Abstract
Purpose of Review
New more stable formulations of glucagon have recently become available, and these provide an opportunity to expand the clinical roles of this hormone in the prevention and management of insulin-induced hypoglycemia. This is applicable in type 1 diabetes, hyperinsulinism, and alimentary hypoglycemia. The aim of this review is to describe these new formulations of glucagon and to provide an overview of current and future therapeutic opportunities that these may provide.
Recent Findings
Four main categories of glucagon formulation have been studied: intranasal glucagon, biochaperone glucagon, dasiglucagon, and non-aqueous soluble glucagon. All four have demonstrated similar glycemic responses to standard glucagon formulations when administered during hypoglycemia. In addition, potential roles of these formulations in the management of congenital hyperinsulinism, alimentary hypoglycemia, and exercise-induced hypoglycemia in type 1 diabetes have been described.
Summary
As our experience with newer glucagon preparations increases, the role of glucagon is likely to expand beyond the emergency use that this medication has been limited to in the past. The innovations described in this review likely represent early examples of a pending large repertoire of indications for stable glucagon.
Keywords: Glucagon, Hypoglycemia, Diabetes, Hyperinsulinism, Alimentary, Formulation
Introduction
Glucagon is secreted by the pancreatic islet α cells in response to hypoglycemia [1] and contributes to the correction of low blood glucose predominantly through increasing glycogenolysis and to a lesser extent through increasing gluconeogenesis. This effect to increase endogenous (primarily hepatic) glucose production plays a counter-regulatory role to insulin in maintaining normal plasma glucose concentrations, and has an established clinical role in the emergency treatment of insulin-induced hypoglycemia in type 1 diabetes (T1D) [2], hyperinsulinism [3 •], and alimentary hypoglycemia [4]. The instability of glucagon in aqueous solution has limited the expansion of its clinical role outside of the emergency setting, as prompt utilization after reconstitution has been required. Even in its current role in the emergency management of insulin-induced hypoglycemia, this reconstitution procedure can be challenging and associated with errors when performed in a stressful environment by non-healthcare providers [5, 6].
New, more stable, formulations of glucagon have recently become available, and these provide an opportunity to expand the role of glucagon in the prevention and management of hypoglycemia in T1D, hyperinsulinism, and other conditions in which hypoglycemia occurs in the setting of excess circulating insulin, such as post-gastric bypass hypoglycemia. The aim of this review is to describe these new formulations of glucagon and to provide an overview of current and future therapeutic opportunities that these may provide.
Physiology of Glucose Homeostasis
Glucose is essential for brain metabolism, and numerous physiological mechanisms work in concert to prevent the development of hypoglycemia. In normal circumstances, β-cell insulin secretion is suppressed as plasma glucose concentrations fall below 80 mg/dL [1]. As glucose concentrations fall further, counter-regulatory mechanisms are activated to mobilize glucose from endogenous glycogen stores, as well as from gluconeogenesis [7].
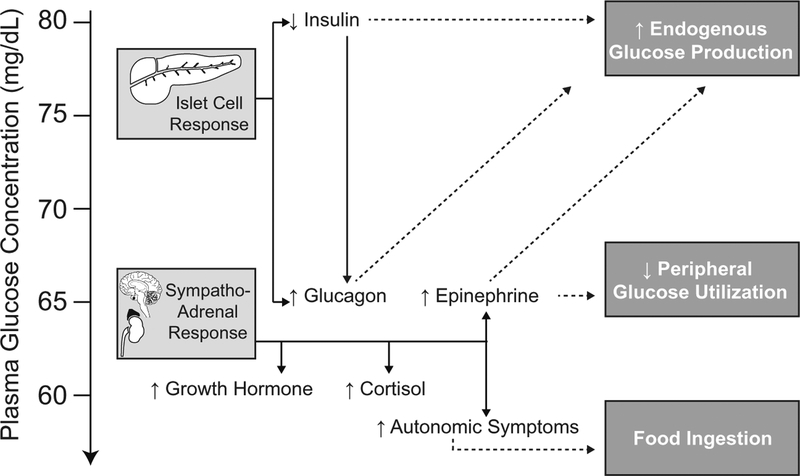
As plasma glucose concentrations approach 70 mg/dL, α-cell glucagon secretion is activated and normally prevents the development of hypoglycemia [1, 7]. As plasma glucose concentrations fall below 65 mg/dL, epinephrine levels begin to rise as part of the sympathoadrenal response [1, 7]. Further decline in plasma glucose levels to below 60 mg/dL is associated with an increase in serum cortisol [1, 7, 8] and growth hormone [1, 7, 9] concentrations as part of the pituitary-adrenal response (Fig. 1). These hormonal responses are not entirely independent of each other. Insulin inhibits glucagon secretion, thus suppression of insulin secretion is required for glucagon to be released [10]. Glucagon [11] and epinephrine [12] also stimulate growth hormone release. These physiological responses occur at thresholds that are higher than would be generally associated with autonomic symptoms of hypoglycemia [1, 7].
Fig. 1.
The islet cell and sympathoadrenal response to falling glucose concentrations in normal physiology
Defective Counter-regulatory Response to Hypoglycemia in Type 1 Diabetes
In T1D, autoimmune destruction of the pancreatic islet β cell results in insulin deficiency. Exogenous insulin administration is required to replace this deficient hormone in the attempt to control hyperglycemia. Unlike the tightly controlled glucose-dependent process for endogenous insulin secretion, exogenous insulin, once administrated, exerts its action according to pharmacokinetic/pharmacodynamic properties and independently of the plasma glucose concentration. Consequently, insulin levels are not suppressed as plasma glucose concentrations decline. This continued exposure of pancreatic islet α cells to insulin, and absence of an intra-islet decrement in β-cell insulin secretion that normally provides a paracrine signal to the α cell necessary for activation, leads to a defective glucagon response that permits the development of hypoglycemia [10, 13].
In addition to the reduced glucagon response to hypoglycemia in T1D, the sympathoadrenal response may also be attenuated. This can be seen following acute or recurrent episodes of hypoglycemia. When a single episode of hypoglycemia occurs in the afternoon, hypoglycemia the following morning is associated with reduced symptoms of hypoglycemia as well as lower epinephrine concentrations. This effect can be seen both in normal subjects [14] and in patients with T1D [15]. Longer duration of hypoglycemia is associated with increased blunting of the sympathoadrenal response [16]. The term hypoglycemia-associated autonomic failure (HAAF) describes the combination of a blunted epinephrine response to hypoglycemia and reduced autonomic symptoms of hypoglycemia leading to hypoglycemia unawareness [17].
