Abstract
Malaria is a deadly infectious disease caused by parasites of the Plasmodium spp. that takes an estimated 435,000 lives each year primarily among young African children. For most children, malaria is a febrile illness that resolves with time but in approximately 1% of cases, for reasons we do not understand, malaria becomes severe and life-threatening. Cerebral malaria (CM) is the most common form of severe malaria, accounting for the vast majority of childhood deaths from malaria despite highly-effective anti-parasite chemotherapy. Thus, CM is one of the most prevalent lethal brain diseases and one for which we have no effective therapy. CM is, in part, an immune-mediated disease and to fully understand CM it is essential to appreciate the complex relationship between the malarial parasite and the human immune system. Here we provide a primer on malaria for immunologists and, in this context, review progress identifying targets for therapeutic intervention.
Introduction
Malaria is a mosquito-borne infectious disease caused by parasites of the Plasmodium spp, the deadliest of which, P. falciparum, is endemic in sub-Saharan Africa. Malaria takes an enormous toll on some of the world’s most vulnerable populations with over two hundred million cases of malaria each year and over 400,000 deaths in Africa alone, mostly among young children (1). In most cases, malaria is an uncomplicated febrile illness that is self-limiting, even in the absence of anti-malarial drugs. However, in approximately 1–2% of cases, mostly among children under the age of five, in endemic areas, malaria becomes life-threatening (2). These lethal forms of malaria, termed severe malaria, are, in many respects, overlapping diseases defined by different pathologies, including severe anemia, respiratory distress caused by lactic acidosis, acute kidney injury, and cerebral malaria (CM) (2). CM is the most prevalent and deadly of these manifestations, with a case fatality rate of 15–25% despite treatment with highly effective anti-malarial chemotherapy (3). Tragically, 14–25% of children who recover from CM suffer from long-term neurological sequalae, including cognitive, vision, and hearing impairments (4). At present we have neither an effective vaccine to protect children from malaria nor a therapy to treat those children who progress from uncomplicated to severe disease.
We do not yet know the molecular basis of the susceptibility of young children to severe complications and consequently it is not possible to predict which children with malaria will progress to severe disease. Children with CM present acutely in coma often precipitated by seizure and with a one- to three-day history of fever, vomiting, and anorexia. Within 36–48 hours, most children will have either recovered from or succumbed to the disease (5, 6). Thus, realistically, the window of opportunity to treat CM is narrow and begins when a child is already suffering impaired consciousness. An effective therapy must not only halt the progression of the disease but must also promote reversal of brain pathologies. In contemplating potential targets of adjunctive therapies (therapies used in conjunction with anti-parasite chemotherapy) it may be helpful to understand the complex life cycle of P. falciparum and its interaction with the human immune system, a relationship that has evolved over 100,000 years, since the speciation of humans (7, 8). Consequently, in this review we first provide a primer for immunologists on parasite-human host interactions in malaria. In this context, we then describe our current understanding of the mechanisms underlying the brain pathology of CM in both humans and in a mouse model of malaria and underscore the validity and importance of the mouse model of CM in the search for adjunctive therapies for CM in African children. We conclude with a discussion of adjunctive therapies in the pipeline.
In 2009 we published a Brief Review in this Journal entitled “What Malaria Knows About the Immune System that Immunologists Still Do Not” (9) in which we made a call to arms of basic immunologists to advance our knowledge of malaria immunity and to aid in malaria vaccine development. We renew that call now, ten years later, to immunologists, particularly neuroimmunologists, to aid in the search for adjunctive therapies for one of the most prevalent lethal brain infections on earth.
Parasite and human host interactions in malaria: a primer for immunologists
The P. falciparum parasite has been around and causing malaria for as long as humans have walked the earth, nearly 100,000 years (7, 8). Thus, the human immune system and P. falciparum have co-evolved. Because of the enormous selective pressure imposed by the high mortality rate of P. falciparum in children and pregnant women, malaria has had an enormous impact on shaping the human genome (10). Conversely, the human immune system has exerted a strong selective pressure on P. falciparum. Understanding the mechanisms by which genetic variants modulate risk of severe malaria in children and how genetic variations in the parasite allow escape from the host’s immune system may define targets for adjunctive therapies in both the parasite and the human host.
Perhaps one of the most familiar examples of the evolutionary pressure of malaria on the human genome is the gene that encodes the hemoglobin S (HbS) variant (11). HbS arose in Africa and is maintained in a frequency of 10–20% despite the fact that in a homozygous state HbS causes sickle cell anemia and is lethal in childhood. This risk is balanced by the fact that HbS in the heterozygous state confers nearly 90% reduction in risk of severe malaria in a study of West African children (12). We do not yet know the mechanisms by which the heterozygous sickle cell trait (HbSA) confers protection. Recent studies suggest that innate features of HbSA red blood cells (RBCs) (13) as well as the effect of HbSA RBCs on the balance of induction of pro- and anti-inflammatory cytokines (14) may play roles in protection.
There is also evidence that P. falciparum may have had an impact on shaping the genes that regulate the immune response to malaria. One intriguing example is the observation that American women of African descent are at a 10-fold increased risk for the autoimmune disease, systemic lupus erythematosus (SLE), as compared to women of European descent. Although the genes in the African genome that account for this increased risk in SLE have yet to be identified, in mouse models it has been demonstrated that SLE-susceptible strains (including mice expressing a duplication of Toll-like receptor 7 (TLR7) and/or a deletion of the inhibitory receptor FcγRIIb) are protected from a lethal mouse malaria that causes CM-like symptoms. Such findings suggest that alterations in genes that confer protection from CM (or other forms of severe malaria) may have been selected for in malaria-endemic Africa. We speculate that such gene evolution driven by the selective pressure of malaria may result in dysregulated immune responses in the absence of malaria leading to autoimmune disease. Indeed, mutations in both FcγRIIb and TLR7 are risk factors for human SLE (15, 16).
