Abstract
The main goal of treatment for type 1 diabetes is to control glycaemia with insulin therapy to reduce disease complications. For some patients, technological approaches to insulin delivery are inadequate, and allogeneic islet transplantation is a safe alternative for those patients who have had severe hypoglycaemia complicated by impaired hypoglycaemia awareness or glycaemic lability, or who already receive immunosuppressive drugs for a kidney transplant. Since 2000, intrahepatic islet transplantation has proven efficacious in alleviating the burden of labile diabetes and preventing complications related to diabetes, whether or not a previous kidney transplant is present. Age, body-mass index, renal status, and cardiopulmonary status affect the choice between pancreas or islet transplantation. Access to transplantation is limited by the number of deceased donors and the necessity of immunosuppression. Future approaches might include alternative sources of islets (eg, xenogeneic tissue or human stem cells), extrahepatic sites of implantation (eg, omental, subcutaneous, or intramuscular), and induction of immune tolerance or encapsulation of islets.
Introduction
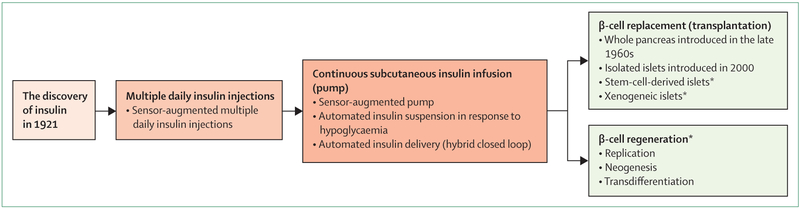
The main goal of treatment for type 1 diabetes is to maintain blood glucose close to the normal range to reduce complications associated with the disease. Many patients can reach this goal with intensive insulin therapy, but have frequent hypoglycaemia episodes associated with exogenous insulin treatment.1–4 By contrast, β-cell replacement therapy aims to maintain normoglycaemia by restoring endogenous and regulated secretion of insulin and other hormones from the islets of Langerhans (figure 1). The proof-of-concept of this approach was shown in the late 1960s by pancreas transplantation. In patients with type 1 diabetes, the transplantation of a pancreas, obtained from a donor without diabetes, can normalise blood glucose. Normoglycaemia is then generally sustained if alloimmune and autoimmune responses can be prevented by immunosuppression. Pancreas transplantation has been associated with improvement in diabetic microangiopathy and macroangiopathy, quality of life, and probably patient survival.5 However, pancreas transplantation carries a substantial risk for surgical complications, most of which are related to the exocrine tissue. A less invasive alternative is islet transplantation, whereby the isolation of islets allows for ectopic transplantation of cells that secrete insulin and glucagon, within only a few millilitres of tissue volume. The efficacy of allogeneic, intrahepatic transplantation of islets to treat diabetes was first shown in patients following pancreatectomy or with type 1 diabetes.6,7 The potential for using islet transplantation as an alternative to insulin therapy for treating type 1 diabetes was shown in 2000, with the consistent achievement of 1-year insulin independence in seven patients.8 Since then, more than 1000 patients with type 1 diabetes have been treated with islet transplantation, alone or after kidney transplantation, for severe hypoglycaemia or poorly controlled glycaemia.9 This worldwide practice has confirmed the overall favourable safety profile of islet transplantation.10–12 The improvement in metabolic outcomes for islet transplantation versus optimised insulin therapy has been confirmed in a randomised clinical trial,13 and these outcomes now approach those of pancreas transplantation.14
Figure 1: Innovating treatments for type 1 diabetes.
Three approaches for treatment of type 1 diabetes: technological improvement of insulin delivery progressively evolving towards a closed loop system; β-cell replacement therapy either with islet transplantation or with pancreas transplantation; and preservation and regeneration of residual native β cells. *Potential future approaches.
This Series paper focuses on the most up-to-date allogeneic islet transplantation, on its indications and limitations, and on the potential ways to overcome the problem of the small numbers of islets and to avoid lifelong immunosuppression.
What is islet transplantation?
Allogeneic islet transplantation aims to restore appropriate insulin, glucagon, and other islet-hormone secretion through the engraftment of pancreatic islets in recipients with insulin-deficient diabetes, most often in patients with type 1 diabetes. Islets are isolated from a deceased-donor pancreas, then infused via the portal vein for delivery to the liver under immunosuppression. Insulin independence might require the infusion of two to three islet preparations (with a goal of at least 9000 islets per kg recipient bodyweight), although a few centres have reported a high proportion of patients gaining insulin independence with islets transplanted from a single donor pancreas when at least 6000 islets per kg were transplanted.15,16
Islet transplantation requires a close collaboration between national regulatory authorities, organ-procurement organisations, tissue-typing laboratories, islet-isolation facilities, and transplant surgery, endocrinology, and interventional radiology departments.
Islet isolation
Islets are isolated from the pancreas of a deceased donor (brain dead or whose heart was not beating), procured through anonymous multi-organ donation. In rare cases, islets have been isolated following partial pancreatectomy from a living, related donor17 but, because of high surgical and metabolic risks, this technique is not used routinely. More than 40% of living, related donors who were initially healthy will develop glucose intolerance following partial pancreatectomy, especially if they gain weight.18
Islets are isolated according to a standardised technique based on enzymatic and mechanical digestion, followed by density gradient purification.19 The resulting product is usually released for transplantation if more than 200 000 islets are obtained, with favourable qualitative assessment of viability, purity, tissue volume, and sterility. However, the number of islets required varies by centre release criteria, patient characteristics, such as bodyweight, and whether it is a first or subsequent infusion.
Islet isolation is done routinely by few institutions worldwide (mainly in North America, Europe, and Australia). Considering the necessary 24 h availability of islet-isolation teams and the complex coordination involved in transplantation, a few highly specialised facilities seem more appropriate than many smaller units. Pancreas procurement and transport should minimise cold ischaemia time, and islet facilities can ship islets to regional centres for transplantation.
Who benefits from islet transplantation?
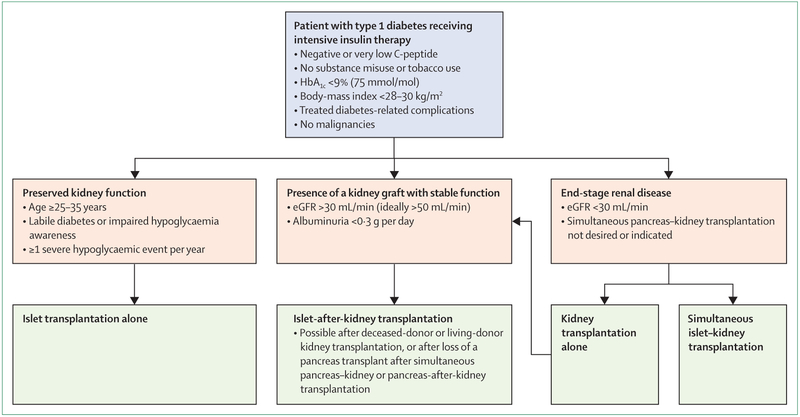
Allogeneic islet transplantation alone is an established therapeutic alternative for patients with type 1 diabetes who have experienced severe hypoglycaemia complicated by impaired hypoglycaemia awareness or excessive glycaemic lability, as defined by current practice recommendations and consensus reports (figure 2).20,21 Addressable causes of hypoglycaemia and glycaemic lability (eg, malabsorption, adrenal insufficiency, and alcohol misuse) should be ruled out, and technological approaches should be attempted before islet transplantation (figure 1).20 Once a decision for transplantation has been made, the choice between islet and pancreas transplantation should be informed on the basis of the patient’s age, body-mass index, renal status, and cardiopulmonary status (figure 2). Islet transplantation alone is usually proposed in cases of insulin-deficient diabetes without clinically relevant insufficient kidney function that are complicated with glycaemic lability, impaired hypoglycaemia awareness, or both.
