Abstract
The study describes the technique of combining microscope-integrated optical coherence tomography (mi-OCT) and proportional reflux hydrodissection (PRH) during pars plana vitrectomy (PPV) in eyes with complex proliferative diabetic retinopathy (PDR) including tractional retinal detachment (TRD), combined retinal detachment (CRD), and taut posterior hyaloid membrane (TPHM). In this technique, PRH is used to create tissue planes between fibrovascular adhesions in areas identified using mi-OCT for insinuating the vitrector, enabling tissue dissection and release of traction. About 46 patients were operated using this technique. 34 eyes had TRD, 9 eyes had CRD, and 3 eyes were diagnosed with TPHM. A second instrument was used only in nine eyes. None of the eyes required use of intraocular scissors. Iatrogenic breaks occurred in six eyes. All patients had successful reattachment at 3-month follow-up. Thus, combination of mi-OCT and PRH is useful incomplete fibrovascular tissue dissection during PPV for complex PDR cases.
Keywords: Diabetic retinopathy, intraoperative OCT, proportional reflux hydrodissection, retinal detachment, vitrectomy
Complications of severe proliferative diabetic retinopathy such as taut posterior hyaloid membrane (TPHM), tractional retinal detachment (TRD), and combined retinal detachment (CRD) result in blinding visual outcomes. Generally, these require surgical management using pars plana vitrectomy (PPV) to relieve the traction, dissect and remove the fibrovascular proliferation, adequately remove the hyaloid membrane, ensure retinal attachment with/without use of tamponade, and to apply laser photocoagulation.[1]
Depending upon the extent of proliferation, techniques such as segmentation and delamination using additional instrumentation such as intraocular forceps, scissors, mini-light source, delaminator, and Tanos's diamond dusted scrapper, among others, may be necessary.[2,3] However, with the availability of improved vitrectomy devices, smaller gauge vitrectors (25 and 27G) and instruments, there has been a reduced need for using additional intraocular instruments.[4] Modern vitrectomy machines provide features such as proportional reflex hydrodissection (PRH) (available in Alcon Constellation® system) that results in controlled egress of fluid from the cutter tip with footswitch control, further aiding tissue dissection.[5]
Microscope-integrated intraoperative optical coherence tomography (mi-OCT) has enabled the surgeon to operate in close proximity to the retina, and appreciate the abnormalities of the vitreoretinal interface, and other retinal abnormalities during surgery itself.[6,7] In this study, we have described the combined use of mi-OCT and PRH in dissecting fibrovascular proliferative tissue, and other membranes in complex severe proliferative diabetic retinopathy.
Surgical Technique
In this retrospective study, consecutive patients with complex severe diabetic retinopathy requiring surgical intervention such as those with TPHM, TRD, and CRD visiting the retina services between July 2018 and December 2018 were included. Since this is a retrospective analysis, Institutional Review Board clearance was waived off. Since the technology was available in our setting since July 2018, it was adopted for all cases of complicated diabetic retinopathy undergoing surgery. The records of the patients, recorded surgical videos, and their operative notes were screened to include only those patients who were operated using mi-OCT (surgical microscope OPMI Lumera 700/Rescan 700, Carl Zeiss Meditec, La Jolla, CA, USA). All the patients were operated by a single surgeon (V.G.) (a senior vitreoretinal surgeon with expertise in tissue dissection with intraocular scissors, and techniques of segmentation and delamination). The extent of fibrovascular proliferation and retinal detachment is described in Table 1. Patients operated for complications such as vitreous hemorrhage in whom mi-OCT was not used were excluded. All the patients were operated using 25G+ valved Alcon Constellation® vitrectomy system with PRH enabled.
Table 1.