In addition to shaping the human genome, P. falciparum itself has evolved mechanisms to evade or disable our immune system. One of the best studied evasion mechanisms is one that allows infected RBCs (iRBCs) to sequester in the tissue vasculature by binding to receptors on the endothelium and thus avoiding clearance in the spleen. Sequestration of iRBCs is mediated by a large family of highly polymorphic genes termed the var genes that encode P. falciparum erythrocyte membrane protein 1 (PfEMP1) expressed on the surface of iRBCs. The variation encoded in the var genes is astounding. There are over 60 var genes in a parasite genome and many of these have multiple polymorphic forms. PfEMP1 are expressed clonally and importantly are targets of naturally acquired antibody immunity. Remarkably, as a parasite clone expands in the blood stage, there is a low level of switching to express new var genes. As antibodies are produced that recognize and eliminate the initial infecting clone, switched clones expressing new var genes expand. This process may be iterated several times over the course of a single P. falciparum infection. It is only after years of chronic malaria exposure that antibodies are acquired to most vars. At present only a relatively small number of such parasite evasive mechanisms are known but given the large size of the P. falciparum genome, over 5,400 genes, it is likely more will be discovered.
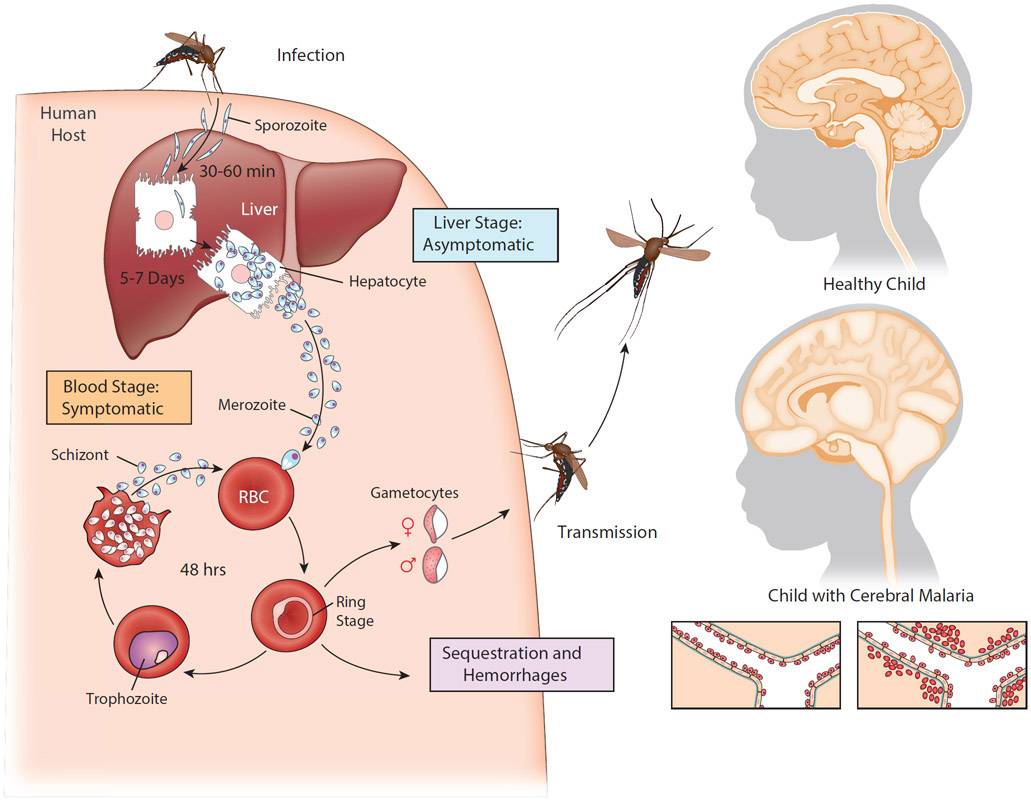
P. falciparum has a complex life cycle that may also reflect the coevolution of the parasite and the human immune system. The P. falciparum life cycle begins in humans with the bite of an P. falciparum infected female Anopheline mosquito that injects a small number of a highly motile form of the parasite, called a sporozoite, into the skin as she takes a blood meal (Fig. 1). The sporozoites do not sequester but rather travel from the bite site through the vasculature to the liver where a small number of hepatocytes are infected. There are no symptoms at this stage of the infection and we now understand that humans rarely develop immunity to the liver stage infection (17). The sporozoites expand enormously in the liver, over 40,000-fold, and differentiate into merozoites that enter the blood and begin rounds of replication in iRBCs that subsequently sequester in the vasculature (18). Triggered by signals that are poorly understood, merozoites differentiate into gametocytes that, when taken up by a blood feeding mosquito, fuse in the insect’s midgut to produce the precursors of sporozoites, the ookinete, thus completing the parasite life cycle. Thus, step by step over 100 millennia, P. falciparum and humans appeared to have co-evolved to ensure that P. falciparum sporozoites escape the host’s immune response and that the host suffers no ill effects for a sufficient period of time to allow merozoites to produce gametocytes and be transmitted by mosquitos. The liver stage of the infection appears to be at a pinnacle of host-parasite interactions. The parasite ensures its transmission by dint of the host suffering no ill effect.
Figure 1. Life cycle of Plasmodium falciparum and a depiction of cerebral malaria pathology.
An infected female Anopheles mosquito takes a blood meal and, in the process, injects a small number of Pf sporozoites in the child’s skin. The sporozoites then migrate rapidly (30–60 min) through the bloodstream to the liver where they invade hepatocytes and, over the course of 5–7 days, proliferate and differentiate into merozoites. This liver stage of the infection is asymptomatic. The infected liver cells rupture releasing large numbers of merozoites into the bloodstream where they promptly invade erythrocytes and, every 48 hours, replicate and release new merozoites that invade uninfected red blood cells (RBCs). This blood stage of infection is symptomatic resulting in periodic fevers and other malaria symptoms. A small number of merozoites are triggered to exit this cycle and develop into male and female gametocytes that are taken up by a blood–feeding female mosquito and where they undergo sexual reproduction in the mosquito mid-gut completing the parasites life cycle. During the blood stage, infected RBCs sequester in the tissue vasculature to avoid splenic clearance. Cerebral malaria results from accumulation of sequestered infected RBCS in the brain vasculature, accompanied by microhemorhages, endothelial activation, blood brain barrier dysfunction and ultimately brain swelling and death.
