Abstract
OBJECTIVE.
To investigate Acinetobacter baumannii infection, colonization, and transmission related to a long-term care facility (LTCF) providing subacute care (facility A).
METHODS.
We reviewed facility A and affiliated local hospital records for facility A residents with A. baumannii isolated during the period January 2009 through February 2010 and compared A. baumannii antimicrobial resistance patterns of residents with those of hospital patients. During March 2010, we implemented a colonization survey of facility A residents who received respiratory support or who could provide sputum samples and looked for A. baumannii colonization risks. Available clinical and survey isolates underwent pulsed-field gel electrophoresis (PFGE); PFGE strains were linked with overlapping stays to identify possible transmission.
RESULTS.
During the period January 2009 through February 2010, 33 facility A residents had A. baumannii isolates; all strains were multidrug resistant (MDR), which was a significantly higher prevalence of MDR strains than that found among isolates from hospital patients (81 [66%] of 122 hospital patient isolates were MDR; P < .001). The sputum survey found that 14 (20%) of 70 residents had A. baumannii colonization, which was associated with ventilator use (adjusted odds ratio, 4.24 [95% confidence interval, 1.06–16.93]); 12 (86%) of 14 isolates were MDR. Four facility A resident groups clustered with 3 PFGE strains and overlapping stays. One of these facility A residents also clustered with 3 patients at an affiliated hospital.
CONCLUSIONS.
We documented substantial MDR A. baumannii infections and colonization with probable intra- and interfacility spread associated with a single LTCF providing subacute care. Given the limited infection prevention and antimicrobial stewardship resources in such settings, regional collaborations among facilities across the spectrum of health care are needed to address this MDR threat.
During February 2010, the California Department of Public Health was notified of clusters of patients with multidrug-resistant Acinetobacter baumannii infections at 2 local hospitals;1 all patients appeared to be admitted predominantly from facility A, a long-term care facility (LTCF) providing both subacute and skilled nursing care.
A. baumannii is an aerobic, gram-negative bacillus often implicated in healthcare-associated infections as an opportunistic pathogen that survives well on and is transmitted via the environment and hands of healthcare personnel, with a propensity for patients who receive mechanical ventilation.2-4 Healthcare-associated Acinetobacter infections have increased in frequency during the past decade as susceptibility to carbapenems has decreased, leaving few if any treatment options, which is even more concerning because there are few antibiotics with novel mechanisms to combat resistance under development.4-8 The epidemiology of A. baumannii healthcare-associated infections is largely understood from the experience in acute care hospital (ACH) intensive care units, although recent evidence suggests that LTCFs may serve as important reservoirs for this pathogen, particularly facilities providing subacute care.9,10
Subacute care, provided within an LTCF, includes ventilatory support, intensive rehabilitation, complex wound care, and postsurgical recovery.11 Patients transferred to ACHs from LTCFs that provide ventilatory care have demonstrated higher percentages of carbapenem-resistant Enterobacteriaceae (CRE) colonization than residents of LTCFs that do not provide ventilatory care.12 A statewide cross-sectional survey of patients who received mechanical ventilation who resided in Maryland ACHs and LTCFs identified A. baumannii in 34% of all patients who received mechanical ventilation; all participating LTCFs had at least 1 patient with A. baumannii isolated.13
The LTCF implicated in this investigation, facility A, has 77 subacute care and 82 skilled nursing beds. Residents in the subacute section are ventilator dependent or require suctioning, air mist, or supplemental oxygen through tracheostomies, whereas those residing in the skilled nursing section do not require ventilatory assistance. The objectives of our investigation were to determine and describe the extent of A. baumannii infection and colonization associated with facility A, identify risk factors for colonization with A. baumannii at facility A, and determine the extent of spread within and beyond facility A.
