Abstract
Background
Stenotrophomonas are ubiquitous gram-negative bacteria, which can survive in a wide range of environments. They can use many substances for their growth and are known to be intrinsically resistant to many antimicrobial agents. They have been tested for biotechnological applications, bioremediation, and production of antimicrobial agents.
Method
Stenotrophomonas sp. Pemsol was isolated from a crude oil contaminated soil. The capability of this isolate to tolerate and degrade polycyclic aromatic hydrocarbons (PAH) such as anthraquinone, biphenyl, naphthalene, phenanthrene, phenanthridine, and xylene was evaluated in Bushnell Hass medium containing PAHs as the sole carbon sources. The metabolites formed after 30-day degradation of naphthalene by Pemsol were analyzed using Fourier Transform Infra-red Spectroscopic (FTIR), Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) and Gas Chromatography-Mass Spectrometry (GC-MS). The genome of Pemsol was also sequenced and analyzed.
Results
Anthraquinone, biphenyl, naphthalene, phenanthrene, and phenanthridine except xylene can be used as sole carbon sources for Pemsol’s growth in Bushnell Hass medium. The degradation of naphthalene at a concentration of 1 mg/mL within 30 days was tested. A newly formed catechol peak and the disappearance of naphthalene peak detected on the UPLC-MS, and GC-MS analyses spectra respectively confirmed the complete degradation of naphthalene. Pemsol does not produce biosurfactant and neither bio-emulsify PAHs. The whole genome was sequenced and assembled into one scaffold with a length of 4,373,402 bp. A total of 145 genes involved in the degradation of PAHs were found in its genome, some of which are Pemsol-specific as compared with other 11 Stenotrophomonas genomes. Most specific genes are located on the genomic islands. Stenotrophomonas sp. Pemsol’s possession of few genes that are associated with bio-emulsification gives the genetic basis for its inability to bio-emulsify PAH. A possible degradation pathway for naphthalene in Pemsol was proposed following the analysis of Pemsol’s genome. ANI and GGDH analysis indicated that Pemsol is likely a new species of Stenotrophomonas. It is the first report on a complete genome sequence analysis of a PAH-degrading Stenotrophomonas. Stenotrophomonas sp. Pemsol possesses features that make it a good bacterium for genetic engineering and will be an excellent tool for the remediation of crude oil or PAH-contaminated soil.
Keywords: Stenotrophomonas, Sequencing., Biphenyl, Polycyclic Aromatic Hydrocarbon (PAH), Naphthalene, Degradation
Introduction
Stenotrophomonas are ubiquitous bacteria, occupying various habitats, including harsh environments (Ryan et al., 2009; Hughes et al., 2016). They can use a wide range of substances for their growth (Juhasz, Stanley & Britz, 2000; Pages et al., 2008; Zhang et al., 2009; Urszula et al., 2009; Iyer, Iken & Leon, 2016). The vast metabolic capability of Stenotrophomonas species has encouraged various studies which aimed at find new paths for their biotechnological application, such as bioremediation, biodegradation, plant growth promotion, removal of organophosphate and synthesis of new antimicrobial agents (Ryan et al., 2009; Rajkumar et al., 2010; Iyer, Iken & Leon, 2016; Arulazhagan et al., 2017). In particular, several studies have focused on the use of Stenotrophomonas maltophilia for the remediation of Polycyclic Aromatic Hydrocarbons (PAHs) or crude oil contaminated sites (Boonchan, Britz & Stanley, 1998; Juhasz, Stanley & Britz, 2000; Arulazhagan et al., 2017).
PAHs are the compounds formed from two or more fused aromatic rings. PAHs often get into released into the environment either through natural or manmade combustion sources. PAHs range from naphthalene (two fused benzene rings) to coronene (seven fused benzene rings). Accidental petroleum spillage is one of the ways through which PAHs get into the environment. Human exposure to PAH or its analogs in the environment poses a high risk to health. Cancer resulting from previous exposure to PAHs has been demonstrated in animal models (Kim et al., 2013). Risks associated with PAH exposure validate the importance of an adequate cleanup strategy of a PAH polluted environment. Microbes are said to be the best agents for the bioremediation in oil-spilled sites (Haritash & Kaushik, 2009).
The degradation of PAH by bacteria is usually via the activities of some enzymes such as oxygenases and peroxidases. These enzymes include, but not limited to, alkane monooxygenases, such as AlkB from Pseudomonas, Alkm from Acinetobacter sp. Strain, ADP-1, AlkB1 and AlkB2 from Rhodococcus sp; XylE, catechol 2, 3 dioxygenases from Pseudomonas putida; NdoB, naphthalene monooxygenase from P. putida; and NidA, pyrene dioxygenase large subunit from Mycobacterium sp. strain PYR-1, as well as various dehydrogenases and protocatechuate dioxygenases in Stenotrophomonas spp. (GUNSALUS, 1951; Seo, Keum & Li, 2009; Urszula et al., 2009; Das & Chandran, 2011). Bacterial degradation of PAHs can also involve the production of bio-surfactants (Van Beilen & Funhoff, 2007; Fritsche & Hofrichter, 2001). Biosurfactants or surface-active substances decrease the surface tension of water molecules, thereby making entrapped PAH on surfaces available for the use of bacteria (Boonchan, Britz & Stanley, 1998).
Genome sequencing has been used to explain detailed characteristics of many bacteria including their metabolic behavior (Makarova et al., 2001; Oyedara et al., 2018). Genome sequencing of some bacteria with the potentials to degrade hydrocarbons and PAHs has given profound insight into the genes involved in the degradation and mineralization of PAHs (Gunsalus, 1951; Schneiker et al., 2006; Kim et al., 2008, Das & Chandran, 2011; Pal et al., 2017). This genome sequence analysis has also provided information on the peripheral pathways associated with the PAH degradation process by bacteria including Stenotrophomonas species (Elufisan et al., 2019). Several bacteria with good potentials for hydrocarbon degradation have been sequenced (Kim et al., 2008; Das et al., 2015; Pal et al., 2017). Although there were reports of PAHs-degrading Stenotrophomonas strains, no such genome was sequenced and analyzed so far.
In this study, Stenotrophomonas sp. Pemsol isolated from crude oil-contaminated soil in the state of Tabasco, Mexico was evaluated for its ability to tolerate and degrade PAH as the sole carbon source in a minimal medium. The genome analysis of Stenotrophomonas sp. Pemsol revealed that it has many genes that are responsible for the degradation of PAHs and other hydrocarbons. The aim of this study is to elucidate and understand the genetic basis involved in the uptake and degradation of PAHs in Stenotrophomonas sp. Pemsol.
Material & Methods
Sampling, isolation, and cultivation of Stenotrophomonassp. Pemsol
Stenotrophomonas sp. Pemsol was isolated from a crude oil-contaminated soil, Tabasco, Mexico (17°52′26.9″N 92°29′12.4″W). One gram of soil sample was added into 10 mL of Luria-Bertani broth, and the mix was incubated at 30 °C overnight. one mL of the bacterial culture was serially diluted from 10−1to 10−8 in phosphate buffer (pH = 6.5). One hundred microliters of each dilution were spread on selective medium (StenoVIA agar, Himedia, India) plates. Colonies formed on plates were selected for further identification.
Biochemical characterization
Biochemical tests were carried out based on the Bergey’s manual of determinative Bacteriological studies.
Sugar utilization test
The use of various sugars as a sole source of carbon was evaluated in liquid medium containing the test sugar as the only source of carbon. The liquid medium contains 2 g peptone, 5 g, Sodium chloride (NaCl) 0.3 g dibasic potassium phosphate (K2HPO4), 3 mg bromocresol purple, 10 g sugar in 1 liter of distilled water. Isolates ability to use different sugars as a sole carbon source were determined by the color changed in the liquid culture from purple to yellow following incubation for 24–48 h at 35 °C. E. coli ATCC (8739), Pseudomonas aeruginosa (ATCC 27853) and uninoculated sugar medium were used as control in the experimental set-up.
Other biochemical tests
Oxidase test was performed by using a commercially available oxidase test kit (Sigma Aldrich, USA). Catalase activity was determined by immersing a loopful of an isolate in hydrogen peroxides. Bile Aesculin hydrolysis was determined by streaking bacteria on Bile Aesculin plate and incubated at 37° C for 24 h. Decarboxylase and Deaminase enzyme activity was evaluated by inoculating isolates in basal medium with 1% amino acid and Lactose (Lysine, Serine, Ornithine). Decarboxylase tests were implemented as described by Elston (1971). Urease activity was evaluated as described by Brink (2010). Proteus mirabilis CDBB-B-1343(ATCC 21100) was used as positive control while E coli DH5 α was used as negative control for the analysis. Gelatin hydrolysis was evaluated in a gelatin agar plate containing nutrient agar and 0.8% gelatin. The gelatin agar was inoculated with a loopful of suspected Stenotrophomonas culture and incubated at 30 °C for 24 h. Hydrolysis of starch was tested on nutrient agar as described by Tindall (1990). Hydrolysis of Tween 80 was analyzed using the method described by Sierra (1957).