Figure 2: Indications for allogeneic islet β-cell replacement therapy in type 1 diabetes.
Pancreas transplantation alone or pancreas-after-kidney transplantation might be indicated in patients aged up to 55 years without cardiovascular morbidity. A threshold for C-peptide of <0·3 ng/mL (100 pmol/L) is often considered but it might vary according to kidney function and the technique of measurement. In some centres, an insulin requirement of <60 units per day is used as a cutoff point. HbA1C=glycated haemoglobin. eGFR=estimated glomerular filtration rate.
When renal insufficiency is present, simultaneous pancreas–kidney transplantation is the reference treatment in patients without cardiovascular morbidity, most of whom are aged up to 55 years. In patients with severe comorbidities, islet transplantation after (or simultaneous to) kidney transplantation might be proposed even if the diabetes is not unstable, because the patient will already be receiving immunosuppressive drugs. Islet-after-kidney transplantation can be proposed in cases of living-donor or deceased-donor kidney transplantation, or after the loss of a pancreas transplant after simultaneous pancreas–kidney transplantation or pancreas-after-kidney transplantation. Pancreas transplantation alone or pancreas-after-kidney transplantation can also be considered in patients aged up to 55 years without cardiovascular morbidity.23
The stages of islet transplantation
Timetable and constraints for the patient
As for all transplantations, islet transplantation first requires a pretransplantation patient evaluation to assess the benefit–risk ratio and ensure that the patient is well informed and provides consent. The patient is then added to the transplantation waiting list, and eventually has an islet transplantation and repeated visits to the transplantation team for safety and efficacy monitoring.
The pretransplantation evaluation includes an assessment for impaired hypoglycaemia awareness and glycaemic lability, diabetes-related complications, the presence of kidney or liver disease, malignancies, and chronic infection. Losing excess bodyweight, with a reduction of insulin requirement (in some studies, a cutoff value of <60 units per day is used), discontinuation of smoking, and lowering the level of glycated haemoglobin (HbA1c) below 9% (<75 mmol/mol) are required to ensure the efficacy of the islet transplantation and to minimise the risk for deterioration of retinopathic or neuropathic complications when blood glucose normalises following transplantation.
The transplantation itself
When an ABO-compatible and crossmatch-negative islet preparation of sufficient quantity is available, a wait-listed patient has either image-guided surgical transmesenteric islet infusion (under a general anaesthetic) or image-guided percutaneous transhepatic islet infusion (with a local anaesthetic), into the main portal circulation with heparinisation.8,24 Islets can be transplanted freshly or after culture. Islet culture means that the transplantation can be planned within a period of 12–72 h after the induction of immunosuppression. Islet culture might result in a modest decrease in islet numbers, but probably enhances the quality and might reduce the immunogenicity of the preparation.
Patency of the main portal vein is assessed by monitoring portal pressure during each infusion of islets, and doppler ultrasonography after each infusion. 1–2 days after the islet infusion, intravenous insulin instituted during the transplantation is transitioned back to a subcutaneous route, before tapering the insulin dose to keep blood glucose concentrations in the normal range. The immunosuppressive drug regimen is also adjusted.
Immunosuppression
Immunosuppression includes an induction phase at each islet infusion, then a maintenance phase during the entire lifespan of the islet transplant. Glucocorticoids are not usually used.
The first clinically successful protocol for islet transplantation, the so-called Edmonton protocol, includes an anti-interleukin-2 (IL-2)-receptor antibody (a T-cell activation inhibitor) before each islet infusion, combined with sirolimus (an mTOR inhibitor) and low-dose tacrolimus (a calcineurin inhibitor).8 Variants of this protocol have been published since 2000. These variants include using different induction agents, such as T-cell depleting agents (eg, antilymphocyte immunoglobulins, alemtuzumab, or teplizumab) or lymphocyte-tracking inhibitors (eg, efalizumab);9,12,15,25–28 using other combinations of maintenance therapy (eg, mycophenolate, everolimus, ciclosporin, azathioprine, belatacept, or abatacept); and adding steroids, or, most often, steroid-sparing anti-inflammatory agents that inhibit TNF-α (eg, etanercept or infliximab), or IL-1β (eg, anakinra or gusperimus hydrochloride [also known as deoxyspergualin]).9,15,29
The Edmonton protocol is often replaced by an immunosuppressive drug regimen that includes antithymocyte immunoglobulin with the first dose, and is premedicated with 1 mg/kg methylprednisolone to mitigate any side-effects from cytokines released from the lysed T cells.11 Subsequent islet infusions are supported with an anti-IL-2-receptor antibody, with all infusions including an etanercept course. Maintenance therapy combines tacrolimus with either sirolimus or mycophenolate. No randomised trial has assessed the superiority of one protocol over the other.
Transplant monitoring
The aim of monitoring is to detect rejection of the transplant and the recurrence of autoimmunity using a minimal immunosuppressive drug regimen; to screen for complications related to diabetes or immunosuppression, especially opportunistic infections and neoplasia; and to assess the effectiveness of the islet transplant for restoring metabolic balance. Consistent post-transplantation monitoring is essential, with weekly then monthly visits, and regular visits or contact with the patient thereafter. In contrast with a pancreas transplant, islets are not accessible for biopsy. Therefore, islet rejection is indicated mainly by a decrease in C-peptide concentration, which is associated with a deterioration in metabolic control. Treatment of acute rejection is difficult, although steroids and rituximab have been attempted.30,31 Rejection can be difficult to distinguish from recurrent autoimmunity, although monitoring of donor-specific alloantibodies and islet-specific autoantibodies might be helpful.32 T-cell auto reactivity and alloreactivity assays are not readily available in the clinical setting.33
What can be expected from islet transplantation?
