Abstract
Schistosomiasis is an important Neglected Tropical Disease caused by blood parasites called schistosomes. In sub-Saharan Africa, two major human schistosomes, namely Schistosoma mansoni and S. haematobium, often occur sympatrically and is responsible for almost 90% of the affected 290 million people worldwide. We have utilized a highly sensitive and specific assay by amplifying species-specific cell-free repeat DNA fragments by polymerase chain reaction to detect either single or dual schistosome infection from a single urine sample from a broad age group. In this study, we have tested filtered urine samples collected from 163 individuals aged 3–63 years, mostly children (median age 10), to evaluate the prevalence of single and dual infections for S. mansoni and S. haematobium in Tomefa community in the Greater Accra region of Ghana. 40–50 mL of urine was filtered through a 12.5 cm Whatman # 3 filter paper in the field. The filter papers were dried, packed individually in sealable plastic bags with a desiccant, and shipped to Marquette University, where DNA was isolated and PCR amplification was carried out with species-specific primers. Disease prevalence was found to be 46.6% for S. mansoni and 48.5% for S. haematobium. Most importantly, 23.3% of participants had dual infections. All of the samples were detected without any cross amplification. The data was evaluated for four age groups and infection rate was highest for the age group of 3–12 years, with more S. haematobium infections than S. mansoni infections. We found a high prevalence of both S. haematobium and S. mansoni infection and a significant proportion of dual infection for the Tomefa community, which in most cases would be missed by traditional parasitological examination of urine or stool. Our highly sensitive and specific approach for detecting underlying multiple schistosome infections is an effective means to detect low intensity infections and would enhance the effectiveness of surveillance and Mass Drug Administration control programs of schistosomiasis.
Keywords: Cell-free repeat DNA, PCR, Schistosoma mansoni (S. mansoni), Schistosoma haematobium (S. haematobium), Urine
Graphical abstract
Study detected Schistosoma mansoni and S. haematobium cell-free repeat DNA from urine samples collected from 163 individuals with ages ranging from 3 to 63 years in Tomefa community of Ghana.
Highlights
-
•
WHO recommended gold-standard tests misses low-level schistosomiasis infection.
-
•
Cell-free repeat DNA detection can identify significant number of such infection.
-
•
Both schistosome species can be detected effectively from a single urine sample.
-
•
Infection rate is higher for younger age group.
-
•
Dual infection rate is higher in Tomefa community of Ghana.
1. Introduction
Schistosomiasis is the second deadliest parasitic disease in the world after malaria and has significant economic and public health consequences, particularly in poor communities. It is caused by trematode parasites of the genus Schistosoma, and three main species – Schistosoma japonicum, S. mansoni and S. haematobium commonly infect humans. According to the 2013 Global Burden of Disease study, 290 million people are infected with schistosomiasis and >90% are found in Sub-Saharan Africa (He et al., 2016; Herricks et al., 2017). The two major species, S. mansoni and S. haematobium that are found in Sub-Saharan Africa often share same location (Hotez and Fenwick, 2009). Schistosomes multiply considerably in an intermediate host (snails) and are easily transmitted to humans. Schistosomiasis is estimated to cause death in over 200,000 people every year (Chitsulo et al., 2004), and this prevalent, chronic parasitic disease requires accurate and sensitive diagnosis for patient management, evaluation of treatment efficiency, monitoring disease transmission and to evaluate the success of control strategies (He et al., 2016). A highly sensitive diagnostic tool is also very important as we move towards lower prevalence so we can ultimately reach the elimination target.
The disease burden for schistosomiasis varies among geographical regions, age groups and gender. Infection prevalence, intensity of infection, and transmission intensity are determined by various factors, such as human behavior, ecological and biological factors related to the parasite. Transmission patterns in highly endemic areas commonly show that 60–80% of school-aged children are infected, and 20–40% of adults can be infected and remain actively infected (Shiff, 2015).
The World Health Organization (WHO) recommended diagnostics include detection of eggs by microscopy from stool following Kato-Katz (KK; S. mansoni) and 10 mL urine filtration (S. haematobium) tests respectively, especially for resource poor settings (He et al., 2016; Shiff, 2015). These tests are being regarded as “Gold-Standard” techniques for detection of parasite eggs in stool and urine. Although these traditional parasitological methods are useful in areas of high prevalence, they lack sensitivity for low-level infections and thus result in misdiagnosis. Serological assays may be an effective alternative. The detection of parasite specific circulating cathodic antigen (CCA) in urine allows for detection of active infection but sensitivity of the currently available commercial test is only moderate.