Preliminary identification of isolates based on 16S RNA gene sequence and phylogenetic analysis
Genomic DNA was extracted from five mL bacterial culture grown in Luria broth using Promega wizard genomic DNA purification kit (Promega, Madison, USA) as per the manufacturer’s instruction. The 16S rRNA genes were amplified by PCR using steno1 (5′AGG GAA ACT TAC GCT AAT ACC- 3′) and steno2 (5′CTC TGT CCC TAC CAT TGT AG-3′). The PCR mix contains 0.5 µL, 2.5 U Taq DNA polymerase, 0.5 µL of 10 mM d -NTP mix, 2.5 µL of 10 × PCR buffer, 1 µL (0.5 µM) of each primer, 0.75 µL (50 mM) MgCl2, 16.75 µL double distilled water and 2 µL DNA (10 ng/µL). PCR products were purified and sequenced at the Centro de Biotecnologia Genomica, Instituto Politecnico Nacional (IPN), Mexico using the Thermofisher Applied Biosystems® 3130 Genetic Analyzer. The 16S rRNA gene sequence was analyzed with Seqman software version 13 and subjected to similarity search using the Blastn program from NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequences were then aligned with homologous sequences retrieved from NCBI database using ClustalW on Mega 6.0 (Tamura et al., 2013), and a phylogenetic tree was constructed using the neighbor-joining algorithm. Reliability of tree topologies was confirmed by bootstrap analysis using 1,000 repeat alignment. Stenotrophomonas sp. Pemsol 16S rRNA sequence has been deposited on NCBI with ascension number, KX500117.1.
Cultivation and growth in PAHs-containing media and bio-emulsification
All PAHs (anthraquinone, 97%; biphenyl, 99%; naphthalene, 99%; phenanthrene, 99%; phenanthridine, 98% and xylene, 98.5%) used for this study were purchased from Sigma Aldrich, Mexico. Growth in PAH tests was carried out using Bushnell Haas (BH) medium with one of the PAH compounds.
A preliminary growth test for Stenotrophomonas sp. Pemsol in PAH-containing media showed that it grew at a concentration of 1 mg/ml–5 mg/ml. All the hydrocarbons were dissolved in dimethyl chloride, and the solvent was allowed to evaporate before introducing the hydrocarbons in the experimental system. A 100 µL of the overnight grown culture of bacteria washed in phosphate buffer was inoculated in 100 mL BH medium containing each PAH at a concentration of 1 mg/mL in 250 mL Erlenmeyer flask, which was incubated at 30° C in a rotatory incubator at a revolution of 200 rpm for 8 days. An uninoculated BH medium containing hydrocarbons and a BH medium with Stenotrophomonas sp. Pemsol were controls. Stenotrophomonas’ growth was checked every two days using colony counting. All experiments were in triplicates. Spectrophotometric analysis was also carried out on culture from all experiments to corroborate the observations from the colony counting method. A test with a mix of 5 PAHs (1 mg/ml for each) in the medium was also implemented. Emulsification was tested according to previous reports by Boonchan, Britz & Stanley, 1998 and Sachan & Sachan, 2015, Boonchan, Britz & Stanley, 1998; Panjiar, Sachan & Sachan, 2015. Briefly, 2ml of fresh engine oil were added to 3 ml cell or cell free culture broth in a graduated screw cap test tube. The mix were vortexed vigorously at a high speed for 2 min. Emulsion stability was determined after 2 h, 12 h and 24 h. The emulsification index was calculated by dividing the height of the emulsion layer by total height of the mixture, multiplied by 100. The emulsion activities of Pemsol was compared with other Oil bio-emulsifying Stenotrophomonas species isolated in our laboratory.
Identification of metabolic intermediates from the degradation of naphthalene
Extraction of naphthalene from culture media was performed using an equal volume of hexane in triplicate. Then hexane was eliminated with vacuum pressure for further analysis (FTIR, UPLC-MS, and GC-MS). The metabolite was left to air dry, before doing the FTIR spectrophotometry analysis on them.
Fourier-transform infrared spectroscopy (FTIR)
The air-dried samples were analyzed on Bruker Alpha FT-IR spectrometer with Platinum ATR (AXS Inc., Madison, WI, USA) to determine the presence or absence of specific bonds after degradation.
Ultra-Performance Liquid Chromatographic-Mass Spectrometry (UPLC-MS) and Gas Chromatography-Mass Spectrometry (GC-MS) analysis
A total of 1 mg of extract was dissolved in one mL in dichloromethane. Then, 0.1 mL of this solution was added to 0.9 mL of methanol, and then analyzed with the Ultra-Performance Liquid Chromatographic (UPLC) with an ACQUITY QDa mass detector from Waters (Milford, MA, USA). The following conditions were applied for the UPLC-MS analysis: column, ACQUITY UPLC®BEH C18 1.7 µm 2.1 × 100 mm ; mobile phase A (0.1% formic acid in water), mobile phase B (methanol) and C (Acetonitrile) in a time 0.5-5 min 27%A:25%B; 48 °C; total run time, 5 min; flow rate, 0.3 mL/min; injection volume, 3.0 µL; temperature column, 40 °C.
A total of 1 mg of extract was dissolved in one mL in dichloromethane. Then, 0.1 mL of this solution was added to 0.9 mL of methanol, which was then used for analysis by Gas Chromatographic (7890A GC System) coupled to a Mass detector (5975C inert MSD with Triple-Axis Detector) from Agilent technologies. The following conditions were applied for the GCMS analysis: column, J&W 19091S-433HP-5MS: 30 m × 250 µm ×0.25 µm; oven program 70 °C for 2 min, then 10 °C/min to 160 °C for 2 min, then 5 °C/min to 240 °C for 2 min, then 30 °C/min to 290 °C for 2 min; Run Time, 34.667 min; injection volume, 2µL.
Whole-genome sequencing
The genomic DNA was extracted as described above using the Promega DNA extraction kit (USA) according to the manufacturer’s instruction. The extracted bacterial genomic DNA was sequenced at the Unidad Universitaria de Secuenciación Masiva y Bioinformática at the Instituto de Biotecnología, UNAM with the Illumina MiSeq platform.
Genome assembly and annotation
The reads quality was checked with Fastqc (Andrews, 2010) and the adaptors from the raw reads were trimmed with trim-galore version 4.10, which also filtered out reads with poor quality. De novo genome assembly was carried out with a standalone Spades 3.11.1 genome assembler (Center for Algorithmic biotechnology, St. Petersburg State University, Russia) (Bankevich et al., 2012). The assembly’s quality was checked with QUAST (Gurevich et al., 2013). The assembled contigs were ordered and reduced into a single scaffold with MedusaCombo, an online genome multi-draft scaffolder (Bosi et al., 2015). The assembled genome was annotated with Prokka annotating pipeline version 1.12 and PGAP (Seemann, 2014; Tatusova et al., 2016). Further functional genome annotation was done with online genome analysis server WebMGA (http://weizhong-lab.ucsd.edu/metagenomic-analysis) (Wu et al., 2011). The KEGG functions and COG categories present in the genome were predicted with the WebMGA online server. The presence of transposon and insertion sequences was predicted with a web-based analysis tool software ISsaga (http://issaga.biotoul.fr/ISsaga2/issaga_index.php) (Varani et al., 2011). The Pan core genome analysis for Stenotrophomonas sp. Pemsol and 11 other Stenotrophomonas species was performed to determine the unique genes in Stenotrophomonas sp. Pemsol. These genomes include Stenotrophomonas maltophilia JV3, Stenotrophomonas maltophilia ASS1, Stenotrophomonas pavani LMG, Stenotrophomonas rhizophilia QLP4, Stenotrophomonas pictorium JCM 9942, Stenotrophomonas maltophilia K279a, Stenotrophomonas nitrireducen 2001, Stenotrophomonas panacihumi, Stenotrophomonas maltophilia ATCC 19687, Stenotrophomonas maltophilia R551-3, and Stenotrophomonas sp. SKK. The annotation of the unique genes for Pemsol was done with the eggNOG mapper and blast2go (Conesa et al., 2005; Huerta-Cepas et al., 2017). Furthermore, the homologous unique genes in Pemsol’s genome were analyzed by blast search on the NCBI database and the synteny of the genes or gene fragments in the corresponding genomes were compared with the help of SyntTax and RAST annotation server.
Prediction of genomic islands (GIs)
The genomic islands (GIs) in Pemsol’s genome were predicted with the genomic IslandViewer 4 (Bertelli et al., 2017).
Comparative genome analysis
Genetic relatedness with other Stenotrophomonas species was determined by analyzing the Average Nucleotide Identity (ANI) on J speciesWS (Richter et al., 2016) and Genome-Genome distance hybridization (GGDH) (Auch et al., 2010) tools. Further analysis on Pemsol was carried out in the Integrated Microbial Genome (IMG) server (https://img.jgi.doe.gov) and Kbase Platform (https://narrative.kbase.us/narrative/ws.27061.obj.1).
The complete genome sequence of Pemsol has been deposited on DDBJ/EMBL/GenBank under the accession number CP025780.1.
