SUMMARY
In this narrative review, we discuss the emerging role of innate immunity in OA joint pain. First, we give a brief description of the pain pathway in the context of OA. Then we consider how neuro-immune signaling pathways may promote OA pain. First, activation of neuronal Pattern Recognition Receptors by mediators released in a damaged joint can result in direct excitation of nociceptors, as well as in production of chemokines and cytokines. Secondly, indirect neuro-immune signaling may occur when innate immune cells produce algogenic factors, including chemokines and cytokines, that act on the pain pathway. Neuro-immune crosstalk occurs at different levels of the pathway, starting in the joint but also in the innervating dorsal root ganglia and in the dorsal horn. Synovitis is characterized by recruitment of immune cells, including macrophages, mast cells, and CD4+ lymphocytes, which may contribute to nociceptor sensitization and OA pain through production of algogenic factors that amplify the activation of sensory neurons. We discuss examples where this scenario has been suggested by findings in human OA and in animal models. Overall, increasing evidence suggests that innate immune pathways play an initiating as well as facilitating role in pain, but information on how these pathways operate in OA remains limited. Since these innate pathways are eminently targetable, future studies in this area may provide fruitful leads towards a better management of symptomatic OA.
Keywords: Osteoarthritis, Pain, Sensitization, Innate Immunity, Neuroinflammation, Toll-like receptors
INTRODUCTION
Osteoarthritis (OA) is one of the most rapidly increasing conditions that contribute to global years lived with disability [1], and joint pain is the primary reason why OA patients seek medical treatment. Yet, options for treating OA pain remain relatively limited, and they are often inadequate or associated with adverse effects [1]. Ultimately, many patients need a joint replacement. Presumably, effective new drugs that specifically target OA pain will only result from a clearer understanding of the molecular and cellular basis of this syndrome. This raises the general question “Why exactly does OA hurt?”. While it is clear that joints are innervated by pain transmitting nerves (nociceptors) [2], it is not precisely known which algogenic factors are generated in OA joints that act upon these nerves to produce pain.
A strong biomechanical component underpins OA pathogenesis. In addition, it is increasingly recognized that low-grade inflammation of the entire joint promotes disease progression, including cartilage and meniscus damage, subchondral bone remodeling, osteophytosis, and synovitis [3]. This low-grade inflammation is thought to result from the activity of the innate immune system, which seeks to restore tissue homeostasis in the face of infection or injury. The cells of the innate immune system represent a communicating network throughout the body that coordinates these responses. Cells that participate in innate immunity are localized within the parenchyma of all tissues and include tissue macrophages, mast cells and dendritic cells. In addition, circulating leukocytes can also be recruited as participants if necessary. Innate immune cells express receptors (“Pattern Recognition Receptors” or PRRs) that can respond to signals provided by pathogens (Pathogen Associated Molecular Patterns or PAMPs, such as microbial nucleic acids, lipoproteins, and carbohydrates) or Damage-Associated Molecular Patterns (DAMPs, a.k.a. “alarmins”), released from injured or dying cells. According to the traditional picture, activated PRRs initiate signaling cascades that produce inflammatory cytokines which attempt to bring the tissue back into homeostatic equilibrium. If help is needed in achieving this goal, cytokines and chemokines can also recruit circulating cells that migrate into tissues and amplify the innate immune response [4].
Pain associated with tissue injury is another important signal used to maintain and/or restore tissue homeostasis, by acting as a warning signal to avoid further injury and aid healing. Various cells throughout the nervous system, including sensory neurons, can respond directly to tissue damage through expression of PRRs and, as discussed below, one neuronal signaling pathway includes rapid PRR modulation of ion channel gating in pain transmitting neurons. In addition, prolonged tissue damage may result in interactions between the innate immune system and the nervous system at different levels of the pain neuraxis. This promotes persistent pain through the production of chemokines and cytokines, which can directly activate neurons as well as further recruit immune cells. This process of “neuroinflammation” has received considerable attention over the last decade, including in the context of OA – as we shall discuss in this review.
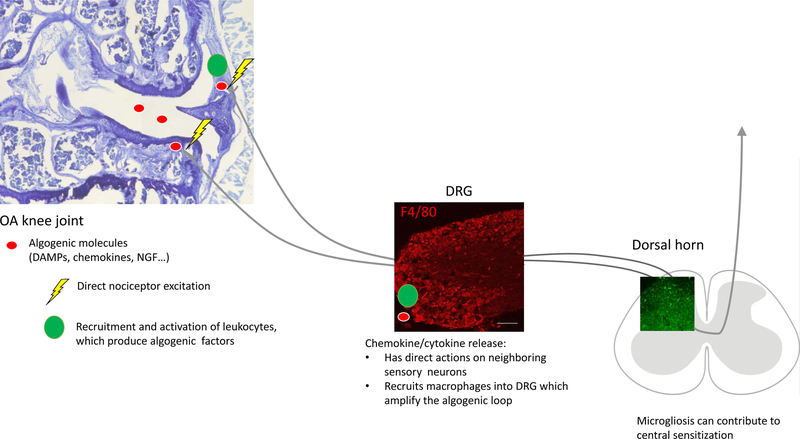
We searched PubMed for the following terms: “osteoarthritis”, “pain”, “sensitization”, “macrophages”, “microglia”, “neuroinflammation”, “synovitis”, “TLR”, DAMPs”, “animal models”, “chemokines”, “nociceptors”, “knock-out mice” in order to provide a narrative review on the emerging role of innate immunity in OA joint pain. First, we give a brief description of the pain pathway in the context of OA. Then we consider how neuro-immune pathways can contribute to OA pain through two mechanisms (Figure 1): first, direct activation of PRRs on sensory neurons can result in excitation as well as production of chemokines and cytokines. Secondly, indirect neuro-immune signaling may occur through production by immune cells of algogenic factors that act on the nociceptive pathway.
Figure 1.
Schematic outlining the direct and indirect actions of innate immune system activity on sensory neurons. In the knee joint, algogenic molecules can directly excite sensory afferents. In addition, leukocytes may be recruited and subsequently produce additional algogenic factors. In the DRG, sensitization of sensory neurons causes chemokine and cytokine release. In the dorsal horn of the spinal cord, activation of microglia supports development of central sensitization. OA=osteoarthritis; DAMP=Disease-associate molecular patterns; NGF=nerve growth factor; DRG=dorsal root ganglion.
JOINT PAIN IN OSTEOARTHRITIS
Anatomy of the pain pathway –
Pain is sensed when specialized peripheral sensory neurons (“nociceptors”) detect a potentially noxious stimulus (mechanical, chemical, or thermal), transduce it into an electrical signal and transmit it to the central nervous system (CNS). These nociceptors are generally thinly myelinated Aδ-fibers or unmyelinated C-fibers, and their cell bodies are located in the dorsal root ganglia (DRG). These neurons extend one axon to the peripheral tissue that they innervate and one axon to the dorsal horn of the spinal cord, where synaptic connections are made [5] (Figure 1).
