Abstract
Objectives:
Medication-related osteonecrosis of the jaw (MRONJ) is a well known side-effect of anti-resorptive drugs. Changes in bone density might potentially constitute the development of ONJ. This study aimed to investigate, to which degree bisphosphonates (bp) and denosumab (db) induce changes in bone density that can be determined from routine diagnostic CT.
Methods:
CT scans of 101 patients were investigated. MRONJ was present in 61 patients (n = 26: db-treated; n = 35 bp-treated). 40 patients were included as a reference group. Bone density was measured at two distinct locations in the mandible (M1: anterior of the mental foramen; M2: retromolar), each on the contralateral side to the necrosis.
Results:
The bone density values measured at both locations were found to be significantly higher in the bp-group compared to the db-group (p = 0.027) and to the reference-group (p = 0.016). Almost no difference (p = 0.84) in bone density value was found between the db- and reference-groups.Investigating the effect of duration of treatment, none of the measured values showed significant differences in both locations of db- and bp-group.
Conclusion:
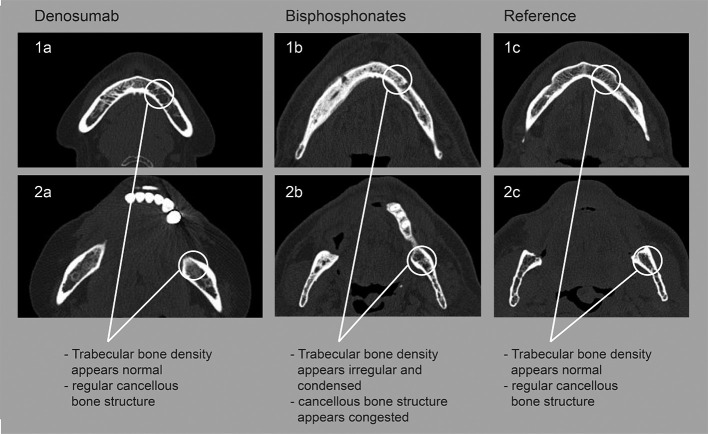
The findings from this study suggest that that bisphosphonates change the microarchitecture of the alveolar bone by being embedded in the mandible, which may subsequently lead to a bp-specific corticalization, and a decrease in vascularization of the lower jaw. This process may be distinctive for bp-treatment and seems to induce the congestion of cancellous bone rather rapidly after the first administrations. This effect could not be determined in denosumab-treated patients.
Keywords: Bone density, medication related osteonecrosis of the jaw, MRONJ, BMD, Osteonecrosis
Introduction
Bisphosphonates (bp) and denosumab (db) are widely and efficiently used in patients with increased bone resorption secondary to metastatic bone disease, osteoporosis, osteolytic lesions in multiple myeloma or Paget’s disease of the bone.1–3 However, concerns are raised by numerous reports of osteonecrosis of the jaw (ONJ) that has become a well-known severe side-effect of db and bp therapy.4 Cumulative incidences of ONJ in patients receiving these agents are currently estimated at around 10% in subjects treated with high doses.5,6 Clinical symptoms include exposed necrotic bone, gingival ulcerations, intra- or extraoral fistulas, swelling, cellulitis and pus exudation.7 In 2014, the American Association of Oral and Maxillofacial Surgeons published a position paper. Here, the presence of medication-related osteonecrosis of the jaw (MRONJ) is defined by current or previous treatment with bp or db, exposed jawbone for at least 8 weeks and no previous radiation therapy of the jaw.4 The pathogenesis of MRONJ is multifactorial but still remains not entirely understood. Several factors have been identified causing MRONJ. It is known that the inhibition of regular osteoclasts resulting in reduced bone remodelling is the fundamental pathophysiological process in the development of ONJ.8 Additional factors have been investigated by numerous authors as being associated with progression and severity of the lesions.9–11
Insufficient vascularization induced by antiresorptive drugs’ impact on bone remodelling inhibition appears to be pivot for the development of necrotic bone.12–14 A recent study by Soares et al demonstrated a bisphosphonate induced corticalization and decrease of vascularization in the jaws of Wistar rats treated with zoledronic acid.15 The authors assumed that zoledronic acid induced corticalization caused congestion of the microstructure of the bone, resulting in a decrease of blood supply and impaired healing capacity. Obliteration of nutritive canals inside the bone and a significant reduction of blood vessels were present. Likewise, other investigators presented additional data showing evidence of bisphosphonate induced decrease of blood vessels and reduction of vessel ramification in the bone.13,14
Dual-energy X-ray absorptiometry (DXA) is the most widely validated technique for bone mineral density (BMD) measurements.16 Nonetheless, several recent studies allege that routine CT measurements, performed for different indications may detect changes in bone density, without additional costs or radiation exposure.17 In CT the bone density is measured in Hounsfield units (HUs) and calculated for a certain region of interest (ROI). This method of bone density evaluation seems feasible and investigations underlined that ancillary application of contrast medium did not enhance CT performance.18
The aim of this study was to investigate changes in the alveolar bone density of patients suffering from MRONJ by analyzing CT scans of the mandible. Therefore, we evaluated differences between the bone density of patients either treated with bp or db. Presuming the hypothesis that we would detect denser bone using HU-based determination of bone density.