METHODS
Descriptive Study of Facility A Residents with A. baumannii Clinical Isolates
We reviewed facility A and affiliated local hospital records to identify patients with laboratory-confirmed A. baumannii isolated from clinical specimens during the period January 1, 2009, through February 28, 2010, classifying them on the basis of residence. Patients were classified as “facility A resident” if culture specimens were obtained at facility A or if the patient was transferred from facility A before culture specimens were obtained, and patients were classified as “hospital patient” if culture specimens were obtained at an affiliated hospital without the patient having been transferred from facility A before culture specimen was obtained. We defined an isolate as multidrug resistant (MDR) if it was nonsusceptible (intermediate or resistant) to 1 or more agents in 3 or more of 9 possible antimicrobial categories.14 All laboratories identified Acinetobacter to the species level and used microbroth dilution and followed Clinical Laboratory Standards Institute (CLSI) standards for antimicrobial susceptibility testing. Among facility A residents, we abstracted information from the facility A medical record as well as hospital records including demographic characteristics, clinical characteristics, hospital course, and outcomes and classified them as A. baumannii infection or incidental colonization on the basis of clinical presentation. We defined an A. baumannii infection as illness in a resident consistent with signs and symptoms of infection according to a 2-physician (E.M. and K.K.T.) chart review. We identified hospital patients as culture positive for A. baumannii via hospital laboratory records during the same time period.
A. baumannii Sputum Colonization Survey at Facility A
On March 15, 2010, we conducted an A. baumannii sputum colonization survey at facility A. We collected sputum specimens from residents with tracheostomies (with or without ventilatory support) or who could cough with sputum production. Staff respiratory therapists collected sputum specimens either through suctioning into sterile Lukens traps or by self-induced cough into sterile cups sent for culture. We classified all A. baumannii isolates by antimicrobial susceptibility as previously described, although testing was limited to 6 of 9 categories. During the survey, we also reviewed demographic information, presence of indwelling devices (eg, central line, urinary catheter, percutaneous endoscopic gastrostomy tube, or gastrostomy tube), and resident location within the facility.
Pulsed-Field Gel Electrophoresis (PFGE) Typing of A. baumannii Isolates from Facility A and Affiliated Hospitals
We sent all available A. baumannii clinical isolates (January 1, 2009–February 28, 2010) and survey isolates (March 15, 2010) collected from facility A and affiliated hospitals to the Centers for Disease Control and Prevention for pulsed-field gel electrophoresis (PFGE) pattern characterization. We included multiple isolates from the same patient(s) to improve the possibility of epidemiologic linkage. We considered strains to be related if 90% or more of their bands matched.15 Two isolates from each PFGE group were selected for antibiotic resistance testing.
On the basis of PFGE pattern clusters, we identified epidemiologic links within each institution. We defined a link between 2 residents in facility A or a local affiliated hospital as overlapping stay by both date and residency in the same room, adjacent room, or room across the hall before at least 1 resident’s first positive culture result. We considered local transmission to be probable if residents or patients with PFGE-related A. baumannii isolates were also epidemiologically linked.
Statistical Methods
Data were analyzed using SAS, version 9.2 (SAS Institute), or OpenEpi, version 2.3.16 Linear regression models were used to evaluate for trend by month in facility A using number of cases, because the precise census was unknown and varied by month but remained relatively stable over the study period. χ2 tests were used to identify factors associated with colonization; age and sex were included a priori with additional variables included when P values less than or equal to 0.1 in the bivariate analysis. P values less than or equal to 0.05 were considered significant.
RESULTS
Descriptive Study of Facility A Residents with A. baumannii from Clinical Isolates
Four local hospitals reported receiving patients directly from facility A; 164 patients at the 4 hospitals and facility A were culture-positive for A. baumannii during January 1, 2009–February 28, 2010. Of these, 33 patients (20%) resided at facility A before their first isolate was obtained, whereas 131 patients (80%) resided at 1 of the 4 hospitals. Of the 33 facility A residents, 31 (94%) had initial culture specimens obtained at hospitals A (6; 18%), B (21; 64%), and C (4; 12%), and 2 residents (6%) had initial culture specimens obtained at facility A but subsequently transferred to hospitals; none had initial culture specimens obtained at the fourth hospital. The temporal distribution of the 33 residents from facility A showed no trend (data not shown). Among the 131 hospital patients with A. baumannii isolates who had not resided at facility A before their first positive isolate, 5 were subsequently transferred to facility A.
Median age of the 33 residents of facility A was 73 years (range, 34–86 years); 18 (55%) of the residents were men; and all had comorbidities. Specimens included 25 sputum samples (76%), 5 urine samples (15%), 2 blood samples (6%), and 1 wound sample (3%). At least 31 residents (94%) had a tracheostomy (with or without receipt of mechanical ventilation). Overall, the median period of hospital stay for the 33 facility A residents was 6 days (range, 1–82 days).