Nucleic acid tests have been shown to be superior in identifying different schistosomes in cases of low-grade infections. The rate of positivity is dramatically higher than using the KK and CCA method for S. mansoni (Hessler et al., 2017; Lodh et al., 2013) and same for urine filtration and hematuria for S. haematobium (Lodh et al., 2013). It has also been seen for zoonotic schistosome parasite, S. japonicum (Cai et al., 2019; Weerakoon et al., 2017, Weerakoon et al., 2018). DNA based assays remarkably reduce the false-negative rate and effectively monitor potential exposure to schistosomiasis. The most widely used body fluids for the isolation of nucleic acids are blood, serum and plasma, however urine is increasingly becoming more popular because it is easier to collect and have a high content of biomolecules originating from different organs and tissues (Manzano-Roman and Siles-Lucas, 2012).
The detection of parasite specific DNA in urine using polymerase chain reaction (PCR) technique is a highly sensitive and specific method available for the diagnosis of schistosomiasis and indicates the presence of the actual parasite even when eggs or antigens are not always detectable. A limited amount of data is currently available on the performance of PCR as a monitoring tool after drug treatment for different age groups, different endemic settings and for different countries. In addition, a simple procedure, which can be adapted to clinical set-up in endemic countries is needed.
We have detected S. mansoni (Lodh et al., 2013) and S. haematobium (Ibironke et al., 2011) parasite-specific cell-free repeat DNA fragment from urine sediment collected on filter paper after urine filtration by PCR. The test is superior in sensitivity and specificity to KK, urine filtration and CCA. We propose to optimize this test for urine samples collected from Ghana across age groups. We have tested urine samples obtained from individuals for either single or dual infection and compare between existing parasitological diagnostic tests and PCR amplification of parasite DNA extracted from urine.
Our overall objective was to evaluate and determine the prevalence via a simplified, sensitive and specific molecular diagnostic approach for detection of two major schistosome parasites (S. mansoni and S. haematobium) from urine samples from different age groups in Tomefa. This was done by amplifying species-specific cell-free repeat DNA fragment via PCR from urine sediment captured on filter paper after urine collection and filtration. The infection prevalence was determined by PCR method and compared against parasitological tests, namely KK (S. mansoni) and 10 mL urine filtration (S. haematobium) to highlight the shortcoming of Gold-Standard techniques in determining the actual prevalence.
2. Materials and methods
2.1. Study region and sample selection
Tomefa, a community in the Greater Accra region of Ghana, was selected for this study due to presence of intermediate host snails Bulinus sp. and Biomphalaria pfeifferi - in the Weija Lake close to community and serving as the main source of water for domestic purpose. Additionally, the presence of high proportion of S. mansoni (90%) and S. haematobium (66%) infections in this area has been confirmed by previous studies conducted by project students supervised in Noguchi Memorial Institute for Medical Research (NMIMR) (Abonie, 2013). Although there is the annual MDA for school age children, the lack of safe drinking water, domestic use, recreational and fishing activities ensures the continuous transmission of the disease.
163 participants from 3 to 63 years old, mostly children (median age 10), were selected to provide stool and urine samples for this study. Each participant was provided with a unique identification (ID) number. The age and sex of each participant was also recorded. Out of 163 participants, 93 were male and 70 were female (Table 1).
Table 1.
Age distribution of the study participants.
| Age groups |
Total |
||||
|---|---|---|---|---|---|
| Gender | 3‐12 years (Group A) | 13‐17 years (Group B) | 18‐39 years (Group C) | 40‐63 years (Group D) | |
| Female | 57 (35.0%a) | 8 (4.9%) | 3 (1.8%) | 2 (1.2%) | 70 (42.9%) |
| Male | 61 (37.4%) | 23 (14.1%) | 4 (2.5%) | 5 (3.1%) | 93 (57.1%) |
| Total | 118 (72.4%) | 31 (19.0%) | 7 (4.3%) | 7 (4.3%) | 163 (100%) |
Percentage is based on 163 participants; whose gender and age information are available
2.2. Sample collection, parasitological testing and DNA extraction
Both stool (approximately 10 g–20 g) and urine samples (approximately 40–50 mL) were collected from each participant. Stool samples were assessed by microscopic examination via KK for S. mansoni eggs (Lodh et al., 2017). Each urine sample was assessed for color, pH, and specific gravity and for the presence of protein, glucose and bilirubin, as well as the presence or absence of blood (macro- and micro-hematuria) using observational and the Urine Reagent Strips (Teco Diagnostics, CA) respectively. The hematuria assessment was done for three categories: 1) blood absent (141), 2) macro-hematuria (10), and 3) micro-hematuria (12) present. Ten milliliter (10 mL) urine filtration was done on each urine sample to microscopically determine the presence of eggs for S. haematobium (World Health Organization, 1991).
Subsequently for each urine sample, a Whatman No. 3 filter paper (Whatman International, Maidstone, England) was labeled with the above-mentioned unique ID for each participant that corresponded to the age, sex, physiological and diagnostic information of the participant. 30–40 mL of urine from each study participant was passed through filter paper placed in a plastic funnel. Filter paper was left to air dry under a fly-proof net before packing into individual Ziploc bags with a desiccant. Filter papers were shipped to Marquette University, Milwaukee, Wisconsin, USA for DNA extraction and molecular diagnosis.