Nociceptor activation in the joint –
Pain is initiated by factors that activate nociceptors innervating the skin, joints and viscera. Nociceptors and immune cells respond to tissue injury by promoting neuro-inflammatory signaling that facilitates pain as well as tissue repair. If damage does not resolve, such as in the course of OA and other chronic arthritides, the persistent inflammatory environment causes peripheral and central sensitization of the nervous system. The result of this sensitization is long-term changes in gene and protein expression in neurons, as well as infiltration of immune cells, further boosting the inflammatory state and promoting chronic pain [6]. An innate immune response in the knee clearly generates a great deal of crosstalk between different joint tissues, mediated by inflammatory cytokines [7]. However, in order to produce pain, either these factors themselves, or other factors generated downstream of their actions, must ultimately act to stimulate nociceptors. Nociceptors express a wide range of receptors for specific ligands, including chemokines, cytokines, nerve growth factor (NGF), neuropeptides, bradykinin, prostaglandins, ATP, etc. [8]. We know, for example, that prostaglandin receptors are expressed by joint nociceptors [9], and drugs that inhibit their synthesis (non-steroidal anti-inflammatory drugs - NSAIDs) can have beneficial effects on OA-associated pain [10]. However, current research suggests that many other molecules, including DAMPs, may also have direct actions on nociceptors and this may be an important way of producing joint pain.
Pain phenotypes in genetically modified mice –
Researchers are increasingly incorporating pain-related outcomes in experimental models of OA [11]. All rodent models of OA (surgical, chemically induced, spontaneous) develop signs of pain, including weightbearing deficits and locomotive or gait changes. Furthermore, increased sensitivity to mechanical stimuli has been reported through behavioral assessments including knee hyperalgesia and referred mechanical allodynia in the hindpaw, as well as by direct neuronal measurements of excitation such as electrophysiology and calcium imaging [11]. In order to evaluate how the innate immune response contributes to OA pain, we started by exploring pain phenotypes reported in OA models in genetically modified mice. We found reports in 13 mutant mouse strains related to the innate immune system (Table 1). From this table, it is quite clear that manipulating essential elements of the innate immune network can have a profound impact on pain associated with OA, with some pathways being essential for both joint damage and pain while others appear to modulate pain without affecting joint damage. How then does innate immunity participate in joint pathology and pain? We might hypothesize that damage to cells and cartilage extracellular matrix (ECM) generates DAMPs that activate the innate immune system producing some degree of chronic inflammation, which is detrimental to knee health. DAMPs might be fragments generated from proteins, proteoglycans, or remnants of cellular breakdown such as uric acid or other inflammation associated alarmins - e.g., heat shock proteins, S100 proteins or HMGB1 [12, 13]. Receptors for DAMPs include Toll-like receptors (TLRs) and NOD-like receptors (NLRs), which are expressed to different degrees by all joint tissues, including sensory neurons [14]. Ligand binding to these receptors results in activation of inflammatory signaling pathways including nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK) and the type I interferon pathways as well as the release of cytokines and chemokines [15, 16].
Table 1.
Transgenic mouse models related to innate immunity that have been tested for OA pain
| Innate Immune Component | Genetic model | Result | Reference |
|---|---|---|---|
| TLRs/DAMPs | Tlr2ko or Chloe mice | Blocking the production (Chloe mice) or action (Tlr2ko) of the 32-mer aggrecan fragment prevented development of knee hyperalgesia but not mechanical allodynia in the hind paw after DMM surgery. | Miller 2018 |
| Tlr4ko mice | Tlr4ko mice were not protected from mechanical allodynia of the hind paw after DMM surgery. | Miller 2015 | |
| Cd14ko mice | Cd14ko mice were protected from decreases in climbing activity observed after DMM surgery. | Sambamurthy 2018 | |
| S100a9ko mice | S100a9ko (which are also functional S100a8ko) mice did not develop weight-bearing deficits or gait changes in the acute synovitis model triggered by streptococcal cell wall intra-articular injection, but they were not protected from mechanical allodynia of the hind paw in this model. | Blom 2018 (abstract) | |
| Chemokines | Ccl2ko or Ccr2ko mice | Weight-bearing deficits in Ccl2ko and Ccr2ko mice were delayed in onset after DMM surgery. | Miotla Zarebska 2017 |
| Ccr2ko mice developed mechanical allodynia of the hind paw after DMM surgery, but this started to resolve from 8 weeks onwards. Ccr2ko mice did not develop decreases in spontaneous locomotion seen in wild-type mice beginning 8 weeks after DMM surgery. | Miller 2012 | ||
| Ccr7ko mice | Locomotor activity decreases in Ccr7ko mice were delayed in onset after DMM surgery. | Sambamurthy 2018 | |
| CCL17 pathway: (Irf4ko, Ccl17 gene deficient, and Ccr4ko mice) | Irf4ko, Ccl17E/E (mice in which both copies of Ccl17 have been replaced by enhanced green fluorescent protein (EGFP)), and Ccr4komice did not develop weight-bearing deficits in the collagenase-induced osteoarthritis mouse model. | Lee 2018 | |
| Cytokines | Tnfko mice | Tnf ko mice developed mechanical allodynia of the hindpaw similar to wild-type mice in the MIA mouse model. | Taniguchi 2015 |
| Tnf ko mice developed weight-bearing deficits similar to wild-type mice in the collagenase-induced osteoarthritis model. | Lee 2018 | ||
| Gm-csfko mice | Gm-csf ko mice were protected from weight-bearing deficits in the collagenase-induced | Cook 2012 |
DIRECT ACTIVATION OF SENSORY NEURONS
Toll-like receptors –
TLRs are one of the most extensive families of PRRs that recognize factors that initiate an innate immune response. TLRs are type I transmembrane receptors with an N-terminal domain containing a leucine-rich repeat motif involved in ligand recognition and accessory molecule interactions [17]. The transmembrane region leads to a C-terminal cytosolic Toll/interleukin-1 receptor (TIR) domain, which initiates downstream signaling processes through interactions with different adaptor proteins. One group of TLRs, including TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 (TLRs 1–9 and 11–13 are expressed in mice) is expressed primarily on the cell surface and recognizes products released from damaged cells (e.g., heat shock proteins, products of degraded ECM) or from the membranes of pathogens (e.g., lipopolysaccharide (LPS)), while members of the second group (TLR3, TLR7, TLR8, and TLR9) are normally expressed on intracellular compartments such as endosomes, endoplasmic reticulum, or lysosomes and recognize nucleic acids derived from infectious pathogens [18]. Under normal conditions, cell-surface TLRs exist as monomers that form homodimers or heterodimers when activated by a ligand [17]. Differences in receptor dimerization and recruitment of unique adaptor proteins allow for further distinction between TLRs in tuning downstream signaling outcomes which lead to the production of inflammatory cytokines and ultimately of other downstream inflammatory mediators [17]. Traditionally, TLR signaling is dependent on recruitment of the cytoplasmic adaptor myeloid differentiation 88 (MyD88). In the case of some TLRs, MyD88-dependent signaling at the cell surface is followed by internalization to the endosomal compartment and activation of other signaling motifs such as TRIF-dependent pathways [19].
