (CAC) was assessed by chest computed tomography.24 Agatston scores of CAC were computed from phantom‐adjusted coronary artery plaque density and area from replicate scans taken at the 5 examinations. CAC was stratified according to absolute cut points of Agatston scores <1, 1 to 100, 101 to 400, and >400,25 and COMT association with these categories over the 5 MESA examinations was assessed using a repeated measures analysis.
The medication inventory method was used to assess aspirin use at each examination. Self‐reported frequency of aspirin use at least 3 days per week was recorded at examinations 1 through 5.
COMT SNPs rs4818 and rs4680 were genotyped using the Illumina CARe iSelect (IBC) chip. Of the 6814 participants in MESA, 6316 had genotyping data available for COMT rs4818.
Statistical Analysis
Cox proportional hazard models were used to assess genetic associations with incident CVD, stroke, and myocardial infarction assuming a standard additive genetic model. Models were either adjusted for age, sex, and site or fully adjusted for the latter plus cardiovascular risk factors, which included medical history of hypertension and diabetes mellitus, smoking status, systolic and diastolic BP, cholesterol, triglycerides, high‐density lipoprotein levels, and fibrinogen. In all cases, we performed race/ethnicity‐stratified analyses adjusting for population substructure with 5 race‐specific principal components. COMT association with CVD has not been studied in black and Hispanic populations. Based on the WGHS, we estimated that we would be adequately powered to observe a difference by COMT genotype if the frequency of the rs4680G allele was >0.30 (Table S1). COMT expression is regulated by estrogen and COMT is also a key enzyme in estrogen metabolism. In the WGHS, our initial observation of COMT effects on CVD were among white women, 44% of whom took exogenous estrogen hormone replacement therapy (HRT). In MESA, 68% of white women reported HRT use in examination 1. Hence, in addition to secondary analyses stratified by sex, we conducted sensitivity analyses in white women stratified by ever/never use of HRT and examined potential COMT effect modification of HRT ever/never use. For each model, the proportionality assumption was verified. Analyses were performed in SAS 9.4 (SAS Institute).
Linear regression was used to evaluate COMT cross‐sectional associations with the IMT composite Z score and baseline risk factors: systolic BP, diastolic BP, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, fasting glucose, HbA1c, fibrinogen, and triglycerides for each race/ethnicity subgroup and then meta‐analyzed for the overall associations. For right‐skewed data (triglycerides), we determined SE estimates using robust SEs. Models for BP and lipids were adjusted for hypertensive and lipid‐lowering medication use, respectively. For CAC, Agatston scores were categorized as previously described26 and updated for each examination. Multinomial logistic regression was used to examine COMT cross‐sectional association with CAC in models adjusted for age, sex, site, and the first 5 principal components for race/ethnicity.
Modification of the COMT association with incidence of CVD by aspirin use was tested on the Cox model coefficients with a term corresponding to the cross‐product of allele number and aspirin use categorized as 0 (not currently used or used <3 times per week) or 1 (used currently at least 3 times per week). Because aspirin use changed over time, and because the proposed mechanism of action for aspirin involves irreversible platelet inhibition, we used a simple time‐varying covariate for aspirin use that was updated at each examination. We also conducted sensitivity analyses stratified by HRT ever/never use specifically among white women to approximate most closely the WGHS population previously analyzed. Individual race/ethnicity COMT and COMT by aspirin use interaction estimates were meta‐analyzed across races using inverse variance fixed effects models in Comprehensive Meta‐Analysis, version 3.3.070 (Biostat, New Jersey).
Results
Participant Characteristics
Characteristics of the study sample at baseline stratified by race are reported in Table 1 and those stratified by rs4818 are reported in Table S2. Age and sex distributions did not differ by race. The allele frequencies of rs4818 and rs4680 (Table S3) varied by race/ethnicity. Both SNPs were in Hardy‐Weinberg equilibrium within each race/ethnicity subgroup (P>0.05). In the overall population, the minor allele frequencies for rs4818 (G allele) and rs4680 (A(Met) allele), were 32% and 40%, respectively. Linkage disequilibrium and correlation between the COMT SNPs was strongest for whites (0.72) and lower among others (0.12–0.24).
Table 1.