Among the 33 facility A residents, 21 (64%) had a clinical infection associated with A. baumannii, and 12 were colonized. Among 21 infected residents, 10 (48%) required a ventilator at admission; 11 (52%) were admitted to the intensive care unit; and 3 (14%) died, each within 1 week of having an A. baumannii–positive culture obtained.
All 33 (100%) of the isolates from facility A residents were classified as MDR, compared with 81 (66%) of 122 hospital patient isolates (odds ratio [OR], 33.41 [95% confidence interval (CI), 2.0–559.2; P ≤ .001). Similarly, 18 (60%) of 30 facility A resident isolates tested were carbapenem resistant compared with 18 (17%) of 108 hospital patient isolates (OR, 7.50 [95% CI, 3.08–18.24]; P ≤ .001). Resistance to all antimicrobial agents tested was detected for 10 (30%) of 33 facility A resident isolates and 13 (11%) of 122 hospital patient isolates; polymyxins were not tested.
A. baumannii Sputum Colonization Survey
Of 70 sputum specimens collected from facility A residents (55 from subacute care and 15 from skilled nursing beds), 14 (20%) were positive for A. baumannii, 12 (86%) of which were MDR. Table 1 shows survey results for facility A residents who were identified as colonized with A. baumannii; 2 colonized residents had previously been identified in the descriptive study; all but 1 resident (93%) resided in the subacute care section. Colonization was significantly associated with receiving antibiotics and ventilatory support at the time of the survey (Table 1). Of the factors entered in the multivariate analysis (age, sex, current antibiotics, presence of an indwelling catheter, and presence of a ventilator), only use of a ventilator was independently associated with A. baumannii colonization (adjusted OR, 4.24 [95% CI, 1.06–16.93]). Among the 12 newly identified colonized residents, 2 (17%) had isolates that were susceptible to all agents tested, whereas 10 (83%) were MDR; 4 (33%) had isolates that were carbapenem resistant, 3 (75%) of which were resistant to all agents tested.
TABLE 1.
Comparison of Facility A Residents with Sputum Colonized with Acinetobacter baumannii by Selected Characteristics, Sputum Colonization Survey, California, March 15, 2010
| Characteristic | No. (%) of colonized residents (n = 70) |
OR (95% CI) | P |
|---|---|---|---|
| Female sex | 7/32 (22) | 1.24 (0.38–4.00) | .72 |
| Male sex | 7/38 (18) | Ref | |
| Age 65 years or more | 8/32 (25) | 1.78 (0.54–5.81) | .34 |
| Age less than 65 years | 6/38 (16) | Ref | |
| Subacute care section | 13/57 (23) | 3.55 (0.42–29.89) | .44 |
| Long-term care section | 1/13 (8) | Ref | |
| ADL dependent | 12/53 (23) | 2.20 (0.44–10.98) | .49 |
| Not ADL dependent | 2/17 (12) | Ref | |
| Indwelling catheter | 13/52 (25) | 5.67 (0.69–46.84) | .10 |
| No indwelling catheter | 1/18 (6) | Ref | |
| Antibiotics | 4/7 (57) | 7.07 (1.37–36.52) | .03 |
| No antibiotics | 10/63 (16) | Ref | |
| Ventilator vs no respiratory care | |||
| Ventilator | 9/23 (39) | 10.29 (1.16–91.63) | .03 |
| No respiratory care | 1/17 (6) | Ref | |
| Tracheostomy vs no respiratory care | |||
| Tracheostomy | 4/30 (13) | 2.46 (0.25–24.02) | .64 |
| No respiratory care | 1/17 (6) | Ref | |
| Ventilator vs tracheostomy | |||
| Ventilator | 9/23 (39) | 4.18 (1.09–16.04) | .05 |
| Tracheostomy | 4/30 (13) | Ref |
note.Facility A residents comprised patients with tracheostomies (with or without ventilatory support) or who could cough with sputum production. ADL, activities of daily living; CI, confidence interval; OR, odds ratio.