It is well established that all joint tissues express TLRs and can participate in innate immune responses, but only recently have these observations been extended to the nerves that innervate tissues, raising the possibility that DAMPs and PAMPs may produce pain by directly regulating nociceptor excitability. For example, several reports have demonstrated that nociceptors can express TLR4 and the activation of TLR4 can initiate neuronal signaling that could result in pain [20–26]. In the context of OA, DRG neurons in culture responded to two proteins whose levels are elevated in OA joints, α2-macroglobulin and S100A8, by an increase in [Ca2+]i (a measure of neuronal excitability) and increased synthesis of the pro-algesic chemokine, CCL2, which can in turn further promote neuronal excitation and pain (see below) [23]. The vast majority of these responses occurred in neurons that express transient receptor potential cation channel subfamily V member 1 (TRPV1), the neuronal receptor for capsaicin, which is involved in many types of algogenic signaling. Responses were blocked by a TLR4 antagonist and were absent in Tlr4ko mice [23]. Moreover, in experimental OA induced by surgical destabilization of the medical meniscus (DMM), the increased neuronal CCL2 synthesis observed in DRG neurons cultured from mice with OA was substantially inhibited by a TLR4 antagonist. However, Tlr4 null mice still developed mechanical allodynia in a similar manner as wild-type mice in this model, indicating that removing TLR4 signaling alone is not sufficient to inhibit this measure of sensitization. Similarly, while there is ample evidence that TLR4 signaling promotes pro-inflammatory pathways in many joint cells, Tlr4 null mice are not protected from joint damage in murine OA models tested thus far, including after DMM [23] and partial meniscectomy [27], suggesting that perhaps it is not sufficient to target just one cell-surface expressed TLR [13].
Several studies have examined the expression pattern of TLR4 in rodent sensory neurons and found that, in general, it is colocalized with other markers of nociceptors including TRPV1, P2X3 and calcitonin G-related peptide (CGRP) [21, 24, 28, 29]. The colocalization of TLR4 and TRPV1 may be important when considering the mechanism through which TLR4 activation can produce rapid neuronal excitation, since traditional signaling mechanisms that result in NFkB activation are presumably too slow to explain this phenomenon. For example, it was demonstrated that TLR4 and TRPV1 are actually associated in a complex that can be isolated by co-immunoprecipitation [30]. This association occurs through the intracellular TIR domain of TLR4, and this domain is required for potentiation of TRPV1 signaling. Hence, it is likely that activation of TLR4 can directly co-activate TRPV1. Further studies have shown that TLR4 is colocalized with the low threshold voltage-dependent Ca2+ channel, CaV3.2, and the action of the TLR4 agonist, LPS, could be inhibited by blocking this channel, although the exact mechanism of this effect was not elucidated [31]. Clearly, excitatory effects such as these may well explain the ability of TLR4 agonists to rapidly increase nociceptor excitability, and thus chronic exposure of nociceptors to tissue damage products – as occurs in OA - may lead to persistent pain. While TLR4 alone appears to be non-essential for producing mechanical allodynia associated with experimental OA [23], Tlr4 null mice can display attenuated mechanical allodynia in other models of chronic pain, but this appears to be centrally mediated and will be discussed further below.
TLR4 has been the most intensively investigated TLR with regard to nociception, but it is not the only TLR expressed by nociceptors [32]. According to recent reports, TLR2 is also expressed in populations of neurons including DRG neurons [33, 34], sometimes in a highly selective manner. Pulmonary sensory afferents, for example, were observed to express only TLR2 and TLR5 [33]. There is now compelling evidence for a role of TLR2 expressed by DRG neurons in OA pain [34]. Cartilage degradation in OA is due to the enzymatic cleavage of the major proteoglycan, aggrecan, by ADAMTS-4 and ADAMTS-5, which cleave the protein in the interglobular domain (E373↓374A). One product of aggrecan cleavage, the 70-kDa N-terminal aggrecan fragment, is retained in the cartilage matrix and is subsequently cleaved by MMPs at N341↓342F, releasing a 32-amino acid fragment (“32-mer”, 342F–E373), which can be secreted into the synovial fluid of OA patients [35]. It has been shown that this 32-mer can participate in the OA innate immune response by activating TLR2 receptors on chondrocytes, synovial fibroblasts, and peritoneal macrophages [36]. This raised the interesting possibility that perhaps the 32-mer could link these other features of OA to pain by directly activating TLR2 receptors expressed by DRG neurons. It was observed that DRG neurons in sectioned tissue or in culture expressed TLR2 receptors [34]. TLR2 is normally expressed on the cell membrane but does not necessarily exist as a homodimer and has the capacity to dimerize with either TLR1 or TLR6, which expands the types of ligands it can interact with. Several of the DRG neurons that expressed TLR2 were also shown to express TLR1 and TLR6, possible dimerization partners for TLR2. Activation of these receptors by the 32-mer or the TLR1/TLR2 agonist, Pam3CSK4, increased CCL2 production by cultured DRG neurons and increased [Ca2+]i in a subset of neurons that predominantly corresponded to TRPV1-expressing nociceptors. These effects were absent in Tlr2 but not Tlr4 null mice. Moreover, the 32-mer produced hyperalgesia following its injection into the knee cavity [34]. In the DMM model, Tlr2 null mice developed structural signs of OA, but they were protected from the development of knee hyperalgesia that is a feature of OA in wild-type mice. Similarly, another mouse line that cannot generate the 32-mer (“Chloe mice”, a transgenic line in which the MMP cleavage site (N341↓342F) in the aggrecan interglobular domain is mutated so as to prevent production of the 32-mer [37]) was also protected from knee hyperalgesia after DMM, even though these mice showed accelerated joint damage [34]. These data therefore exemplify how a DAMP that is specific to OA cartilage damage can produce pain behavior through direct activation of DRG neurons. Moreover, they also link these effects to the production of CCL2, which is strongly pro-algesic, as discussed below.
