Introduction
Key Teaching Points.
-
•
A clinical presentation of flecainide toxicity can occur at normal serum levels in the setting of electrolyte disturbances, namely hyponatremia.
-
•
Caution should be used when initiating flecainide in patients on diuretics, and serum electrolytes should be monitored. Use of diuretics may predispose patients to electrolyte abnormalities that can result in flecainide toxicity.
-
•
The combination of hyponatremia with the use of class Ic sodium channel blocking antiarrhythmics, like flecainide, can result in clinically significant pacemaker failure.
Flecainide is a class Ic antiarrhythmic agent that depresses the rate of depolarization of action potentials by blocking sodium channels.1, 2 It is used commonly in the treatment of supraventricular arrhythmias, atrial fibrillation, and atrial flutter, and less commonly in ventricular tachycardias, in patients with normal cardiac structure.3, 4 Flecainide has a narrow therapeutic window. Severe effects in cases of toxicity and overdose include negative inotropy, bradyarrhythmias, atrioventricular nodal block, ventricular tachycardia or fibrillation, and asystole, with overdose mortality rate at approximately 22%.5
There is sparse literature describing precipitating events for flecainide toxicity.6, 7 We report a case with hyponatremia as a precipitating event for flecainide toxicity manifest by severe bradycardia with normal flecainide serum levels in a patient with a pacemaker.
Case report
A 78-year-old African-American woman with sinus node dysfunction requiring pacemaker implantation, chronic kidney disease stage IV, and recent ischemic stroke was admitted directly to the cardiac intensive care unit from a subacute rehabilitation facility owing to symptomatic bradycardia with pacemaker malfunction and prolonged QRS complexes concerning for flecainide toxicity.
She had been on flecainide at a low dose of 50 mg twice daily for the treatment of paroxysmal atrial fibrillation. A recent echocardiogram had shown normal left ventricular function (ejection fraction 60%) without structural heart disease. A myocardial perfusion stress test showed no evidence for coronary ischemia. On the fourth day of rehabilitation, serum creatinine had risen from baseline 1.3–1.5 mg/dL to 2.0 mg/dL and sodium dropped to 128 mmol/L from 133 mmol/L. Renal function and hyponatremia continued to worsen, with creatinine rising to a peak of 3.53 mg/dL and sodium to 121 mmol/L. She then developed symptomatic bradycardia with heart rate to 32 beats per minute with loss of pacemaker capture. Her blood pressure dropped to the 70s systolic with intact respiratory and mental status and the patient was transferred to our tertiary care academic medical center. Potassium (4.2 mmol/L), magnesium (2.3 mg/dL), and lactate (1.5 mmol/L) levels were normal.
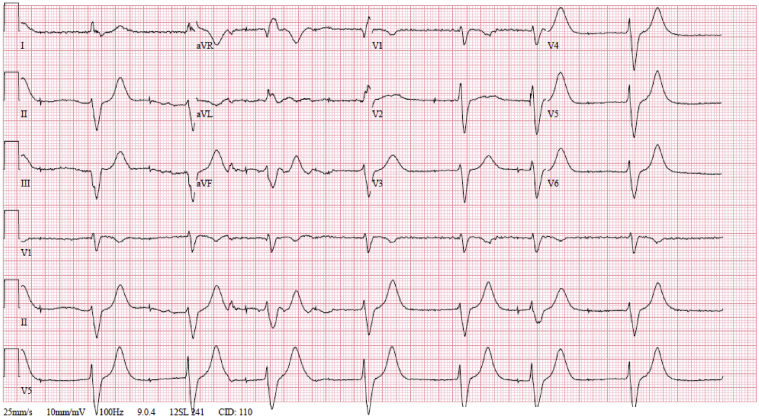
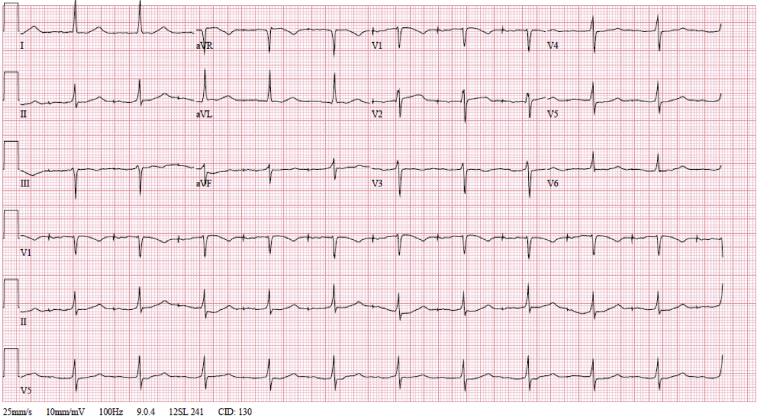
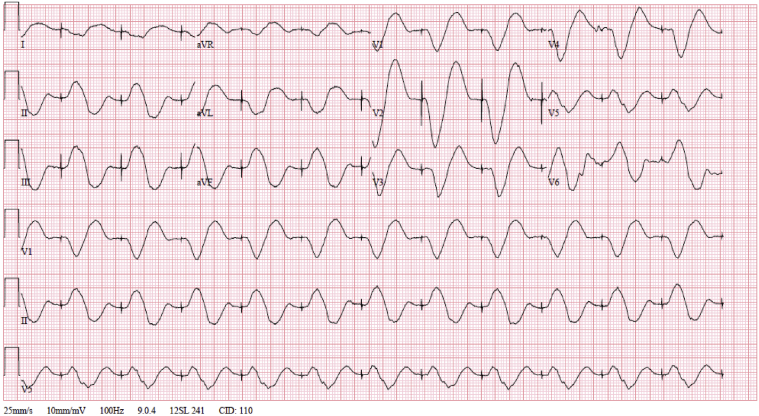
