Abstract
Endovascular therapy (EVT) has been accepted as a minimally invasive treatment for peripheral artery disease, and its applicability has been widened with the development of techniques and devices. A long, totally occluded lesion in the superficial femoral artery (SFA) is one of the most challenging lesions for EVT due to technical difficulties in wire-crossing. Recently, intentional subintimal recanalization is often considered as an alternative option for long SFA occlusions. Previous studies have shown that subintimal approach achieved superior technical success rate and similar patency rate, compared to conventional intraluminal approach. However, there is limited information about complications of the treatment with subintimal approach. Deep vein thrombosis (DVT) due to direct compression by pseudoaneurysm in the SFA, which subsequently develops pulmonary embolism (PE), is considered as a rare complication of subintimal angioplasty for the occlusive SFA lesion. We herein present a case of a patient who developed pseudoaneurysm formation in the SFA after EVT. Although initial EVT was performed successfully with subintimal approach, DVT and PE were caused by the SFA pseudoaneurysm at sub-acute phase following the initial procedure. The pseudoaneurysm was treated with implantation of a covered stent sealing the entry point, disappearing with no endoleak.
<Learning objective: We present a case of the patient who developed pseudoaneurysm formation in the superficial femoral artery (SFA) after endovascular therapy with subintimal approach. At sub-acute phase, deep vein thrombosis and pulmonary embolism were caused by the SFA pseudoaneurysm. The pseudoaneurysm was treated with implantation of a covered stent sealing the entry point. Attention should be paid to pseudoaneurysm following subintimal angioplasty, and a covered stent can be an option as bailout treatment.>
Keywords: Endovascular therapy, Peripheral artery disease, Superficial femoral artery
Introduction
Endovascular therapy (EVT) has been accepted widely as a minimally invasive treatment for peripheral artery disease, with the development of techniques and devices [1], [2]. The applicability of EVT has been widened and EVT has become a first-line therapy not only for stenotic lesions but also totally occluded lesions [3], [4]. For long chronic total occlusion (CTO) femoropopliteal (FP) lesions in which intraluminal crossing of the guidewire resulted in a failure, intentional subintimal recanalization is often considered as an alternative option. Previous studies have shown that subintimal approach achieved superior technical success rate and similar patency rate in long FP occlusions, compared to conventional intraluminal approach [5], [6]. However, complications of EVT with subintimal approach have been unclear. We herein present a case of a patient who developed pseudoaneurysm formation in the superficial femoral artery (SFA) following EVT with subintimal approach. Moreover, deep vein thrombosis (DVT) in the superficial femoral vein (SFV) due to direct compression by SFA pseudoaneurysm subsequently developed pulmonary embolism (PE).
Case report
A 67-year-old woman with intermittent claudication of left lower extremity was admitted to our hospital to receive EVT for the left SFA occlusion. After introduction of a 6-Fr guiding sheath into the left common femoral artery, angiography revealed that the left SFA was totally occluded from the SFA ostium (Fig. 1). A 0.014-inch hydrophilic guidewire with a microcatheter was used to cross the occluded lesion, however, the guidewire was advanced into the subintimal place from the proximal portion of the SFA and could not pass the CTO. A 0.035-inch looped guidewire with conventional subintimal recanalization technique was advanced from