Abstract
Clinical guidelines endorse either a 30 or 20 pack-year smoking history threshold when determining eligibility for lung cancer screening (LCS). However, self-reported smoking history is subject to recall bias that can affect patient eligibility. We examined the reliability of smokers’ self-reported tobacco use and its impact on eligibility for LCS. Current or former smokers aged 55–77 years completed questionnaires requesting demographic information and smoking history. Data were collected between December 2014 and September 2015. Total pack-year smoking history was calculated for each participant based on their responses at baseline and one month later. One hundred and two participants completed the study (mean age = 63.6 years). The intraclass correlation coefficient for the pack-year estimate was 0.93. For the 30 pack-year threshold, eight (7.8%) participants were eligible at one but not both assessment periods. For the 20 pack-year threshold, twelve participants (11.8%) were eligible at one but not both assessment periods. Inconsistent reporting was higher among current compared to former smokers. Smokers’ self-reported tobacco use appears highly reliable over short time periods. Nevertheless, there is some inconsistent reporting. We recommend that clinicians carefully assess smoking history, probe patients’ recall of duration and quantity of smoking, and collect tobacco use information at every encounter.
Keywords: Mass screening, Diagnostic screening programs, Early detection of cancer, Smoking, Self report, Patient reported outcomes
1. Introduction
Smoking history is used to determine a patient’s eligibility for lung cancer screening (LCS) with low-dose computed tomography (LDCT) (Moyer, 2014, Centers for Medicare Medicaid Services, 2015). The Centers for Medicare & Medicaid Services (CMS) and most insurers endorse a 30 pack-year smoking history threshold when determining eligibility for screening with low-dose CT. However, the National Comprehensive Cancer Network (NCCN) endorses a less conservative, 20 pack-year smoking history threshold when one additional risk factor is present (Network NCC, 2017). To calculate pack-year smoking history, smokers are asked to report on the average number of cigarettes they smoked each day multiplied by the total number of years they have smoked. One pack-year is equivalent to smoking an average of 20 cigarettes (one pack) every day for one year.
Recall of smoking history has been used widely in research and epidemiological studies; however, self-reported smoking history is subject to recall bias that has the potential to impact eligibility for LCS. Observational studies have found that retrospective recall of smoking history is reliable (Brigham et al., 2010, Soulakova et al., 2012); however, no known studies have evaluated the reliability of pack-year smoking history among individuals who may be eligible for lung cancer screening based on their age. Here we examine the reliability of smokers’ self-reported tobacco use and how differences in recall using both the 30 pack-year and 20 pack-year smoking history criteria may impact eligibility for LCS.
2. Materials and methods
This study used a repeated-measures design to estimate the test-retest reliability of self-reported smoking history. Data were collected between December 2014 and September 2015. Participants were current or former smokers, ages 55–77 years, recruited from a tobacco treatment program (592 invited), a LCS program (88 invited), and advertisements in the local community (86 responded). To obtain daily and nondaily smokers, the duration and amount of cigarette use was not considered in determining eligibility; however, eligible participants had to have smoked at least 100 cigarettes in their lifetime. Of 766 initially invited to participate, 166 responded, of which 135 were eligible and agreed to participate. Baseline surveys were completed by 120 participants, with 102 providing complete data. Participants were mailed questionnaires (at baseline and one month after baseline) requesting demographic information and smoking history.
Participants responded to questions regarding their smoking history and habits. These questions follow the criteria for enrollment in the National Lung Screening Trial (Aberle et al., 2011), and are consistent with screening guidelines from the United States Preventive Services Task Force (Moyer, 2014) and coverage of screening by CMS (Centers for Medicare Medicaid Services, 2015). The questions given to participants are shown in Table 1.
Table 1.
Questions asked to smokers and non-smokers regarding smoking history.
| 1) “Have you smoked at least 100 cigarettes in your entire lifetime?” |
|
| 2) “How many years have/did you smoke?” |
| 3) “On average, how many cigarettes do/did you smoke per day?” |
| 4) “Do you currently smoke?” |
|
| 5) “How many years ago did you quit smoking?” |
Total pack-year exposure to cigarettes was calculated as average number of packs smoked per day × number of years smoked. Scatterplots were used to display the baseline and one-month follow-up smoking history reports for pack-years. The Kappa coefficient was used to assess agreement of the categorical pack-year smoking history reports (less than 30 pack-years, versus 30 or greater, and less than 20 pack-years, versus 20 of greater) at the two time periods. Agreement was also assessed separately for current and former smokers. Test-retest reliability of the smoking history data was estimated with intraclass correlation coefficients (ICCs) using a two-factor mixed effects model and type consistency (Shrout and Fleiss, 1979).