There are also indications that other TLRs expressed by DRG neurons may be involved in aspects of pain physiology. TLR5, for example, is uniquely expressed in Aβ-fibers and activation of TLR5 with bacterial flagellin produces excitation of these nerves through the activation of sodium channels [38]. In addition, TLR7 receptors were reported to be expressed by small diameter DRG neurons that are involved in pain [39] and itch [40]. Interestingly, rather than being associated with TRPV1 as originally thought [41], TLR7 could be co-immunoprecipitated with transient receptor potential ankyrin 1 (TRPA1) in HEK293 cells, and activation of TLR7 produced TRPA1-dependent excitatory inward currents in DRG neurons [39]. Moreover, the authors of this investigation also showed that certain extracellular miRNAs can activate TLR7 and suggested that these could have a unique role in the control of pain behaviors by this mechanism, a possibility certainly worthy of exploration in the context of OA.
Finally, co-receptors such as lipopolysaccharide binding protein (LBP), MD2, CD44, and CD14 may also modulate TLR signaling. CD14 is expressed with TLR4 on myeloid lineage cells such as monocytes, macrophages, and microglia [42], but non-immune cells such as DRG neurons can also express this receptor [25, 26]. CD14 is broadly expressed within the knee joint – in mice, chondrocytes, meniscal cells, synovium and joint capsular tissues all stained positive for CD14 [43]. Soluble CD14 levels are elevated in the synovial fluid of OA patients, and these levels correlated with severity of knee pain and joint space narrowing [44]. Cd14 knock-out mice were protected from cartilage and subchondral bone damage after DMM surgery, as well as from the decline in spontaneous activity observed in wild-type mice, while overall macrophage infiltration into the joint was not impacted [43]. An interesting recent clinical study provides additional evidence of the potential importance of TLR signaling in OA [45]. Synovial fluid levels of LPS were positively associated with knee joint space narrowing (JSN), osteophyte severity, and total WOMAC score, while synovial fluid levels of LBP were positively associated with self-reported knee pain [45].
In summary, it appears that TLRs expressed by DRG neurons can act as transducers of neuronal activation and pain behavior produced by various DAMPs. In a couple of instances, they have been shown to be involved in OA pain, but in most cases this possibility has not be explored and this will be an important subject for future investigation in the study of OA pain.
Chemokines and their receptors –
Activation of PRRs expressed by DRG neurons or other joint tissues inevitably leads to the production of inflammatory cytokines and chemokines, which then produce the numerous effects associated with the innate immune response. A chief purpose of chemokine production is the recruitment of circulating immune cells (monocytes, NK cells, neutrophils, etc.) to the site of tissue damage [4]. Interestingly, it has now been demonstrated that receptors for many of these molecules, including interleukins, TNF-α and diverse chemokines are also expressed by DRG nociceptors under different pain-related conditions and that activation of these receptors produces rapid excitation of nociceptors [46]. This type of effect would provide a link between pain and other endpoints of the innate immune response and there is a good deal of evidence that this may indeed be the case.
Of all potential mediators that may be involved in the direct control of pain by cytokines, most evidence relates to the effects of chemokines. Receptors for chemokines are all G-protein coupled receptors (GPCRs), and it is well established that certain classes of GPCRs, such as those for kinins and prostaglandins, can excite DRG nociceptors through the transactivation of molecules such as TRP and sodium channels [46]. In 2001, Oh et al. published a report demonstrating that numerous chemokines could produce similar excitatory effects when added to cultured DRG neurons and these chemokines could also elicit pain behavior when injected into the mouse paw [47]. These authors therefore suggested that chemokines might represent an important link between inflammation and pain.
Although this seems like an appealing hypothesis it was important to see whether similar phenomena also occurred in vivo, and under what circumstances. Data were subsequently presented demonstrating that DRG neurons from normal rodents actually only expressed low levels of the chemokine receptor CCR2 [48]. However, in the context of a neuropathic pain model, levels of neuronally expressed CCR2 rose dramatically [48]. Not only that, but numerous CCR2 expressing macrophages were also detected in the DRG. The authors also demonstrated that many DRG neurons now expressed MCP-1/CCL2, the main chemokine ligand for CCR2. In addition, recording from intact DRG nociceptors from experimental, but not control mice, produced rapid depolarization and firing of barrages of spike trains indicating powerful CCL2-induced excitation of these neurons. The method by which CCR2 activation might be engaged in pain signaling was shown to involve a unique mechanism, in that CCL2 could be released from the cell bodies of DRG neurons and act upon neighboring neurons that expressed CCR2, thereby raising the state of overall state of excitation of the ganglion [48]. Together with the CCL2-induced influx of leukocytes into the DRG, it seems likely that the initial expression of CCL2 by DRG neurons, induced as the result of TLR activation (see above), would constitute a trigger for the activation of active pain signaling. There have now been numerous investigations supporting the potential role of CCR2 activation in chronic pain signaling, and similar data sets have been obtained for a number of other chemokines and their receptors (e.g. CXCR4) which can also participate in excitatory DRG signaling and the production of pain behaviors [49].
From the point of view of the present discussion it is of interest to examine data that particularly link chemokine signaling to OA-associated pain behaviors. As discussed, it has been shown that activation of PRRs expressed by DRG neurons can produce increased expression of CCL2. It was observed that there was a marked increase in CCR2 expression in DRG neurons, 8 weeks following DMM surgery, but this had resolved by week 16 [50]. Marked macrophage influx into the DRG was also observed. Interestingly Ccr2 ko mice (which develop similar joint damage after DMM surgery as wild-type mice [50, 51]) displayed initial mechanical allodynia (up to week 8) but this also resolved by 16 weeks rather than being maintained as in wild-type mice. Furthermore, Ccr2 ko mice were protected from locomotive deficits noted in wild-type mice by week 8, and this was accompanied by absence of macrophage infiltration in the DRG [50]. Finally, a CCR2 receptor antagonist (CCR2RA) reversed the locomotive deficits observed in wild-type mice 9 weeks following surgery [50]. Two other studies examined the effects of CCR2 on pain behaviors in this surgical model. In one study, chronic systemic administration of CCR2RA prevented weightbearing deficits in the operated limb [52]. Interestingly, early delivery of the drug over the first four weeks after surgery could inhibit cartilage damage as well as weightbearing deficits up to 12 weeks after surgery. This study also demonstrated expression in the knee joint of another chemokine ligand of CCR2, CCL12. Finally, in another study, both Ccl2 ko and Ccr2 ko mice developed weightbearing deficits 5 to 6 weeks later than wild-type mice, despite the same severity of joint damage [51].
Overall, these data suggest that chemokine signaling in the DRG represents a key component in the establishment of chronic OA pain. Direct effects of DAMPs on DRG neurons may initially induce expression of chemokines and their receptors, which then promote neuronal excitation and pain. These events bear further investigation as they represent a promising direction for therapeutic intervention. In OA patients, a number of chemokines have been reported to be elevated in the synovial fluid [53], and there are several reports that specific chemokines are associated with symptom severity in knee OA, including synovial fluid levels of CCL2 [54, 55].