Clinical findings (preoperative) of patients with complex proliferative diabetic retinopathy
| Number of eyes (%) Total n=46 | |||
|---|---|---|---|
| TRD (n=34) | CRD (n=9) | TPHM (n=3) | |
| Vitreoschisis | 10 (29.4) | 2 (22.2) | 0 |
| Retinal detachment | |||
| Less than 2 quadrants of detachment | 12 (35.3) | 3 (33.3) | - |
| More than 2 quadrants of detachment | 22 (64.7) | 6 (66.6) | - |
| Fibrovascular proliferation | |||
| Adhesions limited to posterior pole and equator | 18 (52.9) | 3 (33.3) | - |
| Adhesions extending beyond equator to the periphery | 16 (47.1) | 6 (66.6) | - |
| Additional macular pathology | 1 (2.9) (LMH) | - | - |
CRD=Combined retinal detachment; LMH=Lamellar macular hole; TRD=Tractional retinal detachment; TPHM=Taut posterior hyaloid membrane
In this study, 46 patients (29 males) with a mean age of 55.63 ± 7.49 years were included. About 34 (73.91%) patients were diagnosed with diabetic TRD. Nine (19.56%) patients had diabetic CRD. Three (6.52%) patients diagnosed with TPHM were also included in the study. Preoperative laser photocoagulation was applied in 30 eyes (65.22%). Intravitreal ranibizumab (0.05 mg/0.01 mL) was administered in seven eyes (15.22%). Three days prior to surgery in order to reduce the vascularity and intraoperative bleeding.
The surgeries were performed under peribulbar anaesthesia. The surgery consisted of standard 3-port pars plana vitrectomy using 25G trocar and cannula. Port-site and core vitrectomy was performed initially. Once core vitreous was cleared, dissection of fibrovascular tissue was initiated under higher magnification. The fibrovascular dissection was begun from the center towards the periphery. Since fibrovascular membranes course from the optic disc to the peripheral retina with adhesions, the dissection was begun perpendicular to the length of the proliferation. At the beginning of dissection, mi-OCT feature of the microscope was activated. Once adequate focusing was achieved, the mi-OCT was used to scan an area where there was a potential space for insinuation of the vitrector safely (between the fibrovascular tissue and retinal surface). After insinuation of the vitrector, PRH was activated, and using foot-switch control, fluid was infused to increase the separation between the fibrovascular tissue and the retina. The separated tissue was then removed using the vitrector [Fig. 1]. This is also explained in a line diagram [Fig. 2]. The fibrovascular tissue was divided into small segments using this technique, till all the tissue dissection was complete [Supplemental Video 1]. The mi-OCT was used to scan the entire posterior pole, peripapillary retina, as well as nasal retina to determine all strong adhesions of the fibrovascular tissue with the retina. This enabled the surgeon to dissect very close to the retina while minimizing the traction and chance of an iatrogenic break. mi-OCT was also used to identify raised neovascularization elsewhere on the retina, and its elevation from the retinal surface.
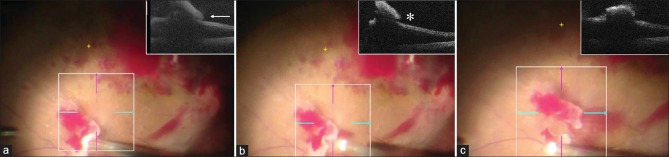
Figure 1.
The surgical steps during dissection of fibrovascular tissue in a case of diabetic tractional retinal detachment using microscope-integrated optical coherence tomography (mi-OCT). (a) The mi-OCT (inset) is used to identify the correct plane of adherence of the fibrovascular tissue with the underlying retina and the vitrector is insinuated in the potential space (white arrow) between the two. (b) Proportional reflux hydrodissection (PRH) is then activated to expand this space and push the retina back, increasing the potential space as seen on the mi-OCT (inset; white asterisk). (c) The cutter is then used to dissect the tissue using low aspiration and high cut rate
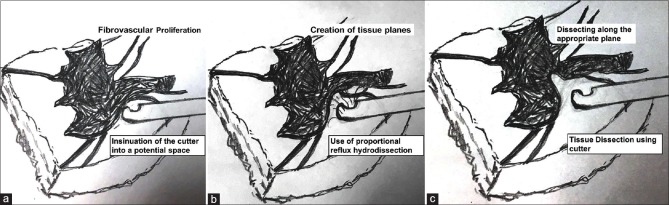
Figure 2.