The research protocol was registered (ClinicalTrials.gov ID: NCT02282969) and approved for use of human subjects by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
3. Results
Mean age of the 102 participants was 63.6 years, with roughly 10% over the age of 70 years. Twenty-four (23.5%) individuals were African American, while 72 (70.6%) identified as White. Fifty-five (53.9%) participants were female, and 19 (18.6%) had a high school degree or less. Half of the participants (n = 51) were current smokers and half were former smokers.
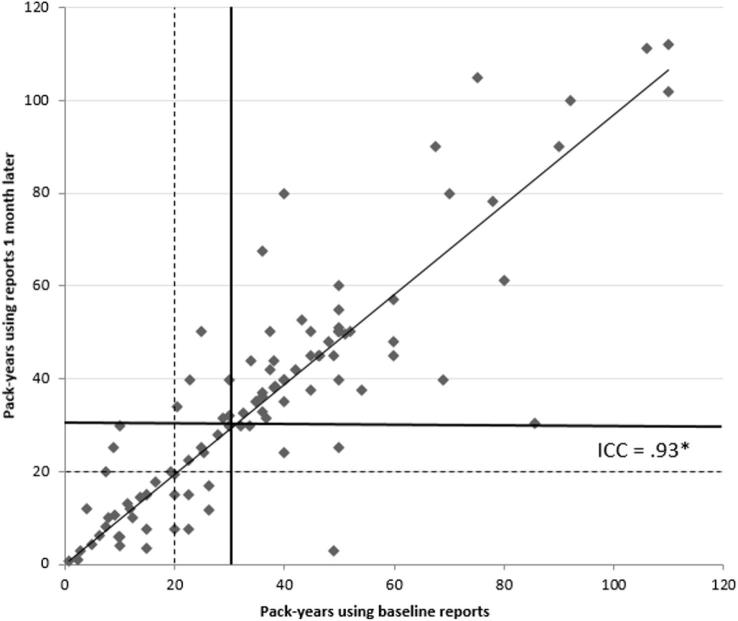
At the baseline and one-month follow-up assessment, 62 (60.8%) participants, versus 64 (62.7%) participants, respectively, reported at least a 30 pack-year smoking history (Kappa = 0.83, p < .001). High test-retest reliability was observed for pack-year estimates, with an ICC greater than 0.90 (see Fig. 1). Based on the 30 pack-year threshold, 35 (34.3%) participants did not meet eligibility at either assessment period, 59 (57.8%) were eligible at both assessments, and 8 (7.8%) were eligible at only one assessment period. Among current smokers, 7 of 51 (13.7%) were eligible at one assessment period but not the other (Kappa = 0.73, p < .001). For former smokers, only 1 of 51 (2.0%) was eligible at only one assessment period (Kappa = 0.95, p < .001).
Fig. 1.
Scatterplot of Pack-Year Smoking History from Baseline and 1-month Reports (n = 102). *ICC = intraclass correlation coefficient. Solid line represents 30 pack-year threshold. Dashed line represents 20 pack-year threshold. Cases in the upper right quadrant exceed the respective 30/20 pack-year threshold at baseline and 1 month periods; cases in lower left quadrant reported less than the respective 30/20 pack-years at both time periods; other cases were inconsistent across time periods.
At the baseline and one-month follow-up assessment, 77 (75.5%) participants, versus 73 (71.6%) participants, respectively, reported at least a 20 pack-year smoking history (Kappa = 0.70, p < .001). Using the 20 pack-year threshold, 21 (20.6%) participants did not meet eligibility at either assessment period, 69 (67.6%) were eligible at both assessments, and 12 (11.8%) were eligible at only one assessment period. Among current smokers, 8 of 51 (15.7%) were eligible at only one assessment period but not the other (Kappa = 0.64, p < .001). For former smokers, 4 of 51 (7.8%) was eligible at only one assessment period (Kappa = 0.77, p < .001).
4. Discussion
Smokers’ self-reported tobacco use history appears highly reliable over short periods of time, when standard questions about smoking history are used. Nevertheless, there is some inconsistent reporting which appears more pronounced among current compared to former smokers (up to 16% of current smokers were inconsistent in reporting their smoking history using the 20 pack-year threshold). Prior survey-based studies have also found that self-reported smoking history is reliable (Soulakova et al., 2012). These findings are encouraging because pack-year smoking history as a measure of smoking intensity has its roots in epidemiologic research and is now being used in clinical settings as an eligibility criterion for LCS.
In clinical practice the medical record is likely the source for determining smoking history. Numerous studies utilizing chart audits and electronic health records show less than half of patient encounters include documentation of tobacco history (Barber et al., 2015, Self et al., 2010, Boyle and Solberg, 2004). Further studies comparing self-reported pack years determined during a shared decision making (SDM) conversation compared to information in the electronic medical record show high levels of discordance between the two, highlighting the importance of the SDM conversation itself for determining LCS eligibility (Modin et al., 2017). Unfortunately, these conversations are happening infrequently (Goodwin et al., 2019) and the opportunity of explore or increase precision of tobacco use history is often lost.