The blood stage of malaria appears to be a different story altogether. The blood stage of the infection is the cause of all the symptoms of malaria from uncomplicated febrile malaria to severe disease and death. Antibodies play a central role in protection from malaria; however, acquisition of protective antibodies has evolved to require years of near chronic parasite exposure in children growing up in malaria endemic Africa, leaving young children at risk of severe disease and death. The ineffective acquisition of immunity is attributed to both the extraordinary variation in parasite var genes as well as to fundamental alterations in the composition and functionality of the host’s memory B cell compartment. As there would not seem to be obvious advantages of febrile disease for the parasite or host and certainly no advantage to either if the host dies, we speculate that P. falciparum-induced disease and death are the result of an imperfect evolution of the parasite and the human host. We imagine that during the evolutionary dance of P. falciparum and humans the attempts of the parasite to escape or block the immune response or the host’s attempts to fully eliminate the parasite backfired leading in most cases to be a febrile but nonlethal disease but in the worst cases to severe malaria with high mortality. This speculation leads to the hypothesis that severe disease may not be a direct repercussion of P. falciparum infection per se but of misguided host immune response provoked by the parasite infection. Thus, we believe that at least some targets of adjunctive therapies for CM may be hidden in the immune response to P. falciparum.
What do we know about CM in African children?
The development of an effective adjunctive therapy for CM in children would clearly benefit from a detailed understanding of the cellular and molecular basis of the disease pathology. Unfortunately, at present our understanding of the mechanisms underlying CM pathology is incomplete. This is due to the difficulty in studying a rapidly progressing disease in children who initially present already suffering from disease pathology. In addition, access to peri-mortem diagnostics and post-mortem samples of brain tissue is limited preventing a detailed correlation of pathological features of CM and clinical symptoms. CM has also been a challenge to study due, in part, to difficulties in accurately diagnosing CM in African children. CM is defined by the World Health Organization as a syndrome that consists of an unrousable coma, not attributable to other neuropathologies and P. falciparum peripheral blood parasitemia (2). However, many children in malaria endemic areas have peripheral parasites regardless of the cause of coma and the cause of coma itself is not always obvious. Several studies provided evidence that the CM could be better defined clinically using fundoscopy to identify CM-specific changes in the retina that mirror changes in the brain vasculature that correlate with disease (19-22). However, recent studies evaluating a variety of clinical parameters suggest that fundoscopy may not provide a binary parameter of CM but rather may reflect a spectrum of CM disease presentation with severe retinal changes indicating late stages of CM (23-25).
A breakthrough in understanding the progression of CM in children came with the use of magnet resonance imaging (MRI) to follow changes in the brains of children during the course of CM. The images showed that brain swelling was associated with increased risk of death in CM and that the most likely cause of death was brain stem herniation (Fig. 1). Brain swelling was subsequently shown by MRI to be vasogenic in nature indicating dysfunction of the blood brain barrier (BBB) (26, 27). Concerning the mechanisms underlying pathology, a hallmark of CM is the heavy sequestration of iRBCs in the brain vasculature of children who died (Fig. 1) (28). The observation that sequestration was often accompanied by intravascular and perivascular pathologies including hemorrhages, lead to the generally accepted hypothesis that mechanical obstruction of the brain vasculature causes CM (29). In addition to obstruction, the binding of iRBCs to the vasculature endothelium could trigger a variety of events leading to brain pathology including impaired vasoregulation, BBB dysfunction and inflammation. Indeed, CM in children is consistently linked to excessive inflammatory responses including the proinflammatory cytokines, TNF-α, INF-γ, IL-1β, and IL-6 and reduced expression of the anti-inflammatory cytokine IL-10 (30). There have been at least 17 trials of 11 therapies targeting mechanisms associated with iRBC sequestration in the brain vasculature and unfortunately none have shown clear efficacy or have been adopted for wider use (31). For example, nitric oxide levels that are essential for endothelia homeostasis decrease in febrile malaria but nitric oxide supplementation in children with CM in clinical trials showed little change in outcome (32).
Recently, there has been renewed interest in adjunctive therapies for CM focusing on new findings concerning the nature of iRBC sequestration in the brain vasculature in CM. The new evidence suggests that expression of particular vars (ones containing domain cassette DC13 and DC8) are associated with severe malaria (33). Of particular interest, DC13- and DC8-containing PfEMP1 were shown to bind to the endothelia cell surface receptor EPCR, a host receptor involved in endothelial cytoprotective pathways (34, 35). Binding of DC-8 and −13 PfEMP1 to endothelial EPCR interfered with EPCR pro-endothelial barrier functions thus suggesting a link between parasite adherence to endothelium and vascular permeability that contributes to the pathogenesis of CM. It is hypothesized that young children may not yet have acquired antibodies specific for these particular PfEMP1 leaving them susceptible to CM (36). This discovery is exciting in offering the possibility that CM could be treated by antagonists that block particular PfEMP1 binding to the brain endothelium as long as the antagonists work rapidly, and the binding and the pathologies caused by PfEMP1 binding are reversible.
In searching for clues for potential targets for CM adjunctive therapy it is worthwhile to also consider what is currently understood about the susceptibility of children to CM. In malaria endemic regions young children are susceptible to CM. After five years of age children typically show resistance to CM and other forms of severe malaria but continue to be susceptible to uncomplicated febrile malaria until adolescence. It is possible that susceptibility to CM is related to unique features of the immaturity of the brains or the immune systems of children. However, nonimmune adults, including travelers, are susceptible to CM, although features of CM differ between children and adults (37). There is also evidence that resistance to CM is determined by a child’s genetic background. For example, HbS confers resistance to CM as discussed above. Casanova and colleagues modeled malaria mortality with age for simple Mendelian inheritance of genetic susceptibility and concluded that a few dozen gene variants could account for the human genetic contribution to severe malaria (38). Although numerous genes that encode important components of immune responses have been associated with resistance or susceptibility to CM, few of these associations have been rigorously tested with consistent results (38). Verification of the link of such genes to protection and an understanding of their function could provide targets for adjunctive therapies for CM in the future.
Another important but poorly studied aspect of CM is the impact of coinfections on the outcome of disease. Indeed, coinfections with Plasmodium including HIV, Mycobacterium tuberculosis, hepatitis virus, and helminths are estimated to affect a third of populations in developing countries. With HIV coinfection the age range of children who are susceptible to CM expands to include significantly older children. One study showed 71% of CM children with HIV coinfection were ≥ 5 years old as compared to only 13% of HIV negative CM children (39). Additionally, the HIV prevalence was elevated in the CM children as compared to the population at large (14.5% and 2% respectively) (39). Moreover, mosquito-borne parasites and viruses are often co-endemic in many malaria-endemic regions. It is likely that potential therapies will need to be tested for their efficacy both in children suffering CM alone and CM with additional coinfections.