The glucose threshold for glucagon secretion in normal physiology is higher than that of the sympathoadrenal response. Consequently, in addition to protecting against hypoglycemia, glucagon protects against recurrent hypoglycemia by preserving the sympathoadrenal response and preventing the development of HAAF.
Defective Counter-regulatory Response to Exercise in Type 1 Diabetes
Exercise provides a significant challenge to glucose homeostasis in patients with T1D. While exercise is a critical promoter of cardiovascular health in this patient population, there are a number of mechanisms through which exercise increases the risk of hypoglycemia.
Muscle uptake of glucose increases during exercise, and the normal physiological response is to reduce insulin secretion and increase glucagon levels [18]. With the intrinsic α-cell defect present in T1D, glucagon secretion fails to increase [19]. Consequently, increased carbohydrate intake and the sympathoadrenal system are required to prevent hypoglycemia. Increased carbohydrate intake prior to, or during, exercise can reduce the likelihood of acute hypoglycemia. However, carbohydrate requirements up to 1 g/min of exercise may be required [20], and this can negate some of the positive effect of exercise on weight management.
As previously mentioned, the epinephrine response to hypoglycemia is blunted in patients with T1D who had hypoglycemia on the previous day [21], and the magnitude of this effect is modified by the degree of hypoglycemia [22]. Exercise can have a similar effect, where repeated prolonged exercise of low intensity or moderate exercise is associated with a reduced epinephrine response to hypoglycemia on the same [23] or subsequent [24] day. This effect of exercise or hypoglycemia on blunting the sympathoadrenal response to falling blood glucose, in combination with increased post-exercise muscle glucose requirements [25], also increases the likelihood of experiencing nocturnal hypoglycemia [26].
Defective Glucose Counter-regulation in Hyperinsulinism
Congenital hyperinsulinism is a rare genetic condition characterized by dysregulated insulin secretion and persistent hypoglycemia. Mutations in ten genes encoding key β-cell factors that regulate insulin secretion have been described, but the genetic etiology remains unknown in over half of infants with this disease. Excess insulin secretion may originate in a pancreatic focus or diffusely affect all islets in the gland. In infants with a focal pancreatic lesion, surgical resection is curative [27]. In those with diffuse disease, medical management with a combination of diazoxide, somatostatin analogs, or continuous enteral dextrose can reduce hypoglycemia occurrence in many infants. However, pancreatectomy may be required when medical management fails. In an effort to avoid surgical resection, treatment with a continuous subcutaneous glucagon infusion [28, 29] has been attempted.
Similar to T1D, insulin levels are not reduced in response to falling blood glucose concentrations in infants with congenital hyperinsulinism [30]. These infants have suppressed glucagon levels during hypoglycemia when compared with infants with non-hyperinsulinemic hypoglycemia [31]. During hypoglycemia, glucagon levels are similarly suppressed in infants with focal and with diffuse disease. In the absence of hypoglycemia in the preceding 48 h, epinephrine levels during hypoglycemia are similar in infants with hyperinsulinism when compared with infants who have non-hyperinsulinemic hypoglycemia [31]. Although the mechanisms of altered counter-regulatory responses to hypoglycemia in infants with congenital hyperinsulinism have been less extensively studied than in T1D, these findings are similar to those seen in patients with T1D and suggest that both intrinsic impairment of glucagon secretion and the development of HAAF also contribute to hypoglycemia in congenital hyperinsulinism.
In patients with congenital hyperinsulinism who have undergone a total pancreatectomy, the subsequent development of diabetes is expected. These children may be at even higher risk of hypoglycemia due the surgical absence of α-cells and inability to secrete glucagon in response to falling glucose concentrations.
Defective Glucose Counter-regulation in Alimentary Hypoglycemia
Postprandial hypoglycemia occurs in up to 75% of patients following gastric bypass surgery [32], with severe hypoglycemia occurring in approximately 1% [33]. In normal anatomy, the incretin response to food intake includes intestinal glucagon-like peptide 1 (GLP-1) secretion, and this augments glucose-dependent insulin secretion, contributing to the regulation of postprandial glucose homeostasis. Alimentary hypoglycemia develops following gastric bypass surgery as a consequence of the Roux-en-Y gastrointestinal reconstruction and is similar to other well-recognized forms of “late dumping syndrome” where altered nutrient transit with rapid delivery to the distal small intestine leads to more rapid glucose absorption and exaggerated GLP-1 and insulin responses [34, 35] with dysregulated islet function that in some individuals leads to late postprandial hypoglycemia [4, 34, 36–40].
Although there is an initial rise in postprandial serum glucagon levels at 30 min in these patients, glucagon levels subsequently fall despite hypoglycemia occurring after 60 to 120 min [41]. Such a defect in glucagon secretion despite lower late post-prandial glucose concentrations may be explained by a combination of the paracrine effect of the increased β-cell response on inhibiting α-cell function [42], with additional contribution from the glucagonostatic effect of exaggerated GLP-1 release [43, 44], and desensitization of central mechanisms for responding to low blood glucose. In fact, individuals studied before and after gastric bypass surgery develop impaired glucagon and epinephrine responses to insulin-induced hypoglycemia [45]. Because exposure to even mild hypoglycemia leads to blunting of subsequent sympathoadrenal responses to hypoglycemia, including sympathetic nervous system augmentation of glucagon release as well as activation of epinephrine secretion [14], it is possible that induction of HAAF contributes to post-gastric bypass hypoglycemia [4].
Formulations of Glucagon
Glucagon is unstable in aqueous solution and spontaneous degradation leads to a loss of bioactivity [46]. The two FDA-approved formulations of standard glucagon are the GlucaGen HypoKit® (Novo Nordisk, Copenhagen, Denmark) [47] and the Glucagon Emergency Kit (Eli Lilly, Indianapolis, IN) [48]. Both formulations are structurally identical to the 29-amino acid human glucagon and are supplied as lyophilized white powder. GlucaGen is reconstituted with sterile water [47], whereas the Glucagon Emergency Kit is reconstituted with a diluent containing glycerin, water for injection and hydrochloric acid [48]. The recommendation for both formulations is that they are used immediately after reconstitution and unused solution discarded. Both preparations are approved for intramuscular, subcutaneous, and intravenous administration.