Methods and materials
Patients
Diagnostic CT scans of 101 patients (49 females, 52 males; mean age: 72.2 ± 10.7 years; age range: 41–91 years) were retrospectively evaluated. A total of 61 patients were diagnosed with MRONJ [n = 26 were treated with denosumab (Table 1); n = 35 were treated with bisphosphonates (Table 2)]. Another 40 patients were included in the study as a reference group. Inclusion criteria were the diagnoses of MRONJ based on the definition by the American Association of Oral and Maxillofacial Surgeons.4 Additionally, we solely included patients that had been treated with either bps or db in the past. Consequently, we excluded patients with a medical history of recieving both groups of substances. Further exclusion criteria were previous radiation therapy of the jaw and osteolytic processes other than MRONJ. The reference group consisted of patients with no previous therapy of any antiresorptive medication. Our search algorithm created a gender balanced and age-matched group of patients with different diagnoses, each not affecting the mandible (Table 3).
Table 1. .
Patient characteristics of db-group
| Db: | |
|---|---|
| n | 26 |
| Age | male = 14/female = 12 |
| Years of treatment | 3.4 ± 1.9 |
| Drug name | |
| XGEVA | n = 25 |
| Prolia | n = 1 |
| Diagnoses | |
| Prostate cancer | n = 11 |
| Breast cancer | n = 10 |
| Lung cancer | n = 1 |
| oOsteoporosis | n = 2 |
| Kidney cancer | n = 1 |
| Cancer of unknown primary | n = 1 |
db, denosumab.
Table 2. .
Patient characteristics of bp-group
| Bps | |
|---|---|
| n | 35 |
| Age | male = 18/female = 17 |
| Years of treatment | 4.6 ± 2.7 |
| Drug name | |
| Zoledronate | n = 24 |
| Bondronate | n = 2 |
| Alendronate | n = 7 |
| Risedronate | n = 1 |
| Ibandronate | n = 1 |
| Diagnoses | |
| Prostate cancer | n = 6 |
| Breast cancer | n = 7 |
| Osteoporosis | n = 9 |
| Kidney cancer | n = 1 |
| Multiple myeloma | n = 12 |
Bp, bisphosphonates.
Table 3. .
Diagnosis of patients from the reference group
| Diagnoses | n |
|---|---|
| Facial skin cancer | 17 |
| Mid face trauma | 11 |
| Intraoral SCC | 4 |
| SCC of the lip | 3 |
| Mucoepidermoid carcinoma | 2 |
| Sinus maxillaris pathology | 2 |
| Orbital tumor | 1 |
SCC, squamos cell carcinoma.
CT
CT scans were performed on a routine clinical 256-slice CT scanner (Philips iCT, Best, The Netherlands). Images were obtained from the local Picture Archiving and Communication System (IMPAX, Agfa Healthcare, Belgium) and reviewed on a standard radiologic workstation. Multiplanar reconstruction and assessment of HU-based determination of bone density was performed using standard clinical applications of the IMPAX software.