the proximal SFA to distal SFA, but failed in distal true lumen re-entry. Distal true lumen re-entry was also attempted applying stiff 0.014-inch guidewires, but failed. Then, a re-entry device (OUTBACK Elite, Cordis, Miami Lakes, FL, USA) was used to achieve distal true lumen re-entry. The re-entry needle was inserted into true lumen at the distal site of the occluded segment using fluoroscopic guidance with multiple projections. Subsequently, a 0.014-inch guidewire was inserted into the distal true lumen through the re-entry needle. Intravascular ultrasound (IVUS) was performed to evaluate the target lesion, showing that the guidewire passed through subintimal space from the proximal to distal SFA along the occluded segment and IVUS-derived lumen diameter at the proximal and distal reference site were 6.7 mm and 4.4 mm. Pre-dilation with a 4 mm balloon was performed, followed by deployment of two self-expanding nitinol stents (SMART, Cordis; 7 × 100 mm and 6 × 150 mm). After post-stent dilation with a 5-mm balloon, final angiography showed an excellent result without any contrast staining outside the stents. She did not complain of any leg pain during EVT, and after EVT as well. Aspirin 100 mg/day and clopidogrel 75 mg/day were started prior to initial EVT and were scheduled to continue at least for 1 month. Two days after EVT, she was discharged from the hospital, with disappearing claudication. Nevertheless, she was re-admitted to our emergency room due to sudden onset dyspnea with leg swelling in the left lower limb 16 days after EVT. Contrast-enhanced computed tomography (CT) showed thrombus in the right pulmonary artery (Fig. 2). Duplex ultrasonography was performed to evaluate DVT and detected thrombus remaining in the distal portion of the left SFV, which incidentally suggested pseudoaneurysm formation in the left SFA. Systemic anticoagulant therapy, including unfractionated heparin with activated partial thromboplastin time monitoring and subsequent edoxaban of 30 mg per day in addition to clopidogrel only, was started immediately under diagnosis of DVT and PE. Ten days after re-admission (26 days after EVT), arteriography and venography were performed to confirm the relationship between the EVT procedure for the left SFA and DVT in the left SFV. Arteriography identified pseudoaneurysm formation outside the stents in the distal part of the SFA, which was not confirmed during initial EVT procedure (Fig. 3). IVUS evaluation was performed subsequently, showing entry point of the pseudoaneurysm at the distal SFA corresponding to the segment in which the re-entry needle was used. Venography and IVUS showed that the SFV was compressed at the point corresponding to the pseudoaneurysm in the SFA, with thrombus formation around the compression. As the pseudoaneurysm in the SFA was considered as a cause of DVT and PE, a 5 × 50 mm covered stent (Viabahn, WL Gore, Flagstaff, AZ, USA) was implanted to seal the entry point. After implantation of the covered stent, arteriography showed that the pseudoaneurysm in the SFA disappeared with no endoleak. Contrast-enhanced CT was performed before discharge, which revealed that both thrombus in the pulmonary artery and in the left lower extremity also disappeared completely.
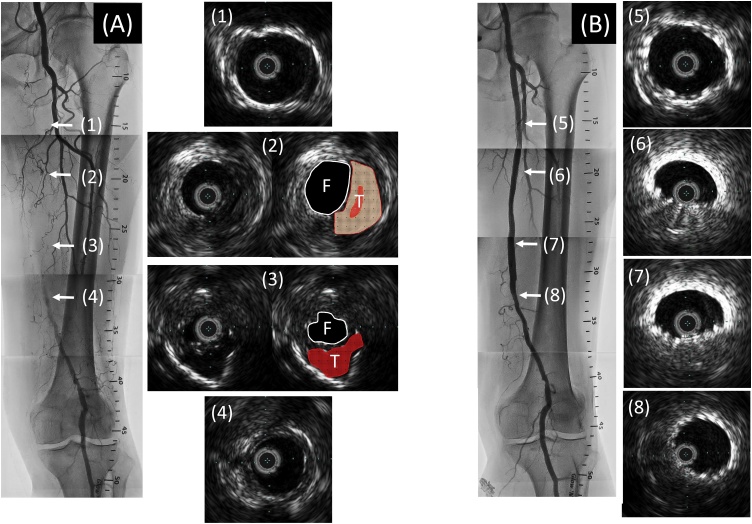
Fig. 1.
(A) Initial angiography showed a total occlusion in the left SFA. IVUS immediately after wire-crossing revealed that the guidewire was passed through subintimal space, false lumen, from the proximal to distal portion. (B) Final angiography showed a good result without any contrast staining outside the stents. IVUS revealed excellent expansion of the stents at the proximal and distal portion of SFA (5, 8), but poor expansion in the segment in which subintimal approach was adopted (6, 7).
