Abstract
Glioma is the most common malignant brain tumor and has high lethality. This tumor generated a robust inflammatory response that results in the deterioration of the disease. However, the prognostic role of systemic cellular inflammatory indicators in gliomas remains controversial. This meta‐analysis aimed to assess the prognostic significance of preoperative neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR), red cell distribution width (RDW), and prognostic nutritional index (PNI) in patients with gliomas. Databases of PubMed, EMBASE, Web of Science, and The Cochrane Library were systematically searched for all studies published up to January 2019. Study screening and data extraction followed established Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. The Newcastle–Ottawa Scale was used to assess the quality of studies. Eighteen studies containing 3,261 patients were included. The analyses showed an increased NLR or RDW was found to be an independent predictor of worse survival in patients with gliomas (hazard ratio (HR): 1.38; 95% confidence interval (CI): 1.09–1.74; P = 0.008; and HR: 1.40; 95% CI: 1.13–1.74; P = 0.002, respectively). Furthermore, a higher PNI indicates a better overall survival (OS; HR: 0.57; 95% CI: 0.42–0.77; P = 0.0002). For the evaluation of PLR and LMR, none of these variables correlated with OS (P = 0.91 and P = 0.21, respectively). Our meta‐analysis indicates the NLR, RDW, and PNI rather than PLR and LMR are the independent index for predicting the OS of gliomas. Pre‐operative NLR, RDW, and PNI can help to evaluate disease progression, optimize treatment, and follow‐up in patients with gliomas.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The prognostic role of systemic cellular inflammatory indicators in gliomas remains controversial.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We present this meta‐analysis to determine if pre‐operative peripheral blood cell count ratios and a nutritional index are prognostic for overall survival in patients with gliomas.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Our meta‐analysis provides incremental understanding on the importance of pre‐operative peripheral neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio, lymphocyte/monocyte ratio, red cell distribution width (RDW), and prognostic nutritional index (PNI) on predicting OS in gliomas.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Preoperative NLR, RDW, and PNI can help to evaluate disease progression and optimize treatment in patients with gliomas.
Gliomas, the most common primary intracranial tumors, arise from glial or precursor cells, representing 81% of malignant brain tumors.1 According to the 2016 World Health Organization (WHO) classification criteria for central nervous system tumors, there are four grades (grades I–IV) to assess the malignant degree of glioma.2 Glioblastoma (GBM) is the most frequent and the highest malignant glioma (grade IV), it usually causes significant mortality and morbidity, and it has a 5‐year overall survival (OS) of no > 5%.3 In the past few decades, the treatment of glioma has made great progress and development, including surgical resection, radiotherapy, chemotherapy, immunotherapy, and molecular targeted therapy.4 However, the prognosis of glioma remains poor, especially for GBM. Such unfavorable prognosis makes it relevant to examine possible preventive strategies.
Numerous studies have indicated that systemic inflammatory responses can dramatically influence tumor growth, progression, and response to treatment.5 Systemic cellular inflammatory markers, such as neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR) or lymphocyte/monocyte ratio (LMR), red cell distribution width (RDW), and prognostic nutritional index (PNI), may be potential prognostic factors for multiple solid tumors, including lung cancer, prostate cancer, gastric cancer, and metastatic melanoma.6, 7, 8 However, the role of those peripheral blood inflammatory indicators in gliomas remains unclear and controversial. In a clinical study with 166 patients with GBM, both NLR and PLR are independent prognostic factors for OS, and a low NLR contributes to a better prognosis.9 Instead of NLR, Kayhan et al.10 suggested that PLR could be a diagnostic value in different metastasis from GBM. Preoperative RDW and PNI levels are also beneficial to evaluate glioma outcomes.11, 12 Inversely, Diaz and his colleague13 have reported that pretreatment systemic inflammatory markers (NLR, MLR, and PLR) are not associated with improved OS in patients with GBM. In addition, an early meta‐analysis of six retrospective studies reported the prognostic role of one inflammatory maker, NLR, in gliomas.14 To our knowledge, there is no meta‐analysis to systematically summarize the potential clinical value of those hematological inflammatory markers in gliomas. Therefore, this meta‐analysis was conducted to assess the prognostic significance of pre‐operative NLR, PLR, LMR or MLR, RDW, and PNI in patients with gliomas.