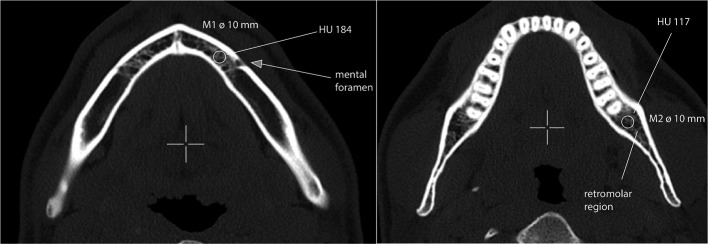
Slice thickness ranged from 1 to 5 mm. Bone density was assessed by placing circular click-and-drag ROIs constrained to the cancellous bone of the jaw in two distinct areas (Figure 1) and measuring mean HU values of the relevant ROI. For each measurement, a rotund ROI with an exact diameter of 10 mm was placed over an area of trabecular bone, excluding teeth, the cortical margins or areas in the course of the inferior alveolar nerve. The bone density was measured in two pre-defined locations of the mandible (M1: 10 mm anterior of the mental foramen; M2: retromolar region) (Figure 2). All measurements were performed on the contralateral side to the necrotic process within the mandible. Patients with necrotic areas on both sides of the mandible were excluded from the analysis. Images were assessed by a craniomaxillofacial surgeon and a radiologist.
Figure 1. .
Location of bone density measurements M1: Measurement 1: anterior of the mental foramen; M2: retromolar region. Circle: ROI. ROI, region of interest.
Figure 2. .
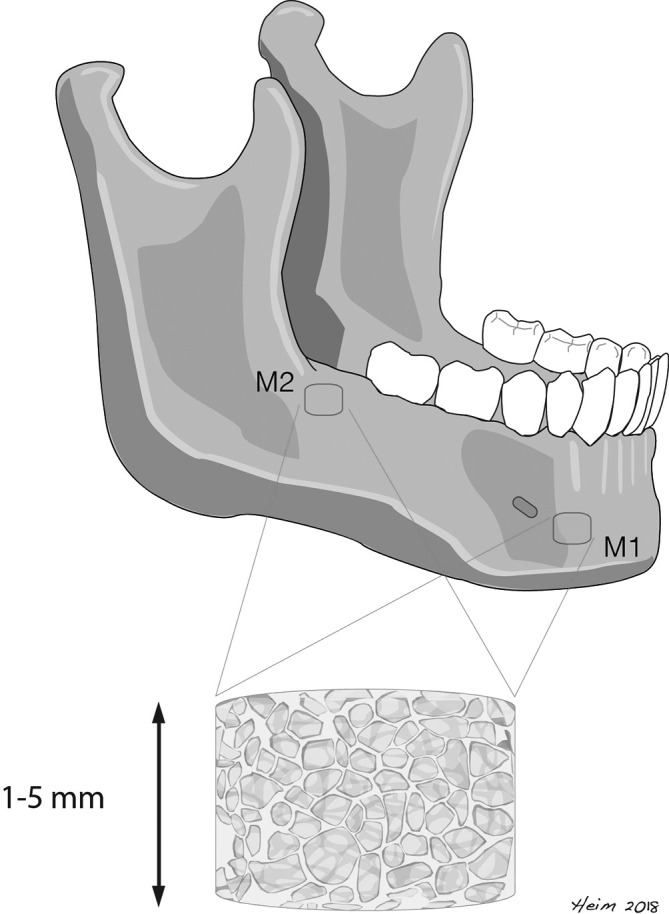
Defined locations of bone density measurements in the mandible Slice thikness in CT ranging from 1 to 5 mm.
Statistical analysis
All information was arranged electronically and analyzed (Microsoft Excel, v. 12.3.6; Microsoft Corporation, Redmond, WA). Measurements were described by the means and corresponding standard deviations (SDs). Unpaired t-tests were used for comparisons between the groups. p values ≤ 0.05 were regarded as statistically significant.
Results
A total of 101 subjects were included in the study. The patients were either diagnosed with db-related osteonecrosis of the jaw (n = 26; 12 female and 14 male) with a mean age of 72.1 ± 12.6 years, ranging from 41 to 91 years; or bp-related osteonecrosis of the jaw (n = 35; 17 female and 18 male) with a mean age of 72.1 ± 9.3 years, ranging from 52 to 91 years; or referred to the reference group with various other diagnoses (n = 40; 20 female; 20 male) with a mean age of 72.5 ± 10.8 years, ranging from 50 to 91 years.
