Abstract
Background
Ex vivo lung perfusion (EVLP) is a relatively new technique that can be used to assess and repair the donor lungs, increasing the utilization of high-risk lungs. However, its effect on outcomes of lung transplantation patients is uncertainty. This meta-analysis is conducted to assess the impact of EVLP on donor lungs and outcomes of recipients compared with the standard lung transplantation.
Material/Methods
We systematically searched for studies comparatively analyzing the efficacy of EVLP and standard cold storage in lung transplantation. The hazard ratio (HR), relative risk (RR), and weighted mean difference (WMD) were used as the effect size (ES) to evaluate the survival outcomes, categorical variables, and continuous variables respectively.
Results
A total of 20 published articles (including 2574 donors and 2567 recipients) were eligible. The chest x-ray manifestations and PaO2/FiO2 100% were more deficient in the EVLP group than the standard group. EVLP improved the function of high-risk donor lungs with the conversion rate ranging from 34% to 100%. The EVLP group had a lower incidence of primary graft dysfunction 3, but longer intensive care unit stay. Other clinical outcomes between the 2 groups were similar.
Conclusions
The pooled results indicated that EVLP could be used to assess and improve high-risk donor lungs and had non-inferior postoperative outcomes compared with the standard cold storage. EVLP not only increased the utilization of marginal donors, but also could extend preservation time and reduce the total ischemia time of donors.
MeSH Keywords: Donor Selection, Lung Transplantation, Perfusion
Background
Lung transplantation (LTx) is an effective treatment for advanced lung disease, which improves patients’ quality of life and extends their life expectancy [1]. However, a profound shortage of donors and underutilization of donor lungs remains a significant challenge in performing LTx [2,3]. In addition to the use of marginal donors and living donors to expand the donor organ pool, ex vivo lung perfusion (EVLP) technology can reduce receptor waiting list mortality by improving donor lung utilization and increasing LTx activity [4,5].
EVLP is a relatively new technology for the procurement of donor lungs which was initially developed as a method for assessing graft quality and improving cardiac death (DCD) donor lung function [6,7]. The first successful use of EVLP to assess and recondition LTx in donor lungs was reported by Steen et al. in 2001, which was the starting point for the “Lund protocol” [7]. In 2008, Cypel et al. [8] in Toronto reported the use of a novel strategy to expand the EVLP assessment of lung function, which laid the foundation for the “Toronto protocol”. In 2012, Warnecke et al. in Hanover reported the first-in-human experience using the portable Organ Care System (OCS) lung device for concomitant preservation, assessment, and transport of donor lungs, which was known as “OCS protocol” [9]. EVLP has evolved to demonstrate that marginal donor lungs could be assessed and treated to achieve similar early outcomes as the standard criteria donor lungs [5]. In addition, due to the process of EVLP not being regarded as “ischemic time”, EVLP might play an essential role in expanding the procurement time and contributing to the long-distance transportation, especially using the portable OCS technique [9]. EVLP technology has attracted more and more attention from transplant centers around the world, but there are still serious concerns about the poor results after transplantation. Although several comparative analyses of clinical outcome between EVLP and traditional cold storage have been reported and some multicenter randomized control clinical trials (RCTs) are being conducted, there are still a lot of uncertainty about EVLP clinical application. Thus, this meta-analysis was performed to determine the short- mid- to long-term results of EVLP compared with that of standard cold storage.
Material and Methods
Literature search strategy
The meta-analysis was performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [10]. The electronic databases, including PubMed, PMC, EMBASE, and Ovid, were comprehensively searched for relevant articles published until March 1, 2019. Search terms included the following: “EVLP or ex vivo lung perfusion” and “lung transplantation”. All references reported in the identified articles were also scanned to identify potentially relevant reports.
Criteria for inclusion and exclusion
The studies included in this meta-analysis need to meet the following criteria: 1) RCTs or cohort studies studying lung transplantation; and 2) studies comparatively analyze the post-transplantation results between EVLP technique and traditional cold storage. The following studies were excluded: 1) articles about animals; 2) single-arm analysis about EVLP technique; and 3) review articles without original data. For duplicate articles reporting the same case population, only the most complete or up-to-date one was included. Two reviewers independently selected eligible studies. Disagreements were settled by discussion.
Data extraction and quality assessment
The following data was collected independently by 2 reviewers using a predesigned form, comprising first author, publication year, study period, country, study design, sample size (donors and recipients), age (donors and recipients), gender (donors and recipients), type of donor, time in ventilation (donors and recipients), chest x-ray abnormalities, PaO2/FiO2 100% (P/F, donors and recipients), indication for LTx, type of LTx, lung allocation score (LAS), extracorporeal membrane oxygenation (ECMO) bridge to LTx, intraoperative extracorporeal circulation (ECC)/ECMO, reason for EVLP, technological type of EVLP, EVLP solution, EVLP duration, the number of accepted donor after EVLP, severe primary graft dysfunction (PGD) after LTx, post-LTx ECMO, residence time in the intensive care unit (ICU), total length of hospital stay, FEV1 of the predicted value (FEV1%), FVC of the predicted value (FVC%), follow-up time and survival data after LTx. The hazard ratio (HR) and 95% confidence intervals (CI) of the EVLP group compared to the traditional cold storage group for OS were primarily collected. If the HR and 95% CI were not explicitly provided, we used Tierney’s methods to extract survival data from the original study data or Kaplan-Meier curve [11]. If the aforementioned items were not reported in the original study, the items were labeled as “not available (NA)”. Inconsistencies in the process were solved by consultations.
The quality of the included cohort studies was independently assessed by 2 reviewers according to the Newcastle-Ottawa Scale (NOS) [12]. The NOS evaluated a study with the score ranged from 0 to 9. A study with a score of 6 or high was considered as a high-quality one. The quality of RCT reports was measured by the Jadad scale [13]. The Jadad scale evaluated a study from 3 perspectives, including randomization, blinding, and withdraw, with scores ranging from 0 to 5. A study achieving a score of 3 or more was identified as a high-quality one.
Statistical analysis
Interest results included improvement of P/F ratio in donor lung after EVLP, total cold ischemic time (CIT) and preservation time in donor lung, P/F ratio after LTx, extubation time, severe incidence of PGD after LTx, requirement of ECMO, residence time in the intensive care unit (ICU), total length of hospital stay, FEV1%, FVC%, survival rate at 30 days, 90 days and 1-year after LTx, and accumulative survival. The HR and 95% CI were used as an effect size (ES) to assess the impact of EVLP on accumulative survival outcomes. Relative risk (RR) with its 95% CI and Mantel-Haenszel model were used to measure the effect of EVLP on categorical variables. For continuous outcomes, weighted mean difference (WMD) was used as the ES to assess the difference between the EVLP group and the traditional group. Heterogeneity across the studies was tested using I-squared statistics [14]. I2≤50% indicated no or moderate heterogeneity, in which case a fixed-effect model was used. I2>50% showed statistically significant heterogeneity, in which case a random-effect model is chosen. To explore the difference among Lund, Toronto, and OCS protocols, subgroup analyses based on different protocols were adopted. Sensitivity analysis by omitting a single study to confirm the robustness of the combined results. By convention, an observed ES>1 implied a more unsatisfactory outcome for the EVLP group compared with the traditional cold storage group. Assessment of potential publication bias was conducted through Begg’s funnel plot and Egger’s test. Data are presented as mean±standard deviations (SD), median (ranges), or median (inter-quartile range, IQR). Stata software version 12.0 (Stata Corporation, TX, USA) was used in the meta-analysis. All the tests were 2-sided, and P<0.05 was considered statistically significant.