These lyophilized glucagon preparations have been administered off-label by continuous subcutaneous infusion via insulin pumps [28, 29]. They have also been used effectively as a continuous intravenous infusion in infants with congenital hyperinsulinism [3]. However, glucagon has a propensity to form fibrils in solution [49, 50], and this can lead to problematic catheter occlusion [29]. Frequent reconstitution with a new catheter three times per day has been described, but this approach is burdensome and has limited its clinical utility [28].
New Glucagon Formulations
Increasing success in bihormonal insulin pump technology has generated a need for more stable glucagon preparations [51], and numerous pharmacological approaches have been taken to achieve this goal. This section will summarize the biochemical modifications made to the currently available preparations, and the pharmacodynamic profiles of each.
Dasiglucagon (Zealand Pharma A/S, Copenhagen, Denmark)
Dasiglucagon is a human glucagon analog that also consists of 29 amino acids, but seven of these have been substituted when compared with native glucagon. This results in an improved stability and reduced the tendency to form fibrils when dissolved in aqueous solution [52].
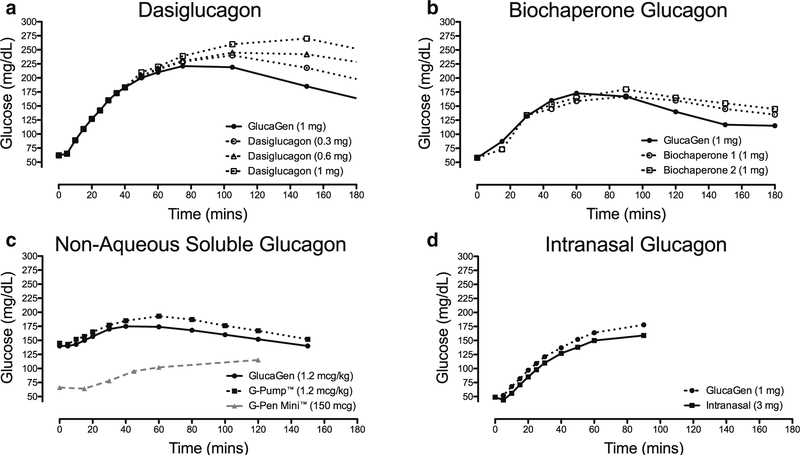
A dose-dependent increase in plasma glucose is seen following administration in patients with T1D who have plasma glucose levels of ~ 55 mg/dL. The time taken to increase glucose concentration to above 70 mg/dL was 6 min with doses of 0.3 mg and 0.6 mg of dasiglucagon, which is comparable to GlucaGen at doses of 0.5 mg and 1 mg. Dasiglucagon does have a longer half-life of approximately 30 min. Peak glucose concentration is seen 35 min after administration, later than the peak with GlucaGen which occurs at 20 min [52] (Fig. 2a).
Fig. 2.
Comparison of the glycemic effect of a dasiglucagon, b biochaperone glucagon, c NAS glucagon, and d intranasal glucagon when compared with standard formulations of glucagon (GlucaGen Hypokit). Mean glucose concentrations at each time-point are represented following administration at t = 0. a Includes 58 patients aged 18 to 50 years with T1D. Hypoglycemia (55 mg/dL ± 10%) was induced by insulin prior to glucagon administration. (adapted from Hovelman et al. [52], with permission from American Diabetes Association). b Includes 27 patients with T1D who received 1 mg of two different biochaperone glucagon formulations when plasma glucose was less than 60 mg/dL. Hypoglycemia was induced by intravenous insulin infusion. (adapted from Glezer et al. [53], with permission from American Diabetes Association). c Black points include 19 adult patients aged 18 to 65 years with T1D. Hypoglycemia was not routinely induced prior to glucagon administration in this study (adapted from Castle et al. [54], with permission from SAGE Publications). Gray points show 62 events of hypoglycemia in 16 adult patients with T1D. In this study, mini-dose (150 mcg) glucagon was used to treat mild hypoglycemia (50 to 69 mg/dL) detected by continuous glucose monitor. (adapted from Haymond et al. [55], with permission from Oxford University Press). d Includes 75 adults with T1D. Hypoglycemia (48 ± 8 mg/dL) was induced by insulin prior to glucagon administration. (adapted from Rickels et al. [56], with permission from American Diabetes Association)
Biochaperone Glucagon (Adocia, Lyon, France)
Biochaperones are polymers, oligomers, and organic compounds that can form a complex with glucagon and improve stability in aqueous solution. Through forming these complexes, biochaperones can protect against degradation and fibril formation. They can also play a role in modifying pharmacokinetic and pharmacodynamic properties [53, 57, 58].
Early studies with these formulations have demonstrated similar early glycemic increases when administered to patients with T1D and hypoglycemia when compared with GlucaGen [53, 59]. More detailed pharmacokinetic and pharmacodynamic studies of two biochaperone glucagon formulations are ongoing [60] (Fig. 2b).
Non-aqueous Soluble Glucagon (Xeris Pharmaceuticals, Chicago, IL, USA)
G-Pump™ or G-Pen Mini™ glucagon are identical formulations that utilize an aprotic solvent strategy to reduce the instability of glucagon in solution and suppress the fibrillation that occurs in aqueous solvents. This approach uses unmodified human glucagon dissolved in dimethylsulfoxide, an aprotic solvent, creating a non-aqueous solution. Concentrations of up to 5 mg/ml in a pre-filled syringe have been described with stability up to 2 years in solution [61, 62].
Non-aqueous soluble (NAS) glucagon and GlucaGen show similar glucose responses following administration at doses of 0.3, 1.2, and 2 mcg/kg in patients with T1D. However, NAS glucagon was associated with more injection site erythema, edema, and discomfort [54] (Fig. 2c).