Patient survival and insulin independence
5-year patient survival after islet transplantation is close to 100%.9,10 Survival tends to be higher in islet transplantation alone than in islet-after-kidney transplantation, but there is no significant difference in survival up to 10 years after transplantation, even though recipients of islet-after-kidney transplants generally have more severe pre-existing diabetes-related complications than recipients of islet transplants alone.14 By comparison, mortality from hypoglycaemic events of patients on waiting lists for islet transplantation is almost 4%.22
We reviewed 22 studies11–16,25,33–47 (table 1) in which transplant function, insulin independence, or the composite criteria of good metabolic balance with HbA1c less than 7% (53 mmol/mol) associated with elimination of severe hypoglycaemia events were clearly reported, with at least 1-year (and up to 5-year) follow-up. 15 of these studies11,12,15,16,25,33,34,37–44 reported data only from recipients of an islet transplant alone: Ryan and colleagues40 included a subgroup analysis on patients who completed the islet transplantation procedure; Froud and colleagues41 included a subgroup analysis on patients who completed the islet transplantation procedure and five out of 14 patients were retransplanted; in the Edmonton international trial,38 insulin independence was associated with HbA1c of less than 6·5% (48 mmol/mol); in Matsumoto and colleagues’ study,43 one of three patients from the anakinra group was retransplanted; in Maffi and colleagues’ study,37 18 of 33 patients had pretransplantation treatment with sirolimus and a subgroup analysis of patients who achieved insulin independence (19 of 33) was done with Kaplan-Meier estimates; in Qi and colleagues’ study,34 three of six patients from the etanercept and exenatide group were retransplanted. Three of the studies35,45,46 reported data from recipients of islet-after-kidney transplants or simultaneous islet and kidney transplants, and four of the studies13,14,36,47 reported data from both recipients of islet transplants alone and islet-after-kidney transplants: Deng and colleagues47 included a subgroup analysis on patients who completed the islet transplantation procedure. All trials were phase 1 or 2, interventional, non-randomised, single arm, open label studies, except those by Froud and colleagues41 (phase 2, randomised, parallel assignment, open label), Hering and colleagues11 (phase 3, non-randomised, single arm, open label), and Lablanche and colleagues13 (phase 3, randomised, parallel assignment, open label). After islet transplantation, 5-year insulin independence might be as high as 50% (table 1).9,14,34–36,48,49 A quarter of patients can remain insulin independent, with HbA1c concentrations of less than or equal to 6·5% (48 mmol/mol), for at least 10 years, with either islet transplantation alone or islet-after-kidney transplantation.14
Table 1:
Summary of 1-year and 5-year metabolic outcomes after islet transplantation since 2004
| 1 year | 5 years | |||||
|---|---|---|---|---|---|---|
| Median (IQR) | Minimum-maximum | Number of study arms (patients) | Median (IQR) | Minimum-maximum | Number of study arms (patients) | |
| Transplant function | ||||||
| ITA recipients | 93% (75–100) | 50–100% | 22 (254) | 81 (78–100) | 74–100% | 6 (34) |
| IAK or SIK recipients | 93% (88–100) | 86–100% | 5 (52) | 80 (74–85) | 74–85% | 2 (24) |
| Insulin independence | ||||||
| ITA recipients | 67% (50–82) | 38–100% | 23 (170) | 40 (14–50) | 8–100% | 7 (20) |
| IAK or SIK recipients | 51% (28–74) | 20–83% | 6 (21) | 32 (9–54) | 9–54% | 3 (7) |
| HbA1c <7% (53 mmol/mol) and no SHEs | ||||||
| ITA recipients | 82% (69–94) | 40–100% | 9 (121) | 54 (50–90) | 50–100% | 4 (13) |
| IAK or SIK recipients | 86% (80–86) | 80–86% | 3 (18) | 59 (58–60) | 58–60% | 2 (7) |
Metabolic control and hypoglycaemia
The main goal of islet transplantation has changed from having insulin independence to eliminating hypoglycaemia and restoring the patient’s awareness of hypoglycaemia. Even if insulin independence can be reached, transplant function progressively declines over time, which nevertheless allows long-term satisfactory metabolic control.
The 5-year persistence of transplant function is around 60–70% when T-cell depletion, TNF-α inhibition, mTOR inhibition, and sufficient islet mass are used, and is 40% in other recipients of islet transplants (table 1).9 10-year transplant function, maintaining both a clinically and statistically significant reduction in hypoglycaemia compared with before transplantation, can reach 75% in the intention-to-treat population.14,35 This decrease of severe hypoglycaemia and reduction of time spent in hypoglycaemia is the earliest and most sustained benefit observed after islet transplantation, and is directly and inversely correlated to C-peptide concentrations, in parallel to the restoration of counter-regulatory hormone responses.9,14,50–52 Several non-randomised studies have also shown better metabolic results with islet transplantation compared with multiple daily injections of insulin,53,54 subcutaneous or intraperitoneal insulin pumps without glucose sensors,24,35,55 and pancreas transplantation (table 2).37,56,57 A randomised study13 has confirmed these results at 6 months after transplantation. A similar study () with a long-term follow-up and comparison with sensor-augmented insulin-pump therapy is ongoing.
Table 2:
Studies comparing islet transplantation with other reference treatments
| Study design | Patient number and type of transplantation | Type 1 diabetes control, number of patients | Islet transplantation immunosuppression | Years of follow-up | Proportion of patients who are insulin free, %* | HbA1c | Severe hypoglycaemia events | Adverse events | Microangiopathy | |
|---|---|---|---|---|---|---|---|---|---|---|
| Islet transplantation vs optimised insulin therapy | ||||||||||
| Vantyghem et al (2009)24 | Non-randomised, retrospective | Seven ITA, six IAK | 17 intraperitoneal pump | Edmonton protocol | 3 | 46% | Lower for islet transplantation vs control: 6·6% vs 8·1% | 0·7 hypoglycaemia events per week for islet transplantation vs 1·7 for insulin-pump therapy at year 3 vs 2·6 before islet transplantation† | Four times higher for islet transplantation vs control | ·· |
| Thompson et al (2011)53 | Prospective, crossover cohort | 32 ITA | 45 intensive medical therapy | Antilymphocyte immunoglobulins plus tacrolimus plus mycophenolate | >5 | 37% | Lower for islet transplantation vs control: 6·7% vs 7·8% | ·· | ·· | Decline of eGFR lower in islet transplantation vs control:−1·5 mL/min per year vs −3·5 mL/min per year; progression of retinopathy lower in islet transplantation vs control: 0% vs 12% of eyes |
| Gerber et al (2015)54 | Non-randomised, retrospective | 22 ITA or IAK | Three control groups: 22 before transplantation; 70 multiple daily injections or continuous subcutaneous insulin infusion; 13 kidney transplantation alone | Edmonton protocol | 7 | 9% at 5 years‡ | Lower for islet transplantation vs control groups: 6·7% vs 8·2%; 6·7% vs 7·6%; 6·7% vs 7·9% | Severe hypoglycaemia events per patient per year lower after islet transplantation vs control: 0·3 vs 4·5 | Higher for islet transplantation vs control: four events vs none | ·· |
| Lablanche et al (2018)13 | Multicentre, open label, randomised, controlled | 25 ITA or IAK | 21 continuous subcutaneous insulin infusion or multiple daily injections | Antilymphocyte immunoglobulins plus steroid bolus plus tacrolimus plus mycophenolate plus etanercept | 1 | 59% | Lower for islet transplantation vs control: 5·8% vs 8·1% | Lower for islet transplantation vs control: 0% vs 2% | Higher for islet transplantation vs control (but one death related to severe hypoglycaemia events on transplantation waiting list) | eGFR lower for islet transplantation vs control: 72 mL/min vs 90 mL/min for ITA, 57 mL/min vs 63 mL/min for IAK |
| Franck et al (2004)57 | Non-randomised, retrospective | Nine ITA, four IAK | 25 simultaneous kidney-pancreas transplantation; five pancreas-after-kidney transplantation | Edmonton protocol | 3 | Lower for islet transplantation vs control: around 20% vs 65% | Higher for islet transplantation vs control: 6·3% vs 5·0% | Same for islet transplantation vs control: both 0% | Lower for islet transplantation vs control: 7·7% vs 23·0% relaparotomy; one death in the control group | ·· |
| Maffi et al (2011)37 | Non-randomised, retrospective | 33 ITA | 33 pancreas transplantation alone | Edmonton protocol; or antilymphocyte immunoglobulins plus mycophenolate plus sirolimus (sirolimus started before transplantation in one subgroup) | 1 | Lower for islet transplantation vs control: 59% vs 75% | ·· | ·· | Lower for islet transplantation vs control: 0% vs 54% relaparotomy | ·· |
| Lehmann et al (2015)35 | Non-randomised, retrospective | 38 ITA or SIK | 94 simultaneous kidney-pancreas transplantation or pancreas-after-kidney transplantation | Edmonton protocol then sirolimus switched to mycophenolate, daclizumab switched to basiliximab or antilymphocyte immunoglobulins | >5 | Lower for islet transplantation vs control: 9% vs 73%‡ | Higher for islet transplantation vs control: 6·5% vs 5·9% | Same for islet transplantation vs control: both dropped by 90% vs before transplantation | Lower for islet transplantation vs control: 10% vs 41% relaparotomy | Decrease in eGFR by year 13 similar for islet transplantation vs control: −13·3 mL/min vs −9·5 mL/min |
All studies are single centre unless otherwise indicated. HbA1C=glycated haemoglobin. ITA=islet transplantation alone. IAK=islet-after-kidney transplantation. eGFR=estimated glomerular filtration rate. SIK=simultaneous islet and kidney transplantation.