The study limitations include inability to determine systematic over and under-reporting of smoking status (Curry et al., 2013). Accuracy of tobacco use in the absence of an objective measure cannot be determined. Additionally, it was not possible to explore reliability among different subgroups of current and former smokers because of the sample size.
We recommend that clinicians carefully assess smoking history, probe patients’ recall of duration and quantity of smoking, and collect tobacco use information at every encounter (US Department of Health and Human Services, 2014, Boyle and Solberg, 2004). Clinical judgment should play a key role in deciding which patients are considered appropriate screening candidates. Most importantly, clinicians should not lose sight of the primary importance of encouraging smoking cessation and abstinence in lowering the risk of lung cancer.
Financial support
This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1306-03385), the National Institute on Drug Abuse at the National Institutes of Health (K23 DA040933 to DSH), a grant from the Cancer Prevention Research Institute of Texas (RP160674), and The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment.
Disclaimer
All statements in this article, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the funding sources or PCORI’s Board of Governors or Methodology Committee.
Author contributions
Study concept and design: RJV, TM; acquisition of data: RJV; analyses and interpretation: all authors; drafting manuscript; all authors; critical revision of the manuscript for important intellectual content; all authors; statistical analysis: TM, RJV; All authors had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Gary Deyter for editing of the manuscript, and Andrea P. Hempstead, Vincent R. Richards, and Viola B. Leal for data collection. We appreciate the helpful comments from Antonia Bennett, PhD, on an earlier version of this manuscript.
References
- Aberle D.R., Adams A.M., Berg C.D., Black W.C., Clapp J.D., Fagerstrom R.M. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. PubMed PMID: 21714641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D., Williamson T., Biro S., Hall Barber K., Martin D., Kinsella L. Data discipline in electronic medical records: Improving smoking status documentation with a standardized intake tool and process. Canad. Family Physic. Med. Famille Canad. 2015;61(12):e570–e576. PubMed PMID: 27035007. [PMC free article] [PubMed] [Google Scholar]
- Boyle R., Solberg L.I. Is making smoking status a vital sign sufficient to increase cessation support actions in clinical practice? Ann. Family Med. 2004;2(1):22–25. doi: 10.1370/afm.38. PubMed PMID: 15053279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham J., Lessov-Schlaggar C.N., Javitz H.S., Krasnow R.E., Tildesley E., Andrews J. Validity of recall of tobacco use in two prospective cohorts. Am. J. Epidemiol. 2010;172(7):828–835. doi: 10.1093/aje/kwq179. PubMed PMID: 20720099; PubMed Central PMCID: PMC2945825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N) 2015 [February 5, 2015]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.
- Curry L.E., Richardson A., Xiao H., Niaura R.S. Nondisclosure of smoking status to health care providers among current and former smokers in the United States. Health Educ. Behav. 2013;40(3):266–273. doi: 10.1177/1090198112454284. [DOI] [PubMed] [Google Scholar]
- Goodwin J.S., Nishi S., Zhou J., Kuo Y.-F. Use of the shared decision-making visit for lung cancer screening among medicare enrollees. JAMA Internal Med. 2019 doi: 10.1001/jamainternmed.2018.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modin H.E., Fathi J.T., Gilbert C.R., Wilshire C.L., Wilson A.K., Aye R.W. Pack-year cigarette smoking history for determination of lung cancer screening eligibility. Comparison of the electronic medical record versus a shared decision-making conversation. Ann. Am. Thoracic Soc. 2017;14(8):1320–1325. doi: 10.1513/AnnalsATS.201612-984OC. PubMed PMID: 28406708. [DOI] [PubMed] [Google Scholar]
- Moyer V.A. U. S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. PubMed PMID: 24378917. [DOI] [PubMed] [Google Scholar]
- Network NCC, 2017. NCCN Guidelines Version 1.2017 Lung Cancer Screening.
- Self T.H., Wallace J.L., Gray L.A., Usery J.B., Finch C.K., Deaton P.R. Are we failing to document adequate smoking histories? a brief review 1999–2009. Curr. Med. Res. Opin. 2010;26(7):1691–1696. doi: 10.1185/03007995.2010.486574. [DOI] [PubMed] [Google Scholar]
- Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Soulakova J.N., Hartman A.M., Liu B., Willis G.B., Augustine S. Reliability of adult self-reported smoking history: data from the tobacco use supplement to the current population survey 2002–2003 cohort. Nicotine Tob. Res. 2012;14(8):952–960. doi: 10.1093/ntr/ntr313. PubMed PMID: 22318688; PubMed Central PMCID: PMC3439867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, 2014. The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.