Results
Isolation and identification of Stenotrophomonas strain Pemsol from crude oil-contaminated soil
The objective of this work is to isolate a Stenotrophomonas species that could be used for bioremediation of oil-polluted soil. Thus, StenoVIA agar medium was used for the selection of Stenotrophomonas strains (Kerr et al., 1996). Several uniform colonies with characteristic yellow color appeared on the selective medium after 48 h of incubation. The colonies that appeared on plates were characterized biochemically (Table 1) and the 16S rRNA gene fragment was amplified with steno1 and steno2 primers. The amplified fragments were sequenced, and blast search analysis of the sequences confirmed that each clone belongs to the genus Stenotrophomonas. The phylogeny produced from the aligned 16S rRNA sequence showed that Pemsol is closely related to Stenotrophomonas maltophilia M27 (Fig. S1).
Table 1. Metabolic characteristics of Stenotrophomonas sp Pemsol.
| Growth substrate | Response |
|---|---|
| Catalase | + + |
| Oxidase | − |
| Galactose | − |
| Fructose | + + |
| Lactose | − |
| Mannitol | − |
| Arabinose | − |
| Maltose | − |
| Mannose | + + |
| Glucose | − |
| Citrate | − |
| Aesculin | + + |
| Trehalose | + + |
| Dulcitol | − |
| Phenylalanine | − |
| Serine | − |
| Lysine | + + |
| Starch | − |
| Tween80 | + + |
| gelatin | + + |
| Hydrogen sulfide | + + |
Preliminary PAH survival test
The clone Pemsol was chosen for further analysis because of its good resistance to PAH. Stenotrophomonas sp. Pemsol ‘s ability to grow and survive in different PAHs was evaluated in BH minimal media with PAHs as a sole carbon source at a concentration ranging from 1 mg/ml to 5 mg/ml. Pemsol showed a good capacity to grow at these concentrations in the 8-day study. Stenotrophomonas sp. Pemsol showed better growth rate at a concentration of 1mg/ml in contrast to the growth at higher concentration of PAHs (Dataset 4).
Utilization of PAHs by Stenotrophomonas sp. Pemsol as a sole carbon source
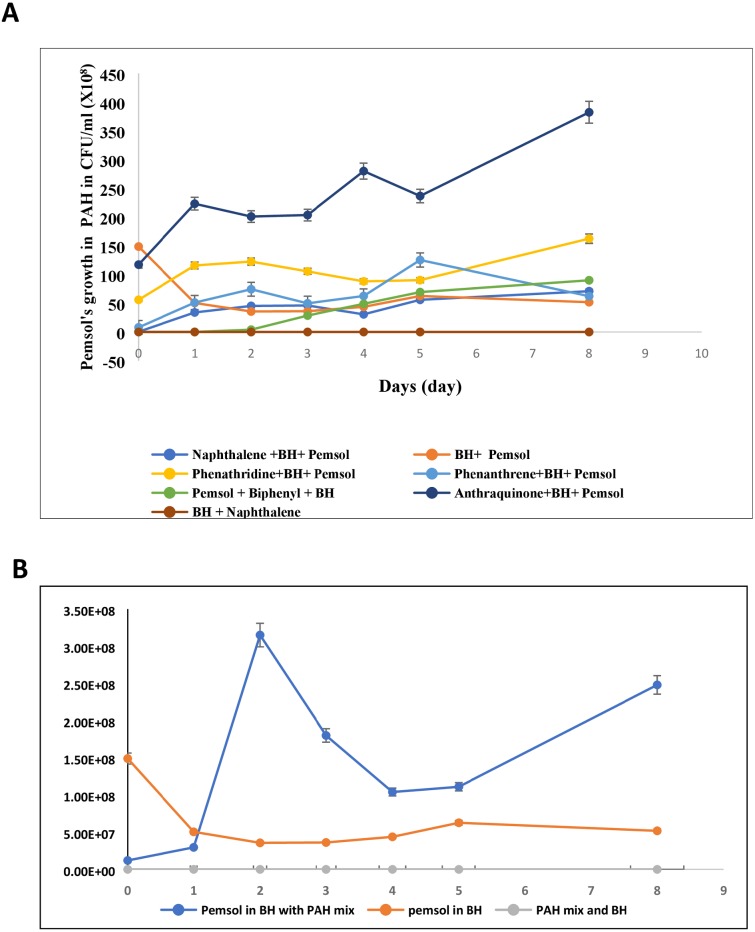
Stenotrophomonas sp. Pemsol grew well in BH medium supplemented with biphenyl, phenanthrene, phenanthridine, naphthalene and anthraquinone as sole carbon source at a concentration of 1 mg/mL but did not exhibit growth in BH medium supplemented with xylene, as shown in Fig. 1 (Fig. S2 showed Pemsol’s growth in BH solid medium). Pemsol also displayed the ability to grow in a mix of the five PAHs at the final concentration of 5 mg/mL (1 mg/mL for each) (Fig. 1B). Pemsol showed two growth peaks in this mix, indicating that this strain may prefer to use some compounds as sole carbon source rather than others.
Figure 1. The growth of Stenotrophomonas sp. Pemsol using different PAHs as unique carbon source.
(A) Pemsol growth in individual PAH; (B) Pemsol’s growth in five PAH mix.
Bio-emulsion and surfactant production in Stenotrophomonas sp. Pemsol
Emulsion and surfactant production can assist in bacterial degradation of PAHs. Bio-surfactant production usually enhances the dislodging of PAH attached to surfaces in water, thereby making hydrophobic hydrocarbon available for the use of bacteria (Cameotra & Bollag, 2003). Thus, the emulsion and surfactant production in Stenotrophomonas sp. Pemsol was evaluated, as described by Boonchan, Britz & Stanley (1998), Panjiar, Sachan & Sachan (2015), Boonchan, Britz & Stanley (1998), Panjiar, Sachan & Sachan (2015). The result showed that Pemsol cannot produce bio-surfactants as it did not bio-emulsify PAHs (Fig. 2).
Figure 2. Bio-emulsification activity of Stenotrophomonas sp. Pemsol.
Analysis of degradation products using FTIR spectroscopy
To confirm if the PAH degradation occurred in the minimal medium using PAH as sole carbon source in which Pemsol grew, one of the PAHs (naphthalene) was chosen for the metabolite analysis. The ability of Stenotrophomonas sp. Pemsol to degrade naphthalene was analyzed using FTIR spectrometry. New peaks observed at wavelengths -OH (3,200–2,800 cm−1), -C = O(CH2) (1,684 cm−1), -C = O(OH) (1,641 cm−1), -CH 2(2911)) after 15th day of degradation study and -OH (3,300–3,100 cm−1), -C = O (1,690 or 1,700 cm−1), -CH2 (3,001 cm−1) after the 30th day provided evidence of degradation of naphthalene by Stenotrophomonas sp. (Figs. S3A–S3C).
UPLC-MS and gas chromatography-MS analyzes of degradation products
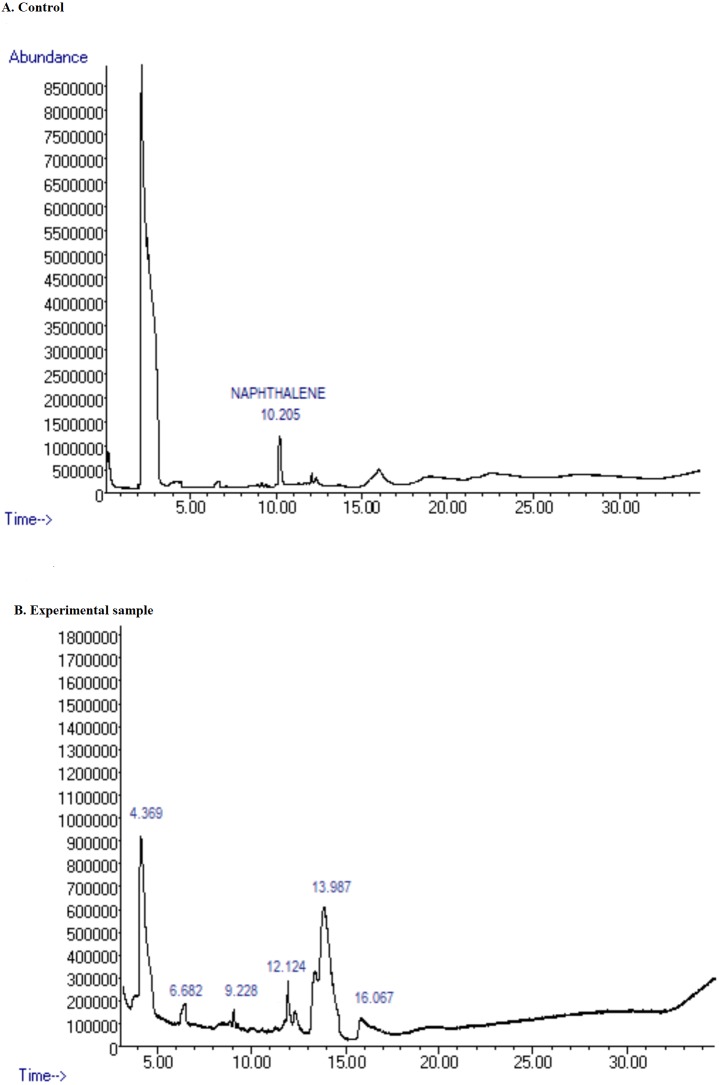
The UPLC-MS and GC-MS analyses were performed to detect the metabolites formed from the degradation of naphthalene after the 30-day experiment. The absence of a peak corresponding to naphthalene on the spectra obtained from GC-MS analysis confirmed the degradation of naphthalene, comparing with the spectra in the control (Fig. 3). Meanwhile, a peak with a molecular weight of 109.98 was seen as the major metabolites in the UPLC-MS spectra. This peak is estimated to be C6H5OH corresponding to the molecular weight for catechol (Figs. S4–S5). It could thus be inferred that the degradation of naphthalene by Pemsol involved the formation of catechol.