Results
Study selection and characteristics
The results of articles selection are shown in Figure 1. A total of 789 articles were identified initially using the search strategy described. We excluded 738 articles because they were duplicate documents, review articles, or irrelevant studies. Afterward, 51 articles were read in full text. Finally, 20 articles were considered suitable for inclusion in the meta-analysis. Among the eligible study, the NOVEL trial [15] is expected to end in 2020, and its 1-year results have been reported in summary form. Considering that the NOVEL trial is an RCT study and can provide some available information, such as necessary patient information, donor lung conversion rate, 30-day survival, and 1-year survival, it was included in our study.
Figure 1.
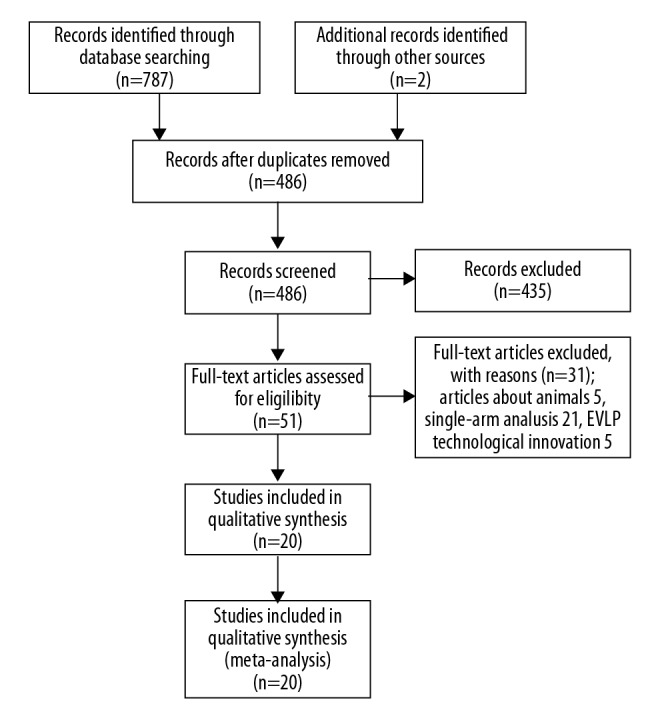
Flow chart of searching the relevant studies included in this meta-analysis.
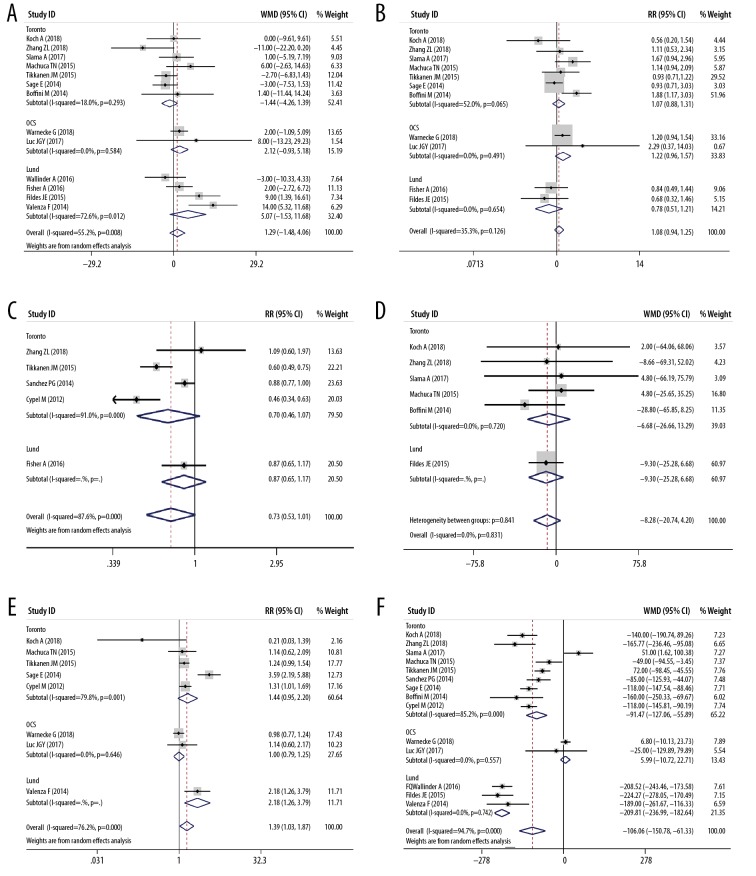
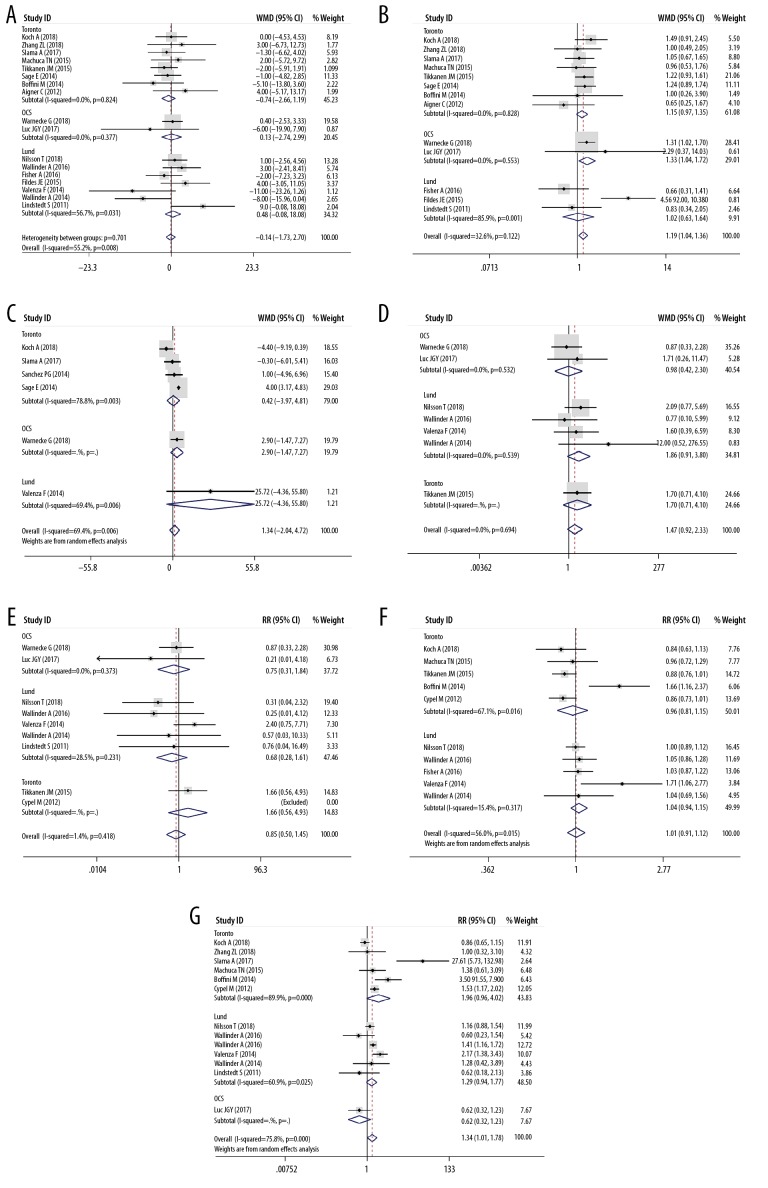
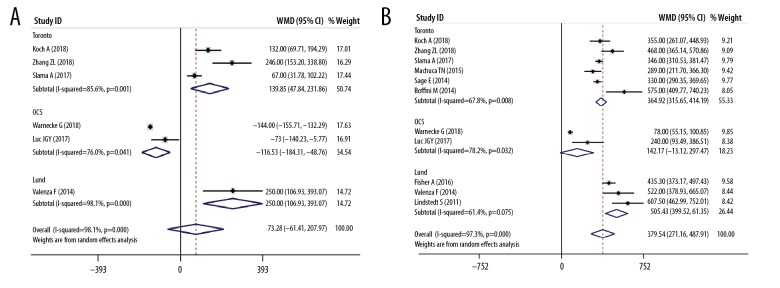
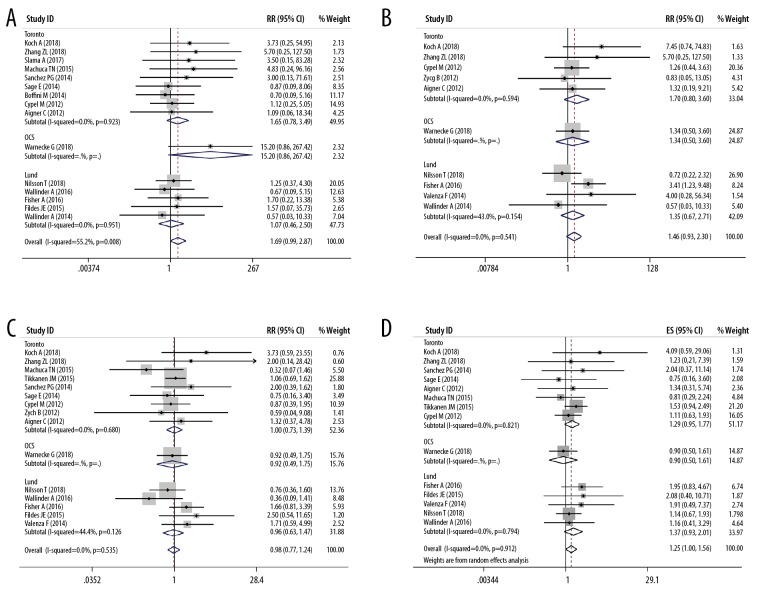
Table 1 listed the main features of the 20 eligible articles [4,15–33] Three RCTs, 3 prospective cohort studies, and 14 retrospective cohort studies were included, and the publication year ranged from 2011 to 2018. The study included a total of 2574 donors and 2567 