PFGE Typing of A. baumannii Isolates from Facility A and Affiliated Hospitals
PFGE analysis of 31 of 36 A. baumannii isolates from facility A and 3 of the 4 affiliated hospitals revealed 4 groups of PFGE strains, from 3 to 15 isolates per group, denoted as A–D among 26 isolates; the remaining 5 isolates were unable to be grouped. All 4 strain groups were identified in facility A residents. Susceptibility testing of representative samples from each group showed them all to be MDR but susceptible to colistin and polymyxin B. All groups included isolates that were resistant to at least 1 carbapenem, but susceptibility did not correlate with PFGE relatedness.
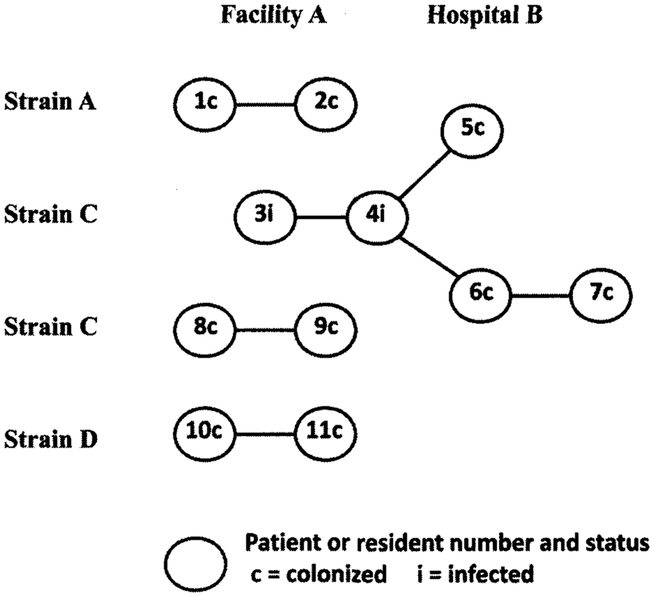
In total, 11 residents and patients were linked both epidemiologically and by PFGE strains A, C, and D (Figure 1). Eight residents (4 groups of 2) were linked at facility A, and 4 patients were linked at hospital B; 2 of those hospital patients never resided in facility A. Patient 4 was linked in both facility A and hospital B, from the isolate obtained at the time of admission. Strain A was identified twice in 1 individual: as a hospital A patient in January and as a facility A resident at the time of the colonization survey in March.
FIGURE 1.
Residents of facility A and patients of hospital B with pulsed-field gel electrophoresis (PFGE) and epidemiological links. PFGE strains were considered to be related if 90% or more of their bands matched. An epidemiological link was defined as overlapping stay in facility A or a local affiliated hospital by both date and residency in the same room, adjacent room, or room across the hall before at least 1 resident’s first positive culture. Patient 4 was transferred from facility A to hospital B, where an infection due to Acinetobacter baumannii was diagnosed; this patient was epidemiologically linked before transfer with facility A resident 3 and after transfer with patients 5 and 6 in hospital B.
DISCUSSION
We documented substantial MDR A. baumannii infections and colonization with probable intra- and interfacility spread associated with a single LTCF providing subacute care. Of 33 residents with A. baumannii isolates identified, 21 (64%) had clinical infection, of whom 11 (52%) were admitted to the intensive care unit. All A. baumannii isolates from facility A residents were MDR, and 60% were carbapenem resistant; the prevalences of MDR isolates and carbapenem-resistant isolates were both significantly higher among facility A residents than among patients not associated with facility A. Of facility A residents surveyed, 20% were colonized with A. baumannii; among these isolates, most were MDR and a third were carbapenem resistant; colonization was independently associated with receipt of mechanical ventilation. Three PFGE strains among 5 clusters of patients with overlapping stays support localized A. baumannii transmission of several clones within facility A and 1 affiliated local hospital.