Figure 3. GC-MS image of the metabolites formed by Pemsol degradation of Naphthalene.
(A) GC-MS spectrum for Naphthalene; (B) GC-MS spectrum for the metabolite formed from the degradation of Naphthalene by Pemsol.
Genome analysis of Stenotrophomonas sp. Pemsol
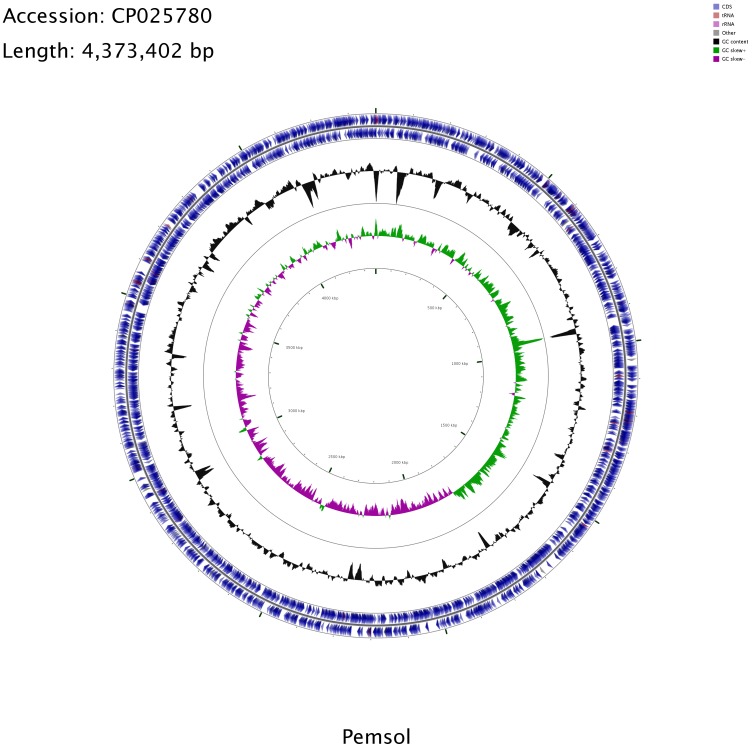
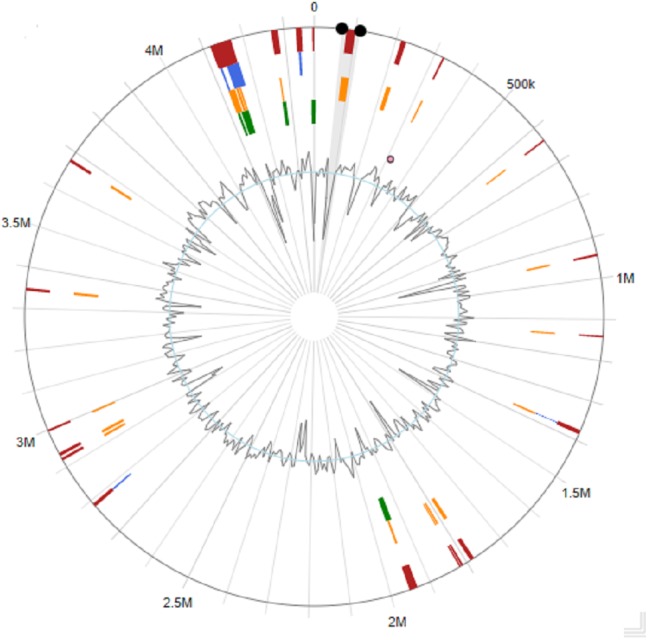
Several strains belonging to the genus Stenotrophomonas have been sequenced, including clinical isolates (Lira et al., 2012; Iyer, Iken & Leon, 2016), but to date, no genome analysis with emphasis on PAH degradation has been reported in this genus. Stenotrophomonas sp. Pemsol was sequenced using the Illumina next generation sequencing technology. The completely sequenced Pemsol’s genome was assembled de novo to 62 contigs. These contigs were then reduced to one contig with Medusa Scaffolder. The genome is composed of a single circular chromosome of 4,373,402 bp (Table 2, Fig. 4).
Table 2. Genome feature.
| Features | Genome |
|---|---|
| DNA, total number of bases | 4,373,402 |
| DNA coding number of bases | 4,370,061 |
| DNA G + C content (%) | 66.59%. |
| Misc_RNA | 39 |
| Protein coding genes | 3,905 |
| rRNA genes | 3 |
| tRNA genes | 67 |
| tmRNA | 1 |
| Genes | 4,037 |
Figure 4. Circular genome map for Stenotrophomonas sp. Pemsol.
Genetic basis for the degradation of PAH by Stenotrophomonas sp. Pemsol
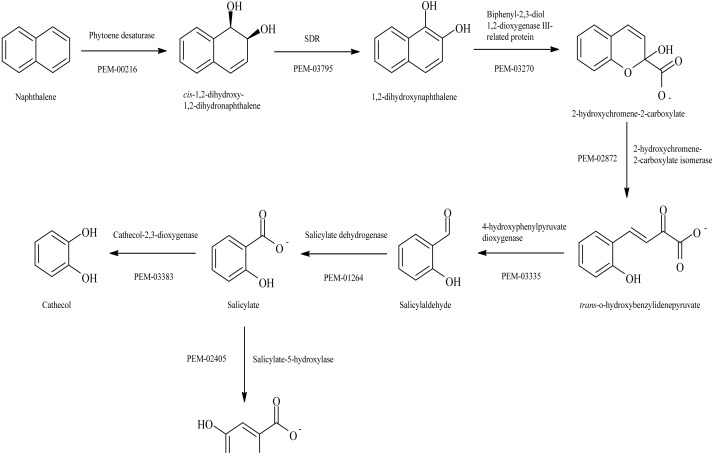
The annotation and deep sequence analysis of the genome showed that it possesses 145 genes that are associated with PAH degradation (Table S1). In this category are nine genes which code for the enzymes in the lactoylglutathione lyase family COG0346 (PEM_01474; 01733; 01959; 02738; 02855; 02960; 02961; 03007; 03383). The lactoylglutathione lyase family protein usually helps in the degradation of aromatic compound (Mesarch, Nakatsu & Nies, 2000). One of the lactoylglutathione (PEM_03383) was predicted to be a catechol 2, 3 dioxygenases on the JGI-IMG analysis platform (Fig. S6). This gene is essential for the conversion of salicylate aldehyde to catechol in the naphthalene’s metabolic degradation pathway (Grund, Denecke & Eichenlaub, 1992). It also has a gene which codes for salicylate hydroxylase (PEM_02405) (COG0654) (EC:1.14.13.1) (nahG). This enzyme catalyzes the removal of the carboxyl group at position 1 of salicylic acid and replaces the carboxyl with a hydroxyl group in the same position. This substitution converts salicylic acid to catechol (Goyal & Zylstra, 1997; Bosch et al., 1999). Two genes, homogentisate 1,2-dioxygenase (PEM_03309) that are involved in the catabolism of aromatic rings (Borowski, Georgiev & Siegbahn, 2005), and 2, 4 dihydroxyacetophenonedioxygenase (PEM_00137), which helps in the cleavage of carbon–carbon bond in a substituent aromatic ring, were also detected (Keegan et al., 2014). The information mentioned above ratifies the capacity of Stenotrophomonas sp. Pemsol to degrade naphthalene as shown in the experiments.
There are several other genes in Stenotrophomonas sp. Pemsol which can enhance the degradation of PAHs. One of such genes is chloromuconate isomerase (PEM_00043, EC:5.5.1.7), which catalyzes the degradation of 1,4-dichlorobenzene degradation (Schmidt, Remberg & Knackmuss, 1980). Others include carboxymethylenebutenolidase which catalyzes the conversion of methyl catechol to 4-carboxymethyl-4-methylbut-2-en-4-olide during the degradation of toluene to 4-oxohex-2-enedioate in Burkholderia (Dobslaw & Engesser, 2015), 4-oxalocrotonate tautomerase (PEM_00595) known to be associated with the degradation of toluene, o-xylene, 3-ethyltoluene, and 1,2,4-trimethylbenzene (Chen et al., 1992). Also present in the genome is biphenyl-2,3-diol 1,2-dioxygenase (PEM_03239, EC:1.13.11.39) that is associated with the degradation of biphenyl and gamma-hexachlorocyclohexane (Yam et al., 2009). Several monooxygenases and dehydrogenases are present in the Pemsol, which could catalyze the degradation of aromatic hydrocarbon and other xenobiotics (Versalovic et al., 2016; Pal et al., 2017) (Table S1).