The mean duration of treatment for db-group was 3.4 ± 1.9 years, ranging from 1 to 8 years. Mean duration for bp-group was 4.7 ± 2.7 years, ranging from 1 to 13 years.
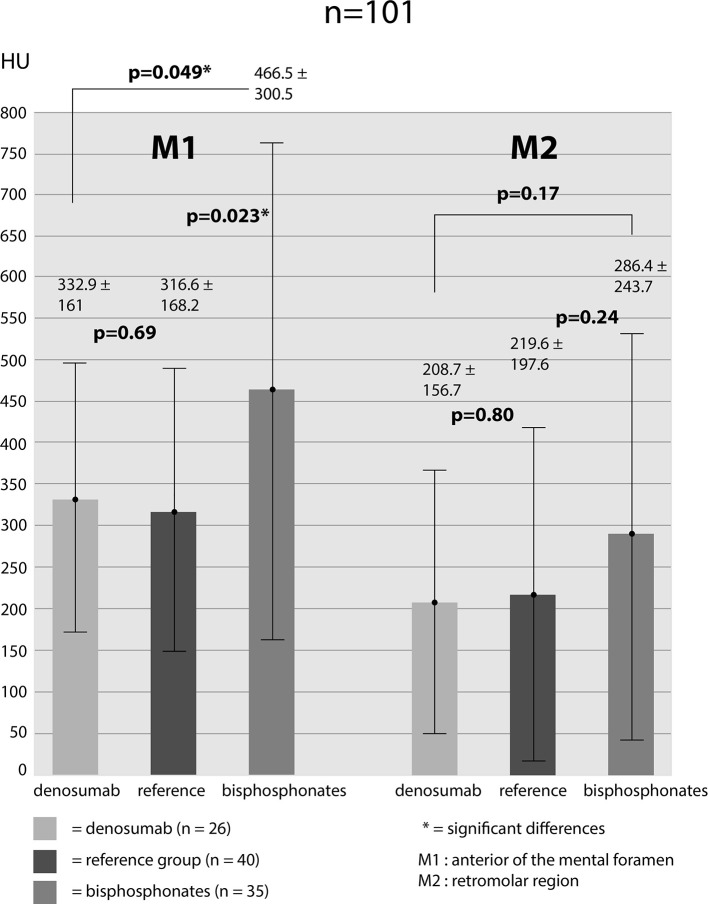
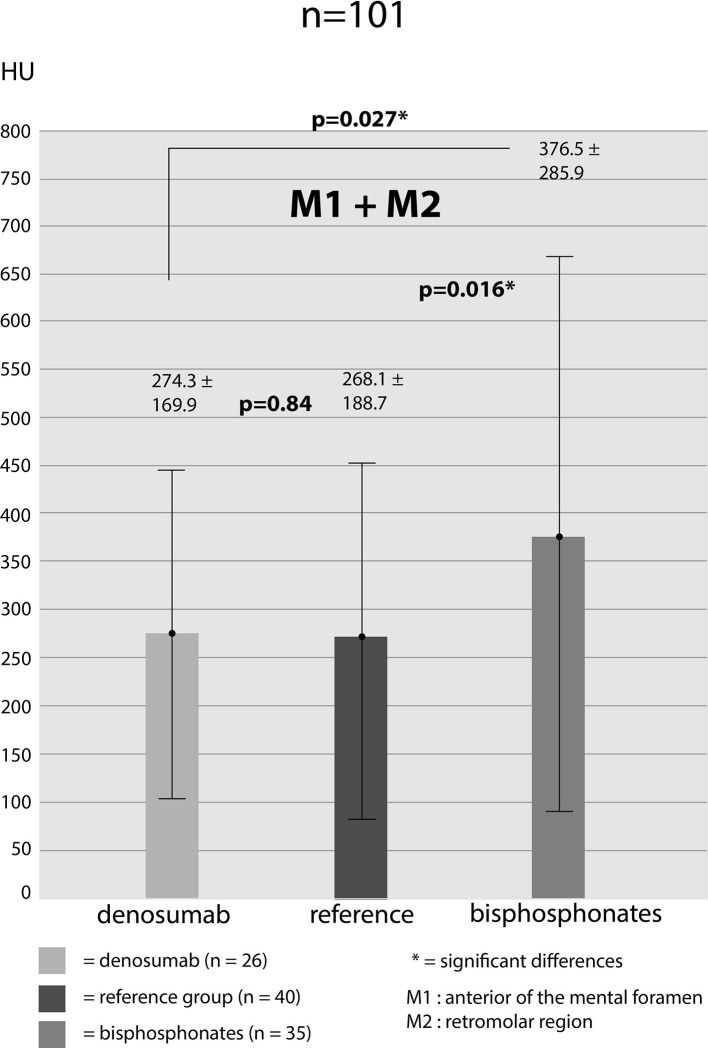
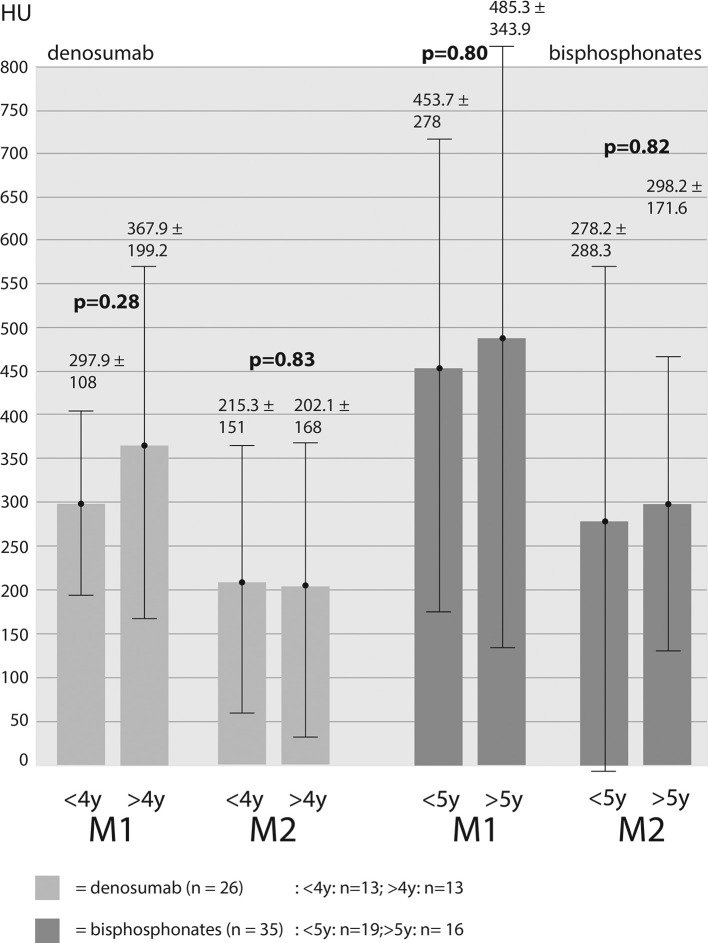
Bone density was measured for M1-location: db-group: 332.9 ± 161 HU, bp-group: 466.5 ± 300.5 HU, reference-group: 316.6 ± 168.2 HU. Measurements for M2-location: db-group: 208.7 ± 156.7 HU, bp-group: 286.4 ± 243.7 HU, reference-group: 219.6 ± 197.6 HU. Bone density values of both areas (M1 and M2) were added up in order to receive average values for the different groups (db-group: 274.3 ± 169.6 HU; bp-group: 376.5 ± 285.9 HU; reference-group: 268.1 ± 188.7 HU). Assessing the bone density values for M1 showed significantly higher HU-values in bp-group compared to db-group (p = 0.049) and bp-group to reference-group (p = 0.023). Differences between db-group and reference group showed no significance (p = 0.69). For M2-location, it emerged a distinct but not significant difference in bone density values between db- and bp-group (p = 0.17) and reference- to bp-group (p = 0.24). Furthermore, there was no significant difference between db- and reference-group (p = 0.80) (Figure 3). Added up values (M1 + M2) again showed a significantly higher bone density value of bp- to db-group (p = 0.027) and to the reference-group (p = 0.016). Bone density values of db- and reference-group showed almost no difference (p = 0.84) (Figure 4). For the purpose of investigating the impact of treatment duration on bone density values we subdivided each of the two groups. db-group was divided into treatment duration less than 4 years (<4y; n = 13) and more than 4 years (>4y; n = 13). None of the measurements at both locations showed significant differences in one density values (M1: 297.9 HU (<4y) vs 367.9 HU (>4y) [p = 0.28]; M2: 215.3 HU (<4y) vs 202.1 HU (<4y) [p = 0.84]). bp-group was subdivided into treatment duration less than 5 years (<5y; n = 19) and more than 5 years (>5y; n = 16). The measured values showed no significant differences in both locations (M1: 453.7 HU (<5y) vs 485.3 HU (>5y) [p = 0.80]; M2: 278.2 (<5y) vs 298.2 HU (>5y) [p = 0.82] (Figure 5).
