Abstract
Background
The incidence and prognostic factors of chondrosarcoma patients have been reported in early studies. However, the association between risk factors and the incidence or prognosis of chondrosarcoma patients with pulmonary metastasis remains unclear. Therefore, we assessed these risk factors among chondrosarcoma patients with pulmonary metastasis.
Material/Methods
From 1365 chondrosarcoma patients in the Surveillance, Epidemiology, and End Results (SEER) database, we collected the information of 69 patients with pulmonary metastasis at the initial diagnosis of chondrosarcoma from 2010 to 2016. We investigated the incidence, risk factors, and prognostic factors for pulmonary metastasis patients by using multivariate logistic regression and multivariate Cox regression analyses.
Results
Data from a total of 69 (6.8%) chondrosarcoma patients with pulmonary metastasis at initial diagnosis were extracted. Patients with the following characteristics were positively associated with higher risk of pulmonary metastasis: dedifferentiated subtype, high grade of malignancy, extracompartmental tumor (Enneking B), presence of regional lymph nodes, local recurrence, large tumor size (larger than 15 cm), and being married. Older patients (older than 67 years), and patients with clear cell chondrosarcoma or large tumor size (larger than 15 cm) exhibited the worse prognosis and survival (overall and cancer-specific). Resection of the primary tumor tended to be correlated with a better prognosis.
Conclusions
The incidence of pulmonary metastasis in chondrosarcoma was approximately 6.8%, with poor prognosis. Identifying risk factors and their associations with the incidence and prognosis in chondrosarcoma patients with pulmonary metastasis could provide a reference for clinical surveillance and guide the design of personalized treatment plans.
MeSH Keywords: Chondrosarcoma, Lung, Neoplasm Metastasis, Prognosis, Risk Factors, SEER Program
Background
Chondrosarcoma (CHS) is a heterogeneous group of malignant tumors consisting of proliferating cartilaginous tissue; it is the second most common primary bone sarcoma after osteosarcoma. CHS constitutes 30% of all primary bone sarcomas. Because neither radiation therapy nor chemotherapy are effective against chondrosarcoma, wide resection with adequate surgical margins is the mainstay of primary treatment [1]. According to previous studies, 8–38% of chondrosarcoma patients developed distant metastasis [2–6]. This has been confirmed to be an independent prognostic factor correlated with poor prognosis of chondrosarcoma patients [2,4,7–11]. Additionally, a majority of the distant metastatic sites were in the lungs [12,13]. A systemic review revealed that sarcoma’s pulmonary metastasis rate was about 18–50% [14]. The development of pulmonary metastasis is a predictor of worse prognosis in CHS patients. The overall survival rate at 10 years by Kaplan-Meier analysis was 17% for patients who developed pulmonary metastasis, with a metastatic rate of approximately 9.6% [9]. Nakamura et al. recently reported that the incidence of pulmonary metastasis in chondrosarcoma patients was 11.2%, and among patients with pulmonary metastasis, the overall survival at 3 years and 5 years was 51.5% and 45.7%, respectively [15]. Early studies reported that older age, tumor site, higher grade of malignancies, and a larger tumor size are established risk factors for distant metastasis in chondrosarcoma patients. Other clinical characteristics have also been found as prognostic factors significantly associated with survival of chondrosarcoma patients, such as age, sex, year of diagnosis, tumor stage, tumor grade, tumor site, tumor size, surgery, and radiation [8,10,16,17]. However, risk factors and prognostic factors for chondrosarcoma patients with pulmonary metastasis remain unclear. Therefore, it is necessary to identify risk factors for the incidence and prognosis in chondrosarcoma patients with pulmonary metastasis.
The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute is a comprehensive source of population-based information on cancer incidence and survival in the United States that was collected from 18 population-based cancer registries covering approximately 27.8% of the U.S. population. We utilized this open database to collect the information of demographic and clinical characteristics to investigate the association of risk factors with the incidence and prognosis in CHS patients with pulmonary metastasis (PM) at initial diagnosis.
Material and Methods
Patient selection
Patient data were abstracted from Incidence-SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying) using SEER*Stat 8.3.5 software, which collects patients’ demographic and clinical characteristics. Since the complete information on distant metastases was not available before 2010, we restricted our study to the period time between 2010 and 2016. The inclusion criteria were as follows: (1) chondrosarcoma patients diagnosed between 2010 and 2016; (2) histologic subtype limited to Chondrosarcoma (9220), Juxtacortical chondrosarcoma (9221), Myxoid chondrosarcoma (9231), Mesenchymal chondrosarcoma (9240), Clear cell chondrosarcoma (9242), Dedifferentiated chondrosarcoma (9243) according to the International Classification of Diseases for Oncology, 3rd Edition codes; (3) tumor sites limited to extremity (C40.0–C40.3), spine (C41.2), thoracic cage (C41.3), others included pelvic bones, sacrum, coccyx and associated joints (C41.4); (4) known survival time, complete follow-up. The exclusion criteria were as follows: (1) diagnosed at autopsy or via death certificate; (2) survival time code 0 months; (3) more than 1 primary tumor; (4) unknown primary tumor surgery information; (5) unknown sequence between radiation and surgery; (6) regional lymph nodes metastasis incomplete information; (7) unknown regional lymph nodes metastasis removed.
As shown in the flow chart (Figure 1), 1365 patients diagnosed with CHS from January 1, 2010, to December 31, 2016, were initially identified. After the exclusion of 351 ineligible patients, a total of 1014 patients remained, both with and without PM. Among those, data on 69 PM patients were collected to analyze the prognostic factors of overall survival for CHS with PM. Eventually, 1 patient who was dead from other causes was excluded, leaving 68 PM patients to predict the prognostic factors of cancer-specific survival.
Figure 1.
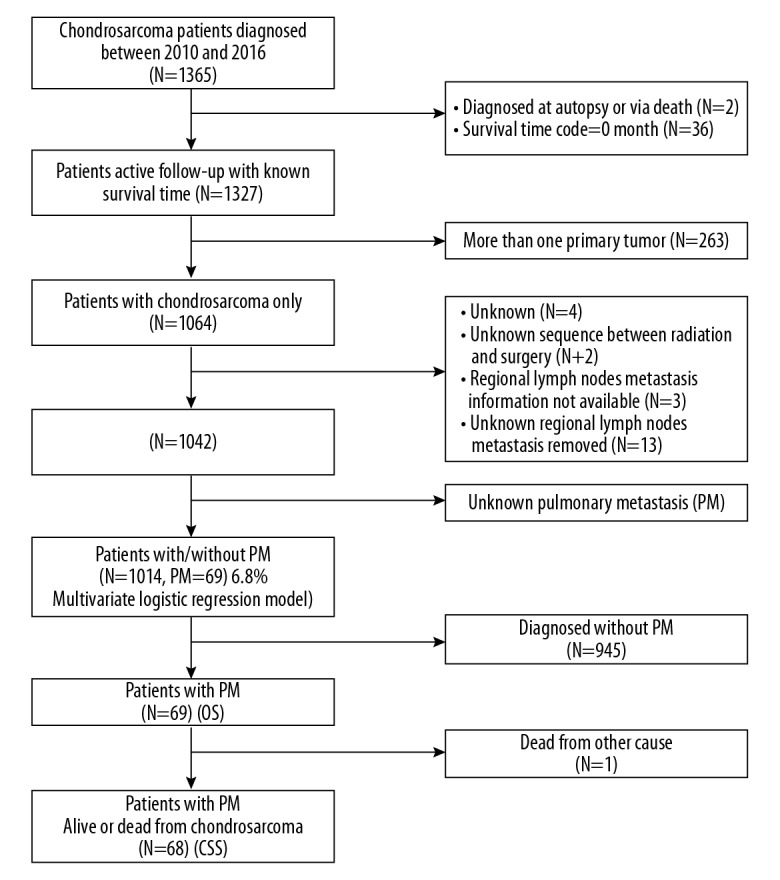
Flow chart of inclusion and exclusion criteria.