Baseline Characteristics of MESA Participants Genotyped for rs4818 by Race
| Race | ||||
|---|---|---|---|---|
| White | Black | Hispanic | Asian | |
| Participants, No. (%) | 2481 | 1639 | 1428 | 768 |
| Age, y | 62.7 (10.3) | 62.2 (10.1) | 61.4 (10.3) | 62.3 (10.4) |
| Women, % | 1304 (52.5) | 903 (54.0) | 740 (51.7) | 389 (50.7) |
| History of diabetes mellitus, % | 150 (6.8) | 285 (20.2) | 255 (21.2) | 101 (16.0) |
| History of hypertension, % | 967 (38.9) | 989 (59.2) | 603 (42.1) | 288 (37.5) |
| Current smoker, % | 286 (11.5) | 306 (18.3) | 192 (13.4) | 44 (5.7) |
| Body mass index, kg/m2 | 27.7 (5.1) | 30.2 (5.9) | 29.5 (5.2) | 24.0 (3.3) |
| Systolic BP, mm Hg | 123.6 (20.5) | 131.8 (21.8) | 126.9 (22.1) | 124.4 (21.7) |
| Diastolic BP, mm Hg | 70.2 (10.0) | 74.6 (10.3) | 71.6 (10.2) | 71.9 (10.3) |
| HDL cholesterol, mg/dL | 52.4 (15.8) | 52.3 (15.2) | 47.5 (13.0) | 49.3 (12.4) |
| Triglycerides, mg/dL | 133.1 (90.5) | 104.9 (69.9) | 158.3 (102.2) | 143.2 (85.9) |
| Total cholesterol, mg/dL | 195.8 (35.4) | 189.5 (36.4) | 198.3 (37.7) | 192.5 (31.5) |
| COMT rs4818 MAF (G) | 0.41 | 0.21 | 0.27 | 0.33 |
| COMT rs4680 MAF (A) | 0.51 | 0.31 | 0.39 | 0.28 |
Numbers in parentheses are expressed as SD unless otherwise indicated. BP indicates blood pressure; COMT, catechol‐O‐methyltransferase; HDL, high‐density lipoprotein; MAF, minor allele frequency; MESA, Multi‐Ethnic Study of Atherosclerosis.
COMT Association With CVD Risk Factors and Subclinical Disease
In meta‐analyses of CVD risk factors across races/ethnicities, only fibrinogen was significantly associated with COMT rs4818 (Table 2 and Table S4). In gene‐dosage models of fibrinogen, the direction of the rs4818 association was consistent across the races/ethnicities (β, −3.65 mg/dL; SE, 1.35 [P=0.007]) and was strongest and statistically significant among Asians. In secondary analyses using sex‐stratified models, the rs4818 association was negative for both women (β, −5.29 mg/dL; SE, 1.93 [P=0.006]) and men (β, −2.28 mg/dL; SE, 2.98 [P=0.44]). In contrast, the overall association of rs4680 with fibrinogen was null, with significant differences in direction between women (β, −2.98 mg/dL; SE, 1.83 [P=0.10]) and men (β, 4.19 mg/dL; SE, 1.75 [P=0.02]) (P interaction=0.005) (Table S4).
Table 2.
Effect Estimates and Standard Error of COMT rs4818 (Per G Allele) and rs4680 (Per Val Allele) Association With Baseline Fibrinogen Levels (mg/dL) in Gene‐Dosage Models
| SNP | Sex | All | White | Black | Hispanic | Asian | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P Value | β | SE | P Value | β | SE | P Value | β | SE | P Value | β | SE | P Value | ||
| rs4818 | All | −3.65 | 1.35 | 0.007 | −0.81 | 2.11 | 0.70 | −2.29 | 3.03 | 0.45 | −4.94 | 3.02 | 0.10 | −9.70 | 3.06 | 0.002 |
| Women | −5.29 | 1.93 | 0.01 | −4.70 | 4.69 | 0.32 | −2.92 | 4.40 | 0.51 | −3.78 | 2.89 | 0.19 | −11.76 | 4.42 | 0.01 | |
| Men | −2.28 | 2.98 | 0.44 | −0.42 | 4.35 | 0.92 | −8.00 | 4.46 | 0.07 | 3.53 | 2.80 | 0.21 | −6.90 | 4.53 | 0.13 | |
| rs4680 | All | 0.53 | 1.29 | 0.68 | 0.84 | 1.96 | 0.67 | 1.70 | 2.78 | 0.54 | −1.43 | 2.89 | 0.62 | 0.54 | 3.31 | 0.87 |
| Women | −2.98 | 1.83 | 0.10 | −3.05 | 2.81 | 0.28 | −0.44 | 4.09 | 0.92 | −7.68 | 4.00 | 0.06 | −0.02 | 4.45 | 0.99 | |
| Men | 4.19 | 1.75 | 0.02 | 5.05 | 2.69 | 0.06 | 4.01 | 3.71 | 0.28 | 5.09 | 4.08 | 0.21 | 1.37 | 4.20 | 0.74 | |
COMT indicates catechol‐O‐methyltransferase; SNP, single nucleotide polymorphism.