An essential tool in the search for therapies: The mouse model of CM
A mouse model for CM, often referred to as experimental CM (ECM), recapitulates many of the features of CM in children (40). Infection of susceptible mice with the mouse parasite P. berghei ANKA (PbA) results in a rapid progression of disease similar to that in children both clinically: including, ataxia, paralysis, and coma, and pathologically: involving BBB dysfunction, brain hemorrhaging, brain swelling and, if left untreated, death within six to seven days, likely by neuronal cell death in the brain stem (41). In addition, mice treated with anti-malarials when neurological symptoms first appear, as is the standard of care for children, show long-term cognitive dysfunction (42, 43). A recent comparison of high resolution cross-sectional and longitudinal MRI images of the progression of ECM in mice with images of children with CM showed similar pathologies, namely vasogenic edema and BBB disruption (44). The accumulation of iRBCs in the brain vasculature, a hallmark of CM in children, is a feature of ECM that is not observed in uncomplicated malaria in mice (40). Moreover, iRBCs appear to occlude brain capillaries suggesting that iRBCs may affect cerebral blood flow in ECM (40). The sequestration of iRBCs is mediated primarily by PfEMP1 in human P. falciparum infections. In mouse PbA parasites express members of polymorphic gene families that are transported to the iRBC membrane, however, these are not orthologs of PfEMP1 and it is not known if these are the functional equivalent of PfEMP1 (45). Recent studies provided evidence that the modification of the iRBC surface by PbA is necessary for CM, showing that the cellular machinery required for the transport of PfEMP1 to iRBC surfaces is conserved in mouse parasites and that PbA parasites deficient in components of this machinery do not cause ECM (46).
An example of the use of the animal model of CM to investigate the novel roles of immune mediators in the development of CM involves complement pathways. The role of complement and polymorphisms in the complement receptor 1 in the susceptibility to CM is well reviewed elsewhere (47, 48). Studies in C5-deficient and C5aR-deficient mouse strains indicated roles for C5a and C5aR in ECM which was supported by the finding that C5a is also elevated in children with CM over those with uncomplicated malaria (49, 50). Additional studies pointed to a role of C5b and the complement membrane attack complex in ECM (51). Although there are complement therapies in the pipeline for other indications (52), to our knowledge these have not yet been tested as therapies in ECM. However, complement based therapies may prove to be a promising avenue of further research.
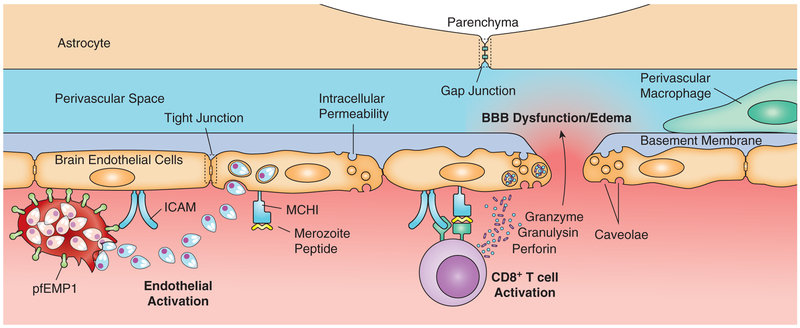
The search for CM therapies that have largely focused on mechanisms implicated in CM pathology, including iRBC sequestration in the brain vasculature or the repercussions of that sequestration, has thus far been unsuccessful. However, findings in the mouse model of CM suggest different modes of pathogenesis involving CD8+ T cells that, by-in-large, have not been explored in human CM. Indeed, ECM is dependent on both parasite antigens in the brain vasculature and the targeting of this vasculature by CD8+ T cells (45). In intravital imaging studies of the brain in ECM, MHC-class I-restricted CD8+ T cells were observed interrogating the brain vasculature and points of contact of CD8+ T cells with the brain endothelium were often associated with transient local leakage of the vessel into the brain parenchyma (41, 53). As in CM in children, vascular leakage in ECM does not appear to be extensive or caused by apoptosis of endothelial cells but is rather associated with vascular junction remodeling and increased levels of caveolae-dependent transcellular transport (40). Other immune cells that were observed accumulating in brains in ECM including macrophages, monocytes, neutrophils, CD4+ T cells, and natural killer cells but did not appear to play an essential role in ECM (45). A current model for ECM (Fig. 2) (45) suggests that iRBCs binding to the brain endothelium both compromises tight junction formation and promotes cross presentation of parasite antigen on MHC-class I molecules on the endothelium. Parasite-specific CD8+ T cells primed in the spleen are recruited to the brain and through perforin-dependent mechanisms damage the endothelium causing BBB dysfunction, brain swelling, and herniation of the brain stem leading to death.
Figure 2. Proposed mechanism of ECM BBB dysfunction.
Parasitized RBCs express PfEMP1 on their cell surfaces that mediate endothelia cell binding and sequestration of the infected RBCs in the brain vasculature. RBC rupture releases merozoites which are endocytosed by endothelia cells that then cross-present parasite antigens on MHC class I molecules to antigen–specific CD8+ T cells. Activated CD8+ T cells release perforin, granzyme, and granulysin which induce apoptotic signaling in endothelial cells, increases in caveolae fluid transport across the endothelium, and alteration of the endothelial cells’ tight junctions. The resultant enhanced permeability of the cerebrovasculature leads to enlargement of the perivascular spaces and edema formation—creating increased intercranial pressure which can ultimately be fatal.
Thus, although ECM has many of the features described in CM in children it also has a dependency on CD8+ T cells. This raises the question, would therapies identified in mice, particularly those targeting CD8+ T cells, be efficacious in children? At present it is difficult to predict as few studies have addressed the recruitment of immune cells to the brains of children who died of CM and those that did focused almost exclusively on neutrophils, platelets, and monocytes that do not appear to be essential for ECM (45). We have recently made an effort to fill this gap in our knowledge of CM by determining if T cells accumulated in the brains of children who died of CM using multiplex immunohistochemistry. In collaboration with Terrie Taylor and her colleagues working in Malawi in the Blantyre Malaria Project along with our NIH colleagues Dorian McGavern and Dragan Maric, we analyzed brain samples from children who died of CM and identified CD8+CD3+ T cells in close contact to the vasculature both within the lumen of venous blood vessels and in the perivascular space on the abluminal surface of the vessels (Riggle et al. unpublished observation). These data suggest that CD3+CD8+ T cells are potentially therapeutic targets for CM adjunctive therapy and that the mouse model may be a highly useful tool for the search for such therapies.