Inflammatory cytokines –
Activation of PRRs expressed by DRG neurons might be expected to increase the production of a variety of nerve-derived inflammatory cytokines in addition to chemokines. Moreover, many such cytokines can also be produced as part of the innate immune response operating in other joint tissues (synovium, bone, cartilage). As is the case for DAMPs and chemokines, it appears that receptors for many of these molecules are expressed by nociceptors and that their direct activation can produce excitation and pain behavior [7]. IL-1β increases the excitability of sensory neurons through p38 MAP kinase by reducing resting slow inactivation of tetrodotoxin (TTX)-resistant voltage-gated sodium channels and by increasing persistent TTX-resistant current near threshold [56]. It is well established that TTX resistant sodium currents are important for nociceptor electrogenesis and results such as these indicate that novel small molecule inhibitors of these channels may be useful for the treatment of pain behaviors, including OA pain [57, 58]. TNFα is another inflammatory cytokine known to directly activate sensory neurons [59], but thus far, Tnf ko mice have not been protected against pain-related behaviors in two different models of OA (MIA and collagenase-induced) [60, 61].
Clinically, cytokines have been detected in the synovial fluid of OA patients to varying degrees and, in some cases, have been shown to correlate with pain [7, 62]. A recent study demonstrated that improvement in pain and function resulting from a diet and exercise intervention in overweight and obese people suffering from knee OA was partly mediated by a change in serum levels of inflammatory cytokines (IL-6, TNF-α, IL-1sR and CRP) [63]. Interestingly, this effect appeared to be independent from changes in body mass index.
INDIRECT NEURO-IMMUNE SIGNALING THROUGH PRODUCTION OF ALGOGENIC FACTORS
In addition to direct neuronal activation by immune mediators, indirect neuro-immune signaling occurs when tissue damage products signal to TLRs on innate immune cells, which then produce algogenic factors that act on the pain pathway and amplify the responses. Several recent reviews discuss these tissue damage products [12, 13, 64], so here we will focus on immune cells that can amplify and modulate pain mechanisms at different levels of the pain pathway, including the joint, the DRG, and the dorsal horn.
Immune cells in the joint
Magnetic resonance imaging (MRI) of OA knee joints has identified two specific pathological structural features that are associated with joint pain: bone marrow lesions and synovitis/effusions, and fluctuations in these MRI findings are associated with fluctuations in pain [65]. Bone marrow lesions (which occur just below the subchondral bone) reflect mechanical load on the bone, while synovitis is evidence of inflammation. In the Multicenter Osteoarthritis Study (MOST), an NIH funded cohort of people with or at risk of knee OA, the association was assessed between sensitization (assessed by quantitative sensory testing) and bone marrow lesions or synovitis. Bone marrow lesions were not associated with sensitization, but synovitis was. The authors therefore suggested that targeting synovitis early on may prevent sensitization and thus reduce pain severity [66], since pain sensitization is associated with pain severity [67], and increased pain sensitization in patients with knee OA is associated with an increased risk of developing persistent knee pain [68].
Synovitis is characterized by changes in the synovium, predominantly influx of inflammatory cells, including macrophages, mast cells, and CD4+ lymphocytes [13]. Recruitment of immune cells into the joint may contribute to nociceptor sensitization and OA pain through production of algogenic factors that chronically activate sensory neurons. Below, we discuss examples where this scenario has been suggested by findings in human OA and in animal models.
Macrophages, Sensitization, and Pain –
An interesting recent study provided the first direct in vivo evidence for macrophage involvement in human OA, through the use of a molecular imaging technique based on 99mTc-EC20 (Etarfolatide) [69]. This study in a small patient sample found that macrophages in the synovium and tibiofemoral joint capsule, but not subchondral bone, were strongly associated with joint pain, and this not only in knee OA but also in wrists, fingers, ankle and great toes. The same group recently reported that levels of soluble CD14 (sCD14) in OA synovial fluid reflected the abundance of activated macrophages in the knee joint capsule and synovium, and were associated with knee pain, further supporting the idea that synovial inflammation driven by macrophages may be a determinant of joint pain [44]. A recent study found that CD14+CD16+ macrophages were enriched in knee OA synovial fluid compared to the circulation, and this macrophage subset correlated with patient-reported outcome measures (stiffness, function, and quality of life) as well as with synovial fluid levels of CCL2 [70].
There have not been many animal studies addressing the role of macrophages in OA pain, but a recent study in rat MIA showed that NSAID-resistant pain (measured by grip strength) could be suppressed by depleting synovial macrophages with clodronate liposomes [71].
Several other innate immune pathways associated with synovitis in OA have been investigated in order to determine effects on both joint damage and pain. Activated macrophages can produce the alarmins S100A8/A9, and OA patients have elevated levels of these proteins in their synovial fluid [72]. S100A8 and S100A9 can promote catabolic signaling in chondrocytes through TLR4 [73], and S100A8 can act on sensory neurons through TLR4 [23]. S100a9ko mice (which are also functional S100a8ko) mice are protected from joint damage in collagenase-induced OA, but not the DMM model [72, 74]. In an acute synovitis model triggered by streptococcal cell wall intra-articular injection, S100a9ko mice did not develop weight-bearing deficits or gait changes, but they were not protected from mechanical allodynia of the hind paw [75]. In addition, a GM-CSF driven pathway has been identified in macrophages that leads to production of CCL17 through Jmjd3 and IRF4 signaling in collagenase-induced OA [60, 76]. Gmcsf ko mice were protected from weight-bearing deficits in this model, accompanied by attenuated synovitis and cartilage damage [77]. In a separate study, Irf4ko, Ccl17E/E, and Ccr4ko (receptor for CCL17) mice were also protected from weight-bearing deficits and joint damage in this model [60]. Finally, synovial expression of CCL19 and its receptor, CCR7, are elevated in human OA [78, 79]. After DMM surgery, Ccr7 ko mice had delayed onset of locomotor activity decreases compared to wild-type mice, accompanied by protection from subchondral bone changes [80]. Together, these studies suggest that modulating synovial activation is likely to impact symptoms, and in some cases OA joint damage, and may represent a target for future therapies.
Mast cells –
Mast cells have also received increased attention because they can produce NGF [81] and respond to it through expression of TrkA [82]. In the MIA model, mice with a TrkA gain-of-function mutation had more rapid onset of mechanical allodynia in the hind paw and immune cell infiltration compared to wild-type mice [83]. It was proposed that NGF induced mast cells to produce elevated levels of prostaglandin D2, which could signal directly to nociceptors to induce mechanical sensitivity [83]. Whether prostaglandin D2 plays a role in human OA is not yet known [84]. In the synovium of OA patients, mast cells are increased, and correlate with radiographic damage, but not with self-reported pain [85]. In addition to mast cells, synovial fibroblasts and macrophages have also been shown to produce NGF in OA synovium [86, 87], and symptomatic chondropathy was associated with higher synovial NGF levels compared to the asymptomatic chondropathy group [86]. Elevated NGF gene expression has also been demonstrated in cartilage and meniscal tissue after DMM surgery in mice [88].