QIAamp DNA Blood Mini Kit (Qiagen, MD) was used for DNA extraction. A regular paper punch was used to make 15 punches (~1 mm diameter of each punch) from one quadrant of the filter paper. The 15 paper discs were placed in a 1.5 mL Eppendorf tube with 700 μL nuclease-free water and then heated at 95 °C for 10 min. The tube was then kept on a shaker at room temperature (22‐25 °C) for overnight. The DNA eluted from the paper was then transferred to a Qiagen QIAamp 2 mL column tube and the manufacturer's protocol was followed for DNA purification. At the end DNA concentration was determined using NanoDrop (Thermo Scientific, DE). Extracted DNA was stored at −20 °C until use.
2.3. Amplification of schistosome cell-free repeat DNA from urine via PCR, gel electrophoresis and sequencing
Species-specific primers (S. mansoni: SmPF: 5′ GAT CTG AAT CCG ACC AAC CG 3′ and SmPR: 5′ ATA TTA ACG CCC ACG CTC TC 3′; S. haematobium: ShDra1F: 5′ TCA CAA CGA TAC GAC CAA C 3′ and ShDra1R: 5′ GAT CTC ACC TAT CAG ACG AAA C 3′) (Hessler et al., 2017) were used to amplify repeat DNA fragments from extracted DNA to confirm the presence of either S. mansoni (121 bp) or S. haematobium (121 bp), or both species. We used an extensive repeat sequence of the schistosome genome, which comprised of ~12% (600,000 copies per cell) of the genome for S. mansoni (Hamburger et al., 1991) and ~15% for S. haematobium (Hamburger et al., 2001). These are non-coding, short tandem repeats (~121 bp) sequences and profoundly different from one another and specific for S. mansoni and S. haematobium.
The 10 μL PCR reaction volume consisted of 5 μL of 2× Master Mix (New England Biolabs, Ipswich, MA), 0.5 μL of each 10 μM primer (forward and reverse), 1.5–2 μL of 25 μM MgCl2, 2 μL of DNA (1 μL for controls), and the rest nuclease-free water. S. mansoni and S. haematobium genomic DNA (BEI Resources, VA) were used as positive controls. Nuclease-free water was used as water control and extracted DNA from urine collected in USA from people who were never been exposed to schistosomiasis was used as no-template negative control. For amplification of S. mansoni, initial denaturation occurred at 95 °C for 10 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 65 °C for 90 s. and extension at 72 °C for 1 min. These were followed by a final extension at 72 °C for 10 min. The same steps were used for amplification of S. haematobium, except that the annealing process was set at 61 °C for 90 s. Amplification of each sample was done at least twice with each set of primers. If there was any contamination issue related to negative and blank controls came out as positive then all the results from the sample run was discarded, and PCR was redone for that entire sample run.
Gel electrophoresis was done on all PCR amplified products to confirm amplification of the correct size amplicon. 5 μl of PCR product for each sample was set into 2% agarose gel stained with SYBR Green I (Life Technologies, NY) and run with a 50 bp DNA ladder (New England BioLabs, MA). Random samples were selected for sequencing to confirm amplification of the targeted species-specific DNA sequence.
A total of 12 samples (a combination of single and dual infections) were sequenced to determine the identity of the amplified cell-free repeat DNA fragments for S. mansoni and S. haematobium. PCR product for these samples were cleaned up with ExoSAP-IT (Affymetrix Inc., OH) and sent to Genewiz (Plainfield, NJ) for sequencing and sequencing reads. The sequenced amplicons were matched against the NCBI GenBank for the identity of the amplified fragments and only the top search result was accepted.
2.4. Statistical tests
The statistical analyses were done by converting the results of PCR amplification to numerical values (1 = positive and 0 = negative). The data was stratified into four age groups (Group A: 3‐12 years, Group B: 13‐17 years, Group C: 18‐39 years and Group D: 40‐63 years) (Table 1). The total positive and negative infections for each schistosome species and the total number of infections for each age group were calculated by JMP 12 (JMP® v12, SAS Institute Inc., Cary, North Carolina, USA). Demographic evaluation of participants was done based on the presence or absence of hematuria by using JMP. Comparison of PCR amplification for both schistosome species against KK for S. mansoni and urine microscopy for S. haematobium was determined. Kappa agreement statistics was calculated to establish the agreement between two diagnostic tests using JMP. The disease prevalence, sensitivity and specificity of PCR amplification for both species were calculated using MedCalc 12.4.0 (MedCalc Software, Ostend, Belgium). Disease prevalence was determined using the number of positive samples by PCR for each schistosome species against the total number of evaluated samples.
3. Results
3.1. Single or dual infection prevalence
Out of 163 individual (93 males and 70 females) samples that were tested (Table 1), a total of 117 (71.8%) individuals were detected with either single or dual schistosome infections. 38(23.3%) individuals were positive for S. mansoni only and 41 (25.2%) individuals were positive for S. haematobium only by PCR. There were 38 (23.3%) individuals infected by both S. mansoni and S. haematobium (Table 2).