Specific PAH degrading genes in genomic islands (GIs)
Microbes have been widely known to acquire new properties via horizontal gene transfer. In Pemsol, 29 genomic islands (GIs) were identified (Fig. 5, Table S2), which is 336,552 bp in length and constituting 7.7% of the genome. Table S3 contains the annotation of the genes in the GIs for Stenotrophomonas sp. Pemsol. Some genes on the GIs showed similarity with genes found in bacteria of other taxa. Most genes were predicted to be of unknown function. Twelve genes encoding transporters and some transcriptional regulators were found in the GIs. For example, a regulatory protein (PEM_01297) required for the regulation of xenobiotics’ degradation is present in the GI (1932729–1952883). Similarly, a Cysteine-liking transporter (PEM_00076) (GI 1816906–1820994) and another sulfite transporter (PEM_03784) (GI, 4135477–4158355) needed for the transport of sulfite molecules were also found in these regions (Takumi & Nonaka, 2016).
Figure 5. Genomic Island distribution in Stenotrophomonas sp. Pemsol.
Analysis of unique genes in adaptation for survival in PAH environment
The Pan-Core genome analysis was done for Stenotrophomonas sp. Pemsol and 11 other Stenotrophomonas species. The result of the analysis showed that Stenotrophomonas sp. Pemsol possesses 154 unique genes. Most genes identified to be unique in Pemsol are present in the genomic islands. The predicted functions for these genes are shown in Table S4. Some of these genes are involved in the degradation of PAH.
A comparative COG category analysis for Stenotrophomonas sp. Pemsol
The COG categories in Stenotrophomonas species was compared in Pemsol and other Stenotrophomonas strains. Stenotrophomonas sp. Pemsol has a higher number of genes in some COG categories than the other 11 Stenotrophomonas species examined. The Fischer test statistical analysis showed that Stenotrophomonas species Pemsol has more genes in the categories: energy production and conversion (C) (6.01%), amino acid transport and metabolism coenzyme transport and metabolism (H) (6.78%), cell motility (N) (3.74%), secondary metabolite biosynthesis, transport and metabolism (Q) (2.58%), general function prediction (R) (8.4%), function unknown (S) (6.4%), signal transduction (T) (6.52%), defense mechanism (V) (3.19%), extracellular mechanism (W) (1.77%) (Table S4).
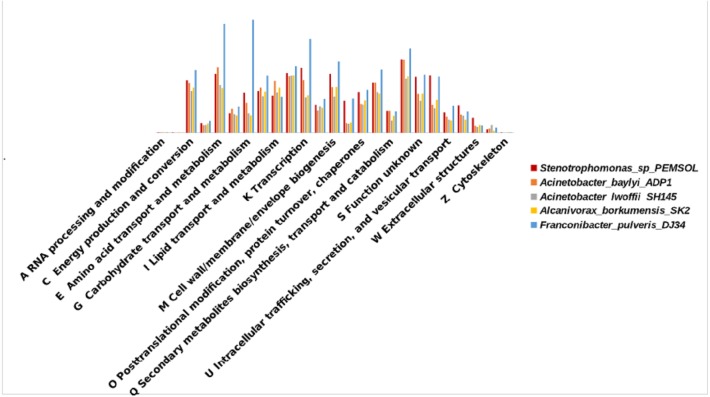
The COG categories in Pemsol was compared with the COGs in four other PAH-degrading bacteria (Acinetobacter_baylyi _ADP1, Acinetobacter_lwoffii _SH145, Alcanivorax_borkumensis _SK2, Franconibacter_pulveris _DJ34) previously reported. The result revealed that Pemsol has more genes in the COG category G, N, T, and W than the other hydrocarbon-degrading bacteria (Fig. 6, Table S5). These categories are associated with carbohydrate transport and metabolism (4.54%), cell movement (3.74%), signal transduction mechanisms (6.52%), and extracellular structure (1.75%).
Figure 6. COG distribution comparison of Pemsol and other hydrocarbon degrading bacteria.
Pemsol’s genomic relatedness with other Stenotrophomonas strains (ANI and GGDH)
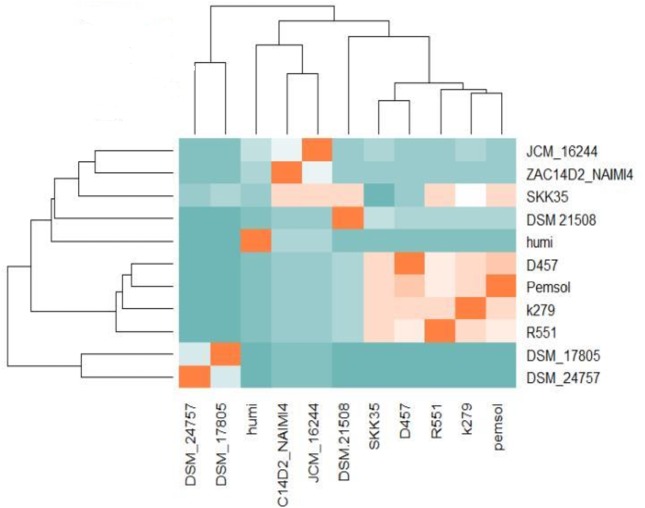
Average Nucleotide Identity blast (ANIb) was employed for the identification of Stenotrophomonas sp. Pemsol to the species level. The ANIb showed that Stenotrophomonas sp is likely a new species as it shares the ANIb similarity closest to S. maltophilia K279a at the level of 91.2% (Fig. 7) and the GGDH analysis result gave the Digital DNA Hybridization (DDH) score at the level of 44.2% using the recommended formula 2 (Table S6).
Figure 7. Average nucleotide identity score for Pemsol and other Stenotrophomonas species.
Discussion
The SVIA selective medium used in this study successfully select Stenotrophomonas strains (Kerr et al., 1996). The biochemical characteristics of the colonies which appear on the SVIA conformed with the features described for members of the genus Stenotrophomonas (Denton & Kerr, 1998; Ryan et al., 2009; Brooke, 2012). Stenotrophomonas sp. Pemsol was obtained by this method from the oil-contaminated site. The sequences of 16S rRNA fragments of bacteria is known to provide valid information about their classification. Stenotrophomonas sp. Pemsol showed 99% similarity with Stenotrophomonas maltophilia M27 (Fig. S1). It could be inferred that Stenotrophomonas sp. Pemsol is likely a S. maltophilia strain. The ANI analysis result, however, showed that Pemsol only shares 91.2% ANI score with S. maltophilia K279a as the closest strain (complete genome sequence is not available for the strain M27). Thus, Stenotrophomonas sp. Pemsol could be a novel species (Figueras et al., 2014) (Fig. 7). Similarly, the DDH (44.2%) value between Pemsol and K279a is below the threshold for the same species (Table S6). Although the 16S RNA gene identity showed a high level of relatedness with S. maltophilia (Fig. S1), the ANIb similarity score and the DDH score suggest Pemsol could be a new species.
Stenotrophomonas are an essential group of bacteria with an enormous capacity to use a wide range of substances for their growth. Stenotrophomonas’ ability to survive in different environments, including those with limited nutrients, has been reported in many studies (Ryan et al., 2009). The strains with such capabilities have been isolated from different environments ranging from common environment to extreme environment such as highly acidic or alkaline environments (Boonchan, Britz & Stanley, 1998; Juhasz, Stanley & Britz, 2000; Samanta, Singh & Jain, 2002; Gao et al., 2013; Tebyanian, Hassanshahian & Kariminik, 2013; Arulazhagan et al., 2017). Thus, the recovery of Pemsol from crude oil-contaminated soil further confirmed the versatility Stenotrophomonas’ versatility (Ryan et al., 2009).
Previous studies have shown that some Stenotrophomonas strains can grow on and degrade xenobiotics such as PAHs and organophosphates (Ryan et al., 2009; Iyer, Iken & Leon, 2016). Stenotrophomonas sp. Pemsol grew effectively in BH medium with different PAHs as the sole carbon source except xylene, implying that Pemsol could possibly degrade these PAHs, making it a potential strain for the remediation of crude oil-contaminated regions.