Demographics and clinical characteristics
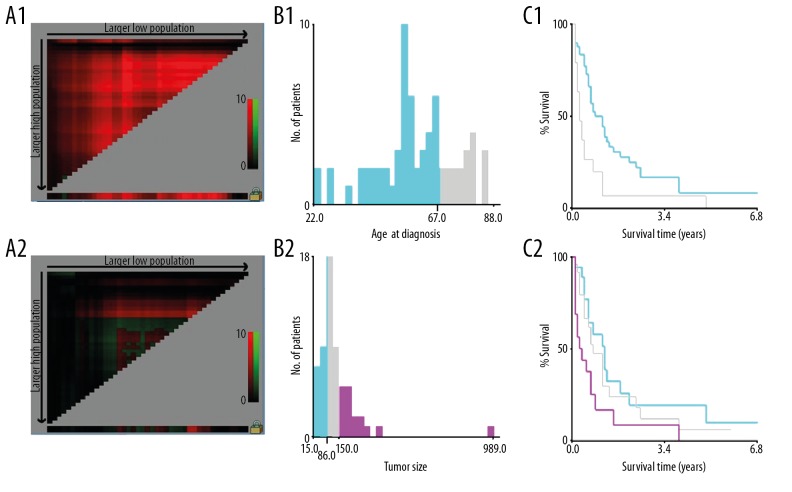
Patients’ demographics included age at diagnosis, sex, race, insurance status, and marital status. Marital status was characterized as unmarried or married. Unmarried included single (never married), widowed, divorced, separated, and unmarried or domestic partner. Clinical characteristics included tumor site, histologic subtype, tumor grade, Enneking staging, the presence or absence of regional lymph nodes metastases, local recurrence and pulmonary metastasis, tumor size, primary tumor surgery, regional lymph nodes removed, chemotherapy, radiation therapy and radiation sequence with surgery to investigate which characteristics are risk and prognostics factors for CHS with PM Primary site was classified as extremity (C40.0–C40.3), spine (C41.2), thoracic cage (C41.3), others included pelvic bones, sacrum, coccyx, and associated joints (C41.4). Histologic subtype was classified as conventional subtype (chondrosarcoma and juxtacortical), myxoid subtype, mesenchymal subtype, clear cell subtype, and dedifferentiated subtype. Regarding tumor grade, Grade I well-differentiated and grade II moderately differentiated lesions (ICD-O-3) were regrouped as low grade, whereas grade III poorly differentiated and grade IV undifferentiated and anaplastic lesions (ICD-O-3) were regrouped as high grade [4,18]. According to Enneking staging system, tumor extension was divided into 2 groups: intracompartmental (A) and extracompartmental (B) [19]. Chemotherapy and radiation therapy were referred to as the treatment for the primary tumor site. X-tile 3.6.1 software (Yale University, New Haven, Connecticut, USA) was employed to identify the optimal cutoff values for continuous variables such as age at diagnosis and tumor size. The optimal cutoff values for age at diagnosis was 67 years old, then age at diagnosis was stratified as younger age ≤67 years old and older age >67 years old. The optimal cutoff values for tumor size was 8.6 cm and 15 cm. Tumor size was categorized as small size (≤8.6 cm), medium size (>8.6–15 cm), and large size (>15 cm) (Figure 2).
Figure 2.
The graphs define the optimal cutoff values of age of diagnosis and tumor size using X-tile analysis. The X-tile analysis of the training cohort is demonstrated with the “lock” symbol indicating that optimal cutoff values of age of diagnosis and tumor size have been identified, respectively (1A, 2A). A histogram (1B, 2B) and survival analysis were developed based on these cutoff values (1C, 2C). For age at diagnosis, optimal cutoff values were identified as 67 years old based on overall survival. For tumor size, optimal cutoff values were identified as 8.6 cm and 15 cm based on overall survival.
Statistical analysis
A chi-square test was used to evaluate the differences between groups for categorical variables. The primary endpoint in this study included overall survival (OS) and CHS cancer-specific survival (CSS). OS was defined as the time interval from the date of diagnosis till the date of death due to any cause. CSS was defined as the time from diagnosis until death due to CHS. Univariate logistic regression was used to determine risk factors for CHS patients with PM. Those characteristics with P-values less than 0.05 in the univariate logistic regression analysis were continually chosen for the multivariate logistic analysis. The odds ratio (OR) and corresponding 95% confidence interval (CI) were used to show the association between clinical characteristics and PM development. Univariate and multivariate Cox proportional hazard models were applied to determine the independent predictors association among several variables for OS and CSS. The hazard ratios (HR) and corresponding 95% confidence interval (CI) were used to show the impact of patient factors on OS and CSS. All statistical analyses were performed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Two-tailed and P-values less than 0.05 were considered statistically significant in all statistical tests.
Results
Demographics and baseline characteristics for CHS patients diagnosed with PM
One thousand and fourteen patients diagnosed as CHS between 2010 and 2016 were collected from the SEER database. The overall cohort was mostly white patients (86%). The sex distribution demonstrated a slight male predominance (56.3%). The mean age for these patients was 52.68 ±17.64 years. Conventional subtype constituted approximately 81.9% of all histologic type chondrosarcomas. Among these CHS patients, 69 patients with PM were retrieved and the mean age of PM patients was 59.54±14.91 years. With regard to tumor primary site, patients with PM were, in ascending order: thoracic cage (5.3%); extremities (6.7%); spine (7%); pelvis, sacrum, coccyx (8.5%). In all histologic subtypes, the dedifferentiated subtype had the highest PM rate of 22.5%. The incidence rate of PM with high-grade tumor and low-grade tumor was 16.9% and 3.1%, respectively. Approximately 87.8% of CHS patients underwent surgery at the primary site, 9.4% had radiation therapy, and 11.9% had chemotherapy. Patients with PM received less primary site surgery and more chemotherapy and radiation therapy. The baseline of demographics and clinical characteristics of patients is presented in Table 1.
Table 1.
Baseline of the demographic and related clinical characteristics for patients diagnosed with chondrosarcoma.
| Characteristics | With PM | Without PM | Entire cohort | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|
| n=69 | 6.8% | n=945 | 93.2% | N=1014 | % | |||
| Sex | χ2=1.086 | 0.297 | ||||||
| Female | 26 | 5.9% | 417 | 94.1% | 443 | 43.7% | ||
| Male | 43 | 7.5% | 528 | 92.5% | 571 | 56.3% | ||