Figure 3. .
CT—measured bone density values in all of the three groups in two locations (M1 + M2). Values given in HU (mean ± standard deviation); *=significant differences (p < 0.05). HU, Hounsfield unit.
Figure 4. .
CT—measured bone density values (added M1 + M2) in all of the three groups. HU, Hounsfield unit.
Figure 5. .
Differences in CT—measured bone density values with regard to duration of medication intake and location of measurement. <4y/<5y=under 4/5 years of treatment;>4y/>5y=over 4/5 years of treatment. HU, Hounsfield unit.
Discussion
Recent studies investigated the development of sclerotic bone architecture with denser cancellous bone, thicker trabeculae and less complex structure in rat jaws treated with zoledronic acid. Subsequently, a significant reduction of blood vessel areas, smaller marrow spaces and nutritive canals were observed. Furthermore, bp-induced bone turnover suppression may induce bone corticalization and impact vascularization.15 It becomes evident that bp-induced changes in bone microstructure plays a key role in ONJ-development.19,20 Against the backdrop of these results, we expected increased bone density measurements in CT-scans of mandibles in patients treated with antiresorptive drugs and suffering from MRONJ, compared to a gender- and age-balanced control group. Although, bisphosphonates and denosumab specifically target osteoclasts and both alendronate and denosumab lead to significant improvement in total bone density,21 differences in structure, specific effects on osteoclasts and molecular targeting have to be respected.8 Key target for the nitrogen-containing bisphosphonates is the farnesyl pyrophosphate synthase, which is needed for prenylation of proteins. Db is a fully human monoclonal antibody that inhibits RANKL to which it binds with high affinity and specificity. Thus, bps need to be taken up from bone matrix into osteoclast cytoplasm during bone resorption, while db works in the extracellular milieu, affects osteoclasts and their precursors which express the RANK protein and does not associate with bone tissue. Furthermore, db is not embedded within bone tissue and is cleared from the bloodstream through the reticuloendothelial system, with a half-life of approximately 26 days.22
Bps have a strong affinity to bone and become incorporated in the bone. There, the substance remains until the process of bone resorption releases the molecules, this process can extend over a period of weeks to years.23 Nevertheless, the site-specific side-effects on the jaw (MRONJ) are similar in both classes of antiresorptive substances.24 Interestingly, we observed no significant bone density differences within both groups (db and bp) regarding the duration of medication intake. In db group, the bone density values for M1 and M2 were higher in >4y-group than in <4y-group, but not significantly. In bp group, the bone density values for M1 were slightly higher in >5y-group than in <5y-group. In M2, values were even slightly lower in >5y-group. Differences showed no significance. The supposition of the authors is that structural changes in the bone that can be detected by CT scans proceed rather rapidly after the initial intake of bisphosphonates.
Comparing bone density values of all three groups showed significantly denser cancellous bone in patients treated with bp than db- and reference-group. However, db- and reference-group bone density values were almost measured equal in both locations (Figure 6). The mean age of the patients in every group was practically equal and the groups were almost gender balanced. Hence, two major biases with generally great effect on bone were eradicated.
Figure 6. .
Examples of radiological findings of patients treated with denosumab (a), bisphosphonates (b) or reference group (c). 1 (a, b, c): anterior of the mental foramen; 2 (a,b,c) retromolar region.