Pemsol grew in the tested PAHs at the concentration ranging from 1–5 mg/ml. Although it can grow in higher concentrations, Pemsol displayed better growth at a concentration of 1 mg/ml. Previous studies have reported the growth of Stenotrophomonas species in other PAHs (Smith, 1990; Johnsen, Wick & Harms, 2005). Juhasz, Stanley & Britz (2000) demonstrated Stenotrophomonas maltophilia strain VUN 10,003’s growth in an experiment trying to degrade and detoxify pyrene, fluoranthene, benz[a]anthracene, benzo[a]pyrene, dibenz[a,h]anthracene and coronene in a 63-day experiment (Juhasz, Stanley & Britz, 2000). In our study, the analysis of the degraded products by GC-MS and UPLC-MS showed that Pemsol completely degraded naphthalene in the minimal medium in a system wrapped with a foil for 30 days. Catechol is the major constituent formed. Catechol is an intermediate product formed from the degradation of PAHs by bacteria before they are mineralized (Gibson, Koch & Kallio, 1968). According to the analysis of the annotated enzymes in Pemsol’s genome, we proposed a naphthalene degradation pathway for Pemsol shown in Fig. 8, which is similar to the pathway described by Eaton & Chapman (1992). The degradation of other PAH compounds was not performed by metabolite analysis, however, the use of other PAH compounds as sole carbon sources for Pemsol’s growth was confirmed, indicating that Pemsol possesses the capacity to degrade these PAHs, although it may employ different metabolic pathways for their degradation. Thus, Stenotrophomonas sp. Pemsol is an important species with the capacity to degrade and metabolize PAHs.
Figure 8. Proposed degradation Pathway for Naphthalene degradation by Pemsol.
Bio-surfactants production in some bacteria is known to enhance PAH degradation (Willumsen & Karlson, 2004; Ruggeri et al., 2009; Das & Chandran, 2011). Bio-surfactant production by bacteria usually ensures the solubilization of hydrophobic PAH. Once hydrophobic PAHs become soluble, bio-degrading bacteria have easier access to use them (Willumsen & Karlson, 2004). Das et al. (2015) reported the presence of 25 genes related to biosurfactant production in a PAH-degrading Pseudomonas aeruginosa N002. However, several bacteria, including Stenotrophomonas, which can degrade PAH without biosurfactant production, have been reported (Bello-Akinosho et al., 2016; Arulazhagan et al., 2017). Pemsol does not produce surfactant, thus it is important to understand the molecular basis for this characteristic. The analysis of Pemsol’s genome showed that it possesses only three genes, phosphomannomutase (PEM_00211) and two glycosyls 2 family transferase genes (PEM_00203, 00204) that can participate in bio-surfactant formation (Pal et al., 2017). Particularly, it lacked some basic genes (such as rhlA, B, R and I which encode rhamnosyltransferase) required for bio-surfactant production in bacteria, 1 (Satpute et al., 2010). Although Pemsol did not bioemuslfiy PAHs, it effectively degraded the PAHs for growth. It can thus be deduced that surfactant production is not essentially required for the uptake of PAHs.
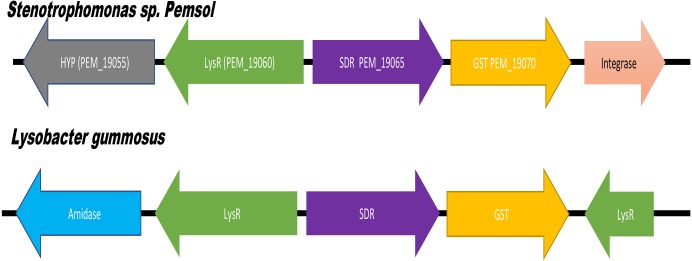
The analysis of Pemsol’s genome with the Island viewer 4 showed that it possesses 29 GIs and several genes or gene clusters in GIs participate in the degradation of PAHs. For example, a gene cluster contains 3 genes which encode the proteins short dehydrogenase reductase (SDR), LysR, and glutathione S-transferases (GST). SDR can catalyze the reduction of C=C bond between an aromatic compound (Kavanagh et al., 2008). The KEGG database clearly showed that GST is directly involved in the degradation of many hydrocarbon compounds (benzopyrene, naphthalene, trichloroethylene, bromobenzene, etc.). The LysR-type transcriptional regulator (LTTR) has been reported to have a significant function in regulating genes that are important for the catabolism of aromatic compound, cell motility, and quorum sensing (Pal et al., 2017). Thus, LysR gene in this gene cluster could be involved in the regulation of SDR and GST for PAH degradation. It is interesting that the closest orthologues of the 3 proteins SDR, LysR, and GST are in the same gene order with the identity of 91.6%, 94.24 and 85.65% respectively in a distant species Lysobacter gummosus on NCBI database, implying that this gene cluster in these two species has a common origin and Pemsol could have obtained this gene cluster by horizontal gene transfer (Fig. 9).
Figure 9. Identified unique gene region that is associated with the degradation of PAH.
Conclusion
Stenotrophomonas sp. Pemsol was isolated from crude oil-contaminated soil from Tabasco, Mexico. It grew in the presence of five PAHs (biphenyl, anthraquinone, phenanthrene, naphthalene, and phenanthridine) as unique carbon source except xylene. The identification of Pemsol confirmed that it is a member of the genus Stenotrophomonas. The ability of Pemsol to degrade PAH was confirmed by its activities on naphthalene as revealed by FTIR, UPLC-MS and GC-MS analysis. The complete genome of Pemsol was sequenced and the analysis revealed that it possesses 145 genes that are involved in the degradation of PAHs but only three genes associated with bio-emulsification, leading to no biosurfactant production. The presence of some genes associated with the degradation of PAHs in the genomic islands inferred that those genes were horizontally acquired. Compared with other four sequenced hydrocarbon-degrading bacteria, Pemsol is much richer in genes for the COG category G, N, T, and W, which are mainly relevant to hydrocarbon utilization and interaction with the environment. These results give insight into the genetic basis involved in the survival of Pemsol in its oil-contaminated site and provide a guide on the possible strategies for the bioremediation of an oil-polluted environment with Stenotrophomonas sp. Pemsol without biosurfactant production.
Supplemental Information
EACH TABLE represents Stenotrophomonas species’ growth in different PAH
COGs category comparison betwen Pemsol and selected member of other Stenotrophomonas species.
COGs category comparison betwen Pemsol and and five other PAH degrading bacteria
The table contains the growth of Stenotrophomonas sp. Pemsol in different PAH studies in the 8 days of tolerance studies
The tables contain the genes associated with PAH degradation by pemsol and their function, the genes that are on the genomic island, and the genes that are unique to Pemsol
Fig. 1 Phylogenetic tree of Stenotrophomonas sp. Pemsol with other members of the genus Stenotrophomonas based on the sequence of 16S rRNA gene, Fig. 2:Pemsols growth in solid media with PAH as sole carbon source Fig. 3 contains FTIR spectrum while Figs. 3 and 4 contain UPLC MS spectra, Fig. 6 Catechol 2, 3 dioxygenase containing region in Pemsol
Funding Statement
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT)-México (Grant No. 168541), Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional, México. Temidayo Oluyomi Elufisan held scholarships from CONACyT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Temidayo O. Elufisan conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Isabel C. Rodríguez-Luna and Alejandro Sánchez-Varela performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Omotayo Opemipo Oyedara and Miguel Angel Villalobos-Lopez conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Armando Hernández-Mendoza conceived and designed the experiments, authored or reviewed drafts of the paper, genome sequencing, and approved the final draft.
Edgar Dantán Gonzalez conceived and designed the experiments, authored or reviewed drafts of the paper, genome sequencing and annotation, and approved the final draft.
Alma D. Paz-González performed the experiments, prepared figures and/or tables, and approved the final draft.
Kashif Muhammad performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Gildardo Rivera analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Xianwu Guo conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The raw data is available at GenBank: CP025780.1.
Data Availability
The following information was supplied regarding data availability:
The data is available at NCBI: NZ_CP025780.1.