SFA, superficial femoral artery; IVUS, intravascular ultrasound; F, false lumen; T, true lumen.
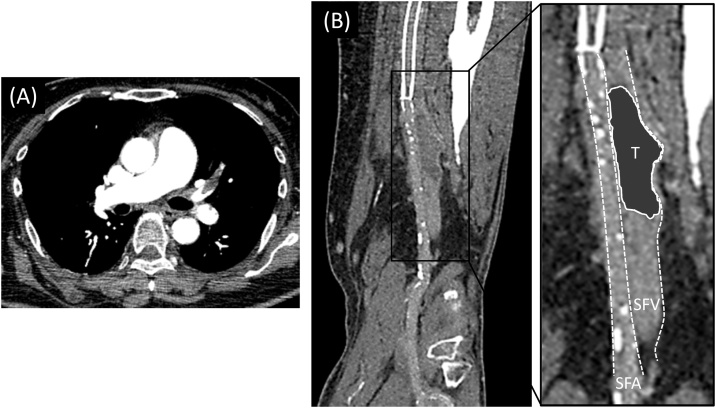
Fig. 2.
(A) Contrast-enhanced CT revealed thrombus formation in the pulmonary artery. (B) CT also showed thrombus in the left SFV locating around at the distal edge of the stents in the left SFA.
CT, computed tomography; SFA, superficial femoral artery; SFV, superficial femoral vein; T, thrombus.
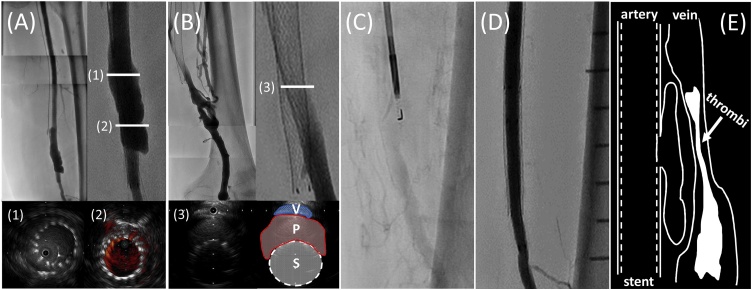
Fig. 3.
(A) Arteriography showed pseudoaneurysm in the distal SFA. IVUS revealed blood flow outside the stents was recognized through stent struts. (B) Venography showed compression of the left SFV. IVUS revealed that the SFV was compressed by pseudoaneurysm in the SFA, having large amounts of thrombus in the SFV. (C) Fluoroscopic image of re-entry needle at the initial procedure. The entry point of the pseudoaneurysm corresponded to the segment in which the re-entry needle was used. (D) Final arteriography revealed that the pseudoaneurysm disappeared after deployment of a covered stent. (E) Schema of this case presenting the relation between the pseudoaneurysm in the SFA and the thrombus in the SFV.
IVUS, intravascular ultrasound; P, pseudoaneurysm; S, stent; SFA, superficial femoral artery; SFV, superficial femoral vein; V, vein.