CM adjunctive therapies in the pipeline
Parents generally do not seek medical care for children with malaria until children show neurological signs of CM and thus are already suffering from brain pathologies. Consequently, an effective adjunctive therapy would both halt the progression of CM and promote healing of brain dysfunction. In ECM, neurological signs appear and intensify throughout day 5 post infection (p.i.) with PbA and ECM progresses rapidly such that by the evening of day 5 p.i. mice show BBB dysfunction, brain swelling, and hemorrhaging on necropsy. Mice begin to die on day 6 p.i. and by day 7 p.i. nearly all mice succumb to the infection. A variety of therapies that target a number of different mechanisms have been tested in the mouse model. Many of these were highly effective in blocking the development of ECM but only if given prior to the appearance of clinical signs, generally before day 4 p.i. For example, we (54) and Mejia et al. (55) targeted T cells in ECM by treatment with rapamycin, an inhibitor of mTOR (the mammalian target of rapamycin) that plays a central role in regulating T cell metabolism. Rapamycin blocked the development of ECM when given on day 4 p.i. but had little effect when given on day 5 p.i. Although results from such studies add to our understanding of the events that lead to ECM, they are disappointing in terms of their potential as adjunctive therapies for CM in children.
Remarkably, two recent therapies that were tested based on their potential impact on T cell function, have proven to be highly effective in rescuing mice from ECM even when provided late in the infection after the appearance of clinical signs. Both therapies, one targeting T cell adhesion to the endothelium (41) and one targeting T cell metabolism (56), were shown to block disease progression and to reverse brain damage.
Concerning T cell adhesion to the endothelium as a target of therapy, ECM and CM are associated with increases in expression of both ICAM-1 and VCAM-1 by endothelia cells by γ-interferon-dependent mechanisms (41, 57, 58). Administration of antibodies specific for LFA-1 and VLA-4, the T cell receptors for ICAM and VCAM-1, on day 5.5 p.i., a time at which mice showed neurological signs of CM, resulted in an immediate displacement of parasite-specific CD8+ T cells from the brain vascular and complete reversal of these pathologies and prevention of death of the infected mice (41). This is a highly promising finding as LFA-1-specific and VLA-4-specific antibodies (also known as efalizumab and natalizumab respectively) are approved for use in humans (59, 60).
Encouraged by the finding that targeting the regulation of T cell metabolism, mTOR, with rapamycin had an effect on ECM albeit only early in the infection, we evaluated the effect of the glutamine antagonist, 6-diazo-5-oxo-L-norleucine (DON), on ECM based on its established inhibitory effect on T cell activation (56). When stimulated through their T cell receptors, T cells become metabolically active taking up large quantities of glutamine that are converted in the cells’ cytoplasm to glutamate by the enzyme glutaminase (61, 62). DON antagonizes glutaminase and subsequent generation of glutamate thereby inhibiting T cell activation.
The response of PbA infected animals to DON was rapid and dramatic. DON was highly effective in rescuing mice from ECM even when delivered late in the infection, as late as the morning of day 6 p.i. When DON was administered, the animals were severely symptomatic. By the afternoon of day 6 p.i. the animals were active and exploring their cages and continued to improve over the following days. Necropsies of the animals revealed that at the time they received initial treatment on the morning of day 6 p.i. their brains were swollen, BBB function was lost, and small hemorrhages had accumulated. Treatment rapidly reversed the swelling and BBB dysfunction and by day 15 p.i. the hemorrhages had resolved (56). Moreover, a longitudinal MRI analysis of PbA infected mice showed that the same pathologies detected by MRI in children with CM, namely brain swelling and BBB dysfunction, were reversed by late stage treatment with a prodrug of DON (44). Although early studies have suggested that DON doesn’t readily cross the BBB in children (63), more recent work in mice found a brain to plasma ratio of approximately 0.1 and speculated that difference arose from detection techniques (64). Regardless, mice (and children) in the throes of this disease do not have an intact BBB so DON may well act directly on the levels of the neurotransmitter, glutamate.
In DON-treated PbA mice, parasite-specific CD8+ T cells continued to be associated with the brain endothelium but were in an inactive state indicating that DON influenced CD8+ T cells in vivo (56). But was the inactivation of CD8+ T cells responsible for the reversal of the disease? DON as a glutamine antagonist has the potential to block any glutamine-dependent event. The balance between glutamine and glutamate is essential for brain function. Glutamate is the principal excitatory neurotransmitter in the brain and excessive activation of glutamate receptors can result in a number of pathological conditions. The process for replenishing glutamate after release involves the glutamate-glutamine cycle in which glutamate is transported to astroglia where it is converted to glutamine by glutamine synthetase. Glutamine is released by the astroglia and transported to neurons where it is converted to glutamate by glutaminase, thus completing the cycle (65). Clearly DON could affect this balance. Furthermore, as a result of structural mimicry to L-glutamine, DON acts as a glutamine antagonist in a wide variety of biological pathways including de novo purine synthesis (66, 67), biosynthesis of D-glucosamine-6-phosphate (68), protein synthesis (69, 70), L-asparagine synthetase (71), and, to a lesser extent, pyrimidine synthesis (72, 73). Finally, glutamate excitotoxicity has been shown to disrupt BBB integrity which may also play a role in CM pathophysiology and be modulated by DON treatment (74-76). The rapidity with which DON reversed the pathologies of PbA infection suggest that its effect, in some part, may be due to an alteration in the balance of glutamine-glutamate in the brain or on other glutamine dependent events. DON has been used in clinical trials in children to treat cancers and although it showed little efficacy it also showed little toxicity (53). Thus, DON appears to be a promising adjunctive therapy for CM in African children.
Conclusion
CM is a deadly disease that takes the lives of hundreds of thousands of young African children each year and leaves CM-survivors with life altering neurological damage. Therapies based primarily on the assumption that CM results, in large part, from the sequestration of iRBCs in the brain vasculature inducing inflammation and brain swelling have thus far shown little efficacy in clinical trials in African children. However, recent studies suggest that CD8+ T cells may be a target of therapies in children with CM and two therapies that target CD8+ T cells, at least in part, have proven highly effective in rescuing PbA infected mice from ECM in the very late stages of the disease in the mouse model. Thus, there is good reason to be optimistic that armed with the mouse model our desperate search for adjunctive therapies for CM just may succeed.