For the end of the follow-up period unless otherwise indicated.
Severe events not recorded.
The study did not aim at gaining insulin independence.
Diabetes complications
The prevention or delay of diabetes-related complications is an important long-term outcome of islet transplantation. The Diabetes Control and Complications Trial1 showed that in patients with type 1 diabetes on a basal-bolus insulin regimen, a degree of sustained residual β-cell function was not only associated with fewer hypoglycaemic episodes but also with reduced risk of microvascular complications compared with no residual β-cell function. These observations led to the rationale that a degree of endogenous insulin secretion, whether from eutopic or transplanted β cells, would reduce the occurrence or progression of diabetes-related complications in the presence of optimised insulin therapy.
With regard to the risk of nephropathy, the long-term benefits of improved glycaemia outweigh the potential nephrotoxic effects of calcineurin inhibitors.14 In a study58 comparing patients on optimised insulin therapy who were on the waiting list for an islet transplantation alone with patients who had already had islet transplantation alone, the islet-transplant recipients experienced less reduction of glomerular filtration rate. In islet-after-kidney transplantation, there was either no change or an improvement in kidney-transplant function,14,35 and no detriment to kidney function has been observed long term after loss of islet function.59 Most of the adverse effects of islet transplantation on kidney function appear to be transient and related to the initiation of calcineurin inhibitors. Close monitoring of the regimen of immunosuppressive drugs is therefore necessary to minimise adverse kidney events.
Compared with patients on optimised insulin therapy, patients who have had islet transplantation alone have a reduced risk for progression of retinopathy.48,49 Nevertheless, in patients with high HbA1c concentrations before islet transplantation, rapid improvement in glycaemic control poses a risk for early vitreal haemorrhage after transplantation. Several studies60,61 have shown stabilisation or improvement of diabetic peripheral sensory neuropathy after islet transplantation compared with before islet transplantation.
In cross-sectional studies comparing patients with type 1 diabetes with a functioning kidney-and-islet transplant with patients with islet-transplant loss, or with patients with a kidney-only transplant with optimised insulin therapy, the overall survival and cardiovascular mortality were significantly better in patients with a functional islet transplant during the 7 years following transplantation.62 Islet transplantation alone is associated with stabilisation of macroangiopathy, or even with improvement of different cardiovascular risk markers, such as carotid intima-media thickness and coronary calcifications, compared with before transplantation.9,63 A 10-year retrospective study suggests the importance of systematic screening of silent ischaemic cardiopathy, especially in patients who received their islet-after-kidney transplant more than 5 years earlier.14
Quality of life
Islet transplantation is associated with long-term improvement in quality of life, especially after islet transplantation alone, largely owing to the resolution of hypoglycaemic events. Chronic diabetic complications, side-effects of immunosuppression, and non-achievement or loss of insulin independence might attenuate this otherwise positive effect on quality of life.64 Of note are reports of successful pregnancies after islet transplantation, although the possible side-effects of immunosuppressive drugs must be taken into account.65
To summarise what can be expected from islet transplantation, in selected patients with type 1 diabetes, this treatment is associated with long-term improvement in metabolic control, with less frequent hypoglycaemia and stabilised, improved, or delayed chronic microvascular complications of diabetes compared with intensive insulin therapy.
Risks
The main risks of islet transplantation are related to the procedure, the immunosuppressive drugs, and hypersensitisation.38,66 The procedural risks of percutaneous transhepatic-portal-vein catheterisation followed by islet infusion include bleeding and portal-vein thrombosis. Although peritransplant heparin is used to reduce the risk of thrombosis and increase islet survival, heparin increases the risk of hepatic bleeding. Hepatic bleeding is estimated to happen in less than 10% of procedures and includes perihepatic haematomas and intra-abdominal or, rarely, intrapleural haemorrhages. When severe, these events might require blood transfusion or, occasionally, surgical interventions.
The use of different types of haemostatic plugs in the catheter tracts has reduced the bleeding risk.67 Some centres do laparoscopic or minisurgical procedures using mesenterial veins to infuse the islets in the portal system.68 Portal-vein thrombosis is a rare complication, and branch-vein clot occurs with an incidence of less than 5%, possibly related to an increased cumulative tissue volume. When portal-branch-vein thrombosis is detected during routine follow-up soon after islet transplantation, longer-term anticoagulation is indicated. Long-term follow-up by ultrasonography of islet-transplant recipients can show patchy hepatic steatosis that is probably related to locally high insulin concentrations.
One of the main barriers to islet transplantation is the requirement for immunosuppressive drugs over the life of the transplant. The most serious risks of immunosuppression are uncommon, but include potentially severe infections and malignancies, including lymphoproliferative disorders induced by the Epstein-Barr virus. The use of T-cell depleting agents leading to more pronounced immunosuppression might increase these risks. Prophylaxis against cytomegalovirus disease is commonly used, as is co-trimoxazole for the prevention of pneumocystis. Preventive measures against malignancies include the avoidance of sun exposure and adherence to routine (especially skin) cancer screening. Individual immunosuppressive drugs can each cause uncommon, severe side-effects.