Table 2.
Detection of positive and negative infection for Schistosoma mansoni and S. haematobium by PCR.
| Schistosoma haematobium |
Schistosoma mansoni |
||
|---|---|---|---|
| Negative | Positive | Total | |
| Negative | 46 (28.2%a) | 38 (23.3%) | 84 (51.5%) |
| Positive | 41 (25.2%) | 38 (23.3%) | 79 (48.5%) |
| Total | 87 (53.4%) | 76 (46.6%) | 163 (100%) |
Bold indicates positive for dual infection.
Percentage is based on total 163 analyzed samples.
The overall disease prevalence rate for S. haematobium infection was 48.5% (CI: 41%–56%) and for S. mansoni was 46.6% (CI: 39%–54%) in Tomefa community. We detected all the positive infections without any cross-amplification (Table 3).
Table 3.
Disease prevalence, sensitivity and specificity measured for PCR detection of either or both schistosome species based on cell-free repeat DNA amplification from urine.
| Disease prevalencea (95% CI) | Sensitivity | Specificity | |
|---|---|---|---|
| Schistosoma mansoni | 46.6% (39%–54%) | 100% | 82.6% |
| Schistosoma haematobium | 48.5% (41%–56%) | 100% | 59.2% |
Disease prevalence is calculated based on the total positive infection of each schistosome species out of total number of urine samples (163 samples) are evaluated by PCR.
For the age groups, children in Group A (3–12 years) had the highest positive infection rate for both species (S. mansoni and S. haematobium). The next highest infection rate was for Group B (13–17 years). The Groups C (18–39 years) and Group D (40–63 years) had less and similar infections (Table 4). For S. haematobium, younger children (Group A) exhibited a higher infection prevalence (39.2%) compared to S. mansoni infection (33.1%). Whereas in older children and adults (Group B, C &D) S. haematobium exhibited lesser infection prevalence (9.2%) compared to S. mansoni infection (13.1%).
Table 4.
Positive and negative infection for both Schistosoma mansoni and S. haematobium for four different age groups determined by PCR.
| Group A | Group B | Group C | Group D | Totala | |
|---|---|---|---|---|---|
| Schistosoma mansoni | |||||
| Negative | 64 | 16 | 4 | 3 | 87 (53.4%) |
| Positive | 54 | 15 | 3 | 4 | 76 (46.6%) |
| Schistosoma haematobium | |||||
| Negative | 54 | 21 | 3 | 6 | 84 (51.5%) |
| Positive | 64 | 10 | 4 | 1 | 79 (48.5%) |
Percentage is based on 163 participants; whose gender and age information are available.
We have also stratified our data for gender specific analysis. The difference in infection prevalence for males and females was not significant (α set at 0.05) for either S. mansoni (Pearson test, p – 0.13) or S. haematobium (Pearson test, p – 0.08).
3.2. Schistosome species identification through sequencing
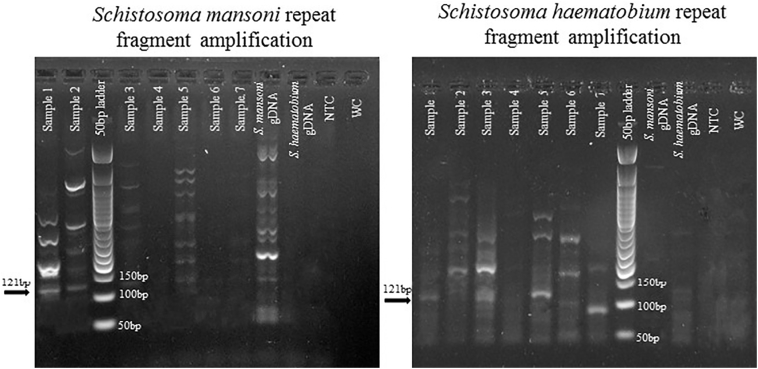
For both S. mansoni and S. haematobium 121 bp fragment (Fig. 1) was yielded. Sequencing of random samples had been done to determine the species identification. Sequences were compared against all the sequences in GenBank. For S. mansoni the 121 bp Sm1‐7 repeat fragment (GenBank: M61098.1) and for S. haematobium the 121 bp Dra1 repeat fragment (GenBank: DQ157698.1) came out as the top match with 98% - 100% identity. The higher homology of the amplified fragment and sequencing also demonstrated the specificity of the species-specific primers and the uniqueness of the repeat fragments of both schistosome species.
Fig. 1.
Species-specific cell-free repeat DNA fragment detection by PCR from filed collected filtered urine samples from Tomefa region of Ghana. Total seven samples are designated by Sample 1 through Sample 7. Abbreviations: gDNA = genomic DNA, NTC = no template control, WC = water control.