Acknowledgements
The authors are grateful to Ryan Kissinger for the expertly prepared graphic images presented in Figures 1 and 2.
Funding
These researchers were supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, Laboratory of Immunogenetics and Laboratory of Malaria Vector Research of the National Institutes of Health.
Footnotes
Disclosures
The authors have no financial conflicts of interest to declare.
References
- 1.2018. World Health Organization. World Malaria Report. World Health Organization, Geneva. [Google Scholar]
- 2.2014. Severe Malaria In Trop. Med. Int. Health. World Health Organization; 7–131. [Google Scholar]
- 3.Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ, Hammond CA, Heyderman RS, Chilingulo CA, Molyneux ME, and Taylor TE. 2015. Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 372: 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmberg D, Franzen-Rohl E, Idro R, Opoka RO, Bangirana P, Sellgren CM, Wickstrom R, Farnert A, Schwieler L, Engberg G, and John CC. 2017. Cerebrospinal fluid kynurenine and kynurenic acid concentrations are associated with coma duration and long-term neurocognitive impairment in Ugandan children with cerebral malaria. Malar. J. 16: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newton CRJC, Hien TT, and White N. 2000. Cerebral malaria. J. Neurol. Neurosurg. Psychiatry 69: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idro R, Jenkins NE, and Newton CRJC. 2005. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 4: 827–840. [DOI] [PubMed] [Google Scholar]
- 7.Mu JB, Duan JH, Makova KD, Joy DA, Huynh CQ, Branch OH, Li WH, and Su XZ. 2002. Chromosome-wide SNPs reveal an ancient origin for Plasmodium falciparum. Nature 418: 323–326. [DOI] [PubMed] [Google Scholar]
- 8.Su XZ, Mu JB, and Joy DA. 2003. The “Malaria’s Eve” hypothesis and the debate concerning the origin of the human malaria parasite Plasmodium falciparum. Microb. Infect. 5: 891–896. [DOI] [PubMed] [Google Scholar]
- 9.Pierce SK, and Miller LH. 2009. World Malaria Day 2009: What malaria knows about the immune system that immunologists still do not. J. Immunol. 182: 5171–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, and et al. 1995. Indicators of life-threatening malaria in African children. N. Engl. J. Med. 332: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SM, and Fairhurst RM. 2014. Malaria parasites and red cell variants: When a house is not a home. Curr. Opin. Hematol. 21: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW, and Marsh K. 2005. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J. Infect. Dis. 192: 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaMonte G, Philip N, Reardon J, Lacsina JR, Majoros W, Chapman L, Thornburg CD, Telen MJ, Ohler U, Nicchitta CV, Haystead T, and Chi JT. 2012. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 12: 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira A, Marguti I, Bechmann I, Jeney V, Chora A, Palha NR, Rebelo S, Henri A, Beuzard Y, and Soares MP. 2011. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 145: 398–409. [DOI] [PubMed] [Google Scholar]
- 15.Nimmerjahn F, and Ravetch JV. 2008. Fc gamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8: 34–47. [DOI] [PubMed] [Google Scholar]
- 16.Souyris M, Mejia JE, Chaumeil J, and Guery JC. 2019. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 41: 153–164. [DOI] [PubMed] [Google Scholar]
- 17.Kori LD, Valecha N, and Anvikar AR. 2018. Insights into the early liver stage biology of Plasmodium. J. Vector Borne Dis. 55: 9–13. [DOI] [PubMed] [Google Scholar]
- 18.Kappe SHI, Vaughan AM, Boddey JA, and Cowman AF. 2010. That was then but this is now: Malaria research in the time of an eradication agenda. Science 328: 862–866. [DOI] [PubMed] [Google Scholar]
- 19.Beare NA, Lewallen S, Taylor TE, and Molyneux ME. 2011. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol. 6: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beare NA, Southern C, Chalira C, Taylor TE, Molyneux ME, and Harding SP. 2004. Prognostic significance and course of retinopathy in children with severe malaria. Arch. Ophthalmol. 122: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 21.Lewallen S, Taylor TE, Molyneux ME, Wills BA, and Courtright P. 1993. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology 100: 857–861. [DOI] [PubMed] [Google Scholar]
- 22.Lewallen S, White VA, Whitten RO, Gardiner J, Hoar B, Lindley J, Lochhead J, McCormick A, Wade K, Tembo M, Mwenechanyana J, Molyneux ME, and Taylor TE. 2000. Clinical-histopathological correlation of the abnormal retinal vessels in cerebral malaria. Arch. Ophthalmol. 118: 924–928. [PubMed] [Google Scholar]
- 23.Villaverde C, Namazzi R, Shabani E, Opoka RO, and John CC. 2017. Clinical comparison of retinopathy-positive and retinopathy-negative cerebral malaria. Am. J. Trop. Med. Hyg. 96: 1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Small DS, Taylor TE, Postels DG, Beare NAV, Cheng J, MacCormick IJC, and Seydel KB. 2017. Evidence from a natural experiment that malaria parasitemia is pathogenic in retinopathy-negative cerebral malaria. Elife 6: e23699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Postels DG, Li CX, Birbeck GL, Taylor TE, Seydel KB, Kampondeni SD, Glover SJ, and Potchen MJ. 2014. Brain MRI of children with retinopathy-negative cerebral malaria. Am. J. Trop. Med. Hyg. 91: 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potchen MJ, Kampondeni SD, Seydel KB, Haacke EM, Sinyangwe SS, Mwenechanya M, Glover SJ, Milner DA, Zeli E, Hammond CA, Utriainen D, Lishimpi K, Taylor TE, and Birbeck GL. 2018. 1.5 Tesla magnetic resonance imaging to investigate potential etiologies of brain swelling in pediatric cerebral malaria. Am. J. Trop. Med. Hyg. 98: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohanty S, Benjamin LA, Majhi M, Panda P, Kampondeni S, Sahu PK, Mohanty A, Mahanta KC, Pattnaik R, Mohanty RR, Joshi S, Mohanty A, Turnbull IW, Dondorp AM, Taylor TE, and Wassmer SC. 2017. Magnetic resonance imaging of cerebral malaria patients reveals distinct pathogenetic processes in different parts of the brain. Msphere 2: e00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NM, Lewallen S, Liomba NG, and Molyneux ME. 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 10: 435–435. [DOI] [PubMed] [Google Scholar]