COMT associations with other CVD risk factors HbA1c, fasting glucose, high‐density lipoprotein cholesterol, and intercellular adhesion molecule were directionally consistent for rs4818 and rs4680 and within 2 SDs of previously reported statistically significant associations (Table S4).1, 14 Adjustment for BP medication or lipid medication use in lipid and systolic and diastolic BP models were similarly null.
No statistically significant associations with carotid artery IMT at examination 1 were observed for COMT rs4818 (β, −0.004; SE, 0.014 [P=0.76]) and rs4680 (β, −0.019; SE, 0.014 [P=0.17]) (Table 3). Further, no statistically significant associations were observed for COMT categories of CAC over the 5 examinations for rs4818 (odds ratio, 1.00; 95% CI, 0.93–1.08 [P=0.93]) or rs4680 (odds ratio, 1.00; 95% CI, 0.93–1.08 [P=0.96]). These results did not differ substantively with stratification by sex.
Table 3.
COMT rs4818 and rs4680 Gene‐Dosage (Per Allele) Association With Carotid IMT Composite Z Scorea and CAC Overall and by Race
| Race/Ethnicity | IMT | CAC | ||
|---|---|---|---|---|
| rs4818 | rs4680 | rs4818 | rs4680 | |
| β (SE), P Value | β (SE), P Value | β (SE), P Value | β (SE), P Value | |
| All | −0.004 (0.014), 0.76 | −0.019 (0.014), 0.17 | 1.00 (0.93–1.08), 0.93 | 1.00 (0.93–1.08), 0.96 |
| White | −0.014 (0.022), 0.52 | −0.003 (0.021), 0.16 | 0.94 (0.85–1.05), 0.28 | 0.95 (0.86–1.06), 0.34 |
| Black | 0.006 (0.031), 0.85 | −0.007 (0.029), 0.81 | 1.06 (0.90–1.25), 0.46 | 1.05 (0.91–1.21), 0.54 |
| Hispanic | −0.011 (0.031), 0.73 | −0.006 (0.027), 0.82 | 1.05 (0.90–1.24), 0.52 | 1.10 (0.95–1.28), 0.20 |
| Asian | 0.018 (0.037), 0.62 | −0.027 (0.038), 0.48 | 1.09 (0.89–1.33), 0.40 | 0.92 (0.75–1.14), 0.45 |
Intima‐media thickness (IMT) composite Z score is based on the standardized 2 carotid IMT site measurement from the common and internal carotid artery. CAC indicates coronary artery calcium; COMT, catechol‐O‐methyltransferase.
COMT Association With Incident CVD
MESA documented 524 primary incident CVD events during 65 957 person‐years of follow‐up. Consistent with our findings in the WGHS and CARDIoGRAM, the rs4818G allele was associated with a 15% lower rate of CVD (hazard ratio [HR], 0.85; 95% CI, 0.74–0.97 [P=0.02]) across the 4 racial/ethnic groups (Table 4 and Figure—Panel A). The association was directionally consistent in each of the race/ethnicity populations and significant among Hispanics. This inverse association with CVD risk was not attenuated by adjustment for CVD risk factors: body mass index, triglycerides, high‐density lipoprotein, cholesterol, systolic BP, history of smoking, diabetes mellitus, hypertension, or fibrinogen. The overall direction of the rs4680 Val allele association with CVD was consistent with rs4818 but was statistically nonsignificant (Table 4 and Figure—Panel B). No substantive changes were observed in models adjusted for risk factors (including fibrinogen) or stratified by sex (Table S5).
Table 4.
COMT rs4818 and rs4680 Gene‐Dosage (Per Allelea) Association With Rates of CVD in MESA
| SNP | Race/Ethnicity | Events/No. | HR (95% CI), P Value |
|---|---|---|---|
| rs4818 | All, model 1b | 498/5984 | 0.85 (0.74–0.97), 0.02 |
| All, model 2c | 497/5961 | 0.85 (0.74–0.98), 0.02 | |
| White | 203/2332 | 0.90 (0.74–1.09), 0.29 | |
| Black | 122/1518 | 0.85 (0.62–1.16), 0.32 | |
| Hispanic | 130/1375 | 0.71 (0.53–0.95), 0.02 | |
| Asian | 43/759 | 0.90 (0.74–1.09), 0.29 | |
| rs4680 | All, model 1b | 524/6157 | 0.95 (0.84–1.08), 0.46 |
| All, model 2c | 495/6066 | 0.96 (0.85–1.09), 0.56 | |
| White | 214/2476 | 1.02 (0.84–1.23), 0.86 | |
| Black | 130/1575 | 0.97 (0.75–1.25). 0.79 | |
| Hispanic | 137/1426 | 0.94 (0.73–1.19), 0.59 | |
| Asian | 43/768 | 0.68 (0.43–1.07), 0.10 |
Allele key: rs4818 coded allele=G, reference=C; rs4680 coded allele=Val (G), reference=Met (A).