recipients. Table 1 and Figure 2 show the features of the donor, and Table 2 and Figure 3 show the characteristics of the recipient. There was no significant difference in the donors’ age (Figure 2A), gender (Figure 2B), donor type (Figure 2C), and mechanical ventilation time (Figure 2D) between the EVLP group and the traditional cold storage group. However, compared with the non-EVLP group, the EVLP group donors had more chest X-ray abnormalities (RR 1.39, 95% CI 1.03–1.87, P<0.05, Figure 2E) and more inferior P/F ratio (WMD −106.06, 95% CI −150.78–61.33 mmHg, P<0.001, Figure 2F). There was also no significant difference for recipients’ age (Figure 3A), LAS (Figure 3C), mechanical ventilation pre-LTx (Figure 3D), ECMO bridging to LTx (Figure 3E), type of LTx (Figure 3F), or total CIT (Figure 4A). Still the EVLP group had more female patients (Figure 3B) and showed more intraoperative ECC/ECMO needs (RR 1.34, 95% CI 1.01–1.78, P<0.05, Figure 3G) and longer preservation time (WMD 379.54, 95% CI 271.16–487.91 minute, P<0.001, Figure 4B) compared with the traditional cold storage group. In the subgroup analysis based on different protocols, the OCS subgroup exhibited equivalence between the 2 groups but the shorter total CIT in the OCS-EVLP group (Figure 4A).
Table 1.
Summary of included studies and donor characteristics.