Methods
This meta‐analysis was conducted according to the recommendations and standards set by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA).15
Study search
Databases of PubMed, EMBASE, Web of Science, and The Cochrane Library were systematically searched for all studies investigating the prognostic significance of pre‐operative inflammatory markers among patients with gliomas published up to January 2019. Three comprehensive search themes were specified. The first theme identified relevant terms for prognosis by combining exploded versions of the Medical Subject Headings terms prognostic indices, prognostic index, prognostic role, prognostic biomarker, prognostic significance, prognostic score, prognostic factor, survival analysis, OS, progression‐free survival, recurrence‐free survival, or hazard ratio (HR). The second theme identified terms related to inflammatory markers by combining inflammatory index, systemic inflammatory, neutrophil lymphocyte ratio (NLR), lymphocyte monocyte ratio (LMR), platelet lymphocyte ratio (PLR), prognostic nutritional index (PNI), red cell distribution width (RDW), or C‐reactive protein. The third theme identified expanded Medical Subject Headings terms by combining glioma, glioblastoma, grade glioma, malignant glioma, glioma prognosis, glial cell tumor, glial cell mixed glioma, mixed glioma, malignant gliomas, high grade glioma, diffuse intrinsic pontine glioma, or GBMs.
The three themes were then combined and further filtered by humans, with no language restrictions. The complete search algorithm is detailed in Table S1 .
Study selection
The study selection process was performed by two reviewers (D.P. and K.K.) independently, with any disagreements being discussed. Studies were included according to the following inclusion criteria: (i) the patients were diagnosed with glioma in any grade and received standard treatments; (ii) pre‐operative systemic inflammatory markers were assessed in patients; (iii) patients were followed up for enough time; and (iv) data of prognostic outcomes were reported. Studies were excluded if: (i) patients were treated with steroid or anti‐inflammatory drugs; (ii) patients showed signs of active infection, bleeding, autoimmune or hematological disorders, hyper or hypothyroidism, as well as uncontrolled hypertension and uncontrolled diabetes, which affect leukocyte (subtypes) counts or function; and (iii) reviews, case reports, conference abstracts, letters, and animal or cell studies. When multiple articles for a single study were present, we used the latest publication and supplemented it, if necessary, with data from the most complete publication.
Data extraction and quality assessment
From each study, information was extracted including: the first author, publication year, geographic location, study design, patient information (sample size, mean/median age, sex distribution, and performance status), inflammatory markers, end point (OS, progression‐free survival), etiology, therapy, follow‐up duration, multivariate factors, HR and corresponding 95% confidence intervals (CIs), or exact P values. When univariate HR and multivariate HR were both reported, only the multivariate HR was used.
The Newcastle–Ottawa Scale was used to assess the quality of studies. A maximum of 9 points can be given for each study in the categories of: selection of patients, comparability of the study groups, and assessment of outcomes. We defined high‐quality studies with scores ≥ 7.
Statistical analysis and exploration of heterogeneity
All data were pooled with the use of classical meta‐analytic methodology, using Review Manager version 5.3 (RevMan 5.3; Cochrane Informatics and Knowledge Management Department). Statistical significance was set at P < 0.05 and 95% CIs were calculated for each investigation and for each outcome variable. The logHR and SE were calculated by the generic inverse‐variance method in RevMan 5.3, and were then used for aggregation of the prognostic role of inflammatory markers in gliomas. Forest plots were used to estimate the pooled HR.