Line diagram explaining the technique of fibrovascular tissue dissection. (a) The initial frame shows a cutter which is inserted into a potential space in the fibrovascular tissue. Using proportional reflux (b), the tissue separation is increased. (c) Finally, the cutter is used to dissect the tissue in the potential space created by reflux
The mi-OCT was also used to identify presence of vitreoschisis [Fig. 3]. Staining of the posterior hyaloid was performed using triamcinolone acetonide. After removal of excessive crystals of triamcinolone, an attempt to visualize remnant posterior hyaloid was done with the help of mi-OCT. TPHM was removed with the help of internal limiting membrane forceps [Supplemental Video 2]. Pre-retinal hemorrhages from bleeding vessels were also displaced using PRH or a 25G soft-tip cannula.
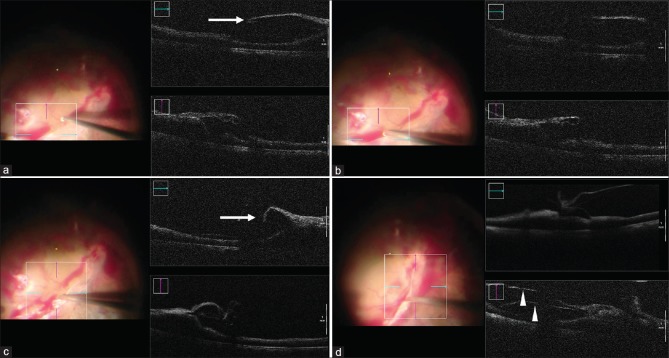
Figure 3.
Microscope-integrated optical coherence tomography (mi-OCT) in a case of diabetic tractional retinal detachment shows an area where the fibrovascular tissue is not adherent to the retina, and space for inserting the cutter is available (white arrow) (a). The vitreous cutter is then used to cut the fibrovascular tissue, first using proportional reflux hydrodissection (PRH) to increase the space, and then activating the cutter mode (b). As the surgery progresses and the fibrovascular tissue (white arrow) is dissected, the retinal elevation and traction reduces (c). In addition, the mi-OCT helps in identifying presence of vitreoschisis near the fibrovascular proliferation (white arrowheads) (d)
Toward the end of surgery, retinal detachment and presence of residual traction was assessed using mi-OCT. It was observed that after the traction was adequately removed, the retinal elevation decreased. Retinal breaks (primary or iatrogenic) were marked with an endocautery and adequately lasered. All peripheral ischemic retina (360°) was lasered. Tamponade, if required, was either C3F8 gas or silicone oil (1,000 cst) depending upon the surgeons preference.
Precautionary measures
When the mi-OCT is activated, care must be taken to achieve a good focus of the retina with appropriate magnification. If there are overlying hemorrhages, these must be cleared to obtain a good view. It is better for the surgeon to have an assistant for adjusting the OCT image during surgery in case there is a defocus. At the beginning of the surgery, while using PRH, one must exercise caution to prevent air bubbles from entering the vitreous cavity and reducing the visualization. Once the core vitrectomy is completed under fluid infusion, air bubbles should not egress from the tubing unless the cutter is used in air. One must be careful not to push the vitrector to avoid retinal breaks, and make sure that the PRH (and not the cutter mode) is activated. Since this technique avoids excessive aspiration forces and pull on the adhesions, lesser bleeding may be encountered during the surgery. However, if the bleeding does occur, it should be cleared with the soft-tip cannula because pre-retinal bleed will preclude view on the mi-OCT. Using vitrector and PRH with active retinal bleed may result in diffusion of the blood and compromise of the view intraoperatively.