In other CT studies of bone at the distal radius, effects in the cortical bone were measured.21 Treatment over 12 months led to a significantly greater bone density increase with db than with alendronate. Other authors report alike results regarding the benefits of db over a greater duration of treatment.8 Nonetheless, density gains of db are usually outlined for cortical bone and are not specifically addressing changes in the alveolar bone of patients suffering from MRONJ. Furthermore, existing studies investigated mostly the impaired bone healing and development of ONJ in the context of bisphosphonate intake.19,20,25
By taking into consideration the similar side-effects of db and bps on alveolar bone,26 two presumptions arise on the backdrop of our results. Firstly, providing that previously demonstrated increased antiresorptive medication induced jaw bone density is directly associated with less blood perfusion in the jaw bone27 and subsequent development of ONJ, this prediction can solely be sustained for bp therapy but not for db treatment. Secondly, significantly greater effects of db compared to bps on bone density and microarchitecture in bones are either constrained to the cortical and not the cancellous bone or elsewise different conditions apply for mandibular bone density development.
However, our results have some limitations. CT scans for measuring bone density levels were used despite the fact that DXA is the most widely validated and preferred technique.28 Although recent studies presented reasonable data comparing DXA and CT scans for bone density measurements,29 alveolar bone was not implied in the settlement. Furthermore, the group of patients with bp-associated osteonecrosis of the jaw was very heterogeneous regarding the intake of different substances. Additionally, the exact doses of intake and administration intervals remained not fully clear in some cases.
In conclusion, we assume that bps change the microarchitecture of the alveolar bone by depositing in the bone in an additional different way than denosumab does. This may subsequently lead to a bp-specific corticalization and a decrease of vascularization in the jaw. Hence, the impairment of vascularization could compromise the alveolar bone and make it more vulnerable to infection and ONJ development.
Footnotes
Acknowledgements: We declare that we have no competing interests. The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Contributor Information
Nils Heim, Email: nils.heim@web.de.
Werner Götz, Email: wgoetz@uni-bonn.de.
Franz-Josef Kramer, Email: franz-josef.kramer@ukbonn.de.
Anton Faron, Email: anton.faron@ukbonn.de.
REFERENCES
- 1.Berenson JR, Lipton A. Bisphosphonates in the treatment of malignant bone disease. Annu Rev Med 1999; 50: 237–48. doi: 10.1146/annurev.med.50.1.237 [DOI] [PubMed] [Google Scholar]
- 2.Hagiwara M, Delea TE, Cong Z, Chung K. Utilization of intravenous bisphosphonates in patients with bone metastases secondary to breast, lung, or prostate cancer. Support Care Cancer 2014; 22: 103–13. doi: 10.1007/s00520-013-1951-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–65. doi: 10.1056/NEJMoa0809493 [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg 2014; 72: 1938–56. doi: 10.1016/j.joms.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 5.Ruggiero SL, Drew SJ. Osteonecrosis of the jaws and bisphosphonate therapy. J Dent Res 2007; 86: 1013–21. doi: 10.1177/154405910708601101 [DOI] [PubMed] [Google Scholar]
- 6.Yarom N, Yahalom R, Shoshani Y, Hamed W, Regev E, Elad S. Osteonecrosis of the jaw induced by orally administered bisphosphonates: incidence, clinical features, predisposing factors and treatment outcome. Osteoporos Int 2007; 18: 1363–70. doi: 10.1007/s00198-007-0384-2 [DOI] [PubMed] [Google Scholar]
- 7.Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol 2012; 23: 1341–7. doi: 10.1093/annonc/mdr435 [DOI] [PubMed] [Google Scholar]
- 8.Baron R, Ferrari S, Russell RGG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48: 677–92. doi: 10.1016/j.bone.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 9.Khan AA, Morrison A, Hanley DA, Felsenberg D, McGauley LK, O’Ryan F, et al. Intermational Task force osteonecrosis of the jaw, diagnosis and management of osteonecrosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res 2015; 30: 3–23. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos MA, Kastritis E, Bamia C, Melakopoulos I, Gika D, Roussou M, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol 2009; 20: 117–20. doi: 10.1093/annonc/mdn554 [DOI] [PubMed] [Google Scholar]