Before calculating the combined results for all trials, statistical heterogeneity was evaluated by using the I 2 statistic and P value, which assessed the appropriateness of pooling the individual study results.16 The I 2 value provided an estimate of the amount of variance across studies because of heterogeneity rather than chance. I 2 values of 25%, 50%, and 75% corresponded to low, moderate, and high levels of heterogeneity, respectively.17 If P ≥ 0.05, the heterogeneity was not substantial. Thus, a fixed‐effects model was used to calculate forest plots. If P < 0.05, however, the heterogeneity was considered substantial. Then a random‐effects model was used.
Subgroup analyses were performed based on region, glioma grade, treatment, cutoff value, and adjustment of HR. The subgroup analysis was assessed using χ2 statistic with a P value of < 0.05 taken to indicate statistical significance. Sensitivity analysis was conducted to evaluate the contribution of each study to heterogeneity by excluding individual studies one at a time. The risk of publication bias was assessed by rendering funnel plots.
This meta‐analysis has been registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42019116307).
Results
Study characteristics
A total of 428 studies were retrieved from the initial search. After removing duplicates, 334 studies were screened. After screening the titles and abstracts, we excluded 275 records. A total of 59 full text manuscripts were examined. Figure 1 provides detailed search selection of studies for this meta‐analysis. In total, 3,261 patients diagnosed with glioma from 18 studies were included.9, 11, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 All of the included studies were published in recent years and were from seven different countries, which were retrospectively designed with 17 single‐center trials9, 11, 18, 19, 20, 22, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34and one multicenter trial.23 There were 15 studies performed multivariate Cox regression analysis and reported adjusted HR.9, 18, 19, 20, 22, 23, 24, 25, 26, 27, 28, 30, 31, 33, 34 The main features of the selected studies are shown in Table 1 .
Figure 1.
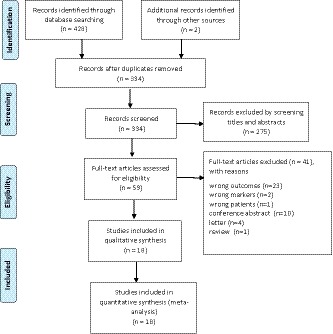
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram showing the selection process for the including studies.
Table 1.
Characteristics of the trials included in the meta‐analysis
| Study | Design, center | Level of evidence | Inflammatory markers | End point | No. of patients (M/F) | Mean/median age | Etiology | Therapy | Follow‐up (months) | Multivariate HR | Adjust factors | PS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Auezova et al.11, Lebanon | Retrospective, one center | 2a | NLR, RDW | OS | 178 (93/85) | 41.6 | Low‐grade gliomas/high‐grade gliomas | Surgery + adjuvant therapy | 80 (period) | No | Age, grade, KPS | KPS < 70 (n = 90) |
| Bambury et al.18, Ireland | Retrospective, one center | 2a | NLR | OS | 84 (65/19) | 58 | GBM | Surgery + adjuvant therapy | 9.3 (median) | Yes | Age, gender, ECOG, location, extent of resection, adjuvant therapy | ECOG < 2 (n = 64) |
| Bao et al.19, China | Retrospective, one center | 2a | NLR, PLR, MLR, RDW | OS | 219 (124/95) | NA | Glioma | Surgery | 60 (period) | Yes | Age, grade | NA |
| Gandhi et al.20, India | Retrospective, one center | 2a | NLR | OS | 72 (58/14) | 42 | Glioma | Surgery | 150 (period) | Yes | Age, location, extent of resection | NA |