Visual and anatomical outcomes
Of the 46 eyes included, anatomical attachment was achieved in all the eyes at 3-month follow-up. Nine eyes (19.56%) had pre-existing retinal breaks and CRD. Iatrogenic breaks developed in additional six eyes (13.04%). All the six eyes that developed iatrogenic breaks had TRD. Thus, overall, internal tamponade was performed in 15 eyes (32.6%) [perflouropropane (C3F8) in 13 eyes (28.26%) and 1,000 cst silicone oil in 2 eyes (4.35%)]. Additional instruments were used in 9 eyes (19.56%), of which internal limiting membrane forceps (25G) was used in 8 eyes (17.39%), and MLS was used in 1 eye (2.17%). In these cases, additional instruments were used since mi-OCT and PRH-aided dissection and identification of cleavage planes could not be performed in the presence of severe anterior proliferation, especially beyond the equator. In these situations, focusing the OCT signal was challenging due to the peripheral location of the adhesions and proliferation. In three eyes with TPHM, internal limiting membrane forceps was used to only peel the taut posterior hyaloid membrane. mi-OCT helped in detecting residual hyaloid membranes in 10 eyes (21.74%). Lamellar macular hole was detected in one eye (which was not observed preoperatively, as the view was obscured by overlying hemorrhages). None of the eyes required use of intraocular scissors or delaminators. Postoperative loose blood was observed in three eyes (6.52%), which cleared spontaneously at 3-month follow-up in two eyes (4.35%), and required additional pars plana vitreous lavage in one eye (2.17%).
No patient in our cohort had recurrence of retinal detachment in the follow-up period.
Discussion
With the introduction of mi-OCT, there has been a generational shift in the visualization of the retina during vitreous surgery. This technology has ushered in an era of state-of-the-art image-guided microsurgery, with a potential of improved outcomes while minimizing complications. The DISCOVER study was performed to assess the feasibility and utility of mi-OCT in the surgical management of PDR (n = 81).[8] The authors included patients undergoing surgical intervention for PDR using mi-OCT and observed that in over 50% cases, the mi-OCT provided additional information, such as identification of retinal planes and retinal holes. This resulted in alterations in surgical management including additional membrane peeling and use of internal tamponade in their series. Gabr et al. also studied the role of mi-OCT along with PPV for management of complex PDR cases such as TRD, vitreous haemorrhage, and CRD (n = 20).[6] The authors concluded that important information was conveyed by mi-OCT that guided the surgeon for optimal management of the patients.
In our study, we have further enhanced the use of mi-OCT along with the new dissection technique of PRH. Dugel et al. demonstrated the role of PRH in aiding dissection of fibrovascular tissue in eyes undergoing PPV for severe PDR.[5] The utility of PRH was also demonstrated by our group in managing complex PDR cases with TRD and CRD (n = 33).[9] PRH increases the working space by allowing insinuation of the vitreous cutter and allows better surgeon control with the foot-switch. In our previous study, the rate of surgical reattachment was 100%, which was comparable to previously published studies (69%–100%).[2,3,10]
We observed that mi-OCT allowed identification of areas where the fibrovascular tissue was strongly adherent, and areas where it was non-adherent, thereby guiding the surgeon to insinuate the vitrector. High cut rates (10,000 cpm) with low aspiration and bevelled cutters further enhance the surgeons' capability of approaching the retina while minimizing the risk of an iatrogenic break. In our series, mi-OCT helped in detecting residual membranes which helped the surgeon to ensure adequate removal. Other abnormalities such as lamellar macular holes were also detected, which were not observed preoperatively. mi-OCT also enabled detection of the posterior vitreoschisis layer which was adherent to the fibrovascular proliferation.
Traditionally, the incidence of iatrogenic breaks is higher while dissecting fibrovascular membranes in a detached retina, compared with an eye with no TRD. These breaks are typically posterior, and result from strong adhesion and tractional forces that act on the retina while dissecting these membranes away using cutters, forceps, or scissors.[1,11,12] When dissection is done using PRH, since it results in egress of fluid from the cutter, there are less chances of the detached retina being pulled into the cutter during dissection. This egress of fluid pushes the retina away from the cutter and provides counter-tractional force aiding the dissection. While dissection of fibrovascular membranes is more challenging in eyes with a detached retina and TRD, we did not encounter any additional challenges and difficulties, over conventional techniques, using our approach of combining mi-OCT with PRH.