References
- Andrews (2010).Andrews S. FastQC a quality control tool for high throughput sequence data. Http://Www.Bioinformatics.Babraham.Ac.Uk/Projects/Fastqc/ 2010
- Arulazhagan et al. (2017).Arulazhagan P, Al-Shekri K, Huda Q, Godon JJ, Basahi JM, Jeyakumar D. Biodegradation of polycyclic aromatic hydrocarbons by an acidophilic Stenotrophomonas maltophilia strain AJH1 isolated from a mineral mining site in Saudi Arabia. Extremophiles. 2017;21:163–174. doi: 10.1007/s00792-016-0892-0. [DOI] [PubMed] [Google Scholar]
- Auch et al. (2010).Auch AF, Von Jan M, Klenk HP, Göker M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Standards in Genomic Sciences. 2010;2:117–134. doi: 10.4056/sigs.531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich et al. (2012).Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Akinosho et al. (2016).Bello-Akinosho M, Makofane R, Adeleke R, Thantsha M, Pillay M, Chirima GJ. Potential of polycyclic aromatic hydrocarbon-degrading bacterial isolates to contribute to soil fertility. BioMed Research International. 2016;2016 doi: 10.1155/2016/5798593. Article 5798593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli et al. (2017).Bertelli C, Laird MR, Williams KP, Lau BY, Hoad G, Winsor GL, Brinkman FSL. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Research. 2017;45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonchan, Britz & Stanley (1998).Boonchan S, Britz ML, Stanley GA. Surfactant-enhanced biodegradation of high molecular weight polycyclic aromatic hydrocarbons by stenotrophomonas maltophilia. Biotechnology and Bioengineering. 1998;59:482–494. doi: 10.1002/(SICI)1097-0290(19980820)59:4<482::AID-BIT11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Borowski, Georgiev & Siegbahn (2005).Borowski T, Georgiev V, Siegbahn PEM. Catalytic reaction mechanism of homogentisate dioxygenase: a hybrid DFT study. Journal of the American Chemical Society. 2005;127:17303–17314. doi: 10.1021/ja054433j. [DOI] [PubMed] [Google Scholar]
- Bosch et al. (1999).Bosch R, Moore ERB, García-Valdés E, Pieper DH. NahW, a novel, inducible salicylate hydroxylase involved in mineralization of naphthalene by Pseudomonas stutzeri AN10. Journal of Bacteriology. 1999;181:2315–2322. doi: 10.1128/jb.181.8.2315-2322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi et al. (2015).Bosi E, Donati B, Galardini M, Brunetti S, Sagot MF, Lió P, Crescenzi P, Fani R, Fondi M. MeDuSa: a multi-draft based scaffolder. Bioinformatics. 2015;31:2443–2451. doi: 10.1093/bioinformatics/btv171. [DOI] [PubMed] [Google Scholar]
- Brink (2010).Brink B. Urease test protocol. Washington, D.C.: American Society for Microbiology; 2010. [Google Scholar]
- Brooke (2012).Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clinical Microbiology Reviews. 2012;25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameotra & Bollag (2003).Cameotra SS, Bollag JM. Biosurfactant-enhanced bioremediation of polycyclic aromatic hydrocarbons. Critical Reviews in Environmental Science and Technology. 2003;33:111–126. doi: 10.1080/10643380390814505. [DOI] [Google Scholar]
- Chen et al. (1992).Chen LH, Kenyon GL, Curtin F, Harayama S, Bembenek ME, Hajipour G, Whitman CP. 4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer. Journal of Biological Chemistry. 1992;267:17716–17721. [PubMed] [Google Scholar]
- Conesa et al. (2005).Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Das et al. (2015).Das D, Baruah R, Sarma Roy A, Singh AK, Deka Boruah HP, Kalita J, Bora TC. Complete genome sequence analysis of Pseudomonas aeruginosa N002 reveals its genetic adaptation for crude oil degradation. Genomics. 2015;105:182–190. doi: 10.1016/j.ygeno.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Das & Chandran (2011).Das N, Chandran P. Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnology Research International. 2011;2011:1–13. doi: 10.4061/2011/941810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton & Kerr (1998).Denton M, Kerr KG. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clinical Microbiology Reviews. 1998;11(1):57–80. doi: 10.1074/jbc.M007003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobslaw & Engesser (2015).Dobslaw D, Engesser KH. Degradation of toluene by ortho cleavage enzymes in Burkholderia fungorum FLU100. Microbial Biotechnology. 2015;8:143–154. doi: 10.1111/1751-7915.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton & Chapman (1992).Eaton RW, Chapman PJ. Bacterial metabolism of naphthalene: construction and use of recombinant bacteria to study ring cleavage of 1, 2-dihydroxynaphthalene and subsequent reactions. Journal of Bacteriology. 1992 doi: 10.1128/jb.174.23.7542-7554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston (1971).Elston HR. Lysine decarboxylase activity in broth and agar media. Applied Microbiology. 1971;22(6):1091–1095. doi: 10.1128/am.22.6.1091-1095.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elufisan et al. (2019).Elufisan TO, Lozano L, Bustos P, Rodríguez-Luna IC, Sánchez-Varela A, Oyedara OO, Villalobos-López MÁ, Guo X. Complete genome sequence of stenotrophomonas maltophilia strain SVIA2, isolated from crude oil-contaminated soil in Tabasco, Mexico. Microbiology Resource Announcements. 2019;8(30):e00529-19. doi: 10.1128/mra.00529-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueras et al. (2014).Figueras MJ, Beaz-Hidalgo R, Hossain MJ, Liles MR. Taxonomic affiliation of new genomes should be verified using average nucleotide identity and multilocus phylogenetic analysis. Genome Announcements. 2014;2(6):e00927-14. doi: 10.1128/genomea.00927-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche & Hofrichter (2001).Fritsche W, Hofrichter M. Biotechnology set. Hoboken: Wiley; 2001. Aerobic degradation by microorganisms; pp. 144–167. [Google Scholar]
- Gao et al. (2013).Gao S, Seo JS, Wang J, Keum YS, Li J, Li QX. Multiple degradation pathways of phenanthrene by Stenotrophomonas maltophilia C6. International Biodeterioration and Biodegradation. 2013;79:98–104. doi: 10.1016/j.ibiod.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, Koch & Kallio (1968).Gibson DT, Koch JR, Kallio RE. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968;7:2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- Goyal & Zylstra (1997).Goyal AK, Zylstra GJ. Genetics of naphthalene and phenanthrene degradation by Comamonas testosteroni. Journal of Industrial Microbiology and Biotechnology. 1997;19:401–407. doi: 10.1038/sj.jim.2900476. [DOI] [PubMed] [Google Scholar]
- Grund, Denecke & Eichenlaub (1992).Grund E, Denecke B, Eichenlaub R. Naphthalene degradation via salicylate and gentisate by Rhodococcus sp. strain B4. Applied and Environmental Microbiology. 1992;58:1874–1877. doi: 10.1128/aem.58.6.1874-1877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus (1951).Gunsalus IC. Bacterial physiology. Dordrecht: Elsevier; 1951. Growth of bacteria; pp. 101–125. [DOI] [Google Scholar]
- Gurevich et al. (2013).Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritash & Kaushik (2009).Haritash AK, Kaushik CP. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. Journal of Hazardous Materials. 2009;169:1–15. doi: 10.1016/j.jhazmat.2009.03.137. [DOI] [PubMed] [Google Scholar]
- Huerta-Cepas et al. (2017).Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, Von Mering C, Bork P. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Molecular Biology and Evolution. 2017;34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes et al. (2016).Hughes GL, Raygoza Garay JA, Koundal V, Rasgon JL, Mwangi MM. Genome sequence of Stenotrophomonas maltophilia strain SmAs1, isolated from the Asian Malaria Mosquito Anopheles stephensi. Genome Announcements. 2016;4:e00086–16. doi: 10.1128/genomeA.00086-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, Iken & Leon (2016).Iyer R, Iken B, Leon A. Characterization and comparison of putative Stenotrophomonas maltophilia methyl parathion hydrolases. Bioremediation Journal. 2016;20:71–79. doi: 10.1080/10889868.2015.1114462. [DOI] [Google Scholar]
- Johnsen, Wick & Harms (2005).Johnsen AR, Wick LY, Harms H. Principles of microbial PAH-degradation in soil. Environmental Pollution. 2005;133:71–84. doi: 10.1016/j.envpol.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Juhasz, Stanley & Britz (2000).Juhasz AL, Stanley GA, Britz ML. Microbial degradation and detoxification of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltophilia strain VUN 10,003. Letters in Applied Microbiology. 2000;30(5):396–401. doi: 10.1046/j.1472-765x.2000.00733.x. [DOI] [PubMed] [Google Scholar]
- Kavanagh et al. (2008).Kavanagh KL, Jörnvall H, Persson B, Oppermann U. Medium- and short-chain dehydrogenase/reductase gene and protein families. Cellular and Molecular Life Sciences. 2008;65:3895–3906. doi: 10.1007/s00018-008-8588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan et al. (2014).Keegan R, Lebedev A, Erskine P, Guo J, Wood SP, Hopper DJ, Rigby SEJ, Cooper JB. Structure of the 2, 4′-dihydroxyacetophenone dioxygenase from Alcaligenes sp. 4HAP. Acta Crystallographica Section D: Biological Crystallography. 2014;70:2444–2454. doi: 10.1107/S1399004714015053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr et al. (1996).Kerr KG, Denton M, Todd N, Corps CM, Kumari P, Hawkey PM. A new selective differential medium for isolation of stenotrophomonas maltophilia. European Journal of Clinical Microbiology and Infectious Diseases. 1996;15:607–610. doi: 10.1007/BF01709373. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2013).Kim KH, Jahan SA, Kabir E, Brown RJC. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environment International. 2013;60:71–80. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2008).Kim SJ, Kweon O, Jones RC, Edmondson RD, Cerniglia CE. Genomic analysis of polycyclic aromatic hydrocarbon degradation in Mycobacterium vanbaalenii PYR-1. Biodegradation. 2008;19:859–881. doi: 10.1007/s10532-008-9189-z. [DOI] [PubMed] [Google Scholar]