Model 1: meta‐analysis of Cox proportional models adjusted for age, sex, race, site, and the first 5 principal components specific to each of the 4 race/ethnicities.
Model 2: meta‐analysis of Cox proportional models adjusted for time‐varying cardiovascular disease (CVD) risk factors from examination 1 to 5: body mass index, triglycerides, high‐density lipoprotein, low‐density lipoprotein, cholesterol, systolic blood pressure and history of smoking, diabetes mellitus, and hypertension in addition to age, sex, race, and the first 5 principal components specific to each of the 4 race/ethnicities. COMT indicates catechol‐O‐methyltransferase; HR, hazard ratio; MESA, Multi‐Ethnic Study of Atherosclerosis; SNP, single nucleotide polymorphism.
Figure 1.
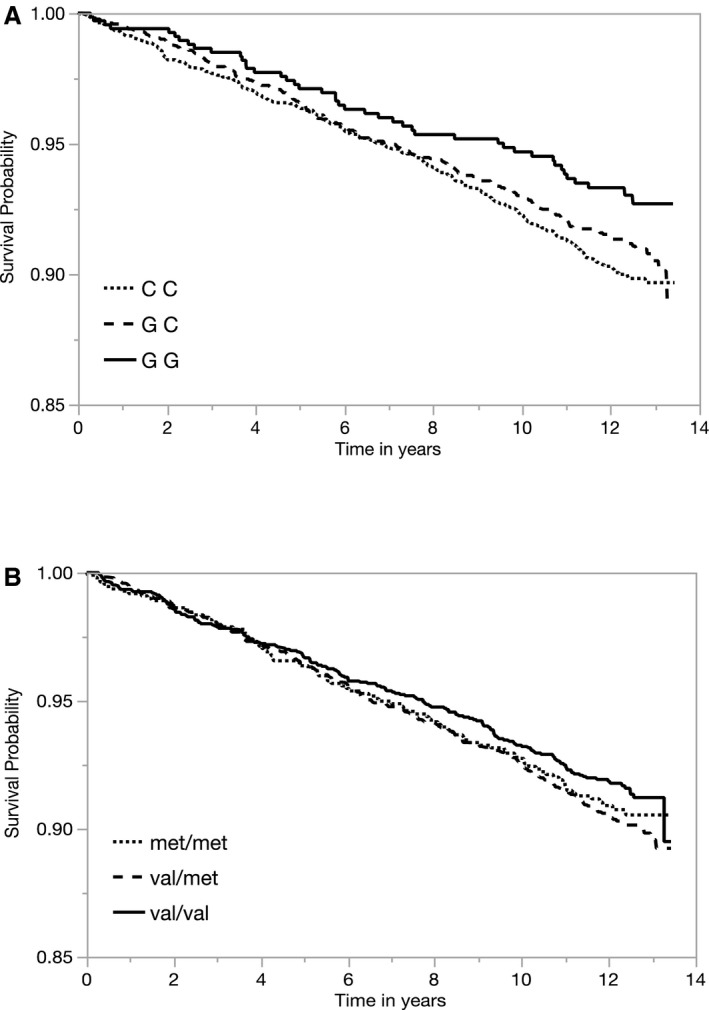
Kaplan–Meier curves of cardiovascular disease event‐free survival probability over the duration of MESA (Multi‐Ethnic Study of Atherosclerosis) (in years) by catechol‐O‐methyltransferase (COMT) (A) rs4818 and (B) rs4680 genotypes.
In the WGHS, our initial observation of COMT effects on CVD were among white women, 43% of whom took exogenous estrogen through HRT. In MESA, use of HRT varied by race, with white women using the most—64.1% used HRT at examination 1 (Table S6). In subset analyses among white women in MESA stratified by HRT use, both COMT rs4680 and rs4818G alleles were associated with higher rates of CVD among women who had never taken HRT. Among white women who had taken HRT, the COMT association was protective for rs4818 and rs4680, but only statistically significant for rs4818 (Table S6). A test for a COMT‐HRT interaction was significant for both SNPs (P interaction<0.05). The COMT‐HRT interaction was not observed among women in the other race/ethnic subpopulations.
COMT and Aspirin
Aspirin use >3 days per week appeared to increase across the 5 examinations, but did not vary by COMT rs4818 genotype (Table S7). When stratified by time‐varying aspirin use over the 5 examinations, the COMT rs4818G allele was statistically significantly associated with lower rates of CVD among individuals who used aspirin <3 days per week (HR, 0.79; 95% CI, 0.65–0.95 [P=0.02]); the corresponding HR among those who used aspirin ≥3 days per week was 0.89 (95% CI, 0.71–1.13; P=0.34 [P interaction=0.39]) (Table 5). There was no difference in rates of CVD associated with COMT rs4680 by aspirin use.