AMG504–1 Intranasal Glucagon/LY900018 (Locemia Solutions, Montreal, Canada)
This preparation of intranasal glucagon comprises 1-mg glucagon per 10-mg dry powder. This powder also contains a phospholipid to enhance absorption and cyclodextrin, a bulking agent [63]. Absorption of the powder occurs across the nasal mucosa and is not affected by nasal congestion. A dose of 3-mg glucagon appears to have the maximal effect, most likely due to saturation of absorption across the nasal mucosa [64] (Fig. 2d). This formulation of glucagon is now FDA-approved under the trade name baqsimiTM (Eli Lilly, Indianapolis, IN) [65].
Other Discontinued or New Products
ZP Glucagon (Zosano Pharma Corp, Freemont, CA, USA)
A transdermal approach has been developed for delivering glucagon [66], and a safety and efficacy study has been completed [67]. However, details on this formulation and the results of this study are not available, and the development of this product has been discontinued [58].
SAR438544 (Sanofi, Paris, France)
This glucagon agonist was also developed for subcutaneous delivery. Escalating doses showed a dose-dependent increase in blood glucose when compared with placebo [68], but this medication is no longer under development [69].
BIOD-961 (Albireo Pharma Inc., Boston, MA, USA)
This is a lyophilized glucagon formulation that does require reconstitution, and an auto-reconstitution device has been developed. Profiles of this formulation are similar to standard glucagon preparations [70].
Published Studies Evaluating Roles for New Formulations of Glucagon
In the preceding sections of this review, we have described three conditions where hyperinsulinemic hypoglycemia is associated with impaired glucagon secretion. We have also discussed that recurrent hypoglycemia in the context of impairment of the glucagon defense mechanism can lead to recurring hypoglycemia with development of HAAF. Increasing availability of glucagon formulations that are more stable and/or more easily administered may expand its clinical role. Here, we will review studies that have explored the use of new glucagon formulations in these conditions.
Mini-Dose Glucagon in Type 1 Diabetes
Mini-dose glucagon administered subcutaneously can be used to prevent hypoglycemia in patients with T1D during periods of reduced oral intake, including episodes of gastroenteritis [71 •]. Doses of 10 mcg per year of age in children with a minimum dose of 20 mcg have been described [71 •]. When standard glucagon was used to provide mini-dose glucagon, reconstitution and use within 24 h was required. Although this approach has been effective, the stability of this reconstituted glucagon over 24 h is not known.
A dose-finding study of NAS glucagon in adult patients demonstrated a dose-dependent rise in glucose concentrations within 20 min of administration, and recommended a dose of 150 mcg due to inconsistent responses to 75 mcg and increased incidence of nausea at a higher dose of 300 mcg [72]. When compared with 16 g glucose tablets, 150 mcg mini-dose glucagon had similar efficacy in the prevention and correction of hypoglycemia (defined as glucose concentration in the 40–70 mg/dL range) in patients with T1D. Mini-dose glucagon resulted in lower maximum glucose concentrations following treatment (102 vs 116 mg/dL, p = 0.01) [55], and so might help to prevent the post-correction hyperglycemia that is common with ad lib use of oral carbohydrate to treat hypoglycemia. A similar approach to using mini-dose NAS glucagon has been used to prevent exercise-induced hypoglycemia and will be described below [73].
Low-dose dasiglucagon has been studied under similar settings in patients with T1D [74]. Doses of 30, 80, 200, and 600 mcg dasiglucagon were compared with the same doses of standard glucagon (Eli Lilly). An increase in glucose concentration by 20 mcg/dL was seen within 20 min of 30 mcg dasiglucagon doses, and the time taken for similar increases in glucose concentration ranged from 9 to 15 min at higher doses [74]. As has been previously described [52], peak glycemic response to dasiglucagon occurred later than with standard glucagon formulations.
The flexibility of stable glucagons, without the need for reconstitution or prompt use, offers new opportunities to include mini-dose glucagon in the standard care for T1D. The development of glucagon pens, for example, may allow for a more predictable response to mild hypoglycemia in T1D in settings where this may be preferable to carbohydrate intake.
Exercise in Type 1 Diabetes
Exogenous glucagon may have a preventative, and therapeutic role in the management of exercise-associated hypoglycemia. In patients with T1D, low-dose glucagon (200 mcg) administration prior to exercise can reduce the decline in blood glucose seen during exercise, and the glycemic response to glucagon administration after exercise is higher than the response at rest [75].
A role for the mini-dose NAS glucagon in the prevention of exercise-associated hypoglycemia has been studied in adults with T1D. A dose of 150 mcg was administered 5 min prior to 45-min aerobic exercise, and this was compared with a 50% reduction in basal insulin and to 40 g oral glucose intake. In this study, mini-dose glucagon completely prevented hypoglycemia that still occurred with reduced insulin intake, and was associated with less post-exercise hyperglycemia than seen with glucose intake [73]. In addition to the effect on hypoglycemia reduction, this approach removes the requirement for extra caloric intake prior to exercise in the patients with T1D.
Emergency Management of Hypoglycemia in Type 1 Diabetes
The required reconstitution of currently available glucagon limits its utility in an emergency situation. Over two-thirds of parents have difficulties during the procedure including opening the pack and mixing the formula, and some may even erroneously inject air or water without glucagon [6]. Comparatively, intranasal glucagon is administered more quickly with almost universal success. In a simulation study, caregivers were significantly more likely to successfully administer the required dose of intranasal glucagon when compared with the Glucagon Emergency Kit (94% vs 13%), and this was completed in a much shorter time (0.3 vs 1.9 min) [76].
When compared with standard glucagon formulations, intranasal glucagon is also similarly efficacious in the emergency management of hypoglycemia. In a study comparing 3 mg intranasal glucagon with 1 mg GlucaGen in adults with T1D during insulin-induced hypoglycemia (plasma glucose concentration ~ 48 mg/dL), there was a similar plasma glucose response with both preparations but the effect of nasal glucagon was delayed by approximately 3 min [56 •] (Fig. 2d). A companion study in children demonstrated similar efficacy and tolerability of 2 mg and 3 mg nasal glucagon, which were as effective as weight-based administration of GlucaGen in increasing plasma glucose by 25 mg/dL from levels < 80 mg/dL without a delay [77]. Children also appeared to tolerate intranasal glucagon better than adults; whereas a minority of adults more often reported head/facial discomfort with intranasal than intramuscular glucagon and a similar minority experienced nausea with both preparations [56 •], children did not report any more head/facial discomfort with intranasal than intramuscular glucagon and experienced less nausea when glucagon was given intranasally [77].