| Han et al.21, China | Retrospective, one center | 2a | NLR, PLR | OS | 152 (95/57) | 50.4 | GBM | Surgery + adjuvant therapy | 13 (median) | Yes | Age, KPS, extent of resection | KPS < 70 (n = 78) |
| He et al.22, China | Retrospective, one center | 2a | PNI | OS, PFS | 188 (116/72) | 45 | High‐grade gliomas (III–IV) | Surgery + adjuvant therapy | 160 (period) | Yes | Age, grade, extent of resection | KPS < 70 (n = 17) |
| Kaya et al.23, Turkey | Retrospective, two centers | 2a | NLR, PLR | OS | 90 (51/39) | 58.5 | GBM | Surgery + adjuvant therapy | 11.3 (median) | Yes | Age, ECOG, adjuvant therapy | ECOG < 2 (n = 50) |
| Liang et al.24, China | Retrospective, one center | 2a | RDW | OS | 109 (67/42) | 54 | GBM | Surgery + adjuvant therapy | 24 (period) | Yes | Age, gender, location, extent of resection, adjuvant therapy | NA |
| Lopes et al.25, Portugal | Retrospective, one center | 2a | NLR, PLR | OS, PFS | 140 (98/42) | 62.9 | GBM | Surgery + adjuvant therapy | 19.4 (median) | Yes | Age, gender, location, extent of resection | KPS (NA) |
| Mason et al.26, Canada | Retrospective, one center | 2a | NLR | OS | 369 (238/131) | 55 | GBM | Surgery + adjuvant therapy | 15.1 (median) | Yes | Age, ECOG, extent of resection | ECOG < 2 (n = 331) |
| McNamara et al.27, Canada | Retrospective, one center | 2a | NLR | OS | 107 (76/31) | 52 | GBM | Surgery + adjuvant therapy | 84 (period) | Yes | Age, ECOG, extent of resection | ECOG < 2 (n = 80) |
| Wang et al.28, China | Retrospective, one center | 2a | NLR, PLR | OS | 112 (70/42) | 50 | Glioma | Surgery | 74 (period) | Yes | Age, KPS, gender, grade, tumor size | KPS < 70 (n = 47) |
| Wang et al.9, China | Retrospective, one center | 2a | NLR, LMR, PLR | OS | 166 (96/70) | 52.1 | GBM | Surgery | NA | Yes | Age, KPS, gender, grade, extent of resection, gene mutant | KPS < 70 (n = 82) |
| Wang et al.29, China | Retrospective, one center | 2a | NLR, PLR, AGR, PNI | OS | 706 (407/299) | 45 | Glioma/GBM | Surgery | 108 (period) | No | Age, gender, gene mutant | NA |
| Weng et al.30, China | Retrospective, one center | 2a | NLR | OS | 239 (108/131) | 48.5 | Glioma | Surgery + adjuvant therapy | 60 (period) | Yes | Age, KPS, gender, extent of resection, gene mutant | KPS < 70 (n = 44) |
| Xu et al.31, China | Retrospective, one center | 2a | AGR, PNI | OS | 166 (84/82) | 50.4 | GBM | Surgery + adjuvant therapy | 14 (median) | Yes | Age, KPS, tumor size, extent of resection, adjuvant therapy | KPS < 70 (n = 75) |
| Yersal et al.32, Turkey | Retrospective, one center | 2a | NLR, PLR | OS, PFS | 80 (39/41) | 56.8 | GBM | Surgery + adjuvant therapy | 60 (period) | No | Age, ECOG, location | ECOG < 2 (n = 50) |
| Zhou et al.33, China | Retrospective, one center | 2a | NLR, PNI, LMR | OS | 84 (50/34) | 53 | GBM | Surgery + adjuvant therapy | 36 (period) | Yes | Age, extent of resection, adjuvant therapy | NA |
AGR, albumin/globulin ratio; ECOG, Eastern Cooperative Oncology Group; GBM, glioblastoma; HR, hazard ratio; KPS, Karnofsky Performance Status; MLR/LMR, monocyte/lymphocyte ratio or lymphocyte/monocyte ratio; NA, not available; NLR, neutrophil/lymphocyte ratio; OS, overall survival; PFS, progression‐free survival; PLR, platelet/lymphocyte ratio; PNI, prognostic nutritional index; PS, performance status; RDW, red cell distribution width.