3.3. Infection prevalence based on presence of blood
From 163 participants, only 22 showed blood presence (macro- and micro-hematuria) in their urine. All 22 were shown to be S. haematobium infected whereas only 12 of them revealed S. mansoni infection. There were 13 male participants as against 9 female participants who were detected with macro- and micro-hematuria. Most blood positive participants (18) belonged to group A. (Table 5).
Table 5.
Demographics of the participants based on presence or absence of blood in urine during sample collection. The calculation is based on 163 participants.
| Variable | Blood absent (N = 141) | Macro-hematuria (N = 10) | Micro-hematuria (N = 12) | |
|---|---|---|---|---|
| Gender | Female | 61 (37.4%) | 2 (1.2%) | 7 (4.3%) |
| Male | 80 (49.1%) | 8 (4.9%) | 5 (3.1%) | |
| Age group | Group A | 100 (61.4%) | 8 (4.9%) | 10 (6.1%) |
| Group B | 28 (17.2%) | 1 (0.6%) | 2 (1.2%) | |
| Group C | 6 (3.7%) | 1 (0.6%) | 0 | |
| Group D | 7 (4.3%) | 0 | 0 | |
| S. mansoni PCR | Negative | 77 (47.2%) | 6 (3.7%) | 4 (2.5%) |
| Positive | 64 (39.2%) | 4 (2.5%) | 8 (4.9%) | |
| S. haematobium PCR | Negative | 84 (51.5%) | 0 | 0 |
| Positive | 57 (35.0%) | 10 (6.1%) | 12 (7.4%) | |
3.4. Comparison of parasitological and molecular diagnostic tests
KK and 10 mL urine filtration microscopy test results were compared against the species-specific PCR results. For S. mansoni, PCR proved to be the best predictor of positive infection by detecting all KK positive infections (45) and 8 individuals who were negative for KK (Table 6). S. mansoni egg was detected from a total of 45 individuals, ranging from 1 to 168. Overall, 38 individuals were negative for both KK and PCR. Kappa values, which show the agreement between two tests, were calculated (−1 = negative association, 0 = random, 1 = complete agreement). There was a high agreement between KK and PCR test positives (Kappa: 0.824; 95% CI: 0.71 – 0.94; P < 0.05; Table 6).
Table 6.
Determination of infection by microscopy using Kato-Katz (KK) for S. mansoni and 10 mL filtration (F) for S. haematobium compared against PCR detection for both species. The agreement statistics determined by Kappa co-efficient has been provided (CI = Confidence interval).
| Schistosome species | KK⁎ +/PCR + | KK −/PCR − | KK +/PCR − | KK −/PCR + | Kappa coefficient (95% CI) |
|---|---|---|---|---|---|
| Schistosoma mansoni | 45 (49.4%) | 38 (41.8%) | 0 | 8 (8.8%) | 0.824 (0.71 – 0.94) |
| Schistosome species | F$ +/PCR + | F −/PCR − | F +/PCR − | F −/PCR + | Kappa coefficient (95% CI) |
|---|---|---|---|---|---|
| Schistosoma haematobium | 21 (12.9%) | 84 (51.5%) | 0 | 58 (35.6%) | 0.271 (0.17 ‐ 0.37) |
KK⁎ data is available for only 91 samples.
F$ data is available for 163 samples.
PCR was the strongest indicator of positive S. haematobium infection with 35.6% (microscopy-/PCR+) more cases compared to just 12.9% positive for microscopy (Table 6). A total of 21 individuals presented eggs and egg count ranged from 10 to 405. PCR was able to detect all of the microscopy positive cases and overall 84 individuals were negative for both tests. The Kappa coefficient was also reflective of our findings. PCR showed a low degree of agreement with microscopy (Kappa: 0.271; 95% CI: 0.17 – 0.37; P < 0.05; Table 6).
4. Discussion
Diagnosis of schistosome infection is crucial for patient management, evaluation of treatment efficiency, monitoring of disease transmission and evaluation of the success of control strategies, as recommended by WHO (Hoy et al., 2014). It is also important to determine the correct infection prevalence for local regions and also to determine the presence of single or dual schistosome species as physiological outcome is different for both species (Koukounari et al., 2009). We have detected both species from the Tomefa community in the Greater Accra region of Ghana with close to 50% of disease prevalence, and most importantly 23% of them presenting co-infections. Most positive infection were detected from individuals with ages ranging from 3 to 63 years with no cross-amplification. These findings were consistent with other research findings as schistosomiasis infection rate was highest for the early age groups irrespective of species.
WHO recommended KK fecal examination for S. mansoni is currently used for mapping and field-based control of schistosomiasis. Due to the typical size and shape of the lateral spine, the egg of S. mansoni can be easily detected and identified. This method is cost-effective and easy to use especially for high infection prevalence areas. However, KK lacks sensitivity for low intensity infections and after control intervention because the eggs occur sporadically. At least three samples are necessary for KK diagnosis in some patients to detect true infection state (C., 2015; Gray et al., 2011; Hoy et al., 2014; Lodh et al., 2013; Lodh et al., 2014; Meningher et al., 2017). We have detected at least 8.8% more infection with cell-free repeat DNA approach, where PCR amplified all the KK positives and more low-level infection due to its increased sensitivity. The proportion of people falsely pronounced as negative because only one standard routine diagnosis was done and therefore parasite egg was missed, can lead to such individuals infecting snails and continuously ensuring disease transmission.