- Lira et al. (2012).Lira F, Hernández A, Belda E, Sánchez MB, Moya A, Silva FJ, Martíneza JL. Whole-genome sequence of Stenotrophomonas maltophilia D457, a clinical isolate and a model strain. Journal of Bacteriology. 2012;194:3563–3564. doi: 10.1128/JB.00602-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova et al. (2001).Makarova KS, Aravind L, Wolf YI, Tatusov RL, Minton KW, Koonin EV, Daly MJ. Genome of the extremely radiation-resistant bacterium deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiology and Molecular Biology Reviews. 2001;65:44–79. doi: 10.1128/mmbr.65.1.44-79.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesarch, Nakatsu & Nies (2000).Mesarch MB, Nakatsu CH, Nies L. Development of catechol 2, 3-dioxygenase-specific primers for monitoring bioremediation by competitive quantitative PCR. Applied and Environmental Microbiology. 2000;66:678–683. doi: 10.1128/AEM.66.2.678-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyedara et al. (2018).Oyedara OO, Segura-Cabrera A, Guo X, Elufisan TO, González RAC, Pérez MAR. Whole-genome sequencing and comparative genome analysis provided insight into the predatory features and genetic diversity of two bdellovibrio species isolated from soil. International Journal of Genomics. 2018;2018 doi: 10.1155/2018/9402073. Article 9402073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages et al. (2008).Pages D, Rose J, Conrod S, Cuine S, Carrier P, Thierry Heulin WA. Heavy metal tolerance in Stenotrophomonas maltophilia. PLOS. 2008;3:1–6. doi: 10.1371/journal.pone.0001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal et al. (2017).Pal S, Kundu A, Das BT, Mohapatra B, Roy A, Manna R, Sar P, Kazy SK. Genome analysis of crude oil degrading Franconibacter pulveris strain DJ34 revealed its genetic basis for hydrocarbon degradation and survival in oil contaminated environment. Genomics. 2017;109:374–382. doi: 10.1016/j.ygeno.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Panjiar, Sachan & Sachan (2015).Panjiar N, Sachan SG, Sachan A. Screening of bioemulsifier-producing micro-organisms isolated from oil-contaminated sites. Annals of Microbiology. 2015;65(2):753–764. doi: 10.1007/s13213-014-0915-y. [DOI] [Google Scholar]
- Rajkumar et al. (2010).Rajkumar M, Ae N, Prasad MNV, Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends in Biotechnology. 2010;28:142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Richter et al. (2016).Richter M, Rosselló-Móra R, Oliver Glöckner F, Peplies J. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics. 2016;32:929–931. doi: 10.1093/bioinformatics/btv681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri et al. (2009).Ruggeri C, Franzetti A, Bestetti G, Caredda P, La Colla P, Pintus M, Sergi S, Tamburini E. Isolation and characterisation of surface active compound-producing bacteria from hydrocarbon-contaminated environments. International Biodeterioration and Biodegradation. 2009;63(7):936–942. doi: 10.1016/j.ibiod.2009.05.003. [DOI] [Google Scholar]
- Ryan et al. (2009).Ryan RP, Monchy S, Cardinale M, Taghavi S, Crossman L, Avison MB, Berg G, Van der Lelie D, Dow JM. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nature Reviews. Microbiology. 2009;7:514–525. doi: 10.1038/nrmicro2163. [DOI] [PubMed] [Google Scholar]
- Samanta, Singh & Jain (2002).Samanta SK, Singh OV, Jain RK. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends in Biotechnology. 2002;20:243–248. doi: 10.1016/S0167-7799(02)01943-1. [DOI] [PubMed] [Google Scholar]
- Satpute et al. (2010).Satpute SK, Bhuyan SS, Pardesi KR, Mujumdar SS, Dhakephalkar PK, Shete AM, Chopade BA. Advances in experimental medicine and biology. 2010. Molecular genetics of biosurfactant synthesis in microorganisms; pp. 14–41. [DOI] [PubMed] [Google Scholar]
- Schmidt, Remberg & Knackmuss (1980).Schmidt E, Remberg G, Knackmuss HJ. Chemical structure and biodegradability of halogenated aromatic compounds. Halogenated muconic acids as intermediates. Biochemical Journal. 1980;192:331–337. doi: 10.1042/bj1920331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiker et al. (2006).Schneiker S, Dos Santos VAM, Bartels D, Bekel T, Brecht M, Buhrmester J, Chernikova TN, Denaro R, Ferrer M, Gertler C, Goesmann A, Golyshina OV, Kaminski F, Khachane AN, Lang S, Linke B, McHardy AC, Meyer F, Nechitaylo T, Pühler A, Regenhardt D, Rupp O, Sabirova JS, Selbitschka W, Yakimov MM, Timmis KN, Vorhölter F-J, Weidner S, Kaiser O, Golyshin PN. Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nature Biotechnology. 2006;24:997–1004. doi: 10.1038/nbt1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann (2014).Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- Seo, Keum & Li (2009).Seo JS, Keum YS, Li QX. Bacterial degradation of aromatic compounds. International Journal of Environmental Research and Public Health. 2009;6:278–309. doi: 10.3390/ijerph6010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra (1957).Sierra G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek. 1957;23(1):15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- Smith (1990).Smith MR. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation. 1990;1:191–206. doi: 10.1007/BF00058836. [DOI] [PubMed] [Google Scholar]
- Takumi & Nonaka (2016).Takumi K, Nonaka G. Bacterial cysteine-inducible cysteine resistance systems. Journal of Bacteriology. 2016;198(9):1384–1392. doi: 10.1128/JB.01039-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura et al. (2013).Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusova et al. (2016).Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Research. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebyanian, Hassanshahian & Kariminik (2013).Tebyanian H, Hassanshahian M, Kariminik A. Hexadecane-degradation by Teskumurella and Stenotrophomonas strains isolated from hydrocarbon contaminated soils. Jundishapur Journal of Microbiology. 2013;6(7):9182. doi: 10.5812/jjm.9182. [DOI] [Google Scholar]
- Tindall (1990).Tindall BJ. A comparative study of the lipid composition of halobacterium saccharovorum from various sources. Systematic and Applied Microbiology. 1990;13(2):128–130. doi: 10.1016/S0723-2020(11)80158-X. [DOI] [Google Scholar]
- Urszula et al. (2009).Urszula G, Izabela G, Danuta W, Sylwia L. Isolation and characterization of a novel strain of Stenotrophomonas maltophilia possessing various dioxygenases for monocyclic hydrocarbon degradation. Brazilian Journal of Microbiology. 2009;40:285–291. doi: 10.1590/S1517-838220090002000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beilen & Funhoff (2007).Van Beilen JB, Funhoff EG. Alkane hydroxylases involved in microbial alkane degradation. Applied Microbiology and Biotechnology. 2007;74:13–21. doi: 10.1007/s00253-006-0748-0. [DOI] [PubMed] [Google Scholar]
- Varani et al. (2011).Varani AM, Siguier P, Gourbeyre E, Charneau V, Chandler M. ISsaga is an ensemble of web-based methods for high throughput identification and semi-automatic annotation of insertion sequences in prokaryotic genomes. Genome Biology. 2011;12(3):R30. doi: 10.1186/gb-2011-12-3-r30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen & Karlson (2004).Willumsen PA, Karlson U. Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation. 2004;7:415–423. doi: 10.1007/bf00056425. [DOI] [Google Scholar]
- Wu et al. (2011).Wu S, Zhu Z, Fu L, Niu B, Li W. WebMGA: a customizable web server for fast metagenomic sequence analysis. BMC Genomics. 2011;12 doi: 10.1186/1471-2164-12-444. Article 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam et al. (2009).Yam KC, D’Angelo I, Kalscheuer R, Zhu H, Wang JX, Snieckus V, Ly LH, Converse PJ, Jacobs WR, Strynadka N, Eltis LD. Studies of a ring-cleaving dioxygenase illuminate the role of cholesterol metabolism in the pathogenesis of Mycobacterium tuberculosis. PLOS Pathogens. 2009;5(3):e1000344. doi: 10.1371/journal.ppat.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2009).Zhang H, Yang C, Zhao Q, Qiao C. Development of an autofluorescent organophosphates-degrading Stenotrophomonas sp., with dehalogenase activity for the biodegradation of hexachlorocyclohexane (HCH) Bioresource Technology. 2009;100:3199–3204. doi: 10.1016/j.biortech.2009.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EACH TABLE represents Stenotrophomonas species’ growth in different PAH
COGs category comparison betwen Pemsol and selected member of other Stenotrophomonas species.
COGs category comparison betwen Pemsol and and five other PAH degrading bacteria
The table contains the growth of Stenotrophomonas sp. Pemsol in different PAH studies in the 8 days of tolerance studies
The tables contain the genes associated with PAH degradation by pemsol and their function, the genes that are on the genomic island, and the genes that are unique to Pemsol
Fig. 1 Phylogenetic tree of Stenotrophomonas sp. Pemsol with other members of the genus Stenotrophomonas based on the sequence of 16S rRNA gene, Fig. 2:Pemsols growth in solid media with PAH as sole carbon source Fig. 3 contains FTIR spectrum while Figs. 3 and 4 contain UPLC MS spectra, Fig. 6 Catechol 2, 3 dioxygenase containing region in Pemsol
Data Availability Statement
The following information was supplied regarding data availability:
The data is available at NCBI: NZ_CP025780.1.