The presence of multiple clones of MDR A. baumannii in facility A and the lack of a trend for culture positivity over time indicates that MDR A. baumannii is endemic in this facility. Although the detection of strain A in a patient in hospital A followed by the later detection of the same strain in facility A suggests that this patient, a resident of facility A since 2008, might have “transported” strain A from hospital A to facility A, we did not examine data from earlier than January 2009. Furthermore, our definition of epidemiological linkage was restricted to placement in adjacent rooms overlapping in time, whereas transmission of A. baumannii via ventilator and respiratory care equipment can occur over longer distances and periods.17 These data are consistent with earlier findings that LTCFs that provide ventilatory care can serve as a potential source for carbapenem-resistant isolates in ACHs and documents likely transmission from a LTCF source in an ACH.12
Additionally, 5 (4%) of 133 A. baumannii–positive hospitalized patients not previously residents of facility A were eventually transferred to facility A, which showed the bidirectional flow of such patients. Furthermore, although not a subject of this investigation, many of the residents who receive ventilatory care in LTCFs are admitted initially from long-term acute care hospitals (LTACHs),18,19 which are increasingly recognized as reservoirs for MDROs because of the higher case mix index of patients cared for in these hospital settings.20-22 Opportunities for and occurrence of multidirectional transmission of MDROs among these levels of care have been demonstrated and are consistent with our findings.2,9,12,18
Compared with other bacterial species, A. baumannii develops resistance rapidly, attributable to its intrinsic characteristics and amplified by inappropriate use of antibiotics and inadequate infection control practices.3 Commonly used antibiotics with limited or no activity against A. baumannii (eg, penicillins, cephalosporins, and macrolides) predispose patients to A. baumannii colonization.23 A majority of LTCFs, such as facility A, do not have antimicrobial stewardship programs.24 Thus, with limited resources for infection control practices, LTCFs have difficulty managing resistant pathogens.24 If facility A is typical, LTCFs providing ventilatory support and other subacute care do not have additional resources to manage the increased risk of infection and resistance that correlates with increased patient acuity compared with a traditional skilled nursing facility.
The 20% sputum colonization rate identified in our survey may be an overestimate for the entire facility, because we only obtained specimens from patients who received mechanical ventilation or had productive coughs. Sampling other sites, such as urine, wounds, and rectum, might have increased the yield.25,26 Although data on A. baumannii colonization prevalence in LTCFs are sparse,12 the prevalence in residents of the subacute section of facility A is comparable to the 28% reported in an LTACH.22 Additional limitations include the retrospective nature of the descriptive study, which may have been subject to incomplete documentation and missing or misclassified cases. Isolates were tested at different laboratories and not always with the same antibiotic susceptibility panel, but all used microbroth dilution and followed CLSI standards. PFGE testing was limited to the specimens available, which were incomplete and did not necessarily represent a patient’s first positive isolate.
In conclusion, MDR A. baumannii was prevalent in this LTCF that provided subacute care, with subsequent adverse impacts on residents and the hospitals to which they are often transferred. Hospitals that receive residents from high-risk LTCFs should consider screening patients on the basis of risk factors for colonization and consider isolating colonized patients, and LTCFs that receive patients from hospitals with high rates of MDROs should also consider screening patients at admission. Healthcare settings with the greatest potential for amplification of resistance, the subacute LTCFs and LTACHs, typically have the fewest resources for infection prevention and antimicrobial stewardship. To address this threat, regional collaborations must be developed among healthcare facilities across the continuum of care,27 supported by public health programs, as has been demonstrated previously for specific MDROs.28,29
ACKNOWLEDGMENTS
We thank the staff of facility A, hospitals A–D, and the Santa Clara Public Health Department for their assistance with this investigation. We gratefully acknowledge Sue Chen, RN; Taranisia MacCannell, PhD; Linda Martinez, PHN; Heather O’Connell, PhD; Rebecca Siiteri, RN; David Stevens, MD; Laura Tang, PHN; and Eddie Weiss, MD, for their help with data or specimen collection, isolate testing, or editorial comments. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
REFERENCES
- 1.Johnson NA, Simpkins SM, Hipona P, Hamilton JR, Stevens DA. Resistant Acinetobacter baumannii hospital outbreak from a community source. In: Program and abstracts of the 38th Annual Education Conference and International Meeting of the Association for Professionals in Infection Control and Epidemiology (APIC) Baltimore, MD: APIC, 2011. Abstract 1–003. [Google Scholar]
- 2.Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F, et al. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol 2004;25(10):819—824. [DOI] [PubMed] [Google Scholar]
- 3.Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev 1996;9(2):148—165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunenshine RH, Wright MO, Maragakis LL, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 2007;13(1):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi WS, Kim SH, Jeon EG, et al. Nosocomial outbreak of carbapenem-resistant Acinetobacter baumannii in intensive care units and successful outbreak control program. J Korean Med Sci 2010;25(7):999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 2008;46(8):1254–1263. [DOI] [PubMed] [Google Scholar]
- 7.Infectious Diseases Society of America. Bad bugs, no drugs. July 2004. http://www.idsociety.org/uploadedfiles/idsa/policy_and_advocacy/current_topics_and_issues/antimicrobial_resistance/10x20/images/bad%20bugs%20no%20drugs.pdf. Accessed October 22, 2013.