As shown in Fig. 2, the effect of other stable glucagon formulations on glucose concentrations in adults with T1D during insulin-induced hypoglycemia has also been studied. Dasiglucagon [52, 74], biochaperone glucagon [59], and NAS glucagon [54] all have comparable effects on glucose concentrations in this setting.
Bihormonal Insulin Pump Therapy in Type 1 Diabetes
Algorithms including the integration of continuous glucose monitoring with subcutaneous insulin infusion have led to the development of artificial pancreas systems for the management of T1D. In dual-hormone artificial pancreas systems, glucagon is used in combination with insulin to maintain glucose in a target range. Both single- and dual-hormone approaches have been shown to improve glycemic control, when compared with conventional therapy [78]. A significant limitation of the dual-hormone approach has been the instability of glucagon in solution and this can be associated with infusion set blockages [29]. Newer glucagon formulations may have less risk of this complication and dasiglucagon is currently being investigated for a possible role [79]. Finally, glucagon may be pumped independently from insulin administration in a continuous fashion [80] or in response to glucose declining below a threshold or when hypoglycemia is predicted by continuous glucose monitoring as is already done with automated suspension of insulin delivery.
Hyperinsulinemic Hypoglycemia
Intravenous glucagon has an established role in the stabilization of infants with hyperinsulinemic hypoglycemia and is effective in reducing the requirement for continuous glucose infusion to prevent hypoglycemia [3•]. In a study including four infants treated with continuous subcutaneous NAS glucagon at 5 to 15 mg/kg/h over 3 days, glucose infusion rate reduced from 10.8 ± 4.9 mg/kg/min to an average of 4.9 ± 1.2 mg/kg/min without any observed blockages of the pump infusion set [81].
Studies are ongoing to determine the efficacy of new glucagon preparations administered subcutaneously in this patient population. A randomized controlled trial to evaluate the effect of 10 mcg/h subcutaneous dasiglucagon aiming to recruit 32 patients is ongoing [82]. A phase 2 multicenter trial is also underway to evaluate the effect of subcutaneous NAS glucagon administered at 5 mcg/kg/h for 48 h on glucose infusion requirement in similar infants [83].
Alimentary Hypoglycemia
As previously described, there is a suboptimal glucagon response to falling glucose levels in patients with post-prandial hypoglycemia [41]. As hypoglycemia in this setting is triggered by an exaggerated GLP-1 response to carbohydrate intake, excess carbohydrate intake in response to hypoglycemia can potentially exacerbate the problem. The timely administration of subcutaneous glucagon has the potential to offset the hypoglycemia-inducing effect of this GLP-1 excess. Using a continuous glucose monitor to detect glucose trends and determine optimal timing for NAS glucagon administration in patients with postprandial hypoglycemia, Laguna Sanz et al. were able to reduce the duration of plasma glucose concentration less than 75 mg/dL in 7 adult patients with postprandial hypoglycemia [84].
Conclusions
Until now, the therapeutic use of glucagon has been limited by its instability in solution, but new formulations are more stable and have demonstrated similar pharmacodynamic properties. As our experience with newer glucagon preparations increases, the role of glucagon is likely to expand beyond the emergency use that this medication has been limited to in the past. Glucagon is likely to play a role in the implementation of bihormonal insulin pump therapy in patients with T1D, and will also allow for innovation in hypoglycemia management in other settings. In this review, we have discussed potential roles for these glucagon formulations in patients with T1D, hyperinsulinemic hypoglycemia, and alimentary hypoglycemia. These innovations likely represent the early examples of a pending large repertoire of indications for stable glucagon.
Funding
Michael R. Rickels is supported in part by Public Health Service Research Grant R01 DK091331. Diva D. De Leon is supported in part by Public Health Service Research Grants R01 DK056268 and R01 DK098517.
Diva D. De Leon reports grants from National Institute of Health, during the conduct of the study, grants and personal fees from Zealand Pharma A/S, grants and personal fees from Crinetics, personal fees from Soleno Therapeutics, non-financial support from Dexcom, personal fees from Novartis Pharmaceuticals, personal fees from NovoNordisk, personal fees from Xoma Corporation, personal fees from ProSciento, other from Merck, outside the submitted work.
Michael R. Rickels reports grants from National Institutes of Health, during the conduct of the study, grants and personal fees from Xeris Pharmaceuticals and personal fees from Hua Medicine, outside the submitted work.
Abbreviations
- T1D
Type 1 diabetes
- GLP-1
Glucagon-like peptide
- HAAF
Hypoglycemia-associated autonomic failure
Footnotes
Conflict of Interest Colin P. Hawkes declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent All procedures performed in studies conducted by the authors involving human participants were in accordance with the ethical standards of the University of Pennsylvania or Children’s Hospital of Philadelphia Institutional Review Boards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Therapies and New Technologies in the Treatment of Diabetes
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. 1987;79(3):777–81. 10.1172/JCI112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham MB, Jones TW, Naranjo D, Karges B, Oduwole A, Tauschmann M, et al. ISPAD clinical practice consensus guidelines 2018: assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl27):178–92. 10.1111/pedi.12698. [DOI] [PubMed] [Google Scholar]
- 3.Hawkes CP, Lado JJ, Givler S, De Leon DD. The effect of continuous intravenous glucagon on glucose requirements in infants with congenital hyperinsulinism. JIMD rep. 2019;45:45–50. 10.1007/8904_2018_140. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrates the effect of a continuous intravenous glucagon infusion on glucose requirement on infants with congenital hyperinsulinism.