Immune cells in the DRG
In nerve injury models, DRGs become infiltrated by macrophages, neutrophils, and T-cells [89, 90]. These cells are recruited by chemokines and cytokines produced by sensory neurons, and – once activated within the DRG- can further stimulate sensory neurons by producing algogenic factors, resulting in persistent peripheral sensitization [89]. After DMM, elevated expression of CCL2 and CCR2 by sensory neurons occurred 8 weeks after surgery, coinciding with increased macrophage infiltration into the DRG [50]. Ccr2 null mice were protected from macrophage infiltration into the DRG as well as the development of persistent pain behaviors [50, 51]. In another study, fewer macrophages were detected in the DRG of mice treated with an anti-ADAMTS-5 antibody, alongside reduced joint damage and mechanical allodynia, providing further evidence that DRG infiltration is a response to joint pathology [91]. Macrophages were also detected in the DRG in the inflammatory rat antigen-induced arthritis (AIA) model [92]. In the murine collagenase-induced OA model, satellite glial cell activation was described, and activated glia appeared to surround neurons that stained positive for the damage marker, ATF3 [93]. Finally, we recently performed a microarray study of DRGs at different time points after DMM surgery. Pathway analysis revealed that many pathways associated with cellular infiltration and activation were increased during the late stage of the model, correlating with the presence of persistent pain behaviors. Immunostaining confirmed increases in macrophage and T-cell populations in the DRG during this time [94].
Innate immune cells, TLRs and Chemokines in the Dorsal Horn
As outlined in the previous sections, peripheral nerves in the joint are stimulated through multiple mechanisms in the course of OA. Like in other chronic diseases, long-term peripheral stimulation can lead to central sensitization. Clinical evidence supporting the occurrence of central sensitization in OA has been obtained by noting that sites away from the affected joints become sensitive to mechanical stimuli over the course of the disease [95].
Preclinically, there is mounting evidence that innate immune mechanisms are participating in this central sensitization process [32, 96]. In particular, the role of non-neuronal cells in the CNS has been investigated in many pain models. Activation and proliferation of tissue-resident microglia and astrocytes occurs alongside the recruitment and infiltration of T-cells, monocytes, and neutrophils [97]. In particular, activation of microglia (“microgliosis”) in the dorsal horn of the spinal cord has been observed in the MIA model [98–100] and after DMM surgery [101]. In nerve injury models, microglial activation has been shown to lead to neuronal excitation through production of cytokines and chemokines. In particular, TLR signaling on microglia can upregulate cytokine release, while upregulation of the enzyme cathepsin S can lead to release of the membrane-bound chemokine, fractalkine [97]. Interestingly, microglia also express the receptor for fractalkine, CX3CR1 [102, 103]. In the DMM model, CX3CR1 reporter mice were used in order to demonstrate that expression was largely constrained to microglia in the dorsal horn, and upregulation of fractalkine by sensory neurons was observed 8 weeks after surgery when chronic pain behaviors first appear [101]. Overall, the exact relationship relating microgliosis and the development of chronic pain behaviors must still be investigated.
In the K/BxN serum transfer model of rheumatoid arthritis, wild-type mice develop persistent mechanical allodynia despite the fact that joint inflammation resolves over time. Tlr4 null mice showed reversal of mechanical allodynia after peripheral inflammation had resolved, and intrathecal administration of a TLR4 antagonist during the inflammatory phase of the model also inhibited persistent allodynia in wild-type mice [104]. In addition, in neuropathic models such as painful response to cancer chemotherapeutic drugs like paxlitel, Tlr4 null mice show diminished mechanical allodynia [22, 105]. These studies raise the idea that targeting the TLR4 signaling pathway centrally in addition to peripherally may be useful for treating chronic pain associated with inflammation, but the role of TLR4 in the spinal cord remains to be tested in the context of OA. An important factor to consider is the differential role of TLR4 in male and female mice. Intrathecal injection of LPS causes mechanical allodynia in male, but not female mice, while LPS applied to the hindpaw induces equivalent allodynia in both sexes [106]. A follow-up study determined that microglia are not required for mechanical pain hypersensitivity in female mice, but instead T-lymphocytes appear to mediate the response [107]. Further work must examine whether these findings translate to humans and to specific disease models.
CONCLUSIONS
In summary, there is increasing evidence that innate immune pathways play an initiating as well as facilitating role in pain associated with OA. While the precise mechanisms of these pathways are being unraveled in pain models, information on how these pathways operate in OA is currently still limited. Since these innate pathways are eminently targetable, it seems that future studies in this area may provide fruitful leads towards a better management of OA.
ACKNOWLEDGMENTS
Anne-Marie Malfait (R01AR064251, R01AR060364, R61AR073576) and Richard Miller (R01AR064251, R61AR073576) were supported by the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS). Rachel Miller was supported by NIAMS (K01AR070328). The funding sources had no role in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS
A.M. Malfait serves as an Associate Editor for Osteoarthritis and Cartilage.