The generation of donor-specific antibodies bears a theoretical risk of sensitisation, and might be a barrier against future transplantation. Nevertheless, the risk of sensitisation after multiple transplantations does not appear to be greater than that of a single transplantation (10% of cases), bearing in mind that alloreactivity with respect to successive mismatches seems to subside with their repetition.69 The maintenance of immunosuppression protects against sensitisation, and it is probably important to withdraw immunosuppressive drugs gradually in cases of islet-transplant failure, unless they are still indicated to support a functioning kidney transplant.
Prognostic factors
Prolonged insulin independence after islet transplantation has been shown in many studies. However, like in all other allogeneic transplantations, islet-transplant function often declines with time, resulting in the progressive deterioration of glucose control and reintroduction of antidiabetic agents or small doses of insulin. Many factors can, in theory, affect the outcomes of islet transplantation, including those factors related to the recipient baseline characteristics, but also to the donors, the techniques used for preparing and administering the islets, as well as to immunosuppression, rejection, and recurrent autoimmunity.
Data from the Collaborative Islet Transplant Registry9 show that the 5-year outcomes of islet transplantation are similar when done alone or after a kidney transplantation, and better results are observed when the recipient is older than 35 years, female, with a low baseline insulin requirement and HbA1c concentration, and has no pre-existing islet-cell autoantibodies. Among the transplant characteristics, a high number of transplanted islets, often in two or three separate infusions, as well as a short period of islet culture before transplantation appear to have favourable outcomes. Long-term islet-transplantation outcomes also appear to be related to the type of immunosuppressive drugs that are administered, with regimens combining induction therapy with T-cell depletion, TNF-α inhibitors, or both, and maintenance therapy with an mTOR inhibitor, calcineurin inhibitors, or both, being the most favourable. In recipients combining all these favourable factors, 5-year transplant survival was 69% and 5-year insulin independence was 47%, both approaching the proportions reported after pancreas transplantation alone.23
Islet transplantation in patients with type 1 diabetes triggers immune reactions that are innate and specific, autoimmune and alloimmune, as well as humoral or cellular.67 Donor-specific antibodies are frequently detected in patients with poor transplant survival, and have been associated with HLA-DR mismatches and pre-existing panel-reactive antibodies.70 However, de-novo donor-specific antibodies in recipients of islet transplants might not directly affect transplant survival.71
As well as their number, qualitative characteristics of the transplanted islets also matter, and various in-vitro72 or invivo73 assays have been proposed to estimate islet function. However, none of these tests is available at point of care, and whether their results indicate clinical outcomes remains to be established. Although islet purification is required to decrease the risk of portal-vein thrombosis after islet transplantation, the persistence of small amounts of non-endocrine cells48 and the presence of trapped islets9 are associated with better 5-year metabolic outcomes compared with transplantation with highly purified islets.
The initial transplant function, which can be estimated with various biological proxies or by the initial decrease of insulin requirement, appears highly predictive of later metabolic outcomes.9 Likewise, optimal primary transplant function, as documented by a near-normal beta-score (a composite index based on C-peptide, fasting glucose, HbA1c and insulin requirements) 1 month after the last islet infusion, is an excellent predictor of long-term transplant survival and insulin independence.74
Approximately less than 10% of recipients of islet transplants have procedure-related complications, which, even when fully reversible, have a clear negative effect on later metabolic outcomes.9,68 Collectively, these results suggest that, when allogeneic and autoimmune reactions can be efficiently prevented with immunosuppressive drugs, the main determinant of long-term islet transplantation outcomes is the overall functional mass of islets that initially survived the transplantation procedure.14
Current limitations and future directions
Alternative islet sources
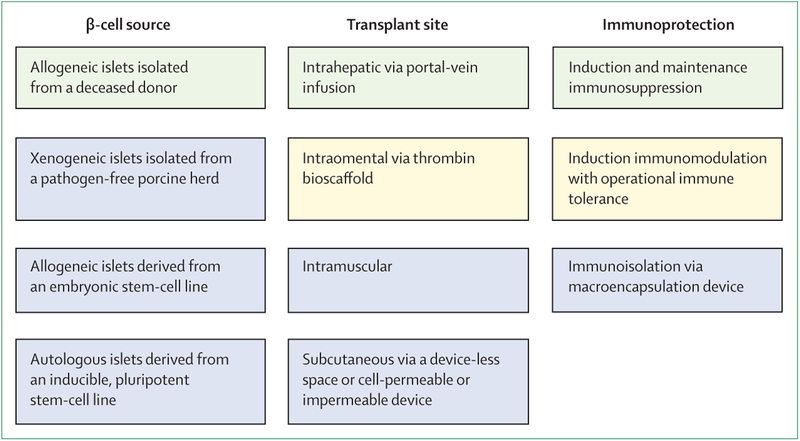
Although allogeneic islet transplantation is an established therapeutic alternative to insulin therapy, access to the procedure is limited by the availability of deceased-donor pancreases for islet isolation and the accessibility of regional isolation facilities to transplantation programmes. The development of an islet source that makes on-demand, limitless manufacturing of cell products possible would substantially expand access to β-cell replacement therapy (figure 3). Nevertheless, this islet source must still overcome important safety concerns and inefficiencies in production.
Figure 3: Current and future approaches to β-cell replacement therapy in type 1 diabetes.
Green=the current clinical approach, which uses allogeneic islets isolated from a deceased donor as the β-cell source, delivery via portal vein infusion for intrahepatic engraftment, and standard T-lymphocyte directed induction and maintenance immunosuppression. Yellow=a case of successful transplantation of allogeneic islets into an omental pouch created with a thrombin bioscaffold has been reported75 and a case of successful intrahepatic transplantation of allogeneic islets has operational islet-transplant tolerance following withdrawal of maintenance immunosuppression (Stock P G, University of California San Francisco, USA, personal communication). These provide proof of concept for the possibility of extrahepatic and immunosuppression-free islet transplantation in humans. Blue=mainly preclinical approaches, with some case studies or phase 1 and 2 studies that do not show reversal of diabetes.
Porcine islets are the most advanced xenogeneic source of islets for transplantation,76 being available from established pathogen-free herds and physiologically maintaining a similar blood glucose concentration to that in humans. However, transplantation of xenogeneic tissue presents a greater immunological barrier than transplantation of allogeneic tissue, with a risk for hyperacute rejection requiring intensive immunosuppression. Xenogeneic transplantation also introduces the potential for transmission of zoonotic infections, such as porcine endogenous retroviruses. Novel genome editing approaches are attempting to engineer porcine islets with lower immunogenicity and retroviral burden than at present, which could enhance the potential efficacy and safety of this cell source.7
The differentiation of human stem cells to insulin-producing islets provides another potentially unlimited cell source for islet transplantation.78 Stem cells from human embryos can be differentiated in vitro to a pancreatic endoderm progenitor stage, which, following transplantation in mouse models, further differentiates in vivo into insulin-producing islet tissue that is capable of reversing diabetes.79,80 However, off-target differentiation of pancreatic ductal structures has been observed with longer-term follow-up of mice that have received transplants.81 Therefore, the uncertain potential for neoplastic transformation requires transplantation in sites where the cells can be readily monitored and retrieved if necessary. Stem cells from human embryos can be further differentiated in vitro to a pancreatic-islet-cell stage, characterised by the capacity for glucose-dependent insulin secretion before transplantation, that might also reverse diabetes in mouse models.82,83 More research is required to determine whether further in-vitro differentiation can lower neoplastic potential and risk for developing non-islet tissue structures with products of embryonic stem cells, as well as to ensure the reproducibility and efficiency of this approach. Islets that are derived from human embryonic stem cells are like islets from deceased donors in that they are allogeneic and require immunoprotection. Islets derived from stem cells have also been differentiated from patients with type 1 diabetes using inducible, pluripotent stem cells84 and somatic-cell nuclear transfer85 that might abrogate alloimmunity. However, autoimmune recurrence is an obstacle faced by any human-tissue source.