- 29.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, and Warrell DA. 1985. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 119: 385–401. [PMC free article] [PubMed] [Google Scholar]
- 30.Grau GE, and Craig AG. 2012. Cerebral malaria pathogenesis: revisiting parasite and host contributions. Future Microbiol. 7: 291–302. [DOI] [PubMed] [Google Scholar]
- 31.White NJ, Turner GDH, Medana IM, Dondorp AM, and Day NPJ. 2010. The murine cerebral malaria phenomenon. Trends Parasitol. 26: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkes MT, Conroy AL, Opoka RO, Hermann L, Thorpe KE, McDonald C, Kim H, Higgins S, Namasopo S, John C, Miller C, Liles WC, and Kain KC. 2015. Inhaled nitric oxide as adjunctive therapy for severe malaria: a randomized controlled trial. Malar. J. 14: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avril M, Brazier AJ, Melcher M, Sampath S, and Smith JD. 2013. DC8 and DC13 var genes associated with severe malaria bind avidly to diverse endothelial cells. PLoS Pathog. 9: e1003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moxon CA, Wassmer SC, Milner DA, Chisala NV, Taylor TE, Seydel KB, Molyneux ME, Faragher B, Esmon CT, Downey C, Toh CH, Craig AG, and Heyderman RS. 2013. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 122: 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ndam NT, Moussiliou A, Lavstsen T, Kamaliddin C, Jensen ATR, Mama A, Tahar R, Wang CW, Jespersen JS, Alao JM, Gamain B, Theander TG, and Deloron P. 2017. Parasites causing cerebral falciparum malaria bind multiple endothelial receptors and express EPCR and ICAM-1-binding PfEMP1. J. Infect. Dis. 215: 1918–1925. [DOI] [PubMed] [Google Scholar]
- 36.Rowe JA, Claessens A, Corrigan RA, and Arman M. 2009. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev. Mol. Med. 11: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Idro R, Marsh K, John CC, and Newton CR. 2010. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr. Res. 68: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcais A, Quintana-Murci L, Thaler DS, Schurr E, Abel L, and Casanova JL. 2010. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Year in Human and Medical Genetics: New Trends in Mendelian Genetics 1214: 18–33. [DOI] [PubMed] [Google Scholar]
- 39.Hochman SE, Madaline TF, Wassmer SC, Mbale E, Choi N, Seydel KB, Whitten RO, Varughese J, Grau GE, Kamiza S, Molyneux ME, Taylor TE, Lee S, Milner DA Jr., and Kim K. 2015. Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. MBio 6: e01390–01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strangward P, Haley MJ, Shaw TN, Schwartz JM, Greig R, Mironov A, de Souza JB, Cruickshank SM, Craig AG, Milner DA, Allan SM, and Couper KN. 2017. A quantitative brain map of experimental cerebral malaria pathology. PLoS Pathog. 13: e1006267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson PA, Hart GT, Russo MV, Nayak D, Yazew T, Pena M, Khan SM, Janse CJ, Pierce SK, and McGavern DB. 2016. CD8(+) T cells induce fatal brainstem pathology during cerebral malaria via luminal antigen-specific engagement of brain vasculature. PLoS Pathog. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carroll RW, Wainwright MS, Kim KY, Kidambi T, Gomez ND, Taylor T, and Haldar K. 2010. A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One 5: e13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reis PA, Comim CM, Hermani F, Silva B, Barichello T, Portella AC, Gomes FC, Sab IM, Frutuoso VS, Oliveira MF, Bozza PT, Bozza FA, Dal-Pizzol F, Zimmerman GA, Quevedo J, and Castro-Faria-Neto HC. 2010. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy. PLoS Pathog. 6: e1000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riggle BA, Sinharay S, Schreiber-Stainthorp W, Munasinghe JP, Maric D, Prchalova E, Slusher BS, Powell JD, Miller LH, Pierce SK, and Hammoud DA. 2018. MRI demonstrates glutamine antagonist-mediated reversal of cerebral malaria pathology in mice. Proc. Natl. Acad. Sci. U. S. A: E12024–E12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riggle BA, Miller LH, and Pierce SK. 2017. Do we know enough to find an adjunctive therapy for cerebral malaria in African children? F1000Res 6: 2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Niz M, Ullrich AK, Heiber A, Blancke Soares A, Pick C, Lyck R, Keller D, Kaiser G, Prado M, Flemming S, Del Portillo H, Janse CJ, Heussler V, and Spielmann T. 2016. The machinery underlying malaria parasite virulence is conserved between rodent and human malaria parasites. Nat Commun 7: 11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoute JA 2011. Complement receptor 1 and malaria. Cell. Microbiol. 13: 1441–1450. [DOI] [PubMed] [Google Scholar]
- 48.Biryukov S, and Stoute JA. 2014. Complement activation in malaria: friend or foe? Trends Mol. Med. 20: 293–301. [DOI] [PubMed] [Google Scholar]