REFERENCES
- 1.Osteoarthritis: A Serious Disease. White Paper Submitted to the U.S. Food and Drug Administration. https://www.oarsi.org/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1.pdf: Pre Competitive Consortium for Osteoarthritis Osteoarthritis Research Society International 2016. [Google Scholar]
- 2.Miller RE, Tran PB, Obeidat AM, Raghu P, Ishihara S, Miller RJ, et al. The Role of Peripheral Nociceptive Neurons in the Pathophysiology of Osteoarthritis Pain. Curr Osteoporos Rep 2015; 13: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlowsky EW, Kraus VB. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol 2015; 42: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 2015; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woller SA, Eddinger KA, Corr M, Yaksh TL. An overview of pathways encoding nociception. Clin Exp Rheumatol 2018; 36: 172. [PubMed] [Google Scholar]
- 7.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine 2014; 70: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaible HG, Schmidt RF. Excitation and sensitization of fine articular afferents from cat’s knee joint by prostaglandin E2. J Physiol 1988; 403: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64: 465–474. [DOI] [PubMed] [Google Scholar]
- 11.Miller RE, Malfait AM. Osteoarthritis pain: What are we learning from animal models? Best Pract Res Clin Rheumatol 2017; 31: 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 2013; 5: 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller RE, Scanzello CR, Malfait AM. An Emerging Role for Toll-like Receptors at the Neuroimmune Interface in Osteoarthritis. Seminars in Immunopathology 2019-In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato J, Agalave NM, Svensson CI. Pattern recognition receptors in chronic pain: Mechanisms and therapeutic implications. Eur J Pharmacol 2016; 788: 261–273. [DOI] [PubMed] [Google Scholar]
- 15.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 2013; 13: 679–692. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov R, Janeway C, Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev 2000; 173: 89–97. [DOI] [PubMed] [Google Scholar]
- 17.Botos I, Segal DM, Davies DR. The structural biology of Toll-like receptors. Structure 2011; 19: 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol 2014; 5: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 2015; 33: 257–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allette YM, Due MR, Wilson SM, Feldman P, Ripsch MS, Khanna R, et al. Identification of a functional interaction of HMGB1 with Receptor for Advanced Glycation End-products in a model of neuropathic pain. Brain Behav Immun 2014; 42: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, et al. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J Neuroinflammation 2012; 9: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain 2014; 15: 712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RE, Belmadani A, Ishihara S, Tran PB, Ren D, Miller RJ, et al. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheumatol 2015; 67: 2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res 2006; 85: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta C, Davies A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J Neurosci Res 2008; 86: 1077–1086. [DOI] [PubMed] [Google Scholar]
- 26.Tse KH, Chow KB, Leung WK, Wong YH, Wise H. Lipopolysaccharide differentially modulates expression of cytokines and cyclooxygenases in dorsal root ganglion cells via Toll-like receptor-4 dependent pathways. Neuroscience 2014; 267: 241–251. [DOI] [PubMed] [Google Scholar]
- 27.Nasi S, Ea HK, Chobaz V, van Lent P, Liote F, So A, et al. Dispensable role of myeloid differentiation primary response gene 88 (MyD88) and MyD88-dependent toll-like receptors (TLRs) in a murine model of osteoarthritis. Joint Bone Spine 2014; 81: 320–324. [DOI] [PubMed] [Google Scholar]
- 28.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 2011; 90: 759–764. [DOI] [PubMed] [Google Scholar]
- 29.Helley MP, Abate W, Jackson SK, Bennett JH, Thompson SW. The expression of Toll-like receptor 4, 7 and co-receptors in neurochemical sub-populations of rat trigeminal ganglion sensory neurons. Neuroscience 2015; 310: 686–698. [DOI] [PubMed] [Google Scholar]
- 30.Min H, Cho WH, Lee H, Choi B, Kim YJ, Lee HK, et al. Association of TRPV1 and TLR4 through the TIR domain potentiates TRPV1 activity by blocking activation-induced desensitization. Mol Pain 2018; 14: 1744806918812636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Tatsui CE, Rhines LD, North RY, Harrison DS, Cassidy RM, et al. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain 2017; 158: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacol Ther 2018; 184: 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung WJ, Lee SY, Choi SI, Kim BK, Lee EJ, In KH, et al. Toll-like receptor expression in pulmonary sensory neurons in the bleomycin-induced fibrosis model. PLoS One 2018; 13: e0193117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller RE, Ishihara S, Tran PB, Golub SB, Last K, Miller RJ, et al. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fosang AJ, Last K, Gardiner P, Jackson DC, Brown L. Development of a cleavage-site-specific monoclonal antibody for detecting metalloproteinase-derived aggrecan fragments: detection of fragments in human synovial fluids. Biochem J 1995; 310 (Pt 1): 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lees S, Golub SB, Last K, Zeng W, Jackson DC, Sutton P, et al. Bioactivity in an Aggrecan 32-mer Fragment Is Mediated via Toll-like Receptor 2. Arthritis Rheumatol 2015; 67: 1240–1249. [DOI] [PubMed] [Google Scholar]
- 37.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, et al. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest 2007; 117: 1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 2015; 21: 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, et al. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014; 82: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci 2010; 13: 1460–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, et al. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol 2011; 186: 6417–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Gioia M, Zanoni I. Toll-like receptor co-receptors as master regulators of the immune response. Mol Immunol 2015; 63: 143–152. [DOI] [PubMed] [Google Scholar]
- 43.Sambamurthy N, Zhou C, Nguyen V, Smalley R, Hankenson KD, Dodge GR, et al. Deficiency of the pattern-recognition receptor CD14 protects against joint pathology and functional decline in a murine model of osteoarthritis. PLoS One 2018; 13: e0206217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol 2015; 67: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage 2016; 24: 1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 2009: 417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 2001; 21: 5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A 2005; 102: 14092–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayaraj ND, Bhattacharyya BJ, Belmadani AA, Ren D, Rathwell CA, Hackelberg S, et al. Reducing CXCR4-mediated nociceptor hyperexcitability reverses painful diabetic neuropathy. J Clin Invest 2018; 128: 2205–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A 2012; 109: 20602–20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miotla Zarebska J, Chanalaris A, Driscoll C, Burleigh A, Miller RE, Malfait AM, et al. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthritis Cartilage 2017; 25: 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Longobardi L, Temple JD, Tagliafierro L, Willcockson H, Esposito A, D’Onofrio N, et al. Role of the C-C chemokine receptor-2 in a murine model of injury-induced osteoarthritis. Osteoarthritis Cartilage 2017; 25: 914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scanzello CR. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J Orthop Res 2017; 35: 735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L, Jiang BE. Serum and synovial fluid chemokine ligand 2/monocyte chemoattractant protein 1 concentrations correlates with symptomatic severity in patients with knee osteoarthritis. Ann Clin Biochem 2015; 52: 276–282. [DOI] [PubMed] [Google Scholar]
- 55.Cuellar VG, Cuellar JM, Kirsch T, Strauss EJ. Correlation of Synovial Fluid Biomarkers With Cartilage Pathology and Associated Outcomes in Knee Arthroscopy. Arthroscopy 2016; 32: 475–485. [DOI] [PubMed] [Google Scholar]