Alternative implantation sites
The liver is the most efficient site for islet engraftment as has been established for allogeneic islet transplantation, and this site is successful in preclinical large-animal models of porcine islet transplantation. For such mature islet-cell products, access for monitoring cellular identity is not necessary, but an alternative site where it is possible to monitor immune responses is desirable. The only case of successful islet engraftment outside of the liver in a patient with type 1 diabetes involved an omental pocket created with a bioartificial thrombin scaffold,75 which might be useful for patients with contraindications to intraportal islet delivery. However, the omental site is no more accessible than is the liver for biopsy monitoring or retrieval that might be required for islets derived from stem cells. Cases of intramuscular transplantation using the brachioradialis muscle in the forearm have shown islet survival for autologous islets.86,87 The development of bioscaffolds capable of inhibiting islet-cell death from hypoxia and of facilitating rapid revascularisation for oxygen delivery will be necessary to support more efficient islet survival in either an intramuscular or a subcutaneous site. Creation of a subcutaneous, so-called device-less space has been successful in mouse models using islets from a deceased donor88 and using pancreatic endoderm cells derived from human embryonic stem cells,81 and requires further testing of diabetes reversal in large animal models.
Alternatives to immunosuppression
The requirement for chronic immunosuppression has limited the current indication for islet transplantation to mainly adult patients who have had a severe hypoglycaemia event complicated by impaired hypoglycaemia awareness; these patients represent less than 10% of the population with type 1 diabetes.89
The incorporation of antibodies that block costimulatory leucocyte functional antigen-1 (ie, efalizumab, now withdrawn from the market in the USA and in Europe) or CD28 on T lymphocytes (eg, belatacept) has allowed for the elimination of calcineurin inhibitors so there is no risk for immunosuppression-related nephrotoxicity.90 The long-term maintenance of insulin independence with minimal and, in one case, no maintenance immunosuppressive drugs supports the possibility of achieving operational islet-transplant tolerance.
Until the induction of immunological tolerance to alloantigens and autoantigens can be consistently established for cellular therapy to treat type 1 diabetes, immunoisolation of islet-cell products is being pursued with encapsulation devices. Pancreatic endoderm cells, derived from human embryonic stem cells, placed subcutaneously in a cell-impermeable device in patients with type 1 diabetes, showed that survival of these cells was substantially reduced by a fibrotic foreign-body response.91 Immunoisolation via macroencapsulation has only maintained human-islet survival in a patient with type 1 diabetes when a refillable oxygen chamber has been incorporated within the device.92
Conclusion
Islet transplantation has proven its long-term efficacy during the last 20 years, both in alleviating the immediate burden of labile diabetes and in preventing long-term diabetes-related complications, whether or not a previous kidney transplant is present. Allogeneic islet transplantation is a safe therapeutic alternative for patients with type 1 diabetes who have had a severe hypoglycaemia event complicated by reduced hypoglycaemia awareness or excessive glycaemic lability. Access to islet transplantation is limited by the availability of deceased-donor pancreases for islet isolation and the necessity of immunosuppression. Future approaches might include alternative sources of islets, extrahepatic sites of implantation, and immunotolerance or immunoisolation, but these approaches still require more advanced phase clinical testing.
Search strategy and selection criteria.
Data for this review were identified by searching MEDLINE, PubMed, PubMed Clinical Trials, and references from relevant articles using the search terms “islet transplantation”, “clinical”, “type 1 diabetes”, “β cell”, “stem cell”, “xenotransplantation”, and “immune tolerance”. Articles published in English between Jan 1, 1990, and March 3, 2019, were included. We mostly selected publications from the past 5 years but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search and selected those we judged relevant. Review articles are cited to provide readers with more details. Pancreas transplantation and islet autotransplantation were excluded from this review.
Acknowledgments
No financial support was provided specifically for the writing of this manuscript. MRR is supported in part by Public Health Services Research Grant R01 DK091331. M-CV and FP are, or have been, supported by the French Ministry of Health, Programme Hospitalier de Recherch Clinique, the European Community (Fond Européen de Développement Régional), Conseil Régional du Nord-Pas-de-Calais, Programme d’investissements d’avenir Labex European Genomic Institute for Diabetes ANR-10-LABX-46, the Société Française d’Endocrinologie, the Association de Recherche pour le Diabète, Santelys, and the Agence de la Biomédecine.
Footnotes
Declaration of interests
M-CV reports non-financial support from Novartis and Ipsen; personal fees from Sanofi, Aegerion, and GlaxoSmithKline; and participation in drug investigation trials supported by Shire and HRA Pharma, all outside the submitted work. MRR reports grants and personal fees from Xeris Pharmaceutical, personal fees from Hua Medicine, and non-financial support from Merck, all outside the submitted work. EJPdK and FP declare no competing interests.
References
- 1.Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications Research Group, Lachin, Genuth S, Cleary P, Davis MD, Nathan DM Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000; 342: 381–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006; 49: 298–305. [DOI] [PubMed] [Google Scholar]
- 3.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019; 21: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012; 35: 1897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruessner AC, Gruessner RW. Long-term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant 2016; 21: 377–85. [DOI] [PubMed] [Google Scholar]
- 6.Tzakis AG, Ricordi C, Alejandro R, et al. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet 1990; 336: 402–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharp DW, Lacy PE, Santiago JV, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes 1990; 39: 515–18. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343: 230–38. [DOI] [PubMed] [Google Scholar]
- 9.Collaborative Islet Transplant Registry. 10th annual Collaborative Islet Transplant Registry report. 2017. https://www.citregistry.org/content/citr-10th-annual-report (accessed March 10, 2019).