Conclusion
By combining mi-OCT with PRH, it may be possible to reduce the surgical challenge of managing complex PDR cases. However, our study has several limitations. It is necessary to compare this current technique with established conventional techniques of tissue dissection in the future so that various outcome measures such as ease of surgery, surgical time, use of additional instrumentation, and anatomical outcomes can be compared. In summary, our study shows that apart from identifying residual membranes and macular abnormalities during PPV, mi-OCT can be also used to guide the surgeon especially when it is combined with the technique of PRH to ensure desirable tissue dissection in cases with diabetic TRD and CRD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos Available on: www.ijo.in
References
- 1.Stewart MW, Browning DJ, Landers MB. Current management of diabetic tractional retinal detachments. Indian J Ophthalmol. 2018;66:1751–62. doi: 10.4103/ijo.IJO_1217_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shroff CM, Gupta C, Shroff D, Atri N, Gupta P, Dutta R. Bimanual microincision vitreous surgery for severe proliferative diabetic retinopathy: Outcome in more than 300 eyes. Retina (Philadelphia, Pa) 2018;38(Suppl 1):S134–45. doi: 10.1097/IAE.0000000000002093. [DOI] [PubMed] [Google Scholar]
- 3.Wang ZY, Zhao KK, Li JK, Rossmiller B, Zhao PQ. Four-port bimanual 23-gauge vitrectomy for diabetic tractional retinal detachment. Acta Ophthalmol. 2016;94:365–72. doi: 10.1111/aos.12951. [DOI] [PubMed] [Google Scholar]
- 4.Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, Tano Y. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116:927–38. doi: 10.1016/j.ophtha.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Dugel PU. Proportional reflux hydrodissection. Retina (Philadelphia, Pa) 2012;32:629–30. doi: 10.1097/IAE.0B013E31824453C7. [DOI] [PubMed] [Google Scholar]
- 6.Gabr H, Chen X, Zevallos-Carrasco OM, Viehland C, Dandrige A, Sarin N, et al. Visualization from intraoperative swept-source microscope-integrated optical coherence tomography in vitrectomy for complications of proliferative diabetic retinopathy. Retina (Philadelphia, Pa) 2018;38(Suppl 1):S110–20. doi: 10.1097/IAE.0000000000002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falkner-Radler CI, Glittenberg C, Gabriel M, Binder S. Intrasurgical microscope-integrated spectral domain optical coherence tomography-assisted membrane peeling. Retina (Philadelphia, Pa) 2015;35:2100–6. doi: 10.1097/IAE.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 8.Khan M, Srivastava SK, Reese JL, Shwani Z, Ehlers JP. Intraoperative OCT-assisted surgery for proliferative diabetic retinopathy in the DISCOVER study. Ophthalmol Retina. 2018;2:411–7. doi: 10.1016/j.oret.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S, Agarwal A, Aggarwal K, Gupta V. The role of proportional reflux during pars plana vitrectomy for tractional retinal detachments. Ophthalmic Surg Lasers Imaging Retina. 2019;50:113–5. doi: 10.3928/23258160-20190129-08. [DOI] [PubMed] [Google Scholar]
- 10.Pessoa B, Dias DA, Baptista P, Coelho C, Beirão JNM, Meireles A. Vitrectomy outcomes in eyes with tractional diabetic macular edema. Ophthalmic Res. 2019;61:94–9. doi: 10.1159/000489459. [DOI] [PubMed] [Google Scholar]
- 11.Han DP, Pulido JS, Mieler WF, Johnson MW. Vitrectomy for proliferative diabetic retinopathy with severe equatorial fibrovascular proliferation. Am J Ophthalmol. 1995;119:563–70. doi: 10.1016/s0002-9394(14)70213-2. [DOI] [PubMed] [Google Scholar]
- 12.Issa SA, Connor A, Habib M, Steel DH. Comparison of retinal breaks observed during 23 gauge transconjunctival vitrectomy versus conventional 20 gauge surgery for proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:109–14. doi: 10.2147/OPTH.S16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.