- 56.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci 2008; 28: 14062–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuelert N, McDougall JJ. Involvement of Nav 1.8 sodium ion channels in the transduction of mechanical pain in a rodent model of osteoarthritis. Arthritis Res Ther 2012; 14: R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rahman W, Dickenson AH. Osteoarthritis-dependent changes in antinociceptive action of Nav1.7 and Nav1.8 sodium channel blockers: An in vivo electrophysiological study in the rat. Neuroscience 2015; 295: 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eitner A, Hofmann GO, Schaible HG. Mechanisms of Osteoarthritic Pain. Studies in Humans and Experimental Models. Front Mol Neurosci 2017; 10: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee MC, Saleh R, Achuthan A, Fleetwood AJ, Forster I, Hamilton JA, et al. CCL17 blockade as a therapy for osteoarthritis pain and disease. Arthritis Res Ther 2018; 20: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taniguchi A, Ishikawa T, Miyagi M, Kamoda H, Sakuma Y, Oikawa Y, et al. Decreased calcitonin gene-related peptide expression in the dorsal root ganglia of TNF-deficient mice in a monoiodoacetate-induced knee osteoarthritis model. Int J Clin Exp Pathol 2015; 8: 12967–12971. [PMC free article] [PubMed] [Google Scholar]
- 62.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 2012; 51: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Runhaar J, Beavers DP, Miller GD, Nicklas B, Loeser R, Bierma-Zeinstra S, et al. Inflammatory cytokines mediate the effects of diet and exercise on pain and function in knee osteoarthritis independent of BMI. Osteoarthritis Cartilage 2019. [DOI] [PubMed] [Google Scholar]
- 64.Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis--finding targets for candidate DMOADs. Nat Rev Rheumatol 2015; 11: 159–170. [DOI] [PubMed] [Google Scholar]
- 65.O’Neill TW, Felson DT. Mechanisms of Osteoarthritis (OA) Pain. Curr Osteoporos Rep 2018; 16: 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, et al. Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol 2016; 68: 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis 2015; 74: 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlesso LC, Segal NA, Frey-Law L, Zhang Y, Na L, Nevitt M, et al. Pain Susceptibility Phenotypes in Those Free of Knee Pain With or at Risk of Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis & Rheumatology 2019; 71: 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage 2016; 24: 1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomez-Aristizabal A, Gandhi R, Mahomed NN, Marshall KW, Viswanathan S. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: a cohort study. Arthritis Res Ther 2019; 21: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakurai Y, Fujita M, Kawasaki S, Sanaki T, Yoshioka T, Higashino K, et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 2019; 160: 895–907. [DOI] [PubMed] [Google Scholar]
- 72.Schelbergen RF, de Munter W, van den Bosch MH, Lafeber FP, Sloetjes A, Vogl T, et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann Rheum Dis 2016; 75: 218–225. [DOI] [PubMed] [Google Scholar]
- 73.Schelbergen RF, Blom AB, van den Bosch MH, Sloetjes A, Abdollahi-Roodsaz S, Schreurs BW, et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum 2012; 64: 1477–1487. [DOI] [PubMed] [Google Scholar]
- 74.van Lent PL, Blom AB, Schelbergen RF, Sloetjes A, Lafeber FP, Lems WF, et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum 2012; 64: 1466–1476. [DOI] [PubMed] [Google Scholar]
- 75.Blom AB, van Lent PL, Abdollahi-Roodsaz S, van der Kraan PM, van den Berg WB. Toll Like Receptor-2 Prevents Cartilage Damage in Osteoarthritis Models that Display Synovial Activation Orthopaedic Research Society Annual Meeting. San Francisco: 2012. [Google Scholar]
- 76.Achuthan A, Cook AD, Lee MC, Saleh R, Khiew HW, Chang MW, et al. Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J Clin Invest 2016; 126: 3453–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cook AD, Pobjoy J, Steidl S, Durr M, Braine EL, Turner AL, et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther 2012; 14: R199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nair A, Gan J, Bush-Joseph C, Verma N, Tetreault MW, Saha K, et al. Synovial chemokine expression and relationship with knee symptoms in patients with meniscal tears. Osteoarthritis Cartilage 2015; 23: 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum 2011; 63: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sambamurthy N, Nguyen V, Smalley R, Xiao R, Hankenson K, Gan J, et al. Chemokine receptor-7 (CCR7) deficiency leads to delayed development of joint damage and functional deficits in a murine model of osteoarthritis. J Orthop Res 2018; 36: 864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A 1994; 91: 3739–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallbook F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol 1997; 27: 2295–2301. [DOI] [PubMed] [Google Scholar]
- 83.Sousa-Valente J, Calvo L, Vacca V, Simeoli R, Arevalo JC, Malcangio M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthritis Cartilage 2018; 26: 84–94. [DOI] [PubMed] [Google Scholar]
- 84.Ioan-Facsinay A Initiating pain in osteoarthritis (OA): is it the mast cell? Osteoarthritis Cartilage 2018; 26: 1–3. [DOI] [PubMed] [Google Scholar]
- 85.de Lange-Brokaar BJ, Kloppenburg M, Andersen SN, Dorjee AL, Yusuf E, Herb-van Toorn L, et al. Characterization of synovial mast cells in knee osteoarthritis: association with clinical parameters. Osteoarthritis Cartilage 2016; 24: 664–671. [DOI] [PubMed] [Google Scholar]
- 86.Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol 2014; 66: 30183027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takano S, Uchida K, Inoue G, Miyagi M, Aikawa J, Iwase D, et al. Nerve growth factor regulation and production by macrophages in osteoarthritic synovium. Clin Exp Immunol 2017; 190: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Driscoll C, Chanalaris A, Knights C, Ismail H, Sacitharan PK, Gentry C, et al. Nociceptive Sensitizers Are Regulated in Damaged Joint Tissues, Including Articular Cartilage, When Osteoarthritic Mice Display Pain Behavior. Arthritis Rheumatol 2016; 68: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 2013; 111: 26–37. [DOI] [PubMed] [Google Scholar]
- 90.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007; 10: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 91.Larkin J, Lohr TA, Elefante L, Shearin J, Matico R, Su JL, et al. Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification. Osteoarthritis Cartilage 2015; 23: 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Massier J, Eitner A, Segond von Banchet G, Schaible HG. Effects of differently activated rodent macrophages on sensory neurons: implications for arthritis pain. Arthritis Rheumatol 2015; 67: 2263–2272. [DOI] [PubMed] [Google Scholar]
- 93.Adaes S, Almeida L, Potes CS, Ferreira AR, Castro-Lopes JM, Ferreira-Gomes J, et al. Glial activation in the collagenase model of nociception associated with osteoarthritis. Mol Pain 2017; 13: 1744806916688219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller RE, Ishihara S, Syx D, Ren D, Miller RJ, Valdes AM, et al. Microarray Analyses Support a Role for Neuroimmune Pathways in the Development of Persistent Pain in Experimental Osteoarthritis. 2018. International Association for the Study of Pain World Congress Boston2018:PTH219. [Google Scholar]
- 95.Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015; 23: 1043–1056. [DOI] [PubMed] [Google Scholar]
- 96.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018; 129: 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018; 19: 138–152. [DOI] [PubMed] [Google Scholar]
- 98.Sagar DR, Burston JJ, Hathway GJ, Woodhams SG, Pearson RG, Bennett AJ, et al. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol Pain 2011; 7: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DL. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS One 2012; 7: e33730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ogbonna AC, Clark AK, Gentry C, Hobbs C, Malcangio M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur J Pain 2013; 17: 514–526. [DOI] [PubMed] [Google Scholar]
- 101.Tran PB, Miller RE, Ishihara S, Miller RJ, Malfait AM. Spinal microglial activation in a murine surgical model of knee osteoarthritis. Osteoarthritis Cartilage 2017; 25: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci 2009; 29: 6945–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci 2004; 20: 1150–1160. [DOI] [PubMed] [Google Scholar]
- 104.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, et al. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain 2011; 152: 2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation 2013; 10: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011; 31: 15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015; 18: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]