The detection of S. haematobium egg is generally performed by urine microscopy. The eggs are released in urine and after concentration of the sample by sedimentation, centrifugation, or filtration eggs can be detected under microscope. Urine test strips for detection of blood (hematuria) in the urine can also be used as an indicator of a potential infection in patients living in endemic areas (Gray et al., 2011). Urine filtration method is also inexpensive and easy to use like KK. However, the reliance of urine filtration on egg detection and non-direct detection of macro- or microhematuria limit their application for moderate and low intensity infection. Furthermore, the success or failure of drug treatment cannot be optimally assessed by urine filtration or hematuria state. This is evident from our study, where missed S. haematobium infection rate is 35.6% and PCR proved to be the best predictor of positive infection (Table 6). The limitation of such traditional techniques have also been highlighted by other authors in large-scale (Knopp et al., 2018) and small-scale studies (Ibironke et al., 2011; Lodh et al., 2014). Clearly our results suggest that PCR is a promising method with very high sensitivity in the detection of S. mansoni and S. haematobium DNA in urine. This appears to be a safer and non-invasive method for the detection of schistosomiasis infection and for treatment follow up especially after mass drug treatment.
We have recorded blood in urine (macro and micro) as an indirect measurement of ailment, most likely for S. haematobium (urogenital schistomiasis). This is because all the 22 individuals who were recorded with either macro- or micro-hematuria included all 21 individuals who were also positive for S. haematobium infection microscopically and mostly within the age group 3–12 years. This was not the case for S. mansoni infection where only 12 with either macro- or micro-hematuria were found from the 45 positive individuals. Interestingly these 12 individuals had dual infections suggesting that presence of blood in urine for S. mansoni infections could be due to dual infection, other infection or physiological complication. So, the use of reagent strip for detecting blood in urine during schistosome infection may be limited to S. haematobium infection only.
Previously S. haematobium DNA in urine and DNA of S. mansoni in feces, sera, plasma or urine has been detected using PCR (Lodh et al., 2014; Manzano-Roman and Siles-Lucas, 2012). Both schistosome species release nuclear material into the blood, which subsequently crossed the trans-renal barrier and passed into the urine. The short life span of this cell-free DNA (ranging from 4 min–12 h) suggests the presence of parasite (Khier and Lohan, 2018). SM1‐7 is a highly repeated, tandemly arranged DNA sequence from S. mansoni, which contains 121 bp tandem repeats (600,000 copies per cell) and comprises at least 12 % of the S. mansoni genome (Hamburger et al., 1991). DraI is a tandem repeat sequence of S. haematobium, accounts for 121 bp in length and contains a restriction site, occupying 15% of the entire genome (Hamburger et al., 2001). These DNA fragments can be detected by the amplification reaction and shows no cross-reactivity to other helminths (Fig. 1) (Pontes et al., 2003) and recently been used for diagnosis of different parasitic diseases (Weerakoon and McManus, 2016). Our previous studies successfully detected these repeat fragment from filtered urine samples collected from Zambia (Hessler et al., 2017; Lodh et al., 2013) and from Ghana (Lodh et al., 2014). The clearance of S. haematobium DNA from urine has been documented after two weeks of praziquantel treatment (Ibironke et al., 2012). Therefore, the presence of DNA in the urine indicates that a viable infection could still be present. We have demonstrated this same approach for Plasmodium falciparum (Mharakurwa et al., 2006) and also for Strongyloides stercoralis (Lodh et al., 2016).
We have introduced a schistosome diagnostic test that greatly simplifies procedures; it is highly sensitive and specific. The convenience of the process involves filtering 40-50 mL urine through coarse filter paper; the paper dried, packed in individual plastic sleeves with desiccant, and is easily and inexpensively transported to a lab where DNA can be extracted from the paper, amplified and the product detected by electrophoresis. There is no need to transport heavy materials, specimens and packages. There is no need to collect and process stool or whole urine in order to scrutinize them for eggs. We aim now to improve this approach by simplifying DNA extraction in a faster and cost-effective way to minimize the overall cost of detection of both species from a single urine sample in clinical set up of the endemic country.
The low sensitivity of current gold-standard tests for both S. mansoni and S. haematobium is a serious deficiency since it failed to determine the actual prevalence in low infection area. Again, when treatment leads to decrease infection prevalence through reduction in the worm load and thereby low egg production/fecundity, the standard microscopy tests will not be sensitive enough to detect most asymptomatic infections. Therefore, without very sensitive diagnostic tests, it will be difficult to ensure any schistosomiasis endemic focus is truly free of transmission. This study addressed the need for molecular based diagnostic test that is highly sensitive and can be used in the African clinical laboratories for detection of schistosomiasis.