Table 5.
COMT rs4818 and rs4680 Gene‐Dosage (Per Allelea) Association With Rates of CVD Stratified by Aspirin Use Overall and by Race
| Race/Ethnicity | Aspirin <3 d/wk | Aspirin ≥3 d/wk | P interaction | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Overallb | 0.79 (0.65–0.95) | 0.02 | 0.89 (0.71–1.13) | 0.34 | 0.39 |
| White | 0.83 (0.61–1.11) | 0.21 | 0.90 (0.66–1.22) | 0.34 | 0.70 |
| Black | 0.65 (0.40–1.04) | 0.07 | 1.27 (0.77–2.11) | 0.38 | 0.06 |
| Hispanic | 0.70 (0.48–1.02) | 0.06 | 0.65 (0.39–1.08) | 0.10 | 0.81 |
| Asian | 1.09 (0.63–1.89) | 0. 76 | 0.19 (0.01–2.40) | 0.20 | 0.21 |
COMT indicates catechol‐O‐methyltransferase; CVD, cardiovascular disease; HR, hazard ratio.
Allele key: rs4818 coded allele=G, reference=C.
Meta‐analysis of Cox proportional models adjusted for age, sex, race, site, and the first 5 principal components specific to each of the 4 race/ethnicities.
In subset analyses of white women stratified by HRT use at examination 1 and aspirin <3 days per week, COMT rs4818 (HR, 0.34; 95% CI, 0.16–0.69 [P=0.004]) and rs4680 (HR, 0.46; 95% CI, 0.25–0.85 [P=0.01]) G alleles were associated with lower rates of CVD. These associations were attenuated among patients using HRT and aspirin ≥3 days per week, with a significant interaction for rs4680 but not rs4818 (Table S8). Among white women who did not use HRT, rates of CVD were higher with or without aspirin use.
Discussion
In MESA, we replicated our previous finding from the WGHS and CARDIoGRAM,5 that the COMT rs4818G allele is associated with lower rates of CVD. This association was similar across race/ethnicity groups and remained significant after adjusting for CVD risk factors. The overall direction of COMT associations with CVD risk factors tended to be consistent with previous findings for systolic BP,1, 10, 11, 12, 13 HbA1c,14 and triglyceride levels,1, 27 but they were not statistically significant in the smaller and more diverse MESA sample. The only risk factor significantly associated with COMT in MESA was fibrinogen. The direction of this association was consistent with a nonsignificant effect observed in the WGHS.1 We did not observe effect modification of the rs4818 association with CVD by aspirin use. With the exception of systolic BP, these findings were directionally similar, but statistically nonsignificant for rs4680. Further, neither COMT SNP was associated with carotid artery IMT or CAC. In contrast to the European ancestry of the WGHS participants, the MESA population is representative of multiple racial/ethnic groups and consists of both men and women. Hence, our findings of significant COMT rs4818 effects on CVD in MESA allow generalization to men, blacks, and Hispanics, where the findings were for the most part consistent. Among Asians, who represented the smallest subpopulation in MESA, the main associations were nonsignificant, although the COMT association with fibrinogen in this group was significant. Whether the modest differences across races represent the play of chance or specific differences in linkage by race will also require dedicated study in larger samples.
These results were not wholly consistent with our original observations in the WGHS where the population we examined consisted of white women, older than 45 years, of whom 43% reported taking estrogen HRT at baseline. In MESA, a majority of white women (64%) reported having used HRT at examination 1. Given the modulatory effects of estrogen on COMT gene expression and COMT's role in estrogen metabolism (converting catechol estrogens to 2‐ and 4‐methoxyestradiol), we conducted stratified analyses among a subset of the MESA cohort most similar to the WGHS population. These subset analyses suggested that the discrepancy between the WGHS and MESA may indeed be attributed to differences in estrogen levels related to sex and HRT use. However, neither the WGHS or MESA were designed to look at the influence of COMT and HRT on the incidence of CVD. Therefore, these findings are hypothesis generating and warrant examination in other cohorts. Further, in the WGHS, the associations of rs4818 and rs4680 with CVD were similar, as the 2 are highly linked in most white populations. However, in the racially diverse MESA sample, where the linkage was much weaker among nonwhite participants, the CVD association with rs4680 was not significant, suggesting that either rs4680 is not the causal locus or its effects on COMT activity or gene expression levels are potentially sensitive to estrogen. Still, much finer mapping, and likely whole genome sequencing, in similarly diverse samples will be needed to determine whether variation at rs4818, which appears to disrupt mRNA stability,18 is the causal locus for the CVD effects observed here.