- 8.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin Infect Dis 2009;48(1):1–12. [DOI] [PubMed] [Google Scholar]
- 9.Sengstock DM, Thyagarajan R, Apalara J, Mira A, Chopra T, Kaye KS. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis 2010;50(12):1611–1616. [DOI] [PubMed] [Google Scholar]
- 10.de Medina T, Carmeli Y. The pivotal role of long-term care facilities in the epidemiology of Acinetobacter baumannii: another brick in the wall. Clin Infect Dis 2010;50(12):1617–1618. [DOI] [PubMed] [Google Scholar]
- 11.California Association of Health Facilities. Guide to long-term care. http://www.cahf.org/AboutCAHF/ConsumerHelp/GuideToLongTermCare.aspx. Accessed October 22, 2013.
- 12.Prabaker K, Lin MY, McNally M, et al. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase–producing Enterobacteriaceae: a multihospital study. Infect Control Hosp Epidemiol 2012;33(12):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thom KA, Maragakis LL, Richards K, et al. Assessing the burden of Acinetobacter baumannii in Maryland: a statewide cross-sectional period prevalence survey. Infect Control Hosp Epidemiol 2012;33(9):883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18(3):268–281. [DOI] [PubMed] [Google Scholar]
- 15.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin Microbiol 1995;33(9):2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean AG, Sullivan KM, Soe MM. OpenEpi: open source epidemiologic statistics for public health, version 2.3.1. http://www.OpenEpi.com. Accessed October 28, 2013. [Google Scholar]
- 17.Bernards AT, Harinck HI, Dijkshoorn L, van der Reijden TJ, van den Broek PJ. Persistent Acinetobacter baumannii? look inside your medical equipment. Infect Control Hosp Epidemiol 2004;25(11):1002–1004. [DOI] [PubMed] [Google Scholar]
- 18.Munoz-Price LS. Long-term acute care hospitals. Clin Infect Dis 2009;49(3):438–443. [DOI] [PubMed] [Google Scholar]
- 19.Kahn JM. The evolving role of dedicated weaning facilities in critical care. Intensive Care Med 2010;36(1):8–10. [DOI] [PubMed] [Google Scholar]
- 20.Lin MY, Lyles-Banks RD, Lolans K. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumonia carbapenemase–producing Enterobacteriaceae. Clin Infect Dis 2013;57(9):1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquez P, Terashita D. Long-term acute care hospitals and carbapenem-resistant Enterobacteriaceae: a reservoir for transmission. Clin Infect Dis 2013. ;57(9):1253–1255. [DOI] [PubMed] [Google Scholar]
- 22.Furuno JP, Hebden JN, Standiford HC, et al. Prevalence of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii in a long-term acute care facility. Am J Infect Control 2008; 36(7):468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunha BA. Acinetobacter. Medscape; October 19, http://emedicine.medscape.com/article/236891-print. Accessed September 9, 2011. [Google Scholar]
- 24.Smith PW, Watkins K, Miller H, et al. Antibiotic stewardship programs in long-term care facilities. Ann Long Term Care 2011;19(4):20–25. [Google Scholar]
- 25.Apisarnthanarak A, Warren DK. Screening for carbapenem-resistant Acinetobacter baumannii colonization sites: an implication for combination of horizontal and vertical approaches. Clin Infect Dis 2013;56(7):1057–1059. [DOI] [PubMed] [Google Scholar]
- 26.Marchaim D, Navon-Venezia S, Schwartz D, et al. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J Clin Microbiol 2007;45(5):1551–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prevention Mielke M. and control of nosocomial infections and resistance to antibiotics in Europe: primum non-nocere: elements of successful prevention and control of healthcare-associated infections. Int J Med Microbiol 2010;300(6):346–350. [DOI] [PubMed] [Google Scholar]
- 28.Schwaber MJ, Lev B, Israeli A, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011;52(7):848–855. [DOI] [PubMed] [Google Scholar]
- 29.Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344(19):1427–1433. [DOI] [PubMed] [Google Scholar]