- 4.Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103(8):2815–26. 10.1210/jc.2018-00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kedia N Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes. 2011;4:337–46. 10.2147/DMSO.S20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris G, Diment A, Sulway M, Wilkinson M. Glucagon administration – underevaluated and undertaught. Practical Diabetes Int. 2001;18(1):22–5. 10.1002/pdi.138. [DOI] [Google Scholar]
- 7.Rickels MR, Schutta MH, Mueller R, Kapoor S, Markmann JF, Naji A, et al. Glycemic thresholds for activation of counterregulatory hormone and symptom responses in islet transplant recipients. J Clin Endocrinol Metab. 2007;92(3):873–9. 10.1210/jc.2006-2426. [DOI] [PubMed] [Google Scholar]
- 8.De Feo P, Perriello G, Torlone E, Ventura MM, Fanelli C, Santeusanio F, et al. Contribution of cortisol to glucose counterregulation in humans. Am J Phys. 1989;257(1 Pt 1):E35–42. [DOI] [PubMed] [Google Scholar]
- 9.De Feo P, Perriello G, Torlone E, Ventura MM, Santeusanio F, Brunetti P, et al. Demonstration of a role for growth hormone in glucose counterregulation. Am J Phys. 1989;256(6 Pt 1):E835–43. [DOI] [PubMed] [Google Scholar]
- 10.Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153(3): 1039–48. 10.1210/en.2011-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkes CP, Grimberg A, Dzata VE, De Leon DD. Adding glucagon-stimulated GH testing to the diagnostic fast increases the detection of GH-sufficient children. Horm Res Paediatr. 2016;85:265–72. 10.1159/000444678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkes CP, Mavinkurve M, Fallon M, Grimberg A, Cody DC. Serial GH measurement after IV placement alone can detect levels above stimulation test thresholds in children. J Clin Endocrinol Metab. 2015:jc20153102. doi: 10.1210/jc.2015-3102. [DOI] [PubMed] [Google Scholar]
- 13.Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes. 2010;59(11):2936–40. 10.2337/db10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40(2):223–6. [DOI] [PubMed] [Google Scholar]
- 15.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91(3):819–28. 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis SN, Mann S, Galassetti P, Neill RA, Tate D, Ertl AC, et al. Effects of differing durations of antecedent hypoglycemia on counterregulatory responses to subsequent hypoglycemia in normal humans. Diabetes. 2000;49(11):1897–903. [DOI] [PubMed] [Google Scholar]
- 17.Rickels MR. Hypoglycemia associated autonomic failure, counterregulatory responses, and therapeutic options in type 1 diabetes. Ann N Y Acad Sci. 2019. 10.1111/nyas.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch IB, Marker JC, Smith LJ, Spina RJ, Parvin CA, Holloszy JO, et al. Insulin and glucagon in prevention of hypoglycemia during exercise in humans. Am J Phys. 1991;260(5 Pt 1):E695–704. 10.1152/ajpendo.1991.260.5.E695. [DOI] [PubMed] [Google Scholar]
- 19.Mallad A, Hinshaw L, Schiavon M, Dalla Man C, Dadlani V, Basu R, et al. Exercise effects on postprandial glucose metabolism in type 1 diabetes: a triple-tracer approach. Am J Physiol Endocrinol Metab. 2015;308(12):E1106–15. 10.1152/ajpendo.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francescato MP, Stel G, Stenner E, Geat M. Prolonged exercise in type 1 diabetes: performance of a customizable algorithm to estimate the carbohydrate supplements to minimize glycemic imbalances. PLoS One. 2015;10(4):e0125220 10.1371/journal.pone.0125220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of antecedent hypoglycemia on counterregulatory responses to subsequent euglycemic exercise in type 1 diabetes. Diabetes. 2003;52(7):1761–9. [DOI] [PubMed] [Google Scholar]
- 22.Galassetti P, Tate D, Neill RA, Richardson A, Leu SY, Davis SN. Effect of differing antecedent hypoglycemia on counterregulatory responses to exercise in type 1 diabetes. Am J Physiol Endocrinol Metab. 2006;290(6):E1109–17. 10.1152/ajpendo.00244.2005. [DOI] [PubMed] [Google Scholar]
- 23.Sandoval DA, Guy DL, Richardson MA, Ertl AC, Davis SN. Acute, same-day effects of antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes mellitus. Am J Physiol Endocrinol Metab. 2006;290(6): E1331–8. 10.1152/ajpendo.00283.2005. [DOI] [PubMed] [Google Scholar]
- 24.Sandoval DA, Guy DL, Richardson MA, Ertl AC, Davis SN. Effects of low and moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes. Diabetes. 2004;53(7):1798–806. [DOI] [PubMed] [Google Scholar]
- 25.Davey RJ, Howe W, Paramalingam N, Ferreira LD, Davis EA, Fournier PA, et al. The effect of midday moderate-intensity exercise on postexercise hypoglycemia risk in individuals with type 1 diabetes. J Clin Endocrinol Metab. 2013;98(7):2908–14. 10.1210/jc.2013-1169. [DOI] [PubMed] [Google Scholar]
- 26.Tsalikian E, Mauras N, Beck RW, Tamborlane WV, Janz KF, Chase HP, et al. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147(4):528–34. 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adzick NS, De Leon DD, States LJ, Lord K, Bhatti TR, Becker SA, et al. Surgical treatment of congenital hyperinsulinism: results from 500 pancreatectomies in neonates and children. J Pediatr Surg. 2019;54(1):27–32. 10.1016/j.jpedsurg.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neylon OM, Moran MM, Pellicano A, Nightingale M, O’Connell MA. Successful subcutaneous glucagon use for persistent hypoglycaemia in congenital hyperinsulinism. J Pediatr Endocrinol Metab. 2013;26(11–12):1157–61. 10.1515/jpem-2013-0115. [DOI] [PubMed] [Google Scholar]
- 29.Mohnike K, Blankenstein O, Pfuetzner A, Potzsch S, Schober E, Steiner S, et al. Long-term non-surgical therapy of severe persistent congenital hyperinsulinism with glucagon. Horm Res. 2008;70(1): 59–64. 10.1159/000129680. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara C, Patel P, Becker S, Stanley CA, Kelly A. Biomarkers of insulin for the diagnosis of Hyperinsulinemic hypoglycemia in infants and children. J Pediatr. 2016;168:212–9. 10.1016/j.jpeds.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Hussain K, Bryan J, Christesen HT, Brusgaard K, Aguilar-Bryan L. Serum glucagon counterregulatory hormonal response to hypoglycemia is blunted in congenital hyperinsulinism. Diabetes. 2005;54(10):2946–51. [DOI] [PubMed] [Google Scholar]