Discussion
Subintimal angioplasty has been introduced as an alternative option to intraluminal angioplasty, especially for long CTO in FP lesions. Because precise re-entry into the true lumen can be challenging for vascular interventionists, they currently use a recently developed re-entry device. Previous studies for the re-entry device have shown that subintimal approach achieved superior technical success rate and similar patency rate in long FP occlusions, compared to conventional intraluminal approach [5], [6]. Moreover, a previous report has shown that use of a re-entry device improves technical success rate reducing the procedural time and fluoroscopy exposure [7]. However, the information on complications in this treatment is not well established. EVT procedure with subintimal approach could be associated with SFA pseudoaneurysm formation [8]. Subintimal angioplasty creates and enlarges subintimal space between the intimal and medial layers of arterial wall, and the structure of the subintimal space would be considered as fragile. In the present case, re-entry into the true lumen was so hard that the re-entry needle was tried several times to achieve re-entry. IVUS revealed that the entry point of the pseudoaneurysm was located at the same segment in which the re-entry needle was used. It is hypothesized that blood flow was exuded through the puncture site of the re-entry needle at sub-acute phase and the exuding blood flow subsequently enlarged subintimal space, which turned into pseudoaneurysm formation. The clinical course of pseudoaneurysm in the SFA remains unclear. Although our group has previously reported a case of rupture of SFA pseudoaneurysm over 4 years after subintimal angioplasty [8], DVT and subsequent PE due to SFA pseudoaneurysm were observed only 16 days after the initial EVT procedure in this case. In the initial procedure, there were no vascular rupture or peri-strut contrast staining outside the stents on angiography after pre-dilation, stent deployment, and subsequent post-dilation. IVUS was also performed at the end of the initial procedure, and there were no pseudoaneurysms on IVUS as well. The exuding blood flow enlarged subintimal space, resulting in pseudoaneurysm formation only in a few days. Only pulmonary arterial phase was obtained in the contrast CT scanning at the re-admission, because we did not have any idea of pseudoaneurysm formation at that time. Arterial phase of contrast CT would be helpful for better understanding of the artery, stent, and pseudoaneurysm.
Reports on treatments for pseudoaneurysm in the SFA have been limited in the previous literature and surgical repair may be an option for pseudoaneurysm [8]. In this case, the pseudoaneurysm was treated successfully with a covered stent sealing the entry point under IVUS estimation. IVUS could visualize the entry point of the pseudoaneurysm and provide a great help in bailout procedure with a covered stent. Although subintimal angioplasty using a re-entry device is sometimes helpful in treatment for tough CTO, operators should pay attention for possible complications including pseudoaneurysm formation. The pseudoaneurysm disappeared completely with a covered stent in this case, however, there are few data on long-term results of covered stents for pseudoaneurysm and further investigations are required.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors thank the staff in the catheterization laboratory at Kawasaki Hospital for their excellent assistance in this case.
References
- 1.Hong M.S., Beck A.W., Nelson P.R. Emerging national trends in the management and outcomes of lower extremity peripheral arterial disease. Ann Vasc Surg. 2011;25:44–54. doi: 10.1016/j.avsg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Sachs T., Pomposelli F., Hamdan A., Wyers M., Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54:1021–1031. doi: 10.1016/j.jvs.2011.03.281. [DOI] [PubMed] [Google Scholar]
- 3.Dormandy J.A., Rutherford R.B. Management of peripheral arterial disease (PAD): TASC Working Group: TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 4.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Haris K.A., Fowkes F.G. TASC II working group: inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;43:S1–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Soga Y., Iida O., Suzuki K., Hirano K., Kawasaki D., Shintani Y. Initial and 3-year results after subintimal versus intraluminal approach for long femoropopliteal occlusion treated with a self-expandable nitinol stent. J Vasc Surg. 2013;58:1547–1555. doi: 10.1016/j.jvs.2013.05.107. [DOI] [PubMed] [Google Scholar]
- 6.Kim K., Ko Y.G., Ahn C.M., Min P.K., Lee J.H., Yoon C.H. Clinical outcomes of subintimal vs. Intraluminal revascularization approaches for long femoropopliteal occlusions in a Korean multicenter retrospective registry cohort. Circ J. 2018;82:1900–1907. doi: 10.1253/circj.CJ-17-1464. [DOI] [PubMed] [Google Scholar]
- 7.Gandini R., Fabiano S., Spano S., Volpi T., Morosetti D., Chiaravalloti A. Randomized control study of the outback LTD reentry catheter versus manual reentry for the treatment of chronic total occlusions in the superficial femoral artery. Catheter Cardiovasc Interv. 2013;82:485–492. doi: 10.1002/ccd.24742. [DOI] [PubMed] [Google Scholar]
- 8.Horimatsu T., Fujii K., Shibuya M., Fukunaga M., Imanaka T., Miki K. Rupture of pseudoaneurysm of the superficial femoral artery over four years after self-expandable nitinol stent implantation. J Cardiol Cases. 2015;12:52–56. doi: 10.1016/j.jccase.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]