- 10.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012; 35: 1436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016; 39: 1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell PJ, Holmes-Walker DJ, Goodman D, et al. On behalf of the Australian Islet Transplant Consortium. Multicenter Australian trial of islet transplantation: improving accessibility and outcomes. Am J Transplant 2013; 13: 1850–58. [DOI] [PubMed] [Google Scholar]
- 13.Lablanche S, Vantyghem MC, Kessler L, et al. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2018; 6: 527–37. [DOI] [PubMed] [Google Scholar]
- 14.Vantyghem MC, Chetboun M, Gmyr V, et al. Ten-year outcome of islet alone or after kidney allotransplantation in type 1 diabetes:a prospective parallel arm cohort study Diabetes Care (in press). [DOI] [PubMed] [Google Scholar]
- 15.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005; 293: 830–35. [DOI] [PubMed] [Google Scholar]
- 16.Rickels MR, Liu C, Shlansky-Goldberg RD, et al. Improvement in β-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 2013; 62: 2890–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto S, Okitsu T, Iwanaga Y, et al. Follow-up study of the first successful living donor islet transplantation. Transplantation 2006; 82: 1629–33. [DOI] [PubMed] [Google Scholar]
- 18.Kumar AF, Gruessner RW, Seaquist ER. Risk of glucose intolerance and diabetes in hemipancreatectomized donors selected for normal preoperative glucose metabolism. Diabetes Care 2008; 31: 1639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricordi C, Goldstein JS, Balamurugan AN, et al. National Institutes of Health-sponsored Clinical Islet Transplantation Consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes 2016; 65: 3418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rickels M, Stock PG, de Koning EJP, et al. Defining outcomes for β-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transplantation 2018; 102: 1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhary P, Rickels MR, Senior PA, et al. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care 2015; 38: 1016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MH, Ward GM, MacIsaac RJ, et al. Mortality in people with type 1 diabetes, severe hypoglycemia, and impaired awareness of hypoglycemia referred for islet transplantation. Transplant Direct 2018; 4: e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojtusciszyn A, Branchereau J, Esposito L, et al. Indications for islet or pancreatic transplantation: statement of the TREPID working group on behalf of the Société francophone du diabète (SFD), Société francaise d’endocrinologie (SFE), Société francophone de transplantation (SFT) and Société française de néphrologie—dialyse—transplantation (SFNDT). Diabetes Metab 2019; 45: 224–37. [DOI] [PubMed] [Google Scholar]
- 24.Vantyghem MC, Marcelli-Tourvieille S, Fermon C, et al. Intraperitoneal insulin infusion versus islet transplantation: comparative study in patients with type 1 diabetes. Transplantation 2009; 87: 66–71. [DOI] [PubMed] [Google Scholar]
- 25.Turgeon NA, Avila JG, Cano JA, et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant 2010; 10: 2082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks AM, Walker N, Aldibbiat A, et al. Attainment of metabolic goals in the integrated UK islet transplant program with locally isolated and transported preparations. Am J Transplant 2013; 13: 3236–43. [DOI] [PubMed] [Google Scholar]
- 27.Nijhoff MF, Engelse MA, Dubbeld J, et al. Glycemic stability through islet-after-kidney transplantation using an alemtuzumab-based induction regimen and long-term triple-maintenance immunosuppression. Am J Transplant 2016; 16: 246–53. [DOI] [PubMed] [Google Scholar]
- 28.Perdigoto AL, Preston-Hurlburt P, Clark P, et al. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia 2019; 62: 655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takita M, Matsumoto S, Shimoda M, et al. Safety and tolerability of the T-cell depletion protocol coupled with anakinra and etanercept for clinical islet cell transplantation. Clin Transplant 2012; 26: e471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreau F, Toti F, Bayle F, et al. Rescue of a pancreatic islet graft after steroid therapy. Transplantation 2012; 93: e10–11. [DOI] [PubMed] [Google Scholar]
- 31.Kessler L, Parissiadis A, Bayle F, et al. Evidence for humoral rejection of a pancreatic islet graft and rescue with rituximab and IV immunoglobulin therapy. Am J Transplant 2009; 9: 1961–66. [DOI] [PubMed] [Google Scholar]
- 32.Korutla L, Rickels MR, Hu RW, et al. Noninvasive diagnosis of recurrent autoimmune type 1 diabetes after islet cell transplantation. Am J Transplant 2019; 19: 1852–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huurman VA, Hilbrands R, Pinkse GG, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS One 2008; 3: e2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi M, Kinzer K, Danielson KK, et al. Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: the UIC experience. Acta Diabetol 2014; 51: 833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehmann R, Graziano J, Brockmann J, et al. Glycemic control in simultaneous islet-kidney versus pancreas-kidney transplantation in type 1 diabetes: a prospective 13-year follow-up. Diabetes Care 2015; 38: 752–59. [DOI] [PubMed] [Google Scholar]
- 36.Lablanche S, Borot S, Wojtusciszyn A, et al. Five-year metabolic, functional, and safety results of patients with type 1 diabetes transplanted with allogenic islets within the Swiss-French GRAGIL network. Diabetes Care 2015; 38: 1714–22. [DOI] [PubMed] [Google Scholar]
- 37.Maffi P, Scavini M, Socci C, et al. Risks and benefits of transplantation in the cure of type 1 diabetes: whole pancreas versus islet transplantation. A single center study. Rev Diabet Stud 2011; 8: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318–30. [DOI] [PubMed] [Google Scholar]
- 39.Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4: 390–401. [DOI] [PubMed] [Google Scholar]
- 40.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54: 2060–69. [DOI] [PubMed] [Google Scholar]
- 41.Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant 2005; 5: 2037–46. [DOI] [PubMed] [Google Scholar]
- 42.Bellin MD, Kandaswamy R, Parkey J, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant 2008; 8: 2463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto S, Takita M, Chaussabel D, et al. Improving efficacy of clinical islet transplantation with iodixanl-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α. Cell Transplant 2011; 20: 1641–47. [DOI] [PubMed] [Google Scholar]
- 44.Maffi P, Berney T, Nano R, et al. Calcineurin inhibitor-free immunosuppressive regimen in type 1 diabetes patients receiving islet transplantation: single-group phase 1/2 trial. Transplantation 2014; 98: 1301–09. [DOI] [PubMed] [Google Scholar]
- 45.Cure P, Pileggi A, Froud T, et al. Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation 2008; 85: 801–12. [DOI] [PubMed] [Google Scholar]
- 46.Tan J, Yang S, Cai J, et al. Simultaneous islet and kidney transplantation in seven patients with type 1 diabetes and end-stage renal disease using a glucocorticoid-free immunosuppressive regimen with alemtuzumab induction. Diabetes 2008; 57: 2666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng S, Markmann JF, Rickels M, et al. Islet alone versus islet after kidney transplantation: metabolic outcomes and islet graft survival. Transplantation 2009; 88: 820–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benomar K, Chetboun M, Espiard S, et al. Purity of islet preparations and 5-year metabolic outcome of allogenic islet transplantation. Am J Transplant 2018; 18: 945–51. [DOI] [PubMed] [Google Scholar]