- 32.Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmuhler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015;11(3):564–9. 10.1016/j.soard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Goldfine AB, Patti ME. How common is hypoglycemia after gastric bypass? Obesity (Silver Spring). 2016;24(6):1210–1. 10.1002/oby.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–14. 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest. 2012;122(12):4667–74. 10.1172/JCI64895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–85. [DOI] [PubMed] [Google Scholar]
- 37.Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–80 e2 10.1053/j.gastro.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–17. 10.1210/jc.2013-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–54. 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 40.Calabria AC, Gallagher PR, Simmons R, Blinman T, De Leon DD. Postoperative surveillance and detection of postprandial hypoglycemia after fundoplasty in children. J Pediatr. 2011;159(4):597–601 e1 10.1016/j.jpeds.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tharakan G, Behary P, Wewer Albrechtsen NJ, Chahal H, Kenkre J, Miras AD, et al. Roles of increased glycaemic variability, GLP-1 and glucagon in hypoglycaemia after Roux-en-Y gastric bypass. Eur J Endocrinol. 2017;177(6):455–64. 10.1530/EJE-17-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon-release inhibitor. J Clin Invest. 1984;74(6):2296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toft-Nielsen M, Madsbad S, Holst JJ. Exaggerated secretion of glucagon-like peptide-1 (GLP-1) could cause reactive hypoglycaemia. Diabetologia. 1998;41(10):1180–6. [DOI] [PubMed] [Google Scholar]
- 44.Rickels MR, Naji A. Reactive hypoglycaemia following GLP-1 infusion in pancreas transplant recipients. Diabetes Obes Metab. 2010;12(8):731–3. 10.1111/j.1463-1326.2010.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrahamsson N, Borjesson JL, Sundbom M, Wiklund U, Karlsson FA, Eriksson JW. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes. 2016;65(9):2667–75. 10.2337/db16-0341. [DOI] [PubMed] [Google Scholar]
- 46.Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Technol. 2010;4(6): 1332–7. 10.1177/193229681000400606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Food and Drug Administration. GlucaGen. NDA 20–918/S-012. 2004. https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20918s012lbl.pdf. Accessed 03/09/2019.
- 48.Food and Drug Administration. Glucagon for Injection. NDA 20–928. 1999. https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20928.pdf. Accessed 03/09/2019. [Google Scholar]
- 49.Ghodke S, Nielsen SB, Christiansen G, Hjuler HA, Flink J, Otzen D. Mapping out the multistage fibrillation of glucagon. FEBS J 2012;279(5):752–65. 10.1111/j.1742-4658.2011.08465.x. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen JS. The nature of amyloid-like glucagon fibrils. J Diabetes Sci Technol. 2010;4(6):1357–67. 10.1177/193229681000400609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taleb N, Haidar A, Messier V, Gingras V, Legault L, Rabasa-LhoretR. Glucagon in artificial pancreas systems: potential benefits and safety profile of future chronic use. Diabetes Obes Metab. 2017;19(1):13–23. 10.1111/dom.12789. [DOI] [PubMed] [Google Scholar]
- 52.Hovelmann U, Bysted BV, Mouritzen U, Macchi F, Lamers D, Kronshage B, et al. Pharmacokinetic and pharmacodynamic characteristics of dasiglucagon, a novel soluble and stable glucagon analog. Diabetes Care. 2018;41(3):531–7. 10.2337/dc17-1402. [DOI] [PubMed] [Google Scholar]
- 53.Glezer S, Hovelmann U, Teng S, Lamers D, Odoul M, Correia J, et al. BioChaperone glucagon (BCG), a stable ready-to-use liquid glucagon formulation, is well tolerated and quickly restores euglycemia after insulin-induced hypoglycemia. Diabetes. 2018;67(Supplement 1):305–OR. 10.2337/db18-305-OR. [DOI] [Google Scholar]
- 54.Castle JR, Youssef JE, Branigan D, Newswanger B, Strange P, Cummins M, et al. Comparative pharmacokinetic/pharmacodynamic study of liquid stable glucagon versus lyophilized glucagon in type 1 diabetes subjects. J Diabetes Sci Technol. 2016;10(5):1101–7. 10.1177/1932296816653141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haymond MW, DuBose SN, Rickels MR, Wolpert H, Shah VN, Sherr JL, et al. Efficacy and safety of mini-dose glucagon for treatment of nonsevere hypoglycemia in adults with type 1 diabetes. J Clin Endocrinol Metab. 2017;102(8):2994–3001. 10.1210/jc.2017-00591. [DOI] [PubMed] [Google Scholar]
- 56.Rickels MR, Ruedy KJ, Foster NC, Piche CA, Dulude H, Sherr JL, et al. Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: a randomized crossover non-inferiority study. Diabetes Care. 2016;39(2):264–70. 10.2337/dc15-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this study, the authors demonstrate the comparable efficacy of intranasal glucagon with standard intramuscular glucagon in managing hypoglycemia in patients with type 1 diabetes.
- 57.Meiffren G, Teng S, Ranson A, Gaudier M, Duracher D, Soula R et al. Preclinical efficacy of a stable aqueous formulation of human glucagon with BioChaperone Technology (BC GLU) American Diabetes Association 77th Scientific Sessions; San Diego, CA: 2017. p. 1150-P. [Google Scholar]
- 58.Wilson LM, Castle JR. Stable liquid glucagon: beyond emergency hypoglycemia rescue. J Diabetes Sci Technol. 2018;12(4):847–53. 10.1177/1932296818757795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ranson A, Hövelmann U, Seroussi C, Lamers D, Correia J, Zijlstra E et al. Biochaperone glucagon, a stable ready-to-use liquid glucagon formulation enabled by biochaperone technology, is well tolerated and quickly restores euglycemia after insulin-induced hypoglycemia Advanced Technologies & Treatment for Diabetes; 20–23bruary 2019; Berlin, Germany: 2019. [Google Scholar]
- 60.A trial to investigate the safety and the pharmacokinetic, pharmacodynamic characteristics of two BioChaperone® glucagon formulations compared to marketed GlucaGen® in Subjects With T1DM. https://ClinicalTrials.gov/show/NCT03176524.