| Age (52.68±17.64) range (4–95) | χ2=2.101 | 0.147 | ||||||
| ≤67 | 50 | 6.2% | 754 | 93.8% | 804 | 79.3% | ||
| >67 | 19 | 9.0% | 191 | 91.0% | 210 | 20.7% | ||
| Race | χ2=1.234 | 0.745 | ||||||
| White | 60 | 6.9% | 812 | 93.1% | 872 | 86.0% | ||
| Black | 6 | 7.9% | 70 | 92.1% | 76 | 7.5% | ||
| Other | 2 | 3.6% | 54 | 96.4% | 56 | 5.5% | ||
| Unknown | 1 | 10.0% | 9 | 90.0% | 10 | 1.0% | ||
| Insurance status | χ2=5.258 | 0.072 | ||||||
| Uninsured | 5 | 15.2% | 28 | 84.8% | 33 | 3.3% | ||
| Insured | 64 | 6.7% | 895 | 93.3% | 959 | 94.6% | ||
| Unknown | 0 | 0.0% | 22 | 100.0% | 22 | 2.2% | ||
| Marital status | χ2=9.640 | 0.008 | ||||||
| Unmarried | 19 | 4.7% | 385 | 95.3% | 404 | 39.8% | ||
| Married | 49 | 9.0% | 497 | 91.0% | 546 | 53.8% | ||
| Unknown | 1 | 1.6% | 63 | 98.4% | 64 | 6.3% | ||
| Primary site | χ2=1.739 | 0.628 | ||||||
| Extremities | 37 | 6.7% | 512 | 93.3% | 549 | 54.1% | ||
| Spine | 4 | 7.0% | 53 | 93.0% | 57 | 5.6% | ||
| Thoracic cage | 11 | 5.3% | 198 | 94.7% | 209 | 20.6% | ||
| Others | 17 | 8.5% | 182 | 91.5% | 199 | 19.6% | ||
| Histologic subtype | χ2=49.181 | <0.001 | ||||||
| Conventional | 39 | 4.7% | 791 | 95.3% | 830 | 81.9% | ||
| Myxoid | 3 | 6.8% | 41 | 93.2% | 44 | 4.3% | ||
| Mesenchymal | 1 | 9.1% | 10 | 90.9% | 11 | 1.1% | ||
| Clear cell | 1 | 5.6% | 17 | 94.4% | 18 | 1.8% | ||
| Dedifferentiated | 25 | 22.5% | 86 | 77.5% | 111 | 10.9% | ||
| Grade | χ2=51.947 | <0.001 | ||||||
| Low grade | 21 | 3.1% | 666 | 96.9% | 687 | 67.8% | ||
| High grade | 36 | 16.9% | 177 | 83.1% | 213 | 21.0% | ||
| Unknown | 12 | 10.5% | 102 | 89.5% | 114 | 11.2% | ||
| Enneking staging | χ2=20.941 | <0.001 | ||||||
| A | 7 | 2.6% | 260 | 97.4% | 267 | 26.3% | ||
| B | 38 | 11.8% | 284 | 88.2% | 322 | 31.8% | ||
| Unknown | 24 | 5.6% | 401 | 94.4% | 425 | 41.9% | ||
| Regional lymph nodes mets | χ2=44.828 | <0.001 | ||||||
| No | 57 | 5.8% | 919 | 94.2% | 976 | 96.3% | ||
| Yes | 6 | 46.2% | 7 | 53.8% | 13 | 1.3% | ||
| Unknown | 6 | 24.0% | 19 | 76.0% | 25 | 2.5% | ||
| Local recurrence | χ2=87.909 | <0.001 | ||||||
| No | 59 | 5.9% | 940 | 94.1% | 999 | 98.5% | ||
| Yes | 9 | 64.3% | 5 | 35.7% | 14 | 1.4% | ||
| Unknown | 1 | 100.0% | 0 | 0.0% | 1 | 0.1% | ||
| Tumor size | χ2=32.863 | <0.001 | ||||||
| ≤8.6 cm | 18 | 3.2% | 550 | 96.8% | 568 | 56.0% | ||
| >8.6–15 cm | 26 | 10.9% | 212 | 89.1% | 238 | 23.5% | ||
| >15 cm | 16 | 15.1% | 90 | 84.9% | 106 | 10.5% | ||
| Unknown | 4 | 3.9% | 98 | 96.1% | 102 | 10.1% | ||
| Primary tumor surgery | χ2=98.059 | <0.001 | ||||||
| No | 34 | 27.4% | 90 | 72.6% | 124 | 12.2% | ||
| Destruction | 0 | 0.0% | 6 | 100.0% | 6 | 0.6% | ||
| Resection | 26 | 3.4% | 731 | 96.6% | 757 | 74.7% | ||
| Amputation | 9 | 7.8% | 107 | 92.2% | 116 | 11.4% | ||
| Surgery, NOS | 0 | 0.0% | 11 | 100.0% | 11 | 1.1% | ||
| Regional lymph nodes removed | χ2=2.579 | 0.108 | ||||||
| No | 68 | 7.1% | 887 | 92.9% | 955 | 94.2% | ||
| Yes | 1 | 1.7% | 58 | 98.3% | 59 | 5.8% | ||
| Chemotherapy | χ2=119.422 | <0.001 | ||||||
| None/unknown | 37 | 4.0% | 882 | 96.0% | 919 | 90.6% | ||
| Yes | 32 | 33.7% | 63 | 66.3% | 95 | 9.4% | ||
| Radiation | χ2=24.117 | <0.001 | ||||||
| None/unknown | 48 | 5.4% | 845 | 94.6% | 893 | 88.1% | ||
| Yes | 21 | 17.4% | 100 | 82.6% | 121 | 11.9% | ||
| Radiation sequence with surgery | χ2=1.772 | 0.412 | ||||||
| None | 61 | 6.5% | 871 | 93.5% | 932 | 91.9% | ||
| Prior | 2 | 14.3% | 12 | 85.7% | 14 | 1.4% | ||
| After | 6 | 8.8% | 62 | 91.2% | 68 | 6.7% | ||
mets – metastasis.
The incidence of PM
Sixty-nine CHS patients were diagnosed with PM, which accounted for 6.8% (69/1014) of the entire cohort. Based on the chi-squared test, as shown in Table 1, the PM incidence of those patients with dedifferentiated subtype (χ2=49.181, P<0.001), high grade of tumor malignancy (χ2=51.947, P<0.001), extracompartmental tumor (χ2=20.941, P<0.001), presence of regional lymph nodes metastases (LNM) (χ2=44.828, P<0.001), presence of local recurrence (χ2=87.909, P<0.001), large tumor size (χ2=32.863, P<0.001), and married (χ2=9.640, P=0.008) were all significantly higher than their counterparts in each group.
Risk factors for developing pulmonary metastasis
Univariate logistic regression analysis showed that factors positively associated with PM development at initial diagnosis included: dedifferentiated subtype (OR=5.896, 95% CI: 3.404–10.212, P<0.001); high grade of tumor malignancy (OR=6.450, 95% CI: 3.673–11.328, P<0.001); extracompartmental tumor (OR=4.970, 95% CI: 2.181–11.324, P<0.001) presence of regional LNM (OR=13.820, 95% CI: 4.497–42.472, P<0.001); presence of local recurrence (OR=28.678, 95% CI: 1.394–4.778, P=0.003); medium tumor size >8.6–15 cm (OR=2.581, 95% CI: 2.013–6.977, P<0.001), large tumor size >15 cm (OR=4.267, 95% CI: 2.182–8.342, P<0.001) and were married (OR=1.998, 95% CI: 1.157–3.449, P=0.013).
As demonstrated in Table 2, multivariate logistic regression indicated that factors positively associated with higher risk of PM development at initial diagnosis included: dedifferentiated subtype (OR=2.207, 95% CI: 1.058–4.603, P=0.035); high grade of tumor malignancy (OR=2.946, 95% CI: 1.415–6.136, P=0.004); extracompartmental tumor (OR=2.783, 95% CI: 1.149–6.743, P=0.023); presence of regional LNM (OR=7.727, 95% CI: 2.117–28.210, P=0.002); presence of local recurrence (OR=22.699, 95% CI: 5.558–92.696, P<0.001), large tumor size >15 cm (OR=2.259, 95% CI: 1.044–4.884, P=0.038), and were married (OR=2.072, 95% CI: 1.115–3.853, P=0.021);
Table 2.
Univariate and multivariate logistic regression for analyzing the demographic and related clinical characteristics for chondrosarcoma patients with pulmonary metastasis at diagnosis.
| Characteristics | With PM | Entire cohort | Incidence | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| n=69 | N=1014 | 6.8 (%) | OR | (95% CI) | P | OR | (95% CI) | P | |
| Sex | NI | ||||||||
| Female | 26 | 443 | 5.9% | R | |||||
| Male | 43 | 571 | 7.5% | 1.306 | 0.789–2.161 | 0.299 | |||
| Age | |||||||||
| ≥67 | 50 | 804 | 6.2% | R | NI | ||||
| >67 | 19 | 210 | 9.0% | 1.500 | 0.864–2.604 | 0.150 | |||
| Race | NI | ||||||||
| White | 60 | 872 | 6.9% | R | |||||
| Black | 6 | 76 | 7.9% | 1.160 | 0.484–2.780 | 0.739 | |||
| Other | 2 | 56 | 3.6% | 0.501 | 0.119–2.106 | 0.346 | |||
| Unknown | 1 | 10 | 10.0% | NA | NA | NA | |||
| Insurance status | NI | ||||||||
| Uninsured | 5 | 33 | 15.2% | R | |||||
| Insured | 64 | 959 | 6.7% | 0.400 | 0.150–1.072 | 0.069 | |||
| Unknown | 0 | 22 | 0.0% | NA | NA | NA | |||
| Marital status | |||||||||
| Unmarried | 19 | 404 | 4.7% | R | R | ||||
| Married | 49 | 546 | 9.0% | 1.998 | 1.157–3.449 | 0.013 | 2.072 | 1.115–3.853 | 0.021 |
| Unknown | 1 | 64 | 1.6% | NA | NA | NA | NA | NA | NA |
| Primary site | |||||||||
| Extremities | 37 | 549 | 6.7% | R | NI | ||||
| Spine | 4 | 57 | 7.0% | 1.044 | 0.358–3.044 | 0.937 | |||
| Thoracic cage | 11 | 209 | 5.3% | 0.769 | 0.385–1.537 | 0.457 | |||
| Others | 17 | 199 | 8.5% | 1.293 | 0.710–2.352 | 0.401 | |||
| Histologic subtype | |||||||||
| Conventional | 39 | 830 | 4.7% | R | R | ||||
| Myxoid | 3 | 44 | 6.8% | 1.484 | 0.440–5.004 | 0.524 | 0.724 | 0.168–3.119 | 0.665 |
| Mesenchymal | 1 | 11 | 9.1% | 2.028 | 0.253–16.245 | 0.505 | 1.065 | 0.124–9.150 | 0.954 |
| Clear cell | 1 | 18 | 5.6% | 1.193 | 0.155–9.196 | 0.865 | 1.144 | 0.137–9.519 | 0.901 |
| Dedifferentiated | 25 | 111 | 22.5% | 5.896 | 3.404–10.212 | <0.001 | 2.207 | 1.058–4.603 | 0.035 |
| Grade | |||||||||
| Low grade | 21 | 687 | 3.1% | R | R | ||||
| High grade | 36 | 213 | 16.9% | 6.450 | 3.673–11.328 | <0.001 | 2.946 | 1.415–6.136 | 0.004 |
| Unknown | 12 | 114 | 10.5% | NA | NA | NA | NA | NA | NA |
| Enneking staging | |||||||||
| A | 7 | 267 | 2.6% | R | |||||
| B | 38 | 322 | 11.8% | 4.970 | 2.181–11.324 | <0.001 | 2.783 | 1.149–6.743 | 0.023 |
| Unknown | 24 | 425 | 5.6% | NA | NA | NA | NA | NA | NA |
| Regional lymph nodes mets | |||||||||
| No | 57 | 976 | 5.8% | R | R | ||||
| Yes | 6 | 13 | 46.2% | 13.820 | 4.497–42.472 | <0.001 | 7.727 | 2.117–28.210 | 0.002 |
| Unknown | 6 | 25 | 24.0% | NA | NA | NA | |||
| Local recurrence | |||||||||
| No | 59 | 999 | 5.9% | R | R | ||||
| Yes | 9 | 14 | 64.3% | 28.678 | 9.316–88.283 | <0.001 | 22.699 | 5.558–92.696 | <0.001 |
| Unknown | 1 | 1 | 100.0% | NA | NA | NA | NA | NA | NA |
| Tumor size | |||||||||
| ≤8.6 cm | 18 | 568 | 3.2% | R | R | ||||
| >8.6–15 cm | 26 | 238 | 10.9% | 2.581 | 1.394–4.778 | 0.003 | 1.491 | 0.742–2.994 | 0.262 |
| >15 cm | 16 | 106 | 15.1% | 4.267 | 2.182–8.342 | <0.001 | 2.259 | 1.044–4.884 | 0.038 |
| Unknown | 4 | 102 | 3.9% | NA | NA | NA | NA | NA | NA |
R – reference; OR – odds ratio; CI – confidence interval; NA – not applicable; NI – not included; mets – metastasis.
Prognostic factors for chondrosarcoma patients
Because the survival rate of CHS patients is over 50%, the median overall survival time could not be calculated by Kaplan-Meier. Univariate and multivariate Cox regression analysis were conducted to determine those clinical characteristics which were associated with the prognosis of CHS patients. We found that male sex (P=0.001), older age (P<0.001), dedifferentiated subtype (P<0.001), high grade of tumor malignancy (P=0.041), extracompartmental tumor (P=0.016), presence of PM (P<0.001), presence of local recurrence (P=0.002), and large tumor size (P=0.001) were associated with poor prognosis of OS. Details of the prognostic factors for CHS patients are listed in Table 3. Remarkably, patients with pulmonary metastasis had worse prognosis than those without PM (Figure 3). Chemotherapy and radiation therapy were not statistically significant associated with prognosis. Resection (P<0.001) and amputation (P=0.026) of the primary tumor were the only prognostic factors that were associated with a better prognosis compared to those without surgery.
Table 3.
Univariate and multivariate Cox regression for analyzing prognostic factors for chondrosarcoma patients (diagnosed 2010–2016).
| Characteristics | Median OS | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | ||
| Sex | |||||||
| Female | 29 (1–83) | R | R | ||||
| Male | 30 (1–83) | 1.356 | 1.019–1.804 | 0.037 | 1.642 | 1.216–2.216 | 0.001 |
| Age | |||||||
| ≤67 | 31 (1–83) | R | R | ||||
| >67 | 18 (1–83) | 3.650 | 2.763–4.821 | <0.001 | 3.115 | 2.266–4.281 | <0.001 |
| Race | NI | ||||||
| White | 30 (1–83) | R | |||||
| Black | 30 (1–82) | 0.653 | 0.346–1.234 | 0.189 | |||
| Others | 24 (1–82) | 0.633 | 0.297–1.346 | 0.235 | |||
| Unknown | NA | NA | NA | NA | |||
| Insurance status | NI | ||||||
| Uninsured | 27 (1–79) | R | |||||
| Insured | 29 (1–83) | 0.705 | 0.361–1.375 | 0.305 | |||
| Unknown | NA | NA | NA | NA | |||
| Marital status | NI | ||||||
| Unmarried | 29 (1–83) | R | |||||
| Married | 29 (1–83) | 0.972 | 0.730–1.294 | 0.845 | |||
| Unknown | NA | NA | NA | NA | |||
| Primary site | |||||||
| Extremities | 28 (1–83) | R | R | ||||
| Spine | 27 (2–83) | 0.987 | 0.531–1.836 | 0.967 | 1.625 | 0.837–3.152 | 0.151 |
| Thoracic cage | 36 (1–83) | 0.684 | 0.461–1.016 | 0.060 | 0.906 | 0.582–1.410 | 0.662 |
| Others | 26 (1–82) | 1.421 | 1.025–1.971 | 0.035 | 1.368 | 0.945–1.980 | 0.097 |
| Histologic subtype | |||||||
| Conventional | 33 (1–83) | R | R | ||||
| Myxoid | 20.5 (1–75) | 1.818 | 0.953–3.469 | 0.070 | 1.571 | 0.784–3.150 | 0.203 |
| Mesenchymal | 25 (4–71) | 2.172 | 0.690–6.832 | 0.185 | 0.688 | 0.192–2.469 | 0.566 |
| Clear cell | 28.5 (1–67) | 0.476 | 0.066–3.407 | 0.460 | 0.761 | 0.103–5.623 | 0.789 |
| Dedifferentiated | 9 (1–68) | 8.546 | 6.332–11.534 | <0.001 | 4.199 | 2.694–6.546 | <0.001 |
| Grade | |||||||
| Low grade | 35 (1–83) | R | R | ||||
| High grade | 16 (1–82) | 5.483 | 4.031–7.460 | <0.001 | 1.594 | 1.046–2.428 | 0.030 |
| Unknown | NA | NA | NA | NA | NA | NA | NA |
| Enneking staging | |||||||
| A | 34 (1–83) | R | R | ||||
| B | 29 (1–83) | 3.747 | 2.475–5.671 | <0.001 | 1.721 | 1.107–2.676 | 0.016 |
| Unknown | NA | NA | NA | NA | NA | NA | NA |
| Regional lymph nodes mets | |||||||
| No | 30 (1–83) | R | R | ||||
| Yes | 8 (1–70) | 3.753 | 1.663–8.468 | 0.001 | 1.010 | 0.420–2.431 | 0.982 |
| Unknown | NA | NA | NA | NA | NA | NA | NA |
| Pulmonary metastasis | |||||||
| No | 31 (1–83) | R | R | ||||
| Yes | 7 (1–82) | 10.672 | 7.736–14.722 | <0.001 | 3.771 | 2.472–5.751 | <0.001 |
| Local recurrence | |||||||
| No | 30 (1–83) | R | R | ||||
| Yes | 6.5 (1–31) | 12.368 | 6.804–22.480 | <0.001 | 3.030 | 1.511–6.077 | 0.002 |
| Unknown | NA | NA | NA | NA | NA | NA | NA |
| Tumor size | |||||||
| ≤8.6 cm | 32 (1–83) | R | R | ||||
| >8.6–15 cm | 24 (1–83) | 2.603 | 1.851–3.659 | <0.001 | 1.163 | 0.794–1.703 | 0.438 |
| >15 cm | 16 (1–80) | 4.743 | 3.262–6.896 | <0.001 | 2.009 | 1.327–3.040 | 0.001 |
| Unknown | NA | NA | NA | NA | NA | NA | NA |
| Primary tumor surgery | |||||||
| No | 13.5 (1–82) | R | R | ||||
| Destruction | 30 (5–77) | NA | NA | 0.930 | NA | NA | NA |
| Resection | 33 (1–83) | 0.173 | 0.126–0.236 | <0.001 | 0.360 | 0.229–0.565 | <0.001 |
| Amputation | 21.5 (1–83) | 0.444 | 0.293–0.672 | <0.001 | 0.540 | 0.315–0.928 | 0.026 |
| Surgery, NOS | 45 (7–75) | 0.103 | 0.014–0.741 | 0.024 | 0.184 | 0.024–1.424 | 0.105 |
| Regional lymph nodes removed | NI | ||||||
| No | 29 (1–83) | R | |||||
| Yes | 28 (1–81) | 1.423 | 0.854–2.371 | 0.175 | |||
| Chemotherapy | |||||||
| None/unknown | 31 (1–83) | R | R | ||||
| Yes | 13 (1–74) | 5.181 | 3.791–7.082 | <0.001 | 1.347 | 0.889–2.041 | 0.159 |
| Radiation | |||||||
| None/unknown | 31 (1–83) | R | R | ||||
| Yes | 19 (1–82) | 3.288 | 2.404–4.496 | <0.001 | 1.104 | 0.649–1.879 | 0.714 |
| Radiation sequence with surgery | |||||||
| None | 30 (1–83) | R | R | ||||
| Prior | 36 (3–76) | 1.630 | 0.605–4.392 | 0.334 | 0.628 | 0.190–2.076 | 0.446 |
| After | 21.5 (2–80) | 1.969 | 1.274–3.044 | 0.002 | 1.480 | 0.724–3.026 | 0.283 |
R – reference; HR – hazard ratio; CI – confidence interval; NA – not applicable; NI – not included; mets – metastasis; OS – overall survival.
Figure 3.
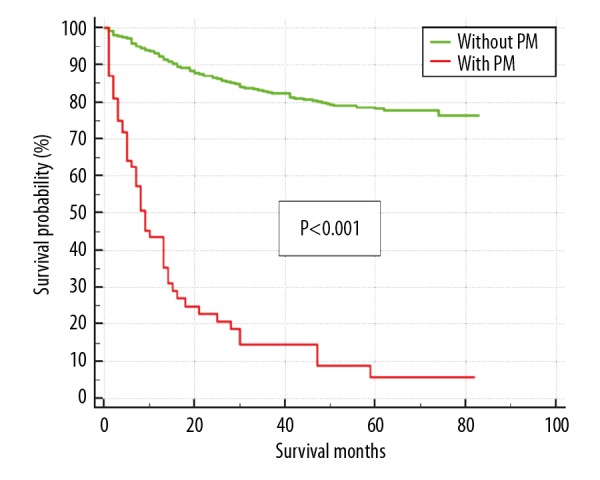
The overall survival curve of chondrosarcoma patients stratified by the presence of pulmonary metastasis.
Prognostic factors for patients with pulmonary metastasis
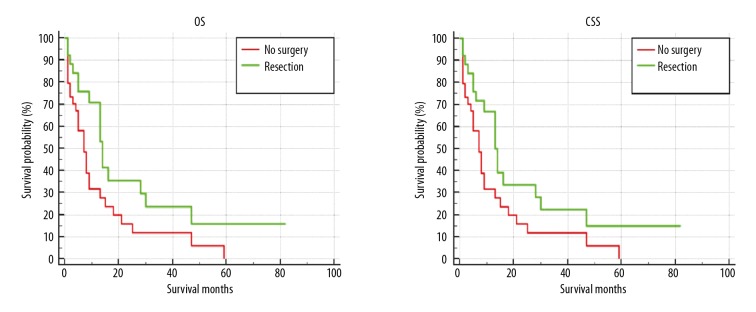
The median overall survival time for CHS patients with PM was 7 months, while the median survival of the cohort was 29 months. At the end of the follow-up, 55 patients with PM (79.71%) had died, of whom 54 died of cancer. The prognostic factors for PM patients are shown in Tables 4 and 5. In the univariate analysis model, factors including older age (HR=2.364, 95% CI: 1.305–4.281, P=0.005); clear cell chondrosarcoma (CCC) subtype (HR=10.437, 95% CI: 1.275–85.470, P=0.029), and large tumor size (HR=2.331, 95% CI: 1.110–4.895, P=0.025) were all associated with poor prognosis of OS and CSS. In contrast, resection of the primary tumor had improved OS and CSS for PM patients (Figure 4). There was no significant impact on OS and CSS by the histological grade of the pulmonary metastasis. Multivariate Cox regression analysis revealed patients with older age (HR=2.668, 95% CI: 1.371–5.192, P=0.004), CCC subtype (HR=10.971, 95% CI: 1.216–98.942, P=0.033), and large tumor size (HR=4.613, 95% CI: 1.857–11.462, P=0.033) were associated with poor prognosis of OS and CSS for PM patients. Moreover, resection of the primary tumor tended to be another prognostic factor which could prolong OS and CSS in PM patients.
Table 4.
Univariate and multivariate Cox regression for analyzing overall survival among chondrosarcoma patients with pulmonary metastasis (diagnosed 2010–2016).
| Characteristics | Median OS | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p | HR | (95% CI) | p | ||
| Sex | NI | ||||||
| Female | 8 (1–82) | R | |||||
| Male | 7 (1–70) | 1.122 | 0.639–1.969 | 0.689 | |||
| Age | |||||||
| ≤67 | 8 (1–82) | R | R | ||||
| >67 | 3 (1–59) | 2.364 | 1.305–4.281 | 0.005 | 2.668 | 1.371–5.192 | 0.004 |
| Race | NI | ||||||
| White | 7.5 (1–82) | R | |||||
| Black | 8.5 (1–15) | 0.838 | 0.258–2.719 | 0.768 | |||
| Other | 4 (3–5) | 1.433 | 0.193–10.652 | 0.725 | |||
| Unknown | NA | NA | NA | NA | |||
| Insurance status | NI | ||||||
| Uninsured | 7 (1–8) | R | |||||
| Insured | 7.5 (1–82) | 0.812 | 0.247–2.671 | 0.732 | |||
| Marital status | NI | ||||||
| Unmarried | 7 (1–47) | R | |||||
| Married | 8 (1–82) | 0.677 | 0.377–1.214 | 0.191 | |||
| Unknown | NA | NA | NA | NA | |||
| Primary site | NI | ||||||
| Extremities | 6 (1–47) | R | |||||
| Spine | 7.5 (3–11) | 0.365 | 0.049–2.692 | 0.323 | |||
| Thoracic cage | 3 (1–70) | 0.741 | 0.342–1.604 | 0.446 | |||
| Others | 8 (1–82) | 0.657 | 0.336–1.285 | 0.220 | |||
| Histologic subtype | |||||||
| Conventional | 7 (1–82) | R | R | ||||
| Myxoid | 10 (9–28) | 0.743 | 0.175–3.148 | 0.687 | 1.069 | 0.223–5.111 | 0.934 |
| Mesenchymal | 47.000 | 0.474 | 0.063–3.542 | 0.467 | 0.085 | 0.018–1.294 | 0.085 |
| Clear cell | 1.000 | 10.437 | 1.275–85.470 | 0.029 | 10.971 | 1.216–98.942 | 0.033 |
| Dedifferentiated | 7 (1–21) | 1.672 | 0.907–3.081 | 0.099 | 1.890 | 0.977–3.655 | 0.059 |
| Grade | NI | ||||||
| Low grade | 7 (1–59) | R | |||||
| High grade | 7 (1–82) | 0.777 | 0.420–1.437 | 0.422 | |||
| Unknown | NA | NA | NA | NA | |||
| Enneking staging | NI | ||||||
| A | 7 (1–30) | R | |||||
| B | 8 (1–82) | 0.818 | 0.359–1.864 | 0.633 | |||
| Unknown | NA | NA | NA | NA | |||
| Regional lymph nodes mets | NI | ||||||
| No | 8 (1–82) | R | |||||
| Yes | 7.5 (1–28) | 1.251 | 0.492–3.183 | 0.638 | |||
| Unknown | NA | NA | NA | NA | |||
| Local recurrence | NI | ||||||
| No | 7 (1–82) | R | |||||
| Yes | 5 (1–13) | 1.676 | 0.737–3.811 | 0.218 | |||
| Unknown | NA | NA | NA | NA | |||
| Tumor size | |||||||
| ≤8.6 cm | 8.5 (1–82) | R | |||||
| >8.6–15 cm | 7.5 (1–70) | 1.232 | 0.615–2.468 | 0.555 | 0.917–4.297 | 0.082 | |
| >15 cm | 3.5 (1–47) | 2.331 | 1.110–4.895 | 0.025 | 1.857–11.462 | 0.001 | |
| Unknown | NA | NA | NA | NA | NA | NA | |
| Primary tumor surgery | |||||||
| No | 7 (1–59) | R | |||||
| Resection | 10.5 (1–82) | 0.507 | 0.274–0.937 | 0.030 | 0.263–1.075 | 0.079 | |
| Amputation | 4 (2–30) | 1.278 | 0.580–2.817 | 0.542 | 0.316–1.767 | 0.506 | |
| Regional lymph nodes removed | NI | ||||||
| No | 7.5 (1–82) | R | |||||
| Yes | 3.000 | 3.825 | 0.507–28.859 | 0.193 | |||
| Chemotherapy | NI | ||||||
| None/unknown | 5 (1–82) | R | |||||
| Yes | 9.5 (1–47) | 0.632 | 0.363–1.101 | 0.106 | |||
| Radiation | NI | ||||||
| None/unknown | 7 (1–82) | R | |||||
| Yes | 8 (1–47) | 0.870 | 0.476–1.588 | 0.650 | |||
| Radiation sequence with surgery | 0.560 | NI | |||||
| None | 7 (1–82) | R | |||||
| Prior | 7 (4–10) | 0.852 | 0.116–6.232 | 0.875 | |||
| After | 10.5 (6–28) | 0.528 | 0.164–1.701 | 0.284 | |||
R – reference; HR – hazard ratio; CI – confidence interval; NA – not applicable; NI – not included; mets – metastasis, OS – overall survival.
Table 5.
Univariate and multivariate Cox regression for analyzing cancer-specific survival among chondrosarcoma patients with pulmonary metastasis (diagnosed 2010–2016).
| Characteristics | Median CSS | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p | HR | (95% CI) | p | ||
| Sex | |||||||
| Female | 8 (1–82) | R | |||||
| Male | 7 (1–70) | 1.108 | 0.631–1.945 | 0.720 | |||
| Age | |||||||
| ≤67 | 8 (1–82) | R | R | ||||
| >67 | 3.5 (1–59) | 2.229 | 1.221–4.070 | 0.009 | 2.446 | 1.250–4.787 | 0.009 |
| Race | |||||||
| White | 8 (1–82) | R | |||||
| Black | 8.5 (1–15) | 0.826 | 0.255–2.682 | 0.751 | |||
| Other | 4 (3–5) | 1.496 | 0.201–11.141 | 0.694 | |||
| Unknown | NA | NA | NA | NA | |||
| Insurance status | |||||||
| Uninsured | 7 (1–8) | R | |||||
| Insured | 8 (1–82) | 0.828 | 0.252–2.723 | 0.756 | |||
| Marital status | |||||||
| Unmarried | 7.5 (1–47) | R | |||||
| Married | 8 (1–82) | 0.728 | 0.402–1.318 | 0.295 | |||
| Unknown | NA | NA | NA | NA | |||
| Primary site | |||||||
| Extremities | 6.5 (1–47) | R | |||||
| Spine | 7.5 (3–11) | 0.750 | 0.177–3.172 | 0.696 | |||
| Thoracic cage | 3 (1–70) | 0.755 | 0.348–1.641 | 0.478 | |||
| Others | 8 (1–82) | 0.672 | 0.343–1.319 | 0.248 | |||
| Histologic subtype | |||||||
| Conventional | 7 (1–82) | R | R | ||||
| Myxoid | 10 (9–28) | 0.709 | 0.168–2.999 | 0.641 | 0.910 | 0.191–4.339 | 0.906 |
| Mesenchymal | 47.000 | 0.462 | 0.062–3.446 | 0.451 | 0.148 | 0.017–1.257 | 0.080 |
| Clear cell | 1.000 | 9.808 | 1.201–80.108 | 0.033 | 9.633 | 1.077–86.191 | 0.043 |
| Dedifferentiated | 7.5 (1–21) | 1.536 | 0.832–2.837 | 0.170 | 1.676 | 0.868–3.237 | 0.124 |
| Grade | |||||||
| Low grade | 7 (1–59) | R | |||||
| High grade | 7 (1–82) | 0.714 | 0.388–1.313 | 0.278 | |||
| Unknown | NA | NA | NA | NA | |||
| Enneking staging | |||||||
| A | 7 (1–30) | R | |||||
| B | 8 (1–82) | 0.823 | 0.361–1.876 | 0.643 | |||
| Unknown | NA | NA | NA | NA | |||
| Regional lymph nodes mets | |||||||
| No | 8 (1–82) | R | |||||
| Yes | 7.5 (1–28) | 1.242 | 0.488–3.160 | 0.649 | |||
| Unknown | NA | NA | NA | NA | |||
| Local recurrence | |||||||
| No | 7.5 (1–82) | R | |||||
| Yes | 5 (1–13) | 1.681 | 0.739–3.823 | 0.215 | |||
| Unknown | NA | NA | NA | NA | |||
| Tumor size | |||||||
| ≤8.6 cm | 11 (1–82) | R | R | ||||
| >8.6–15 cm | 8 (1–70) | 1.100 | 0.552–2.191 | 0.786 | 1.985 | 0.917–4.297 | 0.168 |
| >15 cm | 3.5 (1–47) | 2.173 | 1.048–4.507 | 0.037 | 4.613 | 1.857–11.462 | 0.001 |
| Unknown | NA | NA | NA | NA | NA | NA | NA |
| Primary tumor surgery | |||||||
| No | 7 (1–59) | R | R | ||||
| Resection | 10.5 (1–82) | 0.539 | 0.295–0.986 | 0.045 | 0.532 | 0.298–1.187 | 0.140 |
| Amputation | 5 (3–30) | 1.166 | 0.508–2.679 | 0.717 | 0.701 | 0.283–1.740 | 0.444 |
| Regional lymph nodes removed | |||||||
| No | 8 (1–82) | R | |||||
| Yes | 3.000 | 4.053 | 0.535–30.704 | 0.176 | |||
| Chemotherapy | |||||||
| None/unknown | 5 (1–82) | R | |||||
| Yes | 9.5 (1–47) | 0.623 | 0.358–1.084 | 0.094 | |||
| Radiation | |||||||
| None/unknown | 7 (1–82) | R | |||||
| Yes | 8 (1–47) | 0.949 | 0.526–1.714 | 0.863 | |||
| Radiation sequence with surgery | |||||||
| None | 7 (1–82) | R | |||||
| Prior | 7 (4–10) | 0.873 | 0.119–6.391 | 0.894 | |||
| After | 10.5 (6–28) | 0.711 | 0.255–1.983 | 0.514 | |||
R – reference; HR – hazard ratio; CI – confidence interval; NA – not applicable; NI – not included; mets – metastasis, CSS – cancer-specific survival.
Figure 4.
The overall survival (OS) and cancer-specific survival (CSS) curve of pulmonary metastasis patients stratified by primary surgery site.
In this study, the homogeneous risk factor for the incidence and prognosis of CHS patients with PM was a large tumor size (>15 cm). Patients with dedifferentiated subtype, high grade of malignancy, extracompartmental tumor, presence of regional LNM, presence of local recurrence, and whom were married were significantly associated with high risk of developing PM; however, they were not associated with OS and CSS of PM. Older patients and patients with CCC subtype were significantly correlated with poor prognosis of OS and CSS, but these factors could not predict the risk of developing PM (Figure 5).
Figure 5.
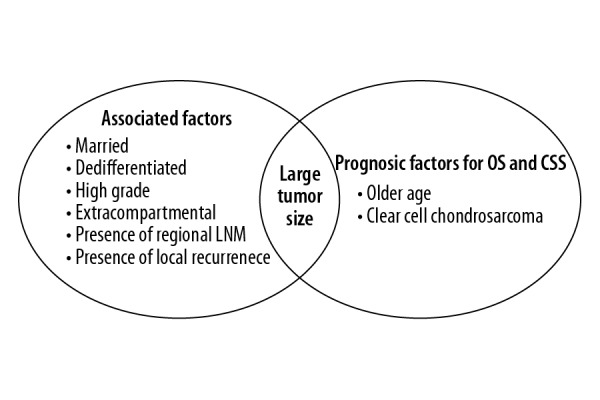
The homogeneous and heterogeneous risk factors for the incidence and prognosis of pulmonary metastasis patients in chondrosarcoma. The left circle was risk factors for developing pulmonary metastasis. The right circle was prognostic factors for PM patients’ OS and CSS. The intersection of 2 circles represent the homogeneous risk factor including large tumor size. OS – overall survival; CSS – cancer-specific survival; LNM – lymph nodes metastasis.
Discussion
To the best of our knowledge, this is the first study using the Surveillance, Epidemiology, and End Results (SEER) database to investigate the risk factors and prognostic factors of CHS patients with PM. According to our findings, 6.8% of CHS patients presented with PM at initial diagnosis. This incidence rate is lower than the rate of 9.6–11.2% previously published [9,15]. In the SEER database, asymptomatic patients are unable to be captured, which might have resulted in underestimation of PM incidence.
Due to the poor prognosis of CHS patients with PM, it is meaningful to determine factors that can identify the clinical characteristics of CHS patients who are at high risk of developing PM. As shown in our study, patients with dedifferentiated subtype, high grade of malignancy, extracompartmental tumor, presence of regional LNM, presence of local recurrence, large tumor size, and whom were married were found to be more likely to have PM. These clinical characteristics are risk factors for developing PM in CHS patients. Our most interesting finding was a statistically significant association in the presence of PM in married patients compared to unmarried patients. The most likely reason for this was that increased social support and encouragement from their spouses resulted in early detection when pulmonary symptoms first manifested. Compared to unmarried patients, married patients also had better treatment adherence and more regular follow-ups [20]. As such, married patients were more likely to be diagnosed with PM. The benefit of marital status on survival of cancer patients had been well studied [21–26]. These studies suggested that marital status was a protective factor for survival and that married patients had significant survival benefits when compared to unmarried patients. Additionally, marital status was also found to be an independent prognostic factor for chondrosarcoma patients in Gao’s study [27], who found that married patients were associated with a better prognosis than unmarried patients. In the tumor stage subgroup analysis, however, marital status was not found to be a significant prognostic factor of survival in chondrosarcoma with distant stage, congruent with the results from our study. The small numbers of unmarried patients (only 19 with PM) in our study may have limited the ability to determine a reliable statistically significant difference in survival between married and unmarried patients. As such, the benefit of marriage in CHS patients with PM still needs further investigating in future studies.
Thorkildsen et al. and Angelini et al. stated that histological grade was correlated with the likelihood of local recurrence and distant metastasis rate [6,28]. Regardless, there was no significant impact of histologic grade on OS in PM patients [29]. We found that PM was more frequent with high-grade tumors than with low-grade tumors. Our study determined that a high-grade tumor was a high-risk factor for developing PM in our study, which is in accordance with previous studies [16,30,31]. Among all histologic subtypes, the dedifferentiated subtype had the highest PM rate, with a statistically significant difference. The dedifferentiated subtype had a high metastatic rate of 65%, but was stable after 2-year follow-up [6]. Malchenko et al. revealed that pulmonary metastases developed within a few months of diagnosis in 90% of dedifferentiated subtype patients. The high rate of dedifferentiated chondrosarcoma metastases is related to expression of a set of “multifunctional” genes, which might explain this phenomenon [32]. In our study, we found that patients with extracompartmental tumors have a higher risk of developing PM than those with intracompartmental tumor. One possible explanations for this is that extracompartmental tumors in CHS may be more aggressive, often presenting in patients who have had inadequate surgical margins [33]. Additionally, neurovascular bundles are located extracompartmentally; thus, when a tumor invades them, it can cause hematogenous metastasis. Identification of the aforementioned high-risk clinical characteristics can help physicians in paying more attention to those with high PM risk and better evaluate the possibility of high PM risk. The 1-year and 3-year disease-free survival rates in CHS patients with PM were 36% and 0%, respectively [29]. Based on the number of new pulmonary metastatic events per patient-year in each grade of sarcomas, Cipriano et al. proposed that pulmonary screening be performed as follows: annually until 5 years for low grade sarcomas; every 3 months for 2 years, every 6 months from 2 to 5 years, then annually from 5 to 10 years [13]. Computed tomography (CT) of the chest had been proven to be more sensitive than positron emission tomography (PET) in detecting pulmonary metastasis from bone sarcomas [34]. Hence, we propose that patients with a high risk of PM should receive a chest radiograph, and CT of the chest needs to be performed every 3–6 months for 5 years, then annually from 5 to 10 years.
Our findings on prognostic factors for CHS patients are consistent with previous studies stating that male sex, older age, high-grade tumor, tumor size, dedifferentiated subtype, presence of PM, presence of local recurrence, and resection or amputation are independent prognostic factors [3,4,6,8,10,11,17,30,31,35,36]. Chemotherapy and radiation therapy were still not able to improve the prognosis of CHS patients [1]. Our study showed that both chemotherapy and radiation were associated with poor prognosis in univariate analysis. Prognostic factors of CHS patients with PM development at initial diagnosis were found to include older age, CCC subtype, and large tumor size. Resection of the primary tumor tended to be another prognostic factor that could prolong OS and CSS in PM patients. This finding is congruent with a study by Song, which suggested that resection of the primary tumor was associated with improved survival for patients with metastatic chondrosarcoma at diagnosis [37]. Furthermore, pulmonary metastasectomy has proven to be effective to prolong survival among patients with pulmonary metastasis [14,38,39]. Information about pulmonary metastasectomy was not included in this study. Regarding age at diagnosis, we determined that older age (>67 years old) was one of the independent prognostic factors resulting in a worse prognosis for CHS patients with PM. This result was similar to that of a recent study, which showed that older age was significantly negatively correlated with OS and CSS in patients with metastatic CHS. For each additional 1-year increase in the age of diagnosis with a reference of 60 years (mean age at diagnosis), the increase in the risk of worse OS and CSS were 1.019 and 1.015, respectively [37]. The presence of comorbidities and worse performance status in older patients can be reasons for a poor prognosis in CHS patients with PM. As mentioned before, the prognosis in patients with clear cell chondrosarcoma tended to be worse than for patients with other chondrosarcomas. CCC is a rare, low-grade, malignant sarcoma with potential to PM. Donati et al. stated that serum alkaline phosphatase levels are often elevated at diagnosis and may provide a useful tumor marker. Once patients are diagnosed with clear cell subtype, they should undergo tumor resection with wide margin and mandatory long-term follow-up [40]. In our study, large tumor size was the homogeneous risk factor for the incidence and prognosis of CHS patients with PM. In univariate analysis, medium and large tumor size were associated with an increase in the risk for having PM, whereas only large tumor size group had a significant association with a high risk of developing PM in multivariate analysis. A possible explanation of this is that a larger tumor size often needs time for tumor growth, increasing the likelihood of metastasis. This highlights the significance of early detection for asymptomatic CHS patients. In survival analysis, large tumor size was associated with a nearly 5-fold increase of the hazard ratio in both OS and CSS. This results in a worse prognosis for patients with large tumor size in both OS and CSS. Our findings were also consistent with recent studies [16,41] that concluded that increasing tumor size was associated with increased of high risk of distant metastasis and mortality. Based on these prognostic factors, physicians can more accurately estimate the prognosis of PM patients. Resection of the primary tumor and pulmonary metastasectomy are recommended to manage PM development.
Potential limitations of our study include an underestimated PM incidence due to the lack of records for asymptomatic PM patients in the SEER database. Secondly, surgical margin status, pathologic fracture, and pulmonary metastasectomy were shown to be independent prognostic factors in CHS patients in previous studies [14,31,38,39,42]. Due to insufficient information about these clinical variables in SEER, we were unable to investigate the association between these factors and PM patients. Finally, our study is retrospective and, as such, selection bias and missing data are inevitable; thus, more prospective studies are needed to further confirm the results.
Conclusions
Based on our retrospective analysis of the SEER database, our study demonstrated risk factors for PM development in CHS patients included: having a dedifferentiated subtype, a high grade of malignancy, extracompartmental tumor, presence of regional LNM, presence of local recurrence, large tumor size (>15 cm), and being married. It was also demonstrated that prognostic factors for CHS patients with PM included older age (>67 years old), CCC subtype, and large tumor size (>15 cm). Additionally, resection of the primary tumor tended to be correlated with a better prognosis. The recognition of these risk factors can potentially be used for clinical surveillance through improving the early detection of PM in CHS patients and in counseling patients regarding the possibility of developing PM. The discovery of prognostic factors can help physicians in making a more accurate prognostic estimation and can be used to design a personized treatment plan for patients with PM.
Acknowledgements
The authors would like to thank all members of the SEER Program tumor registries for their efforts in the establishment of the SEER database.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by the Science and Technology Commission of Shanghai Municipality (grant no. 17411950302) and the National Natural Science Foundation of China (grant no. 81772855)
References
- 1.NCCN Clinical Practice Guidelines in Oncology – Bone Cancer (2019 Version 2)[EB/OL] 2019. https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf.
- 2.Song K, Shi X, Wang H, et al. Can a nomogram help to predict the overall and cancer-specific survival of patients with chondrosarcoma? Clin Orthop Relat Res. 2018;476:987–96. doi: 10.1007/s11999.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nota SP, Braun Y, Schwab JH, et al. The identification of prognostic factors and survival statistics of conventional central chondrosarcoma. Sarcoma. 2015;2015 doi: 10.1155/2015/623746. 623746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 to 2003): An analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–72. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 5.Söderstrom M, Ekfors TO, Böhling TO, et al. No improvement in the overall survival of 194 patients with chondrosarcoma in Finland in 1971–1990. Acta Orthop Scand. 2009;74:344–50. doi: 10.1080/00016470310014292. [DOI] [PubMed] [Google Scholar]
- 6.Thorkildsen J, Taksdal I, Bjerkehagen B, et al. Chondrosarcoma in Norway 1990–2013; An epidemiological and prognostic observational study of a complete national cohort. Acta Oncol. 2019;58:273–82. doi: 10.1080/0284186X.2018.1554260. [DOI] [PubMed] [Google Scholar]
- 7.van Maldegem AM, Gelderblom H, Palmerini E, et al. Outcome of advanced, unresectable conventional central chondrosarcoma. Cancer. 2014;120:3159–64. doi: 10.1002/cncr.28845. [DOI] [PubMed] [Google Scholar]
- 8.Nie Z, Lu Q, Peng H. Prognostic factors for patients with chondrosarcoma: A survival analysis based on the Surveillance, Epidemiology, and End Results (SEER) database (1973–2012) J Bone Oncol. 2018;13:55–61. doi: 10.1016/j.jbo.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin PP, Alfawareh MD, Takeuchi A, et al. Sixty percent 10-year survival of patients with chondrosarcoma after local recurrence. Clin Orthop Relat Res. 2012;470:670–76. doi: 10.1007/s11999-011-2059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreou D, Ruppin S, Fehlberg S, et al. Survival and prognostic factors in chondrosarcoma: Results in 115 patients with long-term follow-up. Acta Orthop. 2011;82:749–55. doi: 10.3109/17453674.2011.636668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fromm J, Klein A, Baur-Melnyk A, et al. Survival and prognostic factors in conventional central chondrosarcoma. BMC Cancer. 2018;18:849. doi: 10.1186/s12885-018-4741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Italiano A, Mir O, Cioffi A, et al. Advanced chondrosarcomas: Role of chemotherapy and survival. Ann Oncol. 2013;24:2916–22. doi: 10.1093/annonc/mdt374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cipriano C, Griffin AM, Ferguson PC, et al. Developing an evidence-based followup schedule for bone sarcomas based on local recurrence and metastatic progression. Clin Orthop Relat Res. 2017;475:830–38. doi: 10.1007/s11999-016-4941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: Aa systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open. 2012:2. doi: 10.1136/bmjopen-2012-001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Matsumine A, Yamada S, et al. Oncological outcome after lung metastasis in patients presenting with localized chondrosarcoma at extremities: Tokai Musculoskeletal Oncology Consortium study. Onco Targets Ther. 2016;9:4747–51. doi: 10.2147/OTT.S107638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song K, Shi X, Liang X, et al. Risk factors for metastasis at presentation with conventional chondrosarcoma: A population-based study. Int Orthop. 2018;42:2941–48. doi: 10.1007/s00264-018-3942-7. [DOI] [PubMed] [Google Scholar]
- 17.van Praag Veroniek VM, Rueten-Budde AJ, Ho V, et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg Oncol. 2018;27:402–8. doi: 10.1016/j.suronc.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Arshi A, Sharim J, Park DY, et al. Chondrosarcoma of the osseous spine: An analysis of epidemiology, patient outcomes, and prognostic factors using the SEER registry from 1973 to 2012. Spine (Phila Pa 1976) 2017;42:644–52. doi: 10.1097/BRS.0000000000001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. 1980. Clin Orthop Relat Res. 2003;(415):4–18. doi: 10.1097/01.blo.0000093891.12372.0f. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SD, Sharma T, Acquaviva K, et al. Social support and chronic kidney disease: An update. Adv Chronic Kidney Dis. 2007;14:335–44. doi: 10.1053/j.ackd.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Jin JJ, Wang W, Dai FX, et al. Marital status and survival in patients with gastric cancer. Cancer Med. 2016;5:1821–29. doi: 10.1002/cam4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Liu Y, Wang Y, et al. The influence of marital status on survival of gallbladder cancer patients: A population-based study. Sci Rep. 2017;7:5322. doi: 10.1038/s41598-017-05545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H, Zhang Y, Song Y, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Clin Res Hepatol Gastroenterol. 2017;41:476–86. doi: 10.1016/j.clinre.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Zhu MX, Qi SH. Marital status and survival in patients with renal cell carcinoma. Medicine (Baltimore) 2018;97:e0385. doi: 10.1097/MD.0000000000010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Cao W, Zheng C, et al. Marital status and survival in patients with rectal cancer: An analysis of the Surveillance, Epidemiology and End Results (SEER) database. Cancer Epidemiol. 2018;54:119–24. doi: 10.1016/j.canep.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang SL, Wang WR, Liu ZJ, et al. Marital status and survival in patients with soft tissue sarcoma: A population-based, propensity-matched study. Cancer Med. 2019;8:465–79. doi: 10.1002/cam4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Z, Ren F, Song H, et al. Marital status and survival of patients with chondrosarcoma: A population-based analysis. Med Sci Monit. 2018;24:6638–48. doi: 10.12659/MSM.911673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angelini A, Guerra G, Mavrogenis AF, et al. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106:929–37. doi: 10.1002/jso.23173. [DOI] [PubMed] [Google Scholar]
- 29.Lin AY, Kotova S, Yanagawa J, et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg. 2015;149:85–92. doi: 10.1016/j.jtcvs.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: A clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–31. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Lee FY, Mankin HJ, Fondren G, et al. Chondrosarcoma of bone: An assessment of outcome. J Bone Joint Surg Am. 1999;81:326–38. doi: 10.2106/00004623-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Malchenko S, Seftor EA, Nikolsky Y, et al. Putative multifunctional signature of lung metastases in dedifferentiated chondrosarcoma. Sarcoma. 2012;2012 doi: 10.1155/2012/820254. 820254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93–99. doi: 10.1302/0301-620x.84b1.11942. [DOI] [PubMed] [Google Scholar]
- 34.Iagaru A, Chawla S, Menendez L, et al. 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl Med Commun. 2006;27:795–802. doi: 10.1097/01.mnm.0000237986.31597.86. [DOI] [PubMed] [Google Scholar]
- 35.Kim HS, Bindiganavile SS, Han I. Oncologic outcome after local recurrence of chondrosarcoma: Analysis of prognostic factors. J Surg Oncol. 2015;111:957–61. doi: 10.1002/jso.23925. [DOI] [PubMed] [Google Scholar]
- 36.Bindiganavile S, Han I, Yun JY, et al. Long-term outcome of chondrosarcoma: A single institutional experience. Cancer Res Treat. 2015;47:897–903. doi: 10.4143/crt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song K, Song J, Chen F, et al. Does resection of the primary tumor improve survival in patients with metastatic chondrosarcoma? Clin Orthop Relat Res. 2019;477:573–83. doi: 10.1097/CORR.0000000000000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: Prognostic factors associated with improved outcomes. Ann Thorac Surg. 2011;92:1780–86. doi: 10.1016/j.athoracsur.2011.05.081. discussion 1786–87. [DOI] [PubMed] [Google Scholar]
- 39.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 40.Donati D, Yin JQ, Colangeli M, et al. Clear cell chondrosarcoma of bone: Long time follow-up of 18 cases. Arch Orthop Trauma Surg. 2008;128:137–42. doi: 10.1007/s00402-007-0353-4. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Chen G, Chen X, et al. Predictors of the survival of patients with chondrosarcoma of bone and metastatic disease at diagnosis. J Cancer. 2019;10:2457–63. doi: 10.7150/jca.30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson JD, Laitinen MK, Parry MC, et al. The role of surgical margins in chondrosarcoma. Eur J Surg Oncol. 2018;44:1412–18. doi: 10.1016/j.ejso.2018.05.033. [DOI] [PubMed] [Google Scholar]