| Author | Year | Period | Country | Design | Samples size | Age (years) | Gender | Type of donor | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EVLP | NEVLP | EVLP | NEVLP | EVLP | NEVLP | EVLP | NEVLP | ||||||
| Koch et al. [20] | 2018 | 2016–2017 | Germany | Cohort | 11 | 41 | 54±14 | 54±16 | F 3, M 8 | F 20, M 21 | DBD 11 | DBD 41 | Jadad 3 |
| Warnecke et al. [16] | 2018 | 2011–2014 | USA, Europe, Australia, and Canada | RCT | 151 | 169 | 42.2±14.4 | 40.2±13.7 | F 72, M 79 | F 67, M 102 | NA | NA | NOS 8 |
| Nilsson et al. [21] | 2018 | 2011–2015 | Sweden | Prospective cohort | 61 | 271 | NA | NA | NA | NA | DBD 61 | DBD 271 | NOS 8 |
| Zhang et al. [22] | 2018 | 2012–2016 | Netherlands | Cohort | 9 | 18 | 41±12.7 | 52±16.3 | F 5, M 4 | F 9, M 9 | DBD6, DCD 3 | DBD 11, DCD 7 | NOS 7 |
| Slama et al. [17] | 2017 | 2013–2015 | Austria | RCT | 35 | 41 | 45 (18–71) | 44 (19–76) | F 18, M 17 | F 12, M 27 | DBD 35 | DBD 41 | Jadad 3 |
| Luc et al. [18] | 2017 | 2011–2015 | Canada | Cohort | 7 | 4 | 48±11 | 40±20 | F 4, M 3 | F 1, M 3 | DCD 7 | DCD 4 | NOS 6 |
| Wallinder et al. [23] | 2016 | 2011–2013 | Sweden | Cohort | 27 | 145 | 47±18 | 50±17 | NA | NA | DBD 27 | DBD 145 | NOS 8 |
| Fisher et al. [24] | 2016 | 2012–2014 | UK (five centers) | Cohort | 18 | 184 | 50.5 (22–61) | 44 (10–68) | F 8, M 10 | F 96, M 86 | DBD 13, DCD 5 | DBD 152, DCD 31 | NOS 7 |
| Machuca et al. [19] | 2015 | 2007–2013 | Canada | Cohort | 28 | 27 | 45±13 | 39±19 | F 13, M 15 | F 11, M 16 | DCD 28 | DCD 27 | NOS 8 |
| Tikkanen et al. [25] | 2015 | 2008–2012 | Canada | Cohort | 63 | 340 | 43.1±14.9 | 45.8±17.6 | F 31, M 32 | F 180, M 160 | DBD 36, DCD 27 | DBD 322, DCD 18 | NOS 8 |
| Fildes et al. [26] | 2015 | 2012–2014 | UK and Sweden | Cohort | 9 | 46 | 54±10.1 | 45±13.1 | F 4, M 5 | F 30, M 16 | NA | NA | NOS 6 |
| Sanchez et al. [15] | 2014 | 2011–2013 | US (six centers) | RCT (abstract) | 42 | 42 | NA | NA | NA | NA | DBD 36, DCD 6 | DBD 41, DCD 1 | NA |
| Sage et al. [29] | 2014 | 2011–2013 | France | Prospective cohort | 31 | 81 | 48 (21–67) | 51 (14–70) | NA | NA | DBD 31 | DBD 81 | NOS 7 |
| Boffini et al. [28] | 2014 | 2011–2013 | Italy | Cohort | 8 | 28 | 44.7±16.2 | 43.3±16.8 | F 7, M 1 | F 13, M 15 | DBD 8 | DBD 28 | NOS 6 |
| Valenza et al. [4] | 2014 | 2011–2013 | Italy | Cohort | 7 | 28 | 54±9 | 40±15 | NA | NA | DBD 7 | DBD 28 | NOS 6 |
| Wallinder et al. [29] | 2014 | 2011–2013 | Sweden | Cohort | 11 | 47 | 56 (19–61) | NA | NA | NA | DBD 11 | DBD 47 | NOS 6 |
| Cypel et al. [30] | 2012 | 2008–2011 | Canada | Cohort | 50 | 253 | Median 45 | Median 45 | NA | NA | DBD 22, DCD 28 | DBD 240, DCD 13 | NOS 8 |
| Zych et al. [31] | 2012 | 2009–2010 | UK | Cohort | 6 | 86 | 43.5±15.1 | NA | F 2, M 4 | NA | DBD 10, DCD 3 | NA | NOS 6 |
| Aigner et al. [32] | 2012 | 2010–2011 | Austria | Prospective cohort | 9 | 119 | 48 (16–58) | NA | NA | NA | DBD 13 | DBD 119 | NOS 6 |
| Lindstedt et al. [33] | 2011 | 2006–2007 | Sweden | Cohort | 6 | 15 | 59 (34–63) | NA | F 3, M 3 | NA | DBD 6 | DBD 15 | NOS 6 |
Data are presented as n/N, mean±SD, median (range). RCT – randomized controlled trial; EVLP – ex vivo lung perfusion; NEVLP – non-EVLP; F – Female; M – Male; DBD – donation after brain death; DCD – donation after cardiac death; NA – not available; SD – standard deviation; NOS – Newcastle-Ottawa Scale.
Figure 2.
Meta-analyses of the characteristics of donors (EVLP group vs. non-EVLP group). (A) Donors’ age; (B) Donors’ gender (Female/Male); (C) The type of donor lungs (DBD/DCD); (D) Ventilation time of donor (hours); (E) Chest X-ray abnormalities of donors (yes/no); (F) PaO2/FiO2 100% of donors (mmHg).
Table 2.
Characteristics of recipients.
| Author | Sample size | Age (years) | Gender | Type of LTx | Indication for LTx | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EVLP | NEVLP | EVLP | NEVLP | EVLP | NEVLP | EVLP | NEVLP | EVLP | NEVLP | ||
| Koch et al. [20] | 11 | 41 | 55±7 | 55±6 | F 13, M 8 | F 17, M 24 | BLT 7, SLT 1, Bilobar 1 | BLT 41 | IPF 2, COPD 8, CPFE 1 | IPF 10, COPD 22, CPFE 3, CF 3, RLTx 1, Sarcoidosis 2 | Up to 500 days |
| Warnecke et al. [16] | 151 | 169 | 50.4± 13.1 | 50.0± 13.6 | F 74, M 77 | F 63, M 106 | BLT 151 | BLT 169 | COPD 46, PF 49, CF 31, IPH 13, Sarcoidosis 4, other 3 | COPD 52, PF 57, CF 40, IPH 6, Sarcoidosis 8, other 6 | Up to 24 months |
| Nilsson et al. [21] | 54 | 271 | 52±12 | 51±13 | NA | NA | BLT 46, SLT 7, Bilobar 1 | BLT 246, SLT 37 | IPF 24, PAH 2, COPD 33, AAD 6, CF 20, other 15 | IPF 25, PAH 6, COPD 28, AAD 13, CF 12, other 16 | Up to 5 years |
| Zhang et al. [22] | 9 | 18 | 53±13.3 | 50±9.5 | F 5, M 4 | F 10, M 8 | BLT 9 | BLT 18 | COPD 6, CF 2, PF 1 | COPD 12, CF 4, PF 2 | Up to 36 months |
| Slama et al. [17] | 35 | 41 | 52.9 (21–68.3) | 54.2 (19.7–66.7) | F 18, M 17 | F 20, M 21 | BLT 35 | BLT 41 | Emphysema 14, PF 9, CF 7, other 5 | Emphysema 21, PF 7, CF 10, other 3 | Up to 90 days |