The initial electrocardiogram (ECG) showed severe sinus bradycardia and pacing spikes without capture, with a wide QRS of 190 ms and a heart rate of 47 beats per minute (Figure 1). An ECG just 1 month prior to presentation notes a narrow QRS complex, without V pacing at that time (Figure 2). Pacemaker interrogation demonstrated a programmed mode of DDDR with outputs set at 4 V @ 1 ms and 3.5 V @ 0.5 ms in the right atrial (RA) and right ventricular (RV) leads, respectively. Documented sensing and capture thresholds from 1 week prior to this encounter were 2.5 mV and 1.1 V @ 1 ms, respectively, in the RA lead and 5.8 mV and 0.6 V @ 0.5 ms in the RV lead. After the current admission, the atrial lead demonstrated no sensing and no ability to capture with stable impedance. The RV lead sensing was 11 mV with increased capture threshold to 3.4 mV @ 1 ms, with stable impedance. The pacemaker was reprogrammed to VVI with lower rate limit of 70 and RV output set to 6 mV @ 1 ms. An ECG obtained following these changes showed ventricular paced rhythm with a markedly prolonged QRS interval of approximately 320 ms (Figure 3). Interrogation of the patient’s device 1 week prior to presentation showed underlying inconsistent severe sinus bradycardia to the 30s or slower with some long pauses and first-degree atrioventricular nodal block with a PR interval of approximately 340 ms. A repeat echocardiogram showed no significant change or wall motion abnormality, with left ventricular ejection fraction remaining at 60%.
Figure 1.
Admission electrocardiogram.
Figure 2.
Electrocardiogram 1 month prior to admission.
Figure 3.
Electrocardiogram after pacemaker adjustments.
Further urine studies suggested a largely prerenal etiology of renal injury. The precipitating insult likely dated back to workup of the stroke with a computed tomography study with intravenous contrast in the setting of hydrochlorothiazide 25 mg daily, which had been given continuously up until the day of transfer. The patient also remained on enalapril 20 mg by mouth 2 times daily, up until the day of transfer to our institution.
She was treated with sodium bicarbonate intravenous injections at a weight-based dosing of 1 mEq/kg, which for our patient resulted in 89 mEq of sodium bicarbonate per injection. An ECG was completed and electrolytes were drawn 3 hours after each dose. A serum flecainide level was drawn before initiation of treatment.
After the administration of 2 doses of sodium bicarbonate 3 hours apart, the ECGs minimally changed, with the QRS complexes still at approximately 320 ms. Serum sodium rose from 121 mmol/L to 127 mmol/L, and serum creatinine improved with each dose. Further sodium bicarbonate injections and diuretics were held in addition to flecainide. The hyponatremia improved with intravenous hydration with 0.9% normal saline at 83 cc/hour. Serum sodium returned to baseline at 134 mmol/L and creatinine to 1.24 mg/dL. Pacemaker function showed improved thresholds daily, with progressive narrowing of the QRS to 110 ms. The previously drawn flecainide level was determined to be within normal therapeutic range (0.2–1.0 mcg/mL) at 0.66 mcg/mL. Approximately 3 days after admission, the patient’s ECGs normalized and the long latency period resolved. The underlying rhythm after recovery was not immediately recorded, but a couple of weeks later it was the same as her preadmission rhythm. Her postrecovery thresholds noted an atrial threshold of 0.9 V @ 1.5 ms with atrial sensing amplitude of 3.2 mV, and a ventricular threshold of 1.2 V @ 0.6 ms with ventricular sensing amplitude of 5.9 mV.