Overall analysis
In the analysis of the NLR for predicting the prognosis of gliomas, 15 studies9, 11, 18, 19, 20, 21, 23, 25, 26, 27, 28, 29, 30, 32, 33 (n = 2,798 patients) were included. The patients with a higher pre‐operative NLR had a worse prognosis (HR: 1.38; 95% CI: 1.09–1.74; P = 0.008; Figure 2 a). Moreover, seven studies9, 19, 21, 25, 28, 29, 32 with 1,575 patients evaluated the PLR for predicting the prognosis of gliomas. There was no significant difference in the prognostic significance of PLR between the patients with low PLR and those with high PLR (HR: 0.99; 95% CI: 0.75–1.29; P = 0.91; Figure 2 b). Compared with a low PNI, a high PNI resulted in a better OS (HR: 0.57; 95% CI: 0.42–0.77; P = 0.0002; Figure 2 c). There were three studies11, 19, 24 with 506 participants that evaluated the prognostic significance of RDW in gliomas. Patients with a higher RDW had poorer OS rates (HR: 1.40; 95% CI: 1.13–1.74; P = 0.002; Figure 2 d). For the pre‐operative LMR, there was no significant difference in the prognosis between the patients with low LMR and those with high LMR (HR: 0.82; 95% CI: 0.60–1.12; P = 0.21; Figure 2 e).
Figure 2.
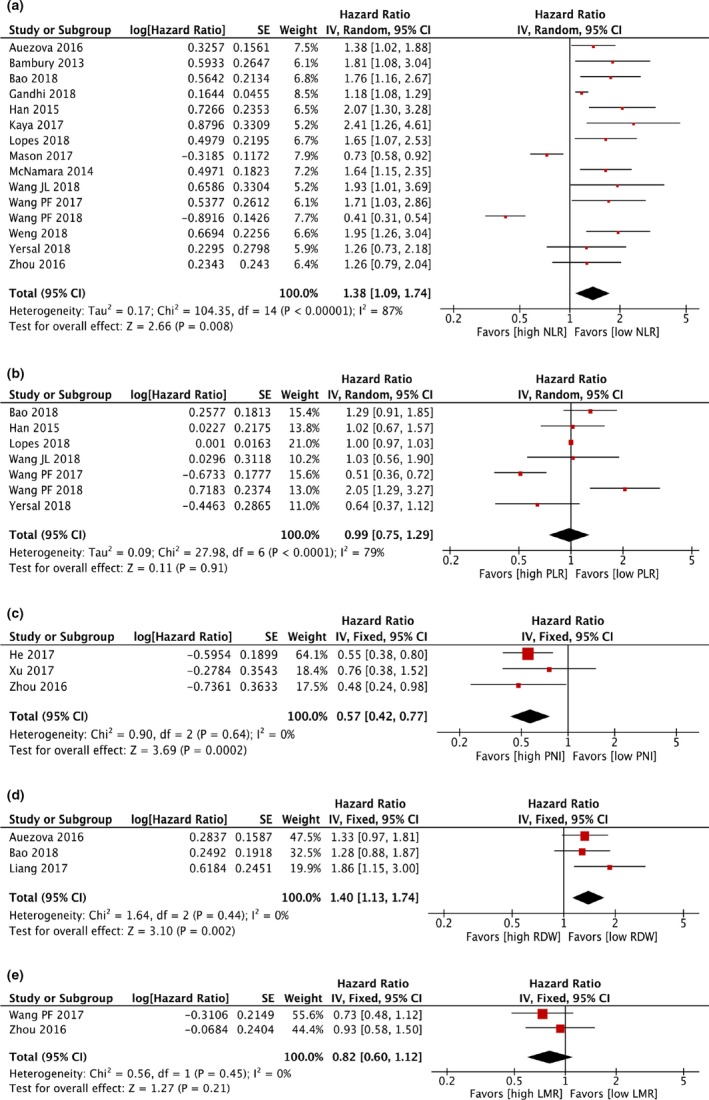
The pooled hazard ratio (HR) of (a) neutrophil/lymphocyte ratio (NLR); (b) platelet/lymphocyte ratio (PLR); (c) prognostