Abstract
Purpose:
To investigate choroidal hyper-reflective foci (HRF) in subjects with retinal dystrophy [Stargardt's disease (SGD) and retinitis pigmentosa (RP)] and their association with demographics, visual acuity, choroidal thickness (CT), and choroidal vascularity index (CVI).
Methods:
Single center retrospective study of subjects with previously diagnosed SGD or RP. Swept-source optical coherence tomography images were analyzed for the presence of choroidal HRFs and CVI using previously validated automated algorithm. A Spearman's rank correlation coefficient was used to evaluate the correlation between the number of HRF and various baseline parameters including age, visual acuity, intraocular pressure, and other optical coherence tomography (OCT) parameters (CT, choroidal area, and CVI) were evaluated in these subjects.
Results:
This study included 46 eyes (23 subjects) and 55 eyes (28 subjects) with previously diagnosed RP and SGD, respectively. In the RP group, the mean number of HRFs was 247.9 ± 57.1 and mean CVI was 0.56 ± 0.04. In SGD group, mean HRF was 192.5 ± 44.3 and mean CVI was 0.41 ± 0.04. Mean HRF was significantly greater in the RP group (0.02), however, the mean CVI was not statistically different. In RP, mean HRF were correlated only with CVI (r = 0.49; P = 0.001), however, in SGD, it correlated with only choroidal area (r = 0.27; P = 0.04).
Conclusion:
Choroidal HRF were present in both RP and SGD subjects with more HRFs in those with RP. These HRFs were associated with alteration in choroidal vascularity, which further adds into the pathogenesis of these diseases.
Keywords: Choroidal vascularity index, hyper-reflective foci, retinal dystrophy
Retinitis pigmentosa (RP) and Stargardt's disease (SGD) are the two most common forms of inherited retinal dystrophy. RP is a group of inherited retinal dystrophies characterized by the development of peripheral bone-spicule pigmentary retinopathy, arteriolar attenuation, and disc pallor. They are associated with a gradual deterioration in peripheral vision and nyctalopia. The progressive loss of rod photoreceptor cells is eventually accompanied by cone dysfunction and associated with chorioretinal atrophy, with varied levels of alteration in visual function. SGD has been associated with a mutation in the ABCA4 gene, which disrupts the processing and transportation of metabolic products of intracellular pathways leading to the alteration of chorioretinal structure and function. Deposition of lipofuscin, degeneration of retinal pigment epithelium cells, and its associated chorioretinal atrophy including loss of photoreceptors leads to a progressive reduction in visual function.
The development of optical coherence tomography (OCT) technology has allowed improved image resolution of deeper chorioretinal structures. Analysis of choroidal morphology and vascularity with spectral-domain OCT (SD-OCT) has demonstrated the alteration in choroidal thickness, morphology, and vascular layers in patients with other retinal dystrophies including RP and SGD.[1] Thinning of the large choroidal vessel layer, in particular, has been demonstrated on examination of choroidal structure using OCT imaging in subjects with RP.[2] Choroidal vascularity index (CVI) is an indirect measure of the density of choroidal vasculature that has been investigated as an alternative measurement of choroidal perfusion in a range of retinal dystrophies including RP, SGD, and cone-rod dystrophy.[3,4] Analysis of choroidal structure may help to improve the clinical staging of these diseases and may be useful in our understanding of disease progression and ultimately the development of treatment strategies for this condition.
Hyper-reflective foci (HRF) have been visualized in the retina and choroid using OCT in a range of chorioretinal diseases including age-related macular degeneration, retinal vein occlusion, and diabetic retinopathy.[5,6,7] HRF development has also been associated with visual acuity variation and treatment outcome after treatment with anti-angiogenic agents.[8] Intraretinal HRF have been described in patients with early RP.[9] Choroidal HRF have also been associated with alteration in choroidal structure. Both retinal and choroidal HRF have been described in patients with RP accompanied with diabetic retinopathy with an associated reduction in visual acuity.[10] SD-OCT analysis of subjects with SGD has demonstrated HRF in the choroid, most prominently in Bruch's/retinal pigment epithelium (RPE) complex, choriocapillaris, and Sattler's layer.[11] It was demonstrated that these choroidal HRF were associated with retinal atrophy, disease duration, and visual function. Further analysis is necessary to determine the association between these hyper-reflective changes and choroidal vascularity to further determine their etiology and perhaps to develop treatment strategies.
Although the presence of HRF has been reported in both RP and SG dystrophy, their direct significance to pathology is unknown, particularly their effect upon visual function. While both are forms of retinal dystrophy, the pathogenesis of these two conditions is markedly different. In fact, while SG dystrophy is predominantly macular dystrophy, RP is progressive rod-cone dystrophy. It is unclear how the presence of these HRF and alteration in choroidal vascularity affects vision in these two different conditions. Comparison, therefore, may help to determine if there are common associations in choroidal remodeling in retinal dystrophy.
The aim of this study was to investigate the presence of choroidal HRF in patients with RP and SGD, and association with demographics, visual acuity, and OCT-derived structural parameters including choroidal thickness and choroidal vascularity index.
Methods
This is a single center retrospective study evaluating patient's with previously diagnosed RP or SGD. The study was approved by the local ethics committee and adhered to the tenets set forth in the Declaration of Helsinki. Approval was obtained from local ethics committee as stated in July 2018.
Study population
All subjects were recruited from retina clinics at LV Prasad Eye Institute, Hyderabad, India with previously diagnosed RP or SGD in this retrospective study. The diagnosis was according to the clinical examination, and ocular investigations were performed to confirm the diagnosis, if required. Exclusion criteria included refractive error of more than 3 dioptres, axial length more than 26 mm, history of other chorioretinal disease, media opacity preventing adequate fundal view or imaging, previous ocular surgery (other than cataract surgery), and any other significant ocular comorbidity.
Imaging protocol
After informed consent, best-corrected visual acuity and disease duration were noted. All patients were adequately dilated with 2.5% phenylephrine and 1% tropicamide. Each patient underwent a SS-OCT (Topcon DRI-OCT-1 Triton) high definition horizontal line scan passing through the fovea.
SS-OCT quantitative analyses
Subfoveal choroidal thickness (CT) was measured manually using the callipers present on the proprietary software as the perpendicular distance between the RPE-Bruch's membrane complex and the choriocapillaris. Manual segmentation of the choroid was consisting of the outer surface of the RPE-Bruch's membrane complex (inner boundary) and the choroidal-scleral junction (outer boundary). This manual method has been utilized previously and reported to have high accuracy to automated segmentation methods.[12]
All SS-OCT macular images were exported in JPEG format, and the presence of HRF was determined using a semi-automated algorithm using a circle Hough transformation in MatLab. Each OCT B scan was first sharpened twice using a Gaussian mark of 4 pixels to increase the contrast between the HRF and surrounding choroid. Thresholding was performed using an empirically chosen threshold of 175 (greyscale intensity). The intensity profile of the HRF and the surrounding choroid post contrast enhancement was uniform across all the images, which were also evident in the intensity histograms. Accordingly, from histogram plots, the threshold was chosen and validated by observer grading. The intraclass correlation for inter observer HRFs counting was 0.89–0.92. Circle Hough transformation was used to detect the HRF using binarized OCT with a radius chosen empirically between 1 and 100 pixels. The observer allows manual extrapolation of choroidal boundaries, which are then extrapolated by the tool. HRF detected outside these boundaries are then excluded to determine choroidal HRF alone.
Subfoveal choroidal area (1500 μm) is segmented into stromal and luminal components using image binarization. Choroidal vascularity index (CVI) was determined as the proportion of luminal areas to the total choroidal area. Shadow-removal refinement of OCT images has been utiltized to reduce the putative reduction in choroidal structure visualization owing to masking of deeper structures including retinal blood vessels.[13] Therefore, CVI with shadow removal (WSR) was obtained from each eye. For CVI-WSR, all scans were shadow-removed using a previously published algorithm prior to image binarization to allow subsequent determination of shadow compensated CVI. Representative images of subject with RP and SGD, including segmentation of choroid, automated detection of HRF, and delineation of luminal and stromal components are shown in Figs. 1 and 2, respectively.
Figure 1.
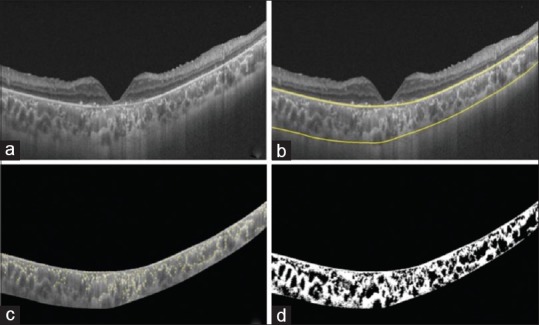
Swept-source optical coherence tomography macula scan of a subject (16-year-old male; right eye with visual acuity 20/400) with retinitis pigmentosa (a); segmentation of choroid using proprietary software (b); automated detection of choroidal hyper-reflective foci (319, yellow dots) using circle Hough transformation (c); delineation of stromal or luminal components to determine choroidal vascularity index (0.45) (d)
Figure 2.

Swept-source optical coherence tomography macula scan of subject (19-year-old male; right eye with visual acuity 20/200) with Stargardt's disease (a); segmentation of choroid using proprietary software (b); automated detection of choroidal hyper-reflective foci (183, yellow dots) using circle Hough transformation (c); delineation of stromal or luminal components to determine choroidal vascularity index (0.38) (d)
Statistical analyses
Spearman's rank correlation coefficient was used to evaluate the correlation between the number of HRF and various baseline parameters including age, visual acuity, intraocular pressure, and other OCT parameters (CT, choroidal area, and CVI) were evaluated in these subjects. P value of less than 0.05 was considered significant. GraphPad prism was used for all the statistical analysis.
Results
Subject characteristics
Forty-six eyes of 23 subjects with previously diagnosed RP were included in this study. There were 9 female and 14 male subjects and 23 left and 23 right eyes in this study. Mean (± SD) age was 27.2 ± 9.8 years and mean (± SD) visual acuity was 0.46 ± 0.49 LogMar (Snellen's equivalent 20/60).
Fifty-five eyes of 28 subjects with previously diagnosed SD were included in this study. This consisted of 10 female and 18 male subjects and 27 left and 28 right eyes. Mean (± SD) age was 26.3 ± 14.2 years and mean (± SD) visual acuity was 0.88 ± 0.33 LogMar (Snellen's equivalent 20/150). Visual acuity was significantly greater in patients with RP (P = 0.03). There were no other significant differences in the demographics between the two subject groups.
Analysis of HRF
The mean number of HRF in the RP group was 247.9 ± 57.1. Mean CVI was 0.56 ± 0.04 [Table 1] in RP group. In SGD group, mean HRF was 192.5 ± 44.3 and mean CVI-WSR was 0.41 ± 0.04. Mean HRF were significantly greater in the RP group (0.02), however, the mean CVI was not statistically different.
Table 1.
Demographics, visual acuity and OCT derived metrics and correlation with number of hyper-reflective foci
| Retinitis pigmentosa | Stargardt’s disease | |||
|---|---|---|---|---|
| Mean | Correlation with hyper-reflective foci (p) | Mean | Correlation with hyper-reflective foci (p) | |
| Age | 27.3±9.8 | –0.26 (0.14) | 26.3±14.2 | –0.07 (0.71) |
| Visual Acuity | 0.46±0.49 | 0.20 (0.21) | 0.88±0.33 | 0.18 (0.19) |
| Intraocular pressure | 13.2±2.1 | 0.14 (0.39) | 13.0±2.5 | 0.10 (0.44) |
| Choroidal thickness | 284.5±97.4 | 0.08 (0.60) | 467.3±55.4 | 0.33 (0.72) |
| Choroidal area | 0.48±0.14 | 0.12 (0.53) | 2.15±0.48 | 0.27 (0.04) |
| Choroidal vascularity index | 0.56±0.04 | 0.49 (0.001) | 0.41±0.04 | –0.02 (0.91) |
In RP, Mean HRF was correlated with CVI (r = 0.49; P = 0.001).HRF were not correlated with demographics (age, visual acuity, and demographics), intraocular pressure, and other OCT parameters (CT and choroidal area). In SGD, mean HRF was correlated with choroidal area alone (r = 0.27; P = 0.04).
Discussion
We report presence of choroidal HRFs in both RP and SGD, with slightly higher occurrence in RP than that of SGD group. We found that choroidal HRFs were not associated with demographic factors, visual acuity, intraocular pressure, or other OCT derived outcomes including CT in either RP or SGD. Our study suggests that choroidal HRF are correlated with CVI (with shadow compensation) in subjects with RP, however, in SGD, choroidal HRF appeared to be correlated with choroidal area alone.
It is interesting that HRF is correlated with CVI as a generation of HRF may be an important factor in chorioretinal remodeling in retinal dystrophies. This CVI alteration reflects the alteration of the inner choroidal layer (including choriocapillaris and medium choroidal vessel layer), especially in RP and SGD, which was described previously.[14] Although it is true that HRF was not correlated with CT, alteration of choroidal vascularity is an important indicator of altered choroidal function. In fact, previous studies have demonstrated that CVI is significantly reduced in SGD patients compared with normal eyes; interestingly, this alteration was more consistent than any change in CT.[3] Further study is of course necessary, including longitudinal studies, which can help to determine how the development of HRF in RP and SGD are associated with chorioretinal function.
Previous analysis of shadow compensation shows that it has the potential to significantly alter CVI measurements by reducing underestimation of choroidal reflectivity.[13] Shadow compensation is known to alter luminal volume measurements and hence CVI calculations with a corresponding reduction in variability.[13] Application of shadow compensation has also been shown to improve image resolution in OCT visualization of other structures including the optic nerve head.[15]
The etiology of choroidal HRF in RP needs further investigation. Alteration in choroidal vasculature, as demonstrated with indocyanine green angiography study in patients with central serous chorioretinopathy may suggest that choroidal HRF may be associated with inflammation generated from delayed choroidal filling.[16] Moreover, HRF may owing to the exudation secondary to delayed vascular filling or choroidal ischemia and the possibly associated change in choroidal blood flow. In fact, both systemic markers of inflammation (c-reactive protein) in patients with RP and analysis of inflammatory cytokines in mouse models of RP appear to be elevated.[17,18]
Choroidal HRF may be associated with choriocapillaris dysfunction, as previously postulated in SGD.[11] Increased choroidal vascularity was associated with high choroidal HRF, perhaps even suggesting that this may be owing to extravasation through damaged capillaries. Reduced perfusion pressure has been demonstrated in eyes with RP. This may well be associated with reduced choroidal vascular impedance associated with damaged blood vessels.[19]
Limitations of this study include its retrospective nature and limited subject numbers. We had clinically diagnosed cases of RP and SGD; however, a genetic profile was not evaluated, which again may have an effect on structural characteristics. A potential limitation is that HRFs could be attributed to hyper-transmission, although shadow compensation should reduce the effect of hyper-transmission.[13] In spite of these weaknesses, our study reports the correlation of choroidal HRFs with demographic, functional, and structural parameters, and especially with the use of shadow removal algorithms, we present authentic information of choroidal vascular changes.
Conclusion
In summary, our study reports the presence of choroidal HRF in both RP and SGD with more HRFs in patients with RP. These HRFs were associated with alteration in choroidal vascularity, which is further involved in the pathogenesis of these diseases. This finding deserves further evaluation to determine how this choroidal vasculature remodeling is associated with both disease activity and disease progression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Adhi M, Read SP, Ferrara D, Weber M, Duker JS, Waheed NK. Morphology and vascular layers of the choroid in stargardt disease analyzed using spectral-domain optical coherence tomography. Am J Ophthalmol. 2015;160:1276–84.e1271. doi: 10.1016/j.ajo.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Adhi M, Regatieri CV, Branchini LA, Zhang JY, Alwassia AA, Duker JS. Analysis of the morphology and vascular layers of the choroid in retinitis pigmentosa using spectral-domain OCT. Ophthalmic Surg Lasers Imaging Retina. 2013;44:252–9. doi: 10.3928/23258160-20130503-08. [DOI] [PubMed] [Google Scholar]
- 3.Ratra D, Tan R, Jaishankar D, Khandelwal N, Gupta A, Chhablani J, et al. Choroidal structural changes and vascularity index in stargardt disease on swept source optical coherence tomography. Retina. 2018;38:2395–400. doi: 10.1097/IAE.0000000000001879. [DOI] [PubMed] [Google Scholar]