- 49.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012; 12: 1576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vantyghem MC, Raverdy V, Balavoine AS, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (beta-score greater than 7) is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (beta-score greater than 3). J Clin Endocrinol Metab 2012; 97: E2078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rickels MR, Fuller C, Dalton-Bakes C, et al. Restoration of glucose counterregulation by islet transplantation in long-standing type 1 diabetes. Diabetes 2015; 64: 1713–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rickels MR, Peleckis AJ, Markmann E, et al. Long-term improvement in glucose control and counterregulation by islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 2016; 101: 4421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson DM, Meloche M, Ao Z, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation 2011; 91: 373–78. [DOI] [PubMed] [Google Scholar]
- 54.Gerber PA, Locher R, Zuellig RA, et al. Glycemia, hypoglycemia, and costs of simultaneous islet-kidney or islet after kidney transplantation versus intensive insulin therapy and waiting list for islet transplantation. Transplantation 2015; 99: 2174–80. [DOI] [PubMed] [Google Scholar]
- 55.Holmes-Walker DJ, Gunton JE, Hawthorne W, et al. Islet transplantation provides superior glycemic control with less hypoglycemia compared with continuous subcutaneous insulin infusion or multiple daily insulin injections. Transplantation 2017; 101: 1268–75. [DOI] [PubMed] [Google Scholar]
- 56.Gerber PA, Pavlicek V, Demartines N, et al. Simultaneous islet–kidney vs pancreas–kidney transplantation in type 1 diabetes mellitus: a 5 year single centre follow-up. Diabetologia 2008; 51: 110–19. [DOI] [PubMed] [Google Scholar]
- 57.Franck A, Deng S, Huang X, et al. Transplantation for type 1 diabetes comparison of vascularized whole-organ pancreas transplantation with isolated pancreatic islets. Ann Surg 2004; 240: 631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Senior PA, Zeman M, Paty BW, Ryan EA, Shapiro AM. Changes in renal function after clinical islet transplantation: four-year observational study. Am J Transplant 2007; 7: 91–98. [DOI] [PubMed] [Google Scholar]
- 59.Peixoto E, Vendrame F, Arnau A, et al. Ten years of preserved kidney function after islet transplant graft failure. Diabetes Care 2016; 39: e209–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fensom B, Harris C, Thompson SE, Al Mehthel M, Thompson DM. Islet cell transplantation improves nerve conduction velocity in type 1 diabetes compared with intensive medical therapy over six years. Diabetes Res Clin Pract 2016; 122: 101–05. [DOI] [PubMed] [Google Scholar]
- 61.Vantyghem MC, Quintin D, Caiazzo R, et al. Improvement of electrophysiological neuropathy after islet transplantation for type 1 diabetes: a 5-year prospective study. Diabetes Care 2014; 37: e141–42. [DOI] [PubMed] [Google Scholar]
- 62.Fiorina P, Folli F, Bertuzzi F, et al. Long-term beneficial effect of islet transplantation on diabetic macro-/microangiopathy in type 1 diabetic kidney-transplanted patients. Diabetes Care 2003; 26: 1129–36. [DOI] [PubMed] [Google Scholar]
- 63.Madrigal JM, Monson RS, Hatipoglu B, et al. Coronary artery calcium may stabilize following islet cell transplantation in patients with type 1 diabetes. Clin Transplant 2017; 31: e13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foster ED, Bridges ND, Feurer ID, et al. Improved health-related quality of life in a phase 3 islet transplantation trial in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2018; 41: 1001–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rickels MR, Markmann E, Naji A. Successful pregnancies after islet transplantation for type 1 diabetes. Am J Transplant 2019;19: 298–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hafiz MM, Faradji RN, Froud T, et al. Immunosuppression and procedure-related complications in 26 patients with type 1 diabetes mellitus receiving allogeneic islet cell transplantation. Transplantation 2005; 80: 1718–28. [DOI] [PubMed] [Google Scholar]
- 67.Villiger P, Ryan EA, Owen R, et al. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant 2005; 5: 2992–98. [DOI] [PubMed] [Google Scholar]
- 68.Caiazzo R, Vantyghem MC, Raverdy V, et al. Impact of procedure-related complications on long-term islet transplantation outcome. Transplantation 2015; 99: 979–84. [DOI] [PubMed] [Google Scholar]
- 69.Naziruddin B, Wease S, Stablein D, et al. HLA class I sensitization in islet transplant recipients: report from the Collaborative Islet Transplant Registry. Cell Transplant 2012; 21: 901–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monti P, Vignali D, Piemonti L. Monitoring inflammation, humoral and cell-mediated immunity in pancreas and islet transplants. Curr Diabetes Rev 2015; 11: 135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen CC, Pouliquen E, Broisat A, et al. Endothelial chimerism and vascular sequestration protect pancreatic islet grafts from antibody-mediated rejection. J Clin Invest 2018; 128: 219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papas KK, Bellin MD, Sutherland DE, et al. Islet Oxygen consumption rate (OCR) dose predicts insulin independence in clinical islet autotransplantation. PLoS One 2015; 10: e0134428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caiazzo R, Gmyr V, Kremer B, et al. Quantitative in vivo islet potency assay in normoglycemic nude mice correlates with primary graft function after clinical transplantation. Transplantation 2008; 86: 360–63. [DOI] [PubMed] [Google Scholar]
- 74.Vantyghem MC, Kerr-Conte J, Arnalsteen L, et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care 2009; 32: 1473–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baidal DA, Ricordi C, Berman DM, et al. Bioengineering of an intraabdominal endocrine pancreas. N Engl J Med 2017; 376: 1887–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? Lancet 2012; 379: 672–83. [DOI] [PubMed] [Google Scholar]
- 77.Denner J Paving the path toward porcine organs for transplantation. N Engl J Med 2017; 377: 1891–93. [DOI] [PubMed] [Google Scholar]
- 78.Odorico J, Markmann J, Melton D, et al. Report of the key opinion leaders meeting on stem cell-derived beta cells. Transplantation 2018; 102: 1223–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008; 26: 443–52. [DOI] [PubMed] [Google Scholar]
- 80.Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012; 61: 2016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pepper AR, Bruni A, Pawlick R, et al. Post-transplant characterization of long-term functional hESC-derived pancreatic endoderm grafts. Diabetes 2019; 68: 953–62. [DOI] [PubMed] [Google Scholar]
- 82.Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32: 1121–33. [DOI] [PubMed] [Google Scholar]
- 83.Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell 2014; 159: 428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat Commun 2016; 7: 11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sui L, Danzl N, Campbell SR, et al. β-Cell replacement in mice using human type 1 diabetes nuclear transfer embryonic stem cells. Diabetes 2018; 67: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rafael E, Tibell A, Rydén M, et al. Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. Am J Transplant 2008; 8: 458–62. [DOI] [PubMed] [Google Scholar]
- 87.Pattou F, Kerr-Conte J, Wild D. GLP-1-receptor scanning for imaging of human beta cells transplanted in muscle. N Engl J Med 2010; 363: 1289–90. [DOI] [PubMed] [Google Scholar]
- 88.Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 2015; 33: 518–23. [DOI] [PubMed] [Google Scholar]
- 89.Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med 2008; 25: 501–04. [DOI] [PubMed] [Google Scholar]
- 90.Posselt AM, Szot GL, Frassetto LA, et al. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation 2010; 90: 1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pullen LC. Stem cell-derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Am J Transplant 2018; 18: 1581–82. [DOI] [PubMed] [Google Scholar]
- 92.Ludwig B, Ludwig S, Steffen A, et al. Favorable outcome of experimental islet xenotransplantation without immunosuppression in a nonhuman primate model of diabetes. Proc Natl Acad Sci USA 2017; 114: 11745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]