| Luc et al. [18] | 7 | 4 | 52±18 | 58±4 | F 4, M 3 | F 1, M 3 | NA | NA | IPF 3, emphysema 2, CF 2 | IPF 2, emphysema 1, scleroderma 1 | 1 year |
| Wallinder et al. 2016 [23] | 27 | 145 | 55±13 | 52±14 | NA | NA | BLT 22, SLT 5 | BLT 113, SLT 32 | IPF 22, COPD 33, AAD 7, RLTx 4, CF 19, other 15 | IPF 24, PAH 8, COPD 24, AAD 13, RLTx 9, CF 7, other 15 | Up to 4 years |
| Fisher et al. [24] | 18 | 184 | 56 (20–64) | 51 (18–70) | F 5, M 13 | F 78, M 106 | BLT 16, SLT 2 | BLT 152, SLT 24 | COPD 5, CF 4, ILD 7, NCFB 1, PAH 1 | COPD 40, CF 47, ILD 47, Emphysema 26, NCFB 8, OB 2, PAH 3, other 9 | Up to 12 months |
| Machuca et al. [19] | 28 | 27 | 52±13 | 50±16 | F 12, M 16 | F 12, M 15 | BLT 21, SLT 7 | BLT 21, SLT 6 | IPF 13, emphysema 8, CF 5, RLTx 1, Scleroderma 1 | IPF 12, Emphysema 9, CF 4, RLTx 4, Scleroderma 1 | Up to 7 years |
| Tikkanen et al. [25] | 63 | 340 | 50.3± 14.6 | 52.3± 14.2 | F 32, M 31 | F 141, M 199 | BLT 48, SLT 15 | BLT 295, SLT 45 | PF 22, COPD 20, CF 14, PAH 3, RLTx 1, other 3 | PF 121, COPD 90, CF 67, PAH 14, RLTx 14, other 34 | Up to 5 years |
| Fildes et al. [26] | 9 | 46 | 3±9.4 | 49±12.0 | F 4, M 5 | F 24, M 222 | NA | NA | COPD 6, CF 1, PAH 1, IPF 1 | COPD 24, Bronchiectasis 7, CF 9, PAH 3, IPF 3 | Up to 12 months |
| Sanchez et al. [15] | 42 | 42 | NA | NA | NA | NA | NA | NA | IPF 19, COPD 13, PPH 1, other 9 | IPF 13, COPD 15, PPH 3, other 11 | Up to 1 year |
| Sage et al. [29] | 31 | 81 | 40 (21–60) | 41 (17–65) | F 20, M 11 | F 42, M 39 | BLT 31 | BLT 81 | CF 15, COPD 9, PF 3, other 4 | CF 40, COPD 16, PF 12, other 13 | Up to 1 year |
| Boffini et al. [28] | 8 | 28 | 46.6±9.8 | 51.7±14.7 | F 2, M 6 | F 7, M 21 | BLT 8 | BLT 16, SLT 12 | PF 4 | PF 13 | 30 days |
| Valenza et al. [4] | 7 | 28 | 38±15 | 49±14 | NA | NA | BLT 6, SLT 1 | BLT 14, SLT 14 | CF 4, other 3 | CF 14, PF 11, other 7 | Up to 800 days |
| Wallinder et al. 2014 [29] | 11 | 47 | 56 (19–61) | 56 (21–70) | NA | NA | BLT 8, SLT 3 | BLT 33, SLT 14 | PF 5, COPD 4, other 2 | PF 14, PAH 2, COPD 13, AAD 6, RLTx 5, other 7 | 3 months |
| Cypel et al. [30] | 50 | 253 | Median 56 | Median 56 | NA | NA | BLT 38, SLT 12 | BLT 223, SLT 30 | Emphysema 19, PF 14, CF 12, other 5 | PF or PAH 98 | Up to 3.5 years |
| Zych et al. [31] | 6 | 86 | 43.5± 15.1 | NA | F 2, M 4 | NA | NA | NA | CF 2, Emphysema 3, HSP 1 | NA | Median 297.5 days |
| Aigner et al. [32] | 9 | 119 | 58 (18–66) | 46 (13–66) | F 3, M 6 | F 61, M 58 | BLT 9 | NA | IPF 4, COPD 3, CF 2 | NA | Up to 16 months |
| Lindstedt et al. [33] | 6 | 15 | 54.5 (35–64) | 41 (24–66) | F 3, M 3 | F 9, M 6 | BLT 6 | BLT 15 | COPD 3, PF 1, CF 1, AAD 1 | COPD 5, CF 7, PF 1, emphysema 1, PAH 1 | NA |
Data are presented as n/N, mean±SD, median (range). LTx – lung transplantation; EVLP – ex vivo lung perfusion; NEVLP – non-EVLP; F – Female; M – Male; BLT – bilateral lung transplantation; SLT – single lung transplantation; IPF – interstitial pulmonary fibrosis; COPD – chronic obstructive pulmonary disease; CPFE – combined pulmonary fibrosis and emphysema; PAH – pulmonary artery hypertension; PF – pulmonary fibrosis; CF – cystic fibrosis; IPH – idiopathic pulmonary hypertension; PAH – pulmonary artery hypertension; AAD – a1-antitrypsin deficiency; RLTx – re-transplantation; ILD – interstitial lung disease; OB – obliterative bronchiolitis; NCFB – non-CF bronchiectasis; HSP – hypersensitivity pneumonitis; NA – not available; SD – standard deviation.
Figure 3.
Meta-analyses of the characteristics of recipients (EVLP group vs. non-EVLP group). (A) Recipients’ age; (B) Recipients’ gender (Female/Male); (C) Lung allocation score of recipients; (D) Mechanical ventilation pre-LTx of recipients (yes/no); (E) ECMO bridge to LTx (yes/no); (F) Type of LTx (bilateral LTx/single LTX); (G) Intraoperative extracorporeal circulation/ extracorporeal membrane oxygenation (yes/no).
Figure 4.
Meta-analyses of donor ischemia time. (A) Total cold ischemic time of donor lungs (min); (B) Total preservative time (min).
The efficacy of EVLP in improving donor lungs
The parameters of EVLP and its role in conversing marginal donor lungs are summarized in Table 3 and Figure 5. Compared with the P/F pre-EVLP, the P/F after EVLP was significantly improved (WMD 184.38, 95% CI 130.17–238.59 mmHg, P<0.001, Figure 5). However, the OCS subgroup did not show significant improvement in P/F (Figure 5), which might be because 1 study in the OCS subgroup involved only standard criteria donors [16]. The conversion rate of donor lungs by EVLP ranged from 34% to 100%. Among those included studies, a total of 1985 cases received traditional cold storage LTx, and 582 cases received EVLP LTx, so it can be said that EVLP made a 29.3% contribution to the LTx activity.
Table 3.