IMT and CAC have both been shown to be associated with incident CVD in MESA and elsewhere.28 Our findings that COMT was not clearly associated with either subclinical measure suggests that this locus is likely to influence risk of CVD through mechanisms other than atherosclerosis. Notably, the effect estimates for intercellular adhesion molecule, SBP, and HbA1c were directionally consistent with the WGHS and the Diabetes Prevention Program,1, 14 although, in the smaller more diverse MESA cohort, the associations were nonsignificant. The decidedly null results for subclinical disease, when combined with the suggestive effects for interaction with aspirin and the observed effect on fibrinogen, tend to suggest that COMT is most likely to exert its effects on the progression of subclinical to clinical CVD rather than on the development of subclinical disease per se.
COMT is expressed in platelets, where catecholamine flux influences development of thrombotic events via platelet activation.29 Thus, genetically derived variation in COMT functionality could modify circulating and platelet levels of catecholamines, in turn shifting the threshold for vascular events. The link between COMT, platelets, and estrogen may also contribute to its effects in cancer development.30, 31 Interestingly, the fibrinogen gene has a corticosteroid response element in its regulatory region,32 and fibrinogen levels are influenced by estrogen in women who use HRT,33, 34 pointing to a potential link between catecholamines, COMT, and fibrinogen.
It is important to acknowledge that some of our results, and especially those in smaller subgroups, had wide CIs, reflecting smaller numbers of participants and events. Results in these smaller subgroups should be treated as hypothesis‐generating and, if possible, confirmed in other multiethnic cohorts that may have similar information.
Conclusions
These results build upon our previous work in the WGHS in demonstrating that common genetic variation in COMT is associated with risk of CVD in a multiracial population of men and women.
Sources of Funding
Hall is supported by the National Heart, Lung, and Blood Institute (NHLBI) K01HL130625 and Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. Kaptchuk is supported by National Center for Complementary and Integrative Health 2K24 AT004095. MESA and the MESA SHARe project are conducted and supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, N01‐HC‐95169, UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 through NHLBI. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, and the National Institute of Diabetes and Digestive and Kidney Diseases Diabetes Research Center grant DK063491 to the Southern California Diabetes Endocrinology Research Center. Funding for SHARe genotyping was provided by NHLBI contract N02‐HL‐64278. Genotyping was performed at Affymetrix (Santa Clara, California) and the Broad Institute of Harvard and MIT (Boston, Massachusetts) using the Affymetrix Genome‐Wide Human SNP Array 6.0.
Disclosures
Psaty serves on the steering committee of the Yale University Open Data Access Project funded by Johnson & Johnson. The remaining authors have no disclosures to report.
Supporting information
Table S1. Power Estimates of HRs for Incident CVD Based on rs4680G(Val) Allele Frequencies Over a Range of GAF From 0.3 to 0.6
Table S2. Demographics, Baseline Characteristics, and Aspirin Use by COMT rs4818 Genotype Use in MESA
Table S3. Distribution of COMT rs4818 and rs4680 Genotypes by Race in MESA
Table S4. Parameter Estimates (β and SE) of COMT rs4818 (Per G Allele) and rs4680 (Per Val Allele) Association With Baseline Fibrinogen Levels (mg/dL) in Gene‐Dosage Models Overall and Stratified by Sex
Table S5. COMT rs4818 and rs4680 Gene‐Dosage (Per Allele*) Association With Rates of CVD Stratified by Race and Sex
Table S6. HRT Use Among Women Assessed at Examination 1 and Rates of CVD by COMT rs4818 and rs4680 Genotype Stratified by HRT Use and Race
Table S7. Aspirin User (%) >3 Days Per Week by COMT rs4818 Genotype at the 5 MESA Examinations
Table S8. COMT rs4818 and rs4680 Gene‐Dosage (Per Allele*) Association With Rates of CVD Stratified by Time‐Varying Aspirin and HRT Ever Use Among White Women
(J Am Heart Assoc. 2019;8:e014986 DOI: 10.1161/JAHA.119.014986.)