- 11.Heim N, Warwas FB, Wilms CT, Reich RH, Martini M, Vitamin D. Vitamin D (25-OHD) deficiency may increase the prevalence of medication-related osteonecrosis of the jaw. J Craniomaxillofac Surg 2017; 45: 2068–74. doi: 10.1016/j.jcms.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Jabbour Z, El-Hakim M, Henderson JE, de Albuquerque RF. Bisphosphonates inhibit bone remodeling in the jaw bones of rats and delay healing following tooth extractions. Oral Oncol 2014; 50: 485–90. doi: 10.1016/j.oraloncology.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 13.Borke JL, McAllister B, Harris T, Neiberg M, Guevarra-Toth C, Fulzele S, et al. Correlation of changes in the mandible and retina/choroid vasculature of a rat model of BRONJ. J Craniomaxillofac Surg 2015; 43: 1144–50. doi: 10.1016/j.jcms.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 14.Guevarra CS, Borke JL, Stevens MR, Bisch FC, Zakhary I, Messer R, et al. Vascular alterations in the Sprague-Dawley rat mandible during intravenous bisphosphonate therapy. J Oral Implantol 2015; 41: e24: e24–9. doi: 10.1563/AAID-JOI-D-13-00074 [DOI] [PubMed] [Google Scholar]
- 15.Soares MQS, Van Dessel J, Jacobs R, da Silva Santos PS, Cestari TM, Garlet GP, et al. Zoledronic acid induces site-specific structural changes and decreases vascular area in the alveolar bone. Journal of Oral and Maxillofacial Surgery 2018; 76: 1893–901. doi: 10.1016/j.joms.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ, Summers RM, et al. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res 2011; 26: 2194–203. doi: 10.1002/jbmr.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budoff MJ, Malpeso JM, Zeb I, Gao YL, Li D, Choi T-Y, et al. Measurement of phantomless thoracic bone mineral density on coronary artery calcium CT scans acquired with various CT scanner models. Radiology 2013; 267: 830–6. doi: 10.1148/radiol.13111987 [DOI] [PubMed] [Google Scholar]
- 18.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 2013; 158: 588–95. doi: 10.7326/0003-4819-158-8-201304160-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmagnola D, Canciani E, Sozzi D, Biglioli F, Moneghini L, Dellavia C. Histological findings on jaw osteonecrosis associated with bisphosphonates (BONJ) or with radiotherapy (Orn) in humans. Acta Odontol Scand 2013; 71: 1410–7. doi: 10.3109/00016357.2013.765592 [DOI] [PubMed] [Google Scholar]
- 20.Kim SM, Eo MY, Kim YS, Lee SK. Histochemical observation of bony reversal lines in bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2017; 123: 220–8. doi: 10.1016/j.oooo.2016.09.225 [DOI] [PubMed] [Google Scholar]
- 21.Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 2010; 25: 1886–94. doi: 10.1002/jbmr.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prolia Product Monograph Amgen Canada Inc. 2011;.
- 23.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone 1999; 25: 97–106. doi: 10.1016/S8756-3282(99)00116-7 [DOI] [PubMed] [Google Scholar]
- 24.Otto S, Baumann S, Ehrenfeld M, Pautke C. Successful surgical management of osteonecrosis of the jaw due to RANK-ligand inhibitor treatment using fluorescence guided bone resection. J Craniomaxillofac Surg 2013; 41: 694–8. doi: 10.1016/j.jcms.2013.05.038 [DOI] [PubMed] [Google Scholar]
- 25.Allen MR, Burr DB, Butt DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg 2009; 67(5 Suppl): 61–70. doi: 10.1016/j.joms.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 26.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. JCO 2011; 29: 1125–32. doi: 10.1200/JCO.2010.31.3304 [DOI] [PubMed] [Google Scholar]
- 27.Córdova LA, Guilbaud F, Amiaud J, Battaglia S, Charrier C, Lezot F, et al. Severe compromise of preosteoblasts in a surgical mouse model of Bisphosphonate-associated osteonecrosis of the jaw. J Craniomaxillofac Surg 2016; 44: 1387–94. doi: 10.1016/j.jcms.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 28.WHO Who scientific group report on the assessment of osteoporosis at primary health care level. Report of a who scientific group summary meeting report. Brussels, Belgium 2004;: 5–7. [Google Scholar]
- 29.Marinova M, Edon B, Wolter K, Katsimbari B, Schild HH, Strunk HM. Use of routine thoracic and abdominal computed tomography scans for assessing bone mineral density and detecting osteoporosis. Curr Med Res Opin 2015; 31: 1871–81. doi: 10.1185/03007995.2015.1074892 [DOI] [PubMed] [Google Scholar]