- 4.Wei X, Mishra C, Kannan NB, Holder GE, Khandelwal N, Kim R, et al. Choroidal structural analysis and vascularity index in retinal dystrophies. Acta Ophthalmol. 2019;97:e116–21. doi: 10.1111/aos.13836. [DOI] [PubMed] [Google Scholar]
- 5.Nassisi M, Fan W, Shi Y, Lei J, Borrelli E, Ip M, et al. Quantity of intraretinal hyperreflective foci in patients with intermediate age-related macular degeneration correlates with 1-year progression. Invest Ophthalmol Vis Sci. 2018;59:3431–9. doi: 10.1167/iovs.18-24143. [DOI] [PubMed] [Google Scholar]
- 6.Mo B, Zhou HY, Jiao X, Zhang F. Evaluation of hyperreflective foci as a prognostic factor of visual outcome in retinal vein occlusion. Int J Ophthalmol. 2017;10:605–12. doi: 10.18240/ijo.2017.04.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuwobi IP, Fan W, Yu C, Yuan S, Liu Q, Zhang Y, et al. Automated segmentation of hyperreflective foci in spectral domain optical coherence tomography with diabetic retinopathy. JMed Imaging (Bellingham) 2018;5:014002. doi: 10.1117/1.JMI.5.1.014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abri Aghdam K, Pielen A, Framme C, Junker B. Correlation between hyperreflective foci and clinical outcomes in neovascular age-related macular degeneration after switching to aflibercept. Invest Ophthalmol Vis Sci. 2015;56:6448–55. doi: 10.1167/iovs.15-17338. [DOI] [PubMed] [Google Scholar]
- 9.Kuroda M, Hirami Y, Hata M, Mandai M, Takahashi M, Kurimoto Y. Intraretinal hyperreflective foci on spectral-domain optical coherence tomographic images of patients with retinitis pigmentosa. Clin Ophthalmol (Auckland, NZ) 2014;8:435–40. doi: 10.2147/OPTH.S58164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi Y, Takahashi A, Nagaoka T, Ishibazawa A, Ishiko S, Yoshida A. Retinal and choroidal hyperreflective foci on spectral-domain optical coherence tomographic images in a patient with retinitis pigmentosa accompanied by diabetic retinopathy. Am J Ophthalmol Case Rep. 2016;3:25–30. doi: 10.1016/j.ajoc.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piri N, Nesmith BL, Schaal S. Choroidal hyperreflective foci in Stargardt disease shown by spectral-domain optical coherence tomography imaging: Correlation with disease severity. JAMA Ophthalmol. 2015;133:398–405. doi: 10.1001/jamaophthalmol.2014.5604. [DOI] [PubMed] [Google Scholar]
- 12.Vupparaboina KK, Nizampatnam S, Chhablani J, Richhariya A, Jana S. Automated estimation of CT distribution and volume based on OCT images of posterior visual section. Comput Med Imaging Graph. 2015;46:315–27. doi: 10.1016/j.compmedimag.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Vupparaboina KK, Dansingani KK, Goud A, Rasheed MA, Jawed F, Jana S, et al. Quantitative shadow compensated optical coherence tomography of choroidal vasculature. Sci Rep. 2018;8:6461. doi: 10.1038/s41598-018-24577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egawa M, Mitamura Y, Niki M, Sano H, Miura G, Chiba A, et al. Correlations between choroidal structures and visual functions in eyes with retinitis pigmentosa. Retina. 2018 doi: 10.1097/IAE.0000000000002285. doi: 10.1097/IAE.0000000000002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard MJ, Strouthidis NG, Ethier CR, Mari JM. Shadow removal and contrast enhancement in optical coherence tomography images of the human optic nerve head. Invest Ophthalmol Vis Sci. 2011;52:7738–48. doi: 10.1167/iovs.10-6925. [DOI] [PubMed] [Google Scholar]
- 16.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 17.Murakami Y, Ikeda Y, Nakatake S, Fujiwara K, Tachibana T, Yoshida N, et al. C-Reactive protein and progression of vision loss in retinitis pigmentosa. Acta Ophthalmol. 2018;96:e174–9. doi: 10.1111/aos.13502. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida N, Ikeda Y, Notomi S, Ishikawa K, Murakami Y, Hisatomi T, et al. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013;120:e5–12. doi: 10.1016/j.ophtha.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt KG, Pillunat LE, Kohler K, Flammer J. Ocular pulse amplitude is reduced in patients with advanced retinitis pigmentosa. Br J Ophthalmol. 2001;85:678–82. doi: 10.1136/bjo.85.6.678. [DOI] [PMC free article] [PubMed] [Google Scholar]