EVLP features and its efficacy of improving donor lungs.
| Author | Reason for EVLP | Technological type | EVLP solution | EVLP duration (min) | PaO2/FiO2 100% (mmHg) | Accepted/total (pair) | Conversion rate | |
|---|---|---|---|---|---|---|---|---|
| Pre EVLP | Post EVLP | |||||||
| Koch et al. [20] | Marginal donor | Toronto | Steen solution with meropenem, dexamethasone and heparin | 240 | 273±70 | 401±35 | 9/11 | 81.8% |
| Warnecke et al. [16] | Random assignment (standard donor) | OCS | OCS/LPD solution with ABO-compatible erythrocyte | 300 | 438.5± 80.0 | 455.5± 111.1 | 150/151 | 99.3% |
| Nilsson et al. [21] | Marginal donor | Lund | Steen solution mixed with red blood cells, heparin and meropenem | 200±94 | 229.52±90 | 438.79±75 | 49.5/61 | 81.0% |
| Zhang et al. [22] | Marginal donor | Toronto | Steen solution with cefuroxime, dexamethasone and heparin | 240 (IQR 84–100.8) | 285.77± 99.76 | NA | 9/10 | 90.0% |
| Slama et al. [17] | Random assignment (standard donor) | Toronto | Steen solution with heparin, cefuroxime and methylprednisolone | 266 (245–329) | 514 (290–626) | NA | 37/39 | 94.9% |
| Luc et al. [18] | Marginal donor | OCS | OCS solution | 210±101 | 367±119 | 500±83 | 7/7 | 100% |
| Wallinder et al. 2016 [23] | Marginal donor | Lund | Steen Solution with red blood cells | 208 (100–577) | 217.52± 85.1 | 477.04 (288.77–594.05) | 24.5/32 | 76.6% |
| Fisher et al. [24] | Marginal donor | Hybrid EVLP (combining Toronto and Lund); Lund | Hybrid: Steen solution; Lund: Steen solution with red cells | NA | 299 (95–535) | 381.5 (74–638) | 18/53 | 34% |
| Machuca et al. [19] | Marginal donor | Toronto | Steen solution with heparin, methylprednisolone and imipenem/cilastatin | 240–360 | 380±103 | NA | 28/35 | 80% |
| Tikkanen et al. [25] | Marginal donor | Toronto | Steen solution | 175 (73–383) | 332.5± 127.0 | 346.1± 104.0 | 63/73 | 86% |
| Fildes et al. [26] | Marginal donor | Lund | Steen solution with blood cells, trometamol and antibiotic | 240 | <300 | >300 | 9/9 | 100% |
| Sanchez et al. [15] | Marginal donor | Toronto | Steen solution | 180–360 | NA | NA | 42/76 | 55% |
| Sage et al. [29] | Marginal donor | Toronto | Steen solution | 243 (124–460) | 274 (162–404) | 511 (378–668) | 31/32 | 96.6% |
| Boffini et al. [28] | Marginal donor | Toronto | Steen solution with antibiotics, heparin and methylprednisolone | 282.8± 57.1 | 200±85 | 438±8 | 8/11 | 73.0% |
| Valenza et al. [4] | Marginal donor | Lund | Steen solution with red blood cells, methylprednisolone, cefazolin, and heparin | 268±104 | 264±78 | 518±55 | 7/8 | 87.5% |
| Wallinder et al. 2014 [29] | Marginal donor | Lund | Steen Solution with red blood cells | 191 (156–577) | 209.27 (68.26–313.53) | 447.04 (303.02–572.3) | 10/11 | 90.9% |
| Cypel et al. [30] | Marginal donor | Toronto | Steen solution with methylprednisolone, imipenem/cilastatin, and heparin | 240–360 | 334 (143–532) | Median 513 | 50/58 | 86.2% |
| Zych et al. [31] | Marginal donor | Toronto | Steen Solution with heparin, methylprednisolone, and antibiotics | 141±28.83 | 317.73±105.98 | 429.94± 68.26 | 6/13 | 46.2% |
| Aigner et al. [32] | Marginal donor | Toronto | Steen solution | 199 (171–290) | 216 (133–271) | 466 (434–525) | 9/13 | 69.2% |
| Lindstedt et al. [33] | Marginal donor | Lund | Steen solution with ABO-compatible erythrocyte, imipenem, insulin, and heparin | 89 (66–121) | 158.26 (86.26–215.27) | 515.29 (387.03–596.3) | 6/8 | 75.0% |
Data are presented as n/N, mean±SD, median (range) or median (IQR). NA – not available; SD – standard deviation; IQR – inter-quartile range.
Figure 5.
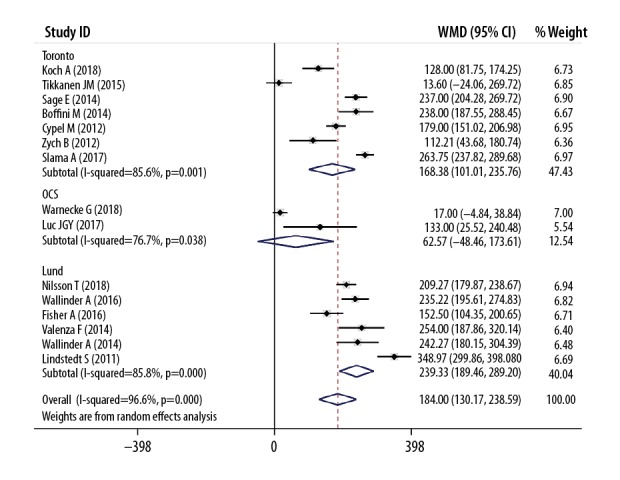
Meta-analyses of conversion results of EVLP (P/F ratio post-EVLP vs. P/F ratio pre-EVLP, mmHg).