- 49.Patel SN, Berghout J, Lovegrove FE, Ayi K, Conroy A, Serghides L, Min-Oo G, Gowda DC, Sarma JV, Rittirsch D, Ward PA, Liles WC, Gros P, and Kain KC. 2008. C5 deficiency and C5a or C5aR blockade protects against cerebral malaria. J. Exp. Med. 205: 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H, Erdman LK, Lu ZY, Serghides L, Zhong K, Dhabangi A, Musoke C, Gerard C, Cserti-Gazdewich C, Liles WC, and Kain KC. 2014. Functional roles for C5a and C5aR but not C5L2 in the pathogenesis of human and experimental cerebral malaria. Infect. Immun. 82: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramos TN, Darley MM, Hu XZ, Billker O, Rayner JC, Ahras M, Wohler JE, and Barnum SR. 2011. Cutting Edge: The Membrane Attack Complex of Complement Is Required for the Development of Murine Experimental Cerebral Malaria. J. Immunol. 186: 6657–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ricklin D, Mastellos DC, Reis ES, and Lambris JD. 2018. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 14: 26–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw TN, Stewart-Hutchinson PJ, Strangward P, Dandamudi DB, Coles JA, Villegas-Mendez A, Gallego-Delgado J, van Rooijen N, Zindy E, Rodriguez A, Brewer JM, Couper KN, and Dustin ML. 2015. Perivascular arrest of CD8+ T cells is a signature of experimental cerebral malaria. PLoS Pathog. 11: e1005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon EB, Hart GT, Tran TM, Waisberg M, Akkaya M, Skinner J, Zinocker S, Pena M, Yazew T, Qi CF, Miller LH, and Pierce SK. 2015. Inhibiting the mammalian target of rapamycin blocks the development of experimental cerebral malaria. MBio 6: e00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mejia P, Trevino-Villarreal JH, Hine C, Harputlugil E, Lang S, Calay E, Rogers R, Wirth D, Duraisingh MT, and Mitchell JR. 2015. Dietary restriction protects against experimental cerebral malaria via leptin modulation and T-cell mTORC1 suppression. Nat. Commun. 6: 6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon EB, Hart GT, Tran TM, Waisberg M, Akkaya M, Kim AS, Hamilton SE, Pena M, Yazew T, Qi CF, Lee CF, Lo YC, Miller LH, Powell JD, and Pierce SK. 2015. Targeting glutamine metabolism rescues mice from late-stage cerebral malaria. Proc. Natl. Acad. Sci. U. S. A. 112: 13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amani V, Vigario AM, Belnoue E, Marussig M, Fonseca L, Mazier D, and Renia L. 2000. Involvement of IFN-gamma receptor-mediated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 30: 1646–1655. [DOI] [PubMed] [Google Scholar]
- 58.Van den Steen PE, Deroost K, Van Aelst I, Geurts N, Martens E, Struyf S, Nie CQ, Hansen DS, Matthys P, Van Damme J, and Opdenakker G. 2008. CXCR3 determines strain susceptibility to murine cerebral malaria by mediating T lymphocyte migration toward IFN-gamma-induced chemokines. Eur. J. Immunol. 38: 1082–1095. [DOI] [PubMed] [Google Scholar]
- 59.Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, Walicke P, Dummer W, Wang X, Garovoy MR, Pariser D, and G. Efalizumab Study. 2003. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N. Engl. J. Med. 349: 2004–2013. [DOI] [PubMed] [Google Scholar]
- 60.Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnalek P, Zadorova Z, Palmer T, Donoghue S, and G. Natalizumab Pan-European Study. 2003. Natalizumab for active Crohn’s disease. N. Engl. J. Med. 348: 24–32. [DOI] [PubMed] [Google Scholar]
- 61.Pollizzi KN, and Powell JD. 2014. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 14: 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, and Frauwirth KA. 2010. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 185: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan MP, Nelson JA, Feldman S, and Vannguyen B. 1988. Pharmacokinetic and phase-I study of intravenous DON (6-diazo-5-oxo-L-norleucine) in children. Cancer Chemother. Pharmacol. 21: 78–84. [DOI] [PubMed] [Google Scholar]
- 64.Alt J, Potter MC, Rojas C, and Slusher BS. 2015. Bioanalysis of 6-diazo-5-oxo-L-norleucine in plasma and brain by ultra-performance liquid chromatography mass spectrometry. Anal. Biochem. 474: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayashi MK 2018. Structure-Function Relationship of Transporters in the Glutamate-Glutamine Cycle of the Central Nervous System. Int. J. Mol. Sci. 19: 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levenberg B, Melnick I, and Buchanan JM. 1957. Biosynthesis of the Purines: 15. The effect of aza-L-serine and 6-diazo-5-oxo-L-norleucine on inosinic acid biosynthesis de novo. J. Biol. Chem. 225: 163–176. [PubMed] [Google Scholar]
- 67.Moore EC, and Lepage GA. 1957. In vivo sensitivity of normal and neoplastic mouse tissues to azaserine. Cancer Res. 17: 804–808. [PubMed] [Google Scholar]
- 68.Ellis DB, and Sommar KM. 1972. Biosynthesis of respiratory tract mucins: II. Control of hexosamine metabolism by L-glutamine-fructose-6-phosphate aminotransferase. Biochim. Biophys. Acta 276: 105–112. [DOI] [PubMed] [Google Scholar]
- 69.Livingston RB, Venditti JM, Cooney DA, and Carter SK. 1970. Glutamine antagonists in chemotherapy. Adv. Pharmacol. Chemother. 8: 57–120. [DOI] [PubMed] [Google Scholar]
- 70.Rosenbluth RJ, Cooney DA, Jayaram HN, Milman HA, and Homan ER. 1976. DON, CONV and DONV-II. Inhibition of L-asparagine synthetase in vivo. Biochem. Pharmacol. 25: 1851–1858. [DOI] [PubMed] [Google Scholar]
- 71.Jayaram HN, Cooney DA, Milman HA, Homan ER, and Rosenbluth RJ. 1976. DON, CONV and DONV : I. Inhibition of L-asparagine synthetase in vitro. Biochem. Pharmacol. 25: 1571–1582. [DOI] [PubMed] [Google Scholar]
- 72.Moore EC, and Hurlbert RB. 1961. Biosynthesis of RNA cytosine and RNA purines: differential inhibition by diazo-oxonorleucine. Cancer Res. 21: 257–261. [PubMed] [Google Scholar]
- 73.Eidinoff ML, Knoll JE, Marano B, and Cheong L. 1958. Pyrimidine Studies: I. Effect of Don (6-diazo-5-oxo-L-norleucine) on incorporation of precursors into nucleic acid pyrimidines. Cancer Res. 18: 105–109. [PubMed] [Google Scholar]
- 74.Janigro D, West GA, Nguyen TS, and Winn HR. 1994. Regulation of blood-brain-barrier endothelial-cells by nitric-oxide. Circ. Res. 75: 528–538. [DOI] [PubMed] [Google Scholar]
- 75.Sharp CD, Fowler M, Jackson TH, Houghton J, Warren A, Nanda A, Chandler I, Cappell B, Long A, Minagar A, and Alexander JS. 2003. Human neuroepithelial cells express NMDA receptors. BMC Neurosci. 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee KE, and Kang YS. 2018. l-Citrulline restores nitric oxide level and cellular uptake at the brain capillary endothelial cell line (TR-BBB cells) with glutamate cytotoxicity. Microvasc. Res 120: 29–35. [DOI] [PubMed] [Google Scholar]