- 61.Newswanger B, Ammons S, Phadnis N, Ward WK, Castle J, Campbell RW, et al. Development of a highly stable, nonaqueous glucagon formulation for delivery via infusion pump systems. J Diabetes Sci Technol. 2015;9(1):24–33. 10.1177/1932296814565131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cersosimo E, Cummins MJ, Kinzell JH, Michalek J, Newswanger BJ, Prestrelski SJ, et al. A phase 2 comparative safety PK/PD study of stable nonaqueous glucagon (g-Pen) vs. Lilly glucagon for treatment of severe hypoglycemia. Diabetes Care. 2014;63(Supplement 1A):LB1. [Google Scholar]
- 63.Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol. 2015;9(1): 38–43. 10.1177/1932296814557518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Locemia Solutions ULC. Safety and efficacy of a novel glucagon formulation in type 1 diabetic patients following insulin-induced hypoglycemia (AMG102). https://clinicaltrials.gov/ct2/show/results/NCT01556594. Accessed 03/13/19 2019. [Google Scholar]
- 65.Food and Drug Administration. BAQSIMI (glucagon) nasal powder. NDA 210134. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210134s000lbl.pdf. Accessed 30 Aug 2019. [Google Scholar]
- 66.Palylyk-Colwell E, Ford C. A transdermal glucagon patch for severe hypoglycemia CADTH Issues in Emerging Health Technologies. Ottawa (ON)2016. p. 1–7. [PubMed] [Google Scholar]
- 67.Safety and efficacy of ZP-glucagon to injectable glucagon for hypoglycemia. https://ClinicalTrials.gov/show/NCT02459938.
- 68.Hompesch M, Grosjean P, Morrow L, Hijazi Y, Ishibai M, Teichert L et al. 1066-P / 1066 - The novel glucagon receptor agonist SAR438544, first in human safety, pharmacokinetic, and pharmacodynamic data from a study in healthy volunteers American Diabetes Association 77th Scientific Sessions; 2017; San Diego: 2017. [Google Scholar]
- 69.Sanofi Announces Q2 2016 Results. http://www.news.sanofi.us/2016-07-29-Sanofi-Announces-Q2-2016-Results.
- 70.Uribe-Bruce L, Morrow L, Canney L, Pichotta P, Hompesch M, Krasner A et al. Pharmacokinetic (Pk) and pharmacodynamic (PD) profiles of BiOD-961 compared with marketed glucagons. American Diabetes Association 2015. [Google Scholar]
- 71.•.Haymond MW, Schreiner B. Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care. 2001;24(4):643–5. [DOI] [PubMed] [Google Scholar]; This seminal study established the mini-dose glucagon approach to managing impending hypoglycemia in pediatric patients with type 1 diabetes.
- 72.Haymond MW, Redondo MJ, McKay S, Cummins MJ, Newswanger B, Kinzell J, et al. Nonaqueous, mini-dose glucagon for treatment of mild hypoglycemia in adults with type 1 diabetes: a dose-seeking study. Diabetes Care. 2016;39(3):465–8. 10.2337/dc15-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rickels MR, DuBose SN, Toschi E, Beck RW, Verdejo AS, Wolpert H, et al. Mini-dose glucagon as a novel approach to prevent exercise-induced hypoglycemia in type 1 diabetes. Diabetes Care. 2018;41(9):1909–16. 10.2337/dc18-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is the first study to demonstrate effective prevention of exercise-induced hypogylcemia with mini-dose glucagon when compared to insulin reduction and glucose ingestion in individuals with type 1 diabetes.
- 74.Hovelmann U, Olsen MB, Mouritzen U, Lamers D, Kronshage B, Heise T. Low doses of dasiglucagon consistently increase plasma glucose levels from hypoglycaemia and euglycaemia in people with type 1 diabetes mellitus. Diabetes Obes Metab. 2019;21(3):601–10. 10.1111/dom.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steineck IIK, Ranjan A, Schmidt S, Clausen TR, Holst JJ, Norgaard K. Preserved glucose response to low-dose glucagon after exercise in insulin-pump-treated individuals with type 1 diabetes: a randomised crossover study. Diabetologia. 2019;62(4):582–92. 10.1007/s00125-018-4807-8. [DOI] [PubMed] [Google Scholar]
- 76.Yale JF, Dulude H, Egeth M, Piche CA, Lafontaine M, Carballo D, et al. Faster use and fewer failures with needle-free nasal glucagon versus injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Technol Ther. 2017;19(7):423–32. 10.1089/dia.2016.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sherr JL, Ruedy KJ, Foster NC, Piche CA, Dulude H, Rickels MR, et al. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39(4):555–62. 10.2337/dc15-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haidar A, Rabasa-Lhoret R, Legault L, Lovblom LE, Rakheja R, Messier V, et al. Single- and dual-hormone artificial pancreas for overnight glucose control in type 1 diabetes. J Clin Endocrinol Metab. 2016;101(1):214–23. 10.1210/jc.2015-3003. [DOI] [PubMed] [Google Scholar]
- 79.The bihormonal ilet bionic pancreas feasibility study. https://ClinicalTrials.gov/show/NCT03840278. [Google Scholar]
- 80.Glucagon infusion in T1D patients with recurrent severe hypoglycemia: effects on counterregulatory responses. https://clinicaltrials.gov/ct2/show/NCT03490942. [Google Scholar]
- 81.Thornton P, Truong L, Reynolds C, Rodriguez L, Cummins M, Junaidi K. Continuous infusion of subcutaneous ready-to-use stable liquid glucagon has similar efficacy to intravenous reconstituted glucagon in children with congenital hyperinsulinism Pediatric Academic Society; Baltimore, MD: 2019. [Google Scholar]
- 82.Open-label trial evaluating efficacy and safety of dasiglucagon in children with congenital hyperinsulinism. https://ClinicalTrials.gov/show/NCT03777176.
- 83.CSI-glucagon for prevention of hypoglycemia in children with congenital hyperinsulinism. https://ClinicalTrials.gov/show/NCT02937558.
- 84.Laguna Sanz AJ, Mulla CM, Fowler KM, Cloutier E, Goldfine AB, Newswanger B, et al. Design and clinical evaluation of a novel low-glucose prediction algorithm with mini-dose stable glucagon delivery in post-bariatric hypoglycemia. Diabetes Technol Ther. 2018;20(2):127–39. 10.1089/dia.2017.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]