The effect of EVLP on outcomes of recipients
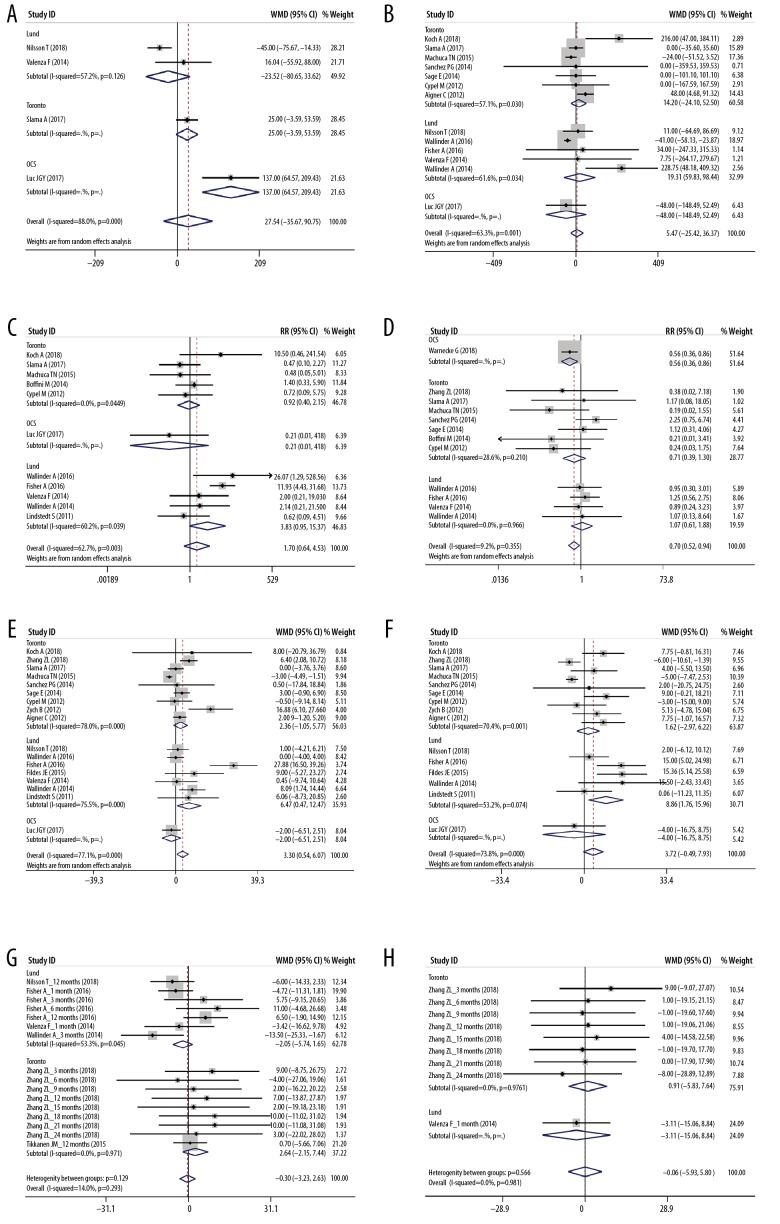
As shown in Figures 6 and 7, there was no significant difference about P/F after LTx (Figure 6A), time to extubation (Figure 6B), postoperative ECMO requirement (Figure 6D), length of hospital stays (Figure 6F), FEV1% (Figure 6G), FVC% (Figure 6H), survival rate at 30 days (Figure 7A), 90 days (Figure 7B) and 1 year (Figure 7C) after LTx, and accumulative survival after LTx (Figure 7D) between the EVLP group and the non-EVLP group. However, compared with the non-EVLP group, the EVLP group showed a lower incidence of PGD 3 (Figure 6C) after LTx, but the longer length of ICU stays (Figure 6E).
Figure 6.
Meta-analyses of perioperative clinical outcomes of recipients. (A) Postoperative PaO2/FiO2 100% of recipients (mmHg); (B) Time to extubation of recipients (hours); (C) PGD3 within 72 h after LTx (yes/no); (D) Postoperative ECMO need (yes/no); (E) ICU stays (days); (F) Hospital stays (days); (G) FEV1% after LTx; (H) FVC% after LTx.
Figure 7.
Meta-analyses of survival outcomes of recipients. (A) Survival rate at 30 days after LTx; (B) Survival rate at 90 days after LTx; (C) Survival rate at 1 year after LTx; (D) Accumulated survival rate after LTx.
Sensitivity analyses and publication bias
The corresponding pooled ESs did not alter significantly during the sensitivity analysis process, suggesting robustness of the results. Publication bias was tested using Begg’s funnel plot and Egger’s test. No significant publication bias was observed in either the visualization of the funnel plot (Figure 8, P=0.381) or Egger’s test (P=0.272).
Figure 8.
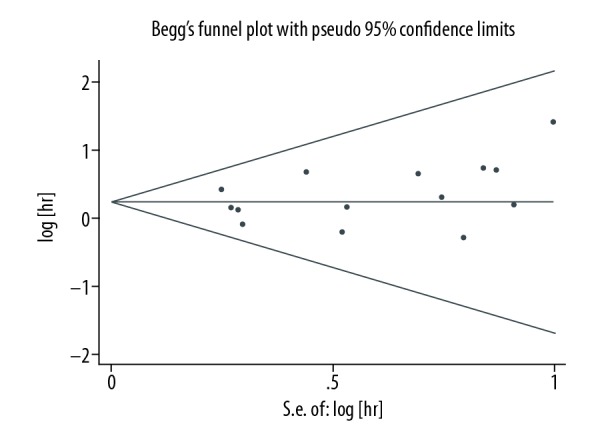
Begg’s test results of the accumulated survival rate.
Discussion
EVLP as a new technique not only could preserve donor lungs but also could be used to assess and recondition/improve the borderline lungs, so it has a great potential to replace the standard cold storage in the procurement of donor lungs. However, synthetic comparative analysis of EVLP technique and standard cold storage in LTx is limited, especially for low-quality donor lungs. This present meta-analysis systematically evaluated the impact of EVLP on LTx outcomes compared with standard cold storage. In the 2 RCTs included, donors in the EVLP group were standard criteria donors [16,17]. Still in other studies, donors in the EVLP group were expanded criteria donors, marginal donors, or initially rejected donors. Combined analyses about donor features showed that the EVLP group had more chest x-ray abnormalities and a poorer P/F ratio than the traditional cold storage group. After the process of EVLP, the poor P/F ratio in the EVLP group was significantly improved with the conversion rate of marginal/rejected donor lungs ranging from 34% to 100%, which promoted the LTx growth by about 29.3%. Luc et al. [18] and Machuca et al. [19] only involved DCD donors and reported the comparison between DCD lungs that underwent EVLP and those transplanted without the use of EVLP, which indicated that EVLP could improve the utilization of extended criteria DCD lungs.
The preservation time in donor lung was much longer in the EVLP group than that in the traditional cold storage group, especially in the Toronto and Lund subgroups. Although the total CIT was similar between the EVLP group and the traditional cold storage group, Toronto and Lund subgroups exhibited longer total CIT in the EVLP group, and the OCS subgroup exhibited shorter total CIT in the EVLP group. Thus, the extra part of preservation time in the EVLP group consisted primarily of the EVLP process, and a longer total CIT. The wide gap between WMD of preservation time (379.54 minutes) and WMD of total CIT (73.28 minutes) could be more approximate to the duration of EVLP, which indicated that EVLP could play an essential role for the expansion of the procurement time [16]. In addition, the OCS protocol based portable EVLP device may allow a significantly shorter CIT and more extended distance transport for donor lungs [16].
The clinical-pathologic features of the recipients between the EVLP and the non-EVLP groups were equivalent, except the EVLP group had more female composition and required more intraoperative ECC/ECMO than the non-EVLP group. There were no significant differences between the 2 groups in using mechanical ventilation/ECMO support after LTx. PGD was graded based on the International Society for Heart and Lung Transplantation (ISHLT) criteria, with grade 3 representing P/F ratio <200 within 72 hours and radiographic infiltrates [34,35]. The EVLP recipients had less incidence of PGD3 throughout the initial 72 hours after LTx than the non-EVLP recipients. However, the length of ICU stays of the EVLP group was longer than the non-EVLP group. This may be probably because the OCS subgroup contributed less incidence of PGD3 (Figure 6C), and the Lund subgroup donated more extended ICU stays (Figure 6E). The peak pulmonary function (FEV1% and FVC%) after LTx, and the short-to long-term survival outcomes were all similar between the 2 groups.