Discussion
Flecainide is a class Ic antiarrhythmic agent used in patients with symptomatic atrial arrhythmias with structurally normal hearts. To our knowledge, there are no prior documented cases of hyponatremia-induced flecainide toxicity with documented normal therapeutic serum flecainide levels.
Previous reports have associated electrolyte abnormalities with flecainide toxicity. Ahmed and colleagues6 described a case of flecainide toxicity as a cause of hyponatremia. When flecainide was discontinued, serum sodium normalized and symptoms resolved. Khavandi and Walker7 demonstrated a presentation of flecainide toxicity with hyponatremia and hypokalemia. The suspected reason for metabolic derangements was from thiazide diuretics, with flecainide toxicity as a result of hyponatremia rather than a definite cause of it.7 In both, no serum flecainide level was drawn. To our knowledge, there are no cases showing hyponatremia-induced flecainide toxicity with a documented normal flecainide level, nor any presenting with pacemaker malfunction. In our patient, renal injury along with use of a diuretic and angiotensin-converting enzyme inhibitor while on flecainide led to cardiotoxic effects due to hyponatremia despite normal (therapeutic) serum flecainide levels.
Flecainide can affect pacing thresholds,8 particularly at supratherapeutic levels. In our case, we also see documentation of pacemaker device malfunction in the setting of flecainide toxicity triggered acutely by hyponatremia rather than supratherapeutic flecainide levels. Pacing thresholds were previously unremarkable on flecainide in this patient. Now, severe QRS widening was seen, and the device had temporary abnormalities in capture and sensing, involving both atrial and ventricular leads—signs typical of flecainide toxicity rather than a pacemaker lead abnormality. Normal pacemaker function recovered with discontinuation of flecainide and resolution of hyponatremia, and the QRS duration began to appropriately decrease.
Flecainide can lead to metabolic abnormalities that lead to toxicity, specifically as it pertains to sodium. However, it appears that hyponatremia can be both a cause and an effect of flecainide toxicity. Being cognizant of medications with potential for sodium disturbances is crucial. Nephrotoxic medications should be monitored in particular—especially diuretics, given their impact on the electrolyte transmembrane channels present in the nephron.
Sodium channels in the myocardium are voltage-gated and generate current to overcome membrane capacitance and resistance, and are the target sites of flecainide. It is possible that the effects of flecainide were potentiated by the hyponatremia through their effects on the myocardium and on other sodium channels in the body. Antiarrhythmics have been shown in animal models to play a role on the sodium channel in locations other than the myocardium, namely the nephron.9, 10 The nephron is responsible for maintenance of total body water and sodium balance. With flecainide also blocking sodium channels on the distal nephron and cortical collecting duct, sodium would not be reabsorbed and thus further potentiate flecainide’s effects on the myocardium.6 The concurrent use of a thiazide diuretic may have further augmented this outcome. In the myocardium, the hyponatremia, possibly augmented through effects on the nephron—as in the case of our patient—may have led to further sodium channel capacitance and blockade, leading to exacerbated effects of flecainide even at therapeutic serum levels.
This case shows that flecainide toxicity can occur even at normal flecainide serum levels in the setting of hyponatremia. Previous cases did not document serum flecainide levels in similar cases of flecainide toxicity with hyponatremia, which may or may not have been attributable to elevated flecainide levels. Hyponatremia alone has not been documented as a cause of pacemaker failure.
Further study would be required to determine at what sodium and flecainide levels these effects take place, and when they precipitate cardiotoxic effects.
Additional studies are also needed to define optimal acute therapy for flecainide toxicity. Limited reports exist on the use of sodium bicarbonate and fat emulsion.11, 12, 13, 14 With use of sodium bicarbonate in this patient, the cardiotoxic effects did not reverse immediately, as the QRS duration on the ECG initially remained prolonged and the pacemaker malfunction persisted. Only with normalization of serum sodium and withholding of flecainide did cardiotoxic changes abate. Further evaluation may need to be performed to determine if the weight-based dosing of 1 mEq/kg is a sufficient dose with which to achieve a desired effect. One case report of neonatal flecainide toxicity documents success with the use of the 1 mEq/kg weight-based dosing, with successful resolution of ECG and electrolyte abnormalities with its use.15 However, this may not be entirely applicable to adult patients.