5. Conclusions
It is apparent that with vector borne diseases, foci of asymptomatic cases due to low infection burden can serve as a significant reservoir for reinfection for the parasite (Stresman et al., 2010). Also, as public health measure, true schistosome prevalence for different age groups in the community and elimination of schistosome infection for a focal area through interventions can only be achieved with accurate and very sensitive diagnosis as transmission is often dependent on residual foci of infection. The PCR has therefore demonstrated to be a more sensitive test than microscopy and urine filtration where treatment results in low infection level and intensity and can be a useful tool in monitoring MDA.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
The authors would like to acknowledge the volunteers who donated their stool and urine for this study. Also, we would like to acknowledge the effort of the field staffs for collecting samples and describing the study to the participants.
Ethics approval
Ethical approval for the study protocol and the informed consent form were obtained from Noguchi Memorial Institute for Medical Research (NMIMR), University of Ghana, Accra, Ghana. IRB study number: 060/15-16. IRB approval from Marquette University was not needed as no human urine samples were collected in USA. Institutional Biosafety Committee (IBC) of Marquette University provided the clearance (BR 166) for doing this study at the University.
References
- Abonie S.D. University of Ghana; Legon: 2013. Concurrent Infections of Schistosoma mansoni and S. haematobium in an Endemic Community in Ghana: Impact of Disease Distribution and Pathology. (Unpublished Master's Thesis) [Google Scholar]
- C. S. Accurate diagnostics for schistosomiasis: a new role for PCR? Rep. Parasitol. 2015;4:23–29. [Google Scholar]
- Cai P., Weerakoon K.G., Mu Y., Olveda R.M., Ross A.G., Olveda D.U., McManus D.P. Comparison of Kato Katz, antibody-based ELISA and droplet digital PCR diagnosis of Schistosomiasis japonica: lessons learnt from a setting of low infection intensity. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitsulo L., Loverde P., Engels D. Schistosomiasis. Nat Rev Microbiol. 2004;2:12–13. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- Gray D.J., Ross A.G., Li Y.S., McManus D.P. Diagnosis and management of schistosomiasis. BMJ (Clinical research Ed.) 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger J., Turetski T., Kapeller I., Deresiewicz R. Highly repeated short DNA-sequences in the genome of Schistosoma-Mansoni recognized by a species-specific probe. Mol. Biochem. Parasitol. 1991;44:73–80. doi: 10.1016/0166-6851(91)90222-r. [DOI] [PubMed] [Google Scholar]
- Hamburger J., He N., Abbasi I., Ramzy R.M., Jourdane J., Ruppel A. Polymerase chain reaction assay based on a highly repeated sequence of Schistosoma haematobium: a potential tool for monitoring schistosome-infested water. Am J Trop Med Hyg. 2001;65:907–911. doi: 10.4269/ajtmh.2001.65.907. [DOI] [PubMed] [Google Scholar]
- He P., Song L.-G., Xie H., Liang J.-Y., Yuan D.-Y., Wu Z.-D., Lv Z.-Y. Nucleic acid detection in the diagnosis and prevention of schistosomiasis. Infectious diseases of poverty. 2016;5(25):1–11. doi: 10.1186/s40249-016-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herricks J.R., Hotez P.J., Wanga V., Coffeng L.E., Haagsma J.A., Basáñez M.-G., Buckle G., Budke C.M., Carabin H., Fèvre E.M., Fürst T., Halasa Y.A., King C.H., Murdoch M.E., Ramaiah K.D., Shepard D.S., Stolk W.A., Undurraga E.A., Stanaway J.D., Naghavi M., Murray C.J.L. The global burden of disease study 2013: what does it mean for the NTDs? PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler M.J., Cyrs A., Krenzke S.C., Mahmoud E.S., Sikasunge C., Mwansa J., Lodh N. Detection of duo-schistosome infection from filtered urine samples from school children in Zambia after MDA. PLoS One. 2017;12 doi: 10.1371/journal.pone.0189400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy A.M., Lundie R.J., Ivens A., Quintana J.F., Nausch N., Forster T., Jones F., Kabatereine N.B., Dunne D.W., Mutapi F., MacDonald A.S., Buck A.H. Parasite-derived MicroRNAs in host serum as novel biomarkers of helminth infection. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibironke O.A., Phillips A.E., Garba A., Lamine S.M., Shiff C. Diagnosis of Schistosoma haematobium by detection of specific DNA fragments from filtered urine samples. The American journal of tropical medicine and hygiene. 2011;84:998–1001. doi: 10.4269/ajtmh.2011.10-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibironke O., Koukounari A., Asaolu S., Moustaki I., Shiff C. Validation of a new test for Schistosoma haematobium based on detection of Dra1 DNA fragments in urine: evaluation through latent class analysis. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khier S., Lohan L. Kinetics of circulating cell-free DNA for biomedical applications: critical appraisal of the literature. Future science OA. 2018;4 doi: 10.4155/fsoa-2017-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp S., Ame S.M., Hattendorf J., Ali S.M., Khamis I.S., Bakar F., Khamis M.A., Person B., Kabole F., Rollinson D. Urogenital schistosomiasis elimination in Zanzibar: accuracy of urine filtration and haematuria reagent strips for diagnosing light intensity Schistosoma haematobium infections. Parasit. Vectors. 2018;11:552. doi: 10.1186/s13071-018-3136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukounari A., Webster J.P., Donnelly C.A., Bray B.C., Naples J., Bosompem K., Shiff C. Sensitivities and specificities of diagnostic tests and infection prevalence of Schistosoma haematobium estimated from data on adults in villages northwest of Accra, Ghana. The American journal of tropical medicine and hygiene. 2009;80:435–441. [PMC free article] [PubMed] [Google Scholar]
- Lodh N., Mwansa J.C., Mutengo M.M., Shiff C.J. Diagnosis of Schistosoma mansoni without the stool: comparison of three diagnostic tests to detect Schistosoma [corrected] mansoni infection from filtered urine in Zambia. The American journal of tropical medicine and hygiene. 2013;89:46–50. doi: 10.4269/ajtmh.13-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodh N., Naples J.M., Bosompem K.M., Quartey J., Shiff C.J. Detection of parasite-specific DNA in urine sediment obtained by filtration differentiates between single and mixed infections of Schistosoma mansoni and S. haematobium from endemic areas in Ghana. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodh N., Caro R., Sofer S., Scott A., Krolewiecki A., Shiff C. Diagnosis of Strongyloides stercoralis: detection of parasite-derived DNA in urine. Acta Trop. 2016;163:9–13. doi: 10.1016/j.actatropica.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodh N., Mikita K., Bosompem K.M., Anyan W.K., Quartey J.K., Otchere J., Shiff C.J. Point of care diagnosis of multiple schistosome parasites: species-specific DNA detection in urine by loop-mediated isothermal amplification (LAMP) Acta Trop. 2017;173:125–129. doi: 10.1016/j.actatropica.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Manzano-Roman R., Siles-Lucas M. MicroRNAs in parasitic diseases: potential for diagnosis and targeting. Mol. Biochem. Parasitol. 2012;186:81–86. doi: 10.1016/j.molbiopara.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Meningher T., Lerman G., Regev-Rudzki N., Gold D., Ben-Dov I.Z., Sidi Y., Avni D., Schwartz E. Schistosomal MicroRNAs isolated from extracellular vesicles in sera of infected patients: a new tool for diagnosis and follow-up of human schistosomiasis. J. Infect. Dis. 2017;215:378–386. doi: 10.1093/infdis/jiw539. [DOI] [PubMed] [Google Scholar]
- Mharakurwa S., Simoloka C., Thuma P., Shiff C., Sullivan D. PCR detection of Plasmodium falciparum in human urine and saliva samples. Malar. J. 2006;5:103. doi: 10.1186/1475-2875-5-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes L.A., Oliveira M.C., Katz N., Dias-Neto E., Rabello A. Comparison of a polymerase chain reaction and the Kato-Katz technique for diagnosing infection with Schistosoma mansoni. Am. J. Trop. Med. Hyg. 2003;68:652–656. [PubMed] [Google Scholar]
- Shiff C. Accurate diagnostics for schistosomiasis: a new role for PCR? Reports in Parasitology. 2015:23. [Google Scholar]
- Stresman G.H., Kamanga A., Moono P., Hamapumbu H., Mharakurwa S., Kobayashi T., Moss W.J., Shiff C. A method of active case detection to target reservoirs of asymptomatic malaria and gametocyte carriers in a rural area in Southern Province, Zambia. Malar. J. 2010;9 doi: 10.1186/1475-2875-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakoon K.G., McManus D.P. Cell-free DNA as a diagnostic tool for human parasitic infections. Trends Parasitol. 2016;32:378–391. doi: 10.1016/j.pt.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Weerakoon K.G., Gordon C.A., Williams G.M., Cai P., Gobert G.N., Olveda R.M., Ross A.G., Olveda D.U., McManus D.P. Droplet digital PCR diagnosis of human schistosomiasis: parasite cell-free DNA detection in diverse clinical samples. J. Infect. Dis. 2017;216:1611–1622. doi: 10.1093/infdis/jix521. [DOI] [PubMed] [Google Scholar]
- Weerakoon K.G., Gordon C.A., Williams G.M., Cai P., Gobert G.N., Olveda R.M., Ross A.G., Olveda D.U., McManus D.P. Co-parasitism of intestinal protozoa and Schistosoma japonicum in a rural community in the Philippines. Infectious diseases of poverty. 2018;7 doi: 10.1186/s40249-018-0504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Parasitology – Laboratory Manuals; World Health Organization Geneva: 1991. Basic Laboratory Methods in Medical Parasitology. [Google Scholar]