Despite our efforts to conduct a comprehensive analysis, there are still some limitations that need to be recognized. First, most of the included studies in our analysis were retrospective cohort studies that provided only weaker statistical power. Second, some studies have shown a relatively small number of patients, which may affect the validity of the statistics. Third, several ESs and its 95% CI were calculated by extracting the data from Kaplan-Meier curves, which might bring statistical deviations inevitably. Finally, donor/recipient characteristics, EVLP processes, and follow-up showed significant heterogeneity. Although random-effect models, subgroup analyses, and sensitivity analyses were performed to address this heterogeneity, these results should still be interpreted with caution.
Conclusions
In our study, EVLP can be used to assess and improve the quality of high-risk donor lungs to expand lung supply and improve donor lung utilization. Additionally, the application of EVLP is non-inferior to standard cold storage regarding postoperative outcomes. Considering an RCT designed for improving low-quality donor lung with EVLP might be problematic from an ethical point, this study can be a rationale for further work.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by the National Key Research and Development Program of China (Project 2017YFC0113500), the Natural Science Foundation of China (Project 81373161), the Major Scientific and Technological Development Program of Zhejiang province (Project 2014C03032); the Natural Science Foundation of Zhejiang province (Project LY19H160054 and LY19H160039), and the Zhejiang Provincial Medical and Health Platform Key Funding Program (Project 2012ZDA017)
References
- 1.Orens JB, Garrity ER., Jr General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009;6(1):13–19. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- 2.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37(10):1169–83. doi: 10.1016/j.healun.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant. 2019;19(Suppl 2):404–84. doi: 10.1111/ajt.15279. [DOI] [PubMed] [Google Scholar]
- 4.Valenza F, Rosso L, Coppola S, et al. Ex vivo lung perfusion to improve donor lung function and increase the number of organs available for transplantation. Transpl Int. 2014;27(6):553–61. doi: 10.1111/tri.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364(15):1431–40. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 6.Steen S, Liao Q, Wierup PN, et al. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76(1):244–52. doi: 10.1016/s0003-4975(03)00191-7. discussion 252. [DOI] [PubMed] [Google Scholar]
- 7.Steen S, Sjoberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357(9259):825–29. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 8.Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319–25. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Warnecke G, Moradiellos J, Tudorache I, et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: A pilot study of 12 patients. Lancet. 2012;380(9856):1851–58. doi: 10.1016/S0140-6736(12)61344-0. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controll Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez PG, Davis RD, D’Ovidio F, et al. The NOVEL lung trial one-year outcomes. J Heart Lung Transplant. 2014;33(4):S71–72. [Google Scholar]
- 16.Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med. 2018;6(5):357–67. doi: 10.1016/S2213-2600(18)30136-X. [DOI] [PubMed] [Google Scholar]
- 17.Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial. J Heart Lung Transplant. 2017;36(7):744–53. doi: 10.1016/j.healun.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Luc JGY, Jackson K, Weinkauf JG, et al. Feasibility of lung transplantation from donation after circulatory death donors following portable ex vivo lung perfusion: A pilot study. Transplant Proc. 2017;49(8):1885–92. doi: 10.1016/j.transproceed.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant. 2015;15(4):993–1002. doi: 10.1111/ajt.13124. [DOI] [PubMed] [Google Scholar]
- 20.Koch A, Pizanis N, Olbertz C, et al. One-year experience with ex vivo lung perfusion: Preliminary results from a single center. Int J Artif Organs. 2018;41(8):460–66. doi: 10.1177/0391398818783391. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson T, Wallinder A, Henriksen I, et al. Lung transplantation after ex vivo lung perfusion in two Scandinavian centres. Eur J Cardiothorac Surg. 2018;55(4):766–72. doi: 10.1093/ejcts/ezy354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZL, van Suylen V, van Zanden JE, et al. First experience with ex vivo lung perfusion for initially discarded donor lungs in the Netherlands: A single-centre study. Eur J Cardiothorac Surg. 2019;55(5):920–26. doi: 10.1093/ejcts/ezy373. [DOI] [PubMed] [Google Scholar]
- 23.Wallinder A, Riise GC, Ricksten SE, et al. Transplantation after ex vivo lung perfusion: A midterm follow-up. J Heart Lung Transplant. 2016;35(11):1303–10. doi: 10.1016/j.healun.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Fisher A, Andreasson A, Chrysos A, et al. An observational study of donor ex vivo lung perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess. 2016;20(85):1–276. doi: 10.3310/hta20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tikkanen JM, Cypel M, Machuca TN, et al. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J Heart Lung Transplantat. 2015;34(4):547–56. doi: 10.1016/j.healun.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 26.Fildes JE, Archer LD, Blaikley J, et al. Clinical outcome of patients transplanted with marginal donor lungs via ex vivo lung perfusion compared to standard lung transplantation. Transplantation. 2015;99(5):1078–83. doi: 10.1097/TP.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 27.Sage E, Mussot S, Trebbia G, et al. Lung transplantation from initially rejected donors after ex vivo lung reconditioning: The French experience. Eur J Cardiothorac Surg. 2014;46(5):794–99. doi: 10.1093/ejcts/ezu245. [DOI] [PubMed] [Google Scholar]
- 28.Boffini M, Ricci D, Bonato R, et al. Incidence and severity of primary graft dysfunction after lung transplantation using rejected grafts reconditioned with ex vivo lung perfusion. Eur J Cardiothorac Surg. 2014;46(5):789–93. doi: 10.1093/ejcts/ezu239. [DOI] [PubMed] [Google Scholar]
- 29.Wallinder A, Ricksten SE, Silverborn M, et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: A case-control study. Eur J Cardiothorac Surg. 2014;45(1):40–44. doi: 10.1093/ejcts/ezt250. discussion 44–45. [DOI] [PubMed] [Google Scholar]
- 30.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144(5):1200–6. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Zych B, Popov AF, Stavri G, et al. Early outcomes of bilateral sequential single lung transplantation after ex-vivo lung evaluation and reconditioning. J Heart Lung Transplant. 2012;31(3):274–81. doi: 10.1016/j.healun.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Aigner C, Slama A, Hotzenecker K, et al. Clinical ex vivo lung perfusion – pushing the limits. Am J Transplant. 2012;12(7):1839–47. doi: 10.1111/j.1600-6143.2012.04027.x. [DOI] [PubMed] [Google Scholar]
- 33.Lindstedt S, Hlebowicz J, Koul B, et al. Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact Cardiovasc Thorac Surg. 2011;12(2):162–65. doi: 10.1510/icvts.2010.244830. [DOI] [PubMed] [Google Scholar]
- 34.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, Part I: Definition and Grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097–103. doi: 10.1016/j.healun.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Porteous MK, Lee JC. Primary graft dysfunction after lung transplantation. Clin Chest Med. 2017;38(4):641–54. doi: 10.1016/j.ccm.2017.07.005. [DOI] [PubMed] [Google Scholar]