Flecainide toxicity from hyponatremia has the potential to cause life-threatening arrhythmias, including loss of pacemaker capture, even with therapeutic flecainide levels. Based on our experience from this case, we recommend regular sodium monitoring in patients on flecainide and particular caution with concomitant diuretic use.
References
- 1.Hopson J.R., Buxton A.E., Rinkenberger R.L., Nademanee K., Heilman J.M., Kienzle M.G. The Flecainide Supraventricular Tachycardia Study Group: Safety and utility of flecainide acetate in the routine care of patients with supraventricular tachyarrhythmias: Results of a multicenter trial. Am J Cardiol. 1996;77:72–82. doi: 10.1016/s0002-9149(97)89121-7. [DOI] [PubMed] [Google Scholar]
- 2.Chimienti M., Cullen M.T., Casadei G. Safety of flecainide versus propafenone for the long-term management of symptomatic paroxysmal supraventricular tachyarrhythmias. Report from the Flecainide and Propafenone Italian Study (FAPIS) Group. Eur Heart J. 1995;16:1943–1951. doi: 10.1093/oxfordjournals.eurheartj.a060852. [DOI] [PubMed] [Google Scholar]
- 3.January C.T., Wann L.S., Alpert J.S. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Am J Cardiol. 2014;64:2246–2280. [Google Scholar]
- 4.Blomstrom-Lundqvist C., Scheinman M.M., Aliot E.M. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology. Cardiology. 2003;108:1871–1909. doi: 10.1161/01.CIR.0000091380.04100.84. [DOI] [PubMed] [Google Scholar]
- 5.Devin R., Garrett P., Anstey C. Managing cardiovascular collapse in severe flecainide overdose without resources to extracorporeal therapy. Emerg Med Australas. 2003;19:155–159. doi: 10.1111/j.1742-6723.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed M., Sra J., Akhtar M., Mortada M.E. A case of flecainide-induced hyponatremia. J Cardiovasc Electrophysiol. 2009;20:1170–1172. doi: 10.1111/j.1540-8167.2009.01450.x. [DOI] [PubMed] [Google Scholar]
- 7.Khavandi A., Walker P.R. Flecainide cardiotoxicity precipitated by electrolyte imbalance. Caution with thiazide diuretics. Emerg Med J. 2007:24–26. doi: 10.1136/emj.2006.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apps A., Miller C.P., Fellows S., Jones M. Cardiac devices with class 1C antiarrhythmics: a potentially toxic combination. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-210598. bcr2015210598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranger S., Sheldon R., Fermini B., Nattel S. Modulation of flecainide’s cardiac sodium channel blockade actions by extracellular sodium: a possible cellular mechanism for the action of sodium salts in flecainide cardiotoxicity. J Pharmacol Exp Ther. 1993;264:1160–1167. [PubMed] [Google Scholar]
- 10.Plass H., Charisius M., Wyskovsky W., Amor F., Turnheim K., Wiener H. Class I antiarrhythmics inhibit Na+ absorption and Cl- secretion in rabbit descending colon epithelium. Naunyn Schmied Arch Pharmacol. 2005;371:492–499. doi: 10.1007/s00210-005-1072-4. [DOI] [PubMed] [Google Scholar]
- 11.Salerno D.M., Murakami M.M., Johnston R.B., Keyler D.E., Pentel P.R. Reversal of flecainide-induced ventricular arrhythmia by hypertonic sodium bicarbonate in dogs. Am J Emerg Med. 1995;13:285–293. doi: 10.1016/0735-6757(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 12.Goldman M.J., Mowry J.B., Kirk M.A. Sodium bicarbonate to correct widened QRS in a case of flecainide overdose. Emerg Med J. 1997;15:183–186. doi: 10.1016/s0736-4679(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 13.Keyler D.E., Pentel P.R. Hypertonic sodium bicarbonate partially reverses QRS prolongation in flecainide in rats. Life Sci. 1989;45:1575–1580. doi: 10.1016/0024-3205(89)90424-4. [DOI] [PubMed] [Google Scholar]
- 14.Ellsworth H., Stellpflug S.J., Cole J.B., Dolan J.A., Harris C.R. A life-threatening flecainide overdose treated with intravenous fat emulsion. Pacing Clin Electrophysiol. 2013;36:87–89. doi: 10.1111/j.1540-8159.2012.03485.x. [DOI] [PubMed] [Google Scholar]
- 15.Jang D.H., Hoffman R.S., Nelson L.S. A case of near-fatal flecainide overdose in a neonate successfully treated with sodium bicarbonate. J Emerg Med. 2013;44:781–783. doi: 10.1016/j.jemermed.2012.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]