References
- 1. Hall KT, Nelson CP, Davis RB, Buring JE, Kirsch I, Mittleman MA, Loscalzo J, Samani NJ, Ridker PM, Kaptchuk TJ, Chasman DI. Polymorphisms in catechol‐O‐methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:2160–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mannisto PT, Kaakkola S. Catechol‐O‐methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 3. Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down‐regulation of human catechol‐O‐methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. [DOI] [PubMed] [Google Scholar]
- 4. Jiang H, Xie T, Ramsden DB, Ho SL. Human catechol‐O‐methyltransferase down‐regulation by estradiol. Neuropharmacology. 2003;45:1011–1018. [DOI] [PubMed] [Google Scholar]
- 5. Tunbridge EM, Harrison PJ. Importance of the COMT gene for sex differences in brain function and predisposition to psychiatric disorders. Curr Top Behav Neurosci. 2011;8:119–140. [DOI] [PubMed] [Google Scholar]
- 6. Yager JD. Catechol‐O‐methyltransferase: characteristics, polymorphisms and role in breast cancer. Drug Discov Today Dis Mech. 2012;9:e41–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stramentinoli G, Gualano M, Algeri S, de Gaetano G, Rossi EC. Catechol‐O‐methyl transferase (COMT) in human and rat platelets. Thromb Haemost. 1978;39:238–239. [PubMed] [Google Scholar]
- 8. Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjostedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlen M. Analysis of the human tissue‐specific expression by genome‐wide integration of transcriptomics and antibody‐based proteomics. Mol Cell Proteomics. 2014;13:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bialecka M, Kurzawski M, Roszmann A, Robowski P, Sitek EJ, Honczarenko K, Gorzkowska A, Budrewicz S, Mak M, Jarosz M, Golab‐Janowska M, Koziorowska‐Gawron E, Drozdzik M, Slawek J. Association of COMT, MTHFR, and SLC19A1(RFC‐1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson's disease. Pharmacogenet Genomics. 2012;22:716–724. [DOI] [PubMed] [Google Scholar]
- 10. Liu C, Kraja AT, Smith JA, Brody JA, Franceschini N, Bis JC, Rice K, Morrison AC, Lu Y, Weiss S, Guo X, Palmas W, Martin LW, Chen YD, Surendran P, Drenos F, Cook JP, Auer PL, Chu AY, Giri A, Zhao W, Jakobsdottir J, Lin LA, Stafford JM, Amin N, Mei H, Yao J, Voorman A; Consortium CHDE, Exome BPC, Go TDC, Consortium TDG , Larson MG, Grove ML, Smith AV, Hwang SJ, Chen H, Huan T, Kosova G, Stitziel NO, Kathiresan S, Samani N, Schunkert H, Deloukas P; Myocardial Infarction G, Consortia CAE , Li M, Fuchsberger C, Pattaro C, Gorski M; Consortium CK , Kooperberg C, Papanicolaou GJ, Rossouw JE, Faul JD, Kardia SL, Bouchard C, Raffel LJ, Uitterlinden AG, Franco OH, Vasan RS, O'Donnell CJ, Taylor KD, Liu K, Bottinger EP, Gottesman O, Daw EW, Giulianini F, Ganesh S, Salfati E, Harris TB, Launer LJ, Dorr M, Felix SB, Rettig R, Volzke H, Kim E, Lee WJ, Lee IT, Sheu WH, Tsosie KS, Edwards DR, Liu Y, Correa A, Weir DR, Volker U, Ridker PM, Boerwinkle E, Gudnason V, Reiner AP, van Duijn CM, Borecki IB, Edwards TL, Chakravarti A, Rotter JI, Psaty BM, Loos RJ, Fornage M, Ehret GB, Newton‐Cheh C, Levy D, Chasman DI. Meta‐analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet. 2016;48:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Annerbrink K, Westberg L, Nilsson S, Rosmond R, Holm G, Eriksson E. Catechol O‐methyltransferase val158‐met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism. 2008;57:708–711. [DOI] [PubMed] [Google Scholar]
- 12. Htun NC, Miyaki K, Song Y, Ikeda S, Shimbo T, Muramatsu M. Association of the catechol‐O‐methyl transferase gene Val158Met polymorphism with blood pressure and prevalence of hypertension: interaction with dietary energy intake. Am J Hypertens. 2011;24:1022–1026. [DOI] [PubMed] [Google Scholar]
- 13. Miyaki K, Htun NC, Song Y, Ikeda S, Muramatsu M, Shimbo T. The combined impact of 12 common variants on hypertension in Japanese men, considering GWAS results. J Hum Hypertens. 2012;26:430–436. [DOI] [PubMed] [Google Scholar]
- 14. Hall KT, Jablonski KA, Chen L, Harden M, Tolkin BR, Kaptchuk TJ, Bray GA, Ridker PM, Florez JC; Diabetes Prevention Program Research G , Mukamal KJ, Chasman DI. Catechol‐O‐methyltransferase association with hemoglobin A1c. Metabolism. 2016;65:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanasaki M, Srivastava SP, Yang F, Xu L, Kudoh S, Kitada M, Ueki N, Kim H, Li J, Takeda S, Kanasaki K, Koya D. Deficiency in catechol‐O‐methyltransferase is linked to a disruption of glucose homeostasis in mice. Sci Rep. 2017;7:7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmer K, Saglam B, Whitehead C, Stock O, Lappas M, Tong S. Severe early‐onset preeclampsia is not associated with a change in placental catechol O‐methyltransferase (COMT) expression. Am J Pathol. 2011;178:2484–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol‐O‐methyltransferase and 2‐methoxyoestradiol is associated with pre‐eclampsia. Nature. 2008;453:1117–1121. [DOI] [PubMed] [Google Scholar]
- 18. Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol‐O‐methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. [DOI] [PubMed] [Google Scholar]
- 19. Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 20. Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE; Women's Genome Health Study Working G . Rationale, design, and methodology of the Women's Genome Health Study: a genome‐wide association study of more than 25,000 initially healthy American women. Clin Chem. 2008;54:249–255. [DOI] [PubMed] [Google Scholar]
- 21. Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi‐Boehm S, Cox D, Dimitriou M, Do R, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco‐Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller‐Nurasyid M, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C, Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ. Large‐scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 23. Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima‐media thickness for cardiovascular disease prediction in the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima‐media thickness in the prediction of cardiovascular disease incidence: the Multi‐Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102:1136–1141, 1141.e1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aladin AI, Al Rifai M, Rasool SH, Dardari Z, Yeboah J, Nasir K, Budoff MJ, Psaty BM, Blumenthal RS, Blaha MJ, McEvoy JW. Relation of coronary artery calcium and extra‐coronary aortic calcium to incident hypertension (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2018;121:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho‐Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee‐Hee Ong R, Croteau‐Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O'Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, Konig IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Doring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O'Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML. Common carotid intima‐media thickness measurements in cardiovascular risk prediction: a meta‐analysis. JAMA. 2012;308:796–803. [DOI] [PubMed] [Google Scholar]
- 29. O'Brien JR. Some effects of adrenaline and anti‐adrenaline compounds on platelets in vitro and in vivo. Nature. 1963;200:763–764. [DOI] [PubMed] [Google Scholar]
- 30. Thompson PA. Finding the responders in the cancer prevention trials. JNCI: Journal of the National Cancer Institute. 2019;111:639–640. Available at: 10.1093/jnci/djy205. [DOI] [PubMed] [Google Scholar]
- 31. Hall KT, Buring JE, Mukamal KJ, Vinayaga Moorthy M, Wayne PM, Kaptchuk TJ, Battinelli EM, Ridker PM, Sesso HD, Weinstein SJ, Albanes D, Cook NR, Chasman DI. COMT and alpha‐tocopherol effects in cancer prevention: gene‐supplement interactions in two randomized clinical trials. JNCI: Journal of the National Cancer Institute. 2019;111:684–694. Available at: 10.1093/jnci/djy204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fish RJ, Neerman‐Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108:419–426. [DOI] [PubMed] [Google Scholar]
- 33. Margarido PF, Bagnoli VR, Maggio da Fonseca A, Maciel GA, Soares JM Jr, D'Amico EA, Baracat EC. Transdermal estrogen therapy effects on fibrinogen levels in women with a past history of venous thromboembolism: a pilot study. Clin Exp Obstet Gynecol. 2011;38:232–235. [PubMed] [Google Scholar]
- 34. Bonnar J. Coagulation effects of oral contraception. Am J Obstet Gynecol. 1987;157:1042–1048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Power Estimates of HRs for Incident CVD Based on rs4680G(Val) Allele Frequencies Over a Range of GAF From 0.3 to 0.6
Table S2. Demographics, Baseline Characteristics, and Aspirin Use by COMT rs4818 Genotype Use in MESA
Table S3. Distribution of COMT rs4818 and rs4680 Genotypes by Race in MESA
Table S4. Parameter Estimates (β and SE) of COMT rs4818 (Per G Allele) and rs4680 (Per Val Allele) Association With Baseline Fibrinogen Levels (mg/dL) in Gene‐Dosage Models Overall and Stratified by Sex
Table S5. COMT rs4818 and rs4680 Gene‐Dosage (Per Allele*) Association With Rates of CVD Stratified by Race and Sex
Table S6. HRT Use Among Women Assessed at Examination 1 and Rates of CVD by COMT rs4818 and rs4680 Genotype Stratified by HRT Use and Race
Table S7. Aspirin User (%) >3 Days Per Week by COMT rs4818 Genotype at the 5 MESA Examinations
Table S8. COMT rs4818 and rs4680 Gene‐Dosage (Per Allele*) Association With Rates of CVD Stratified by Time‐Varying Aspirin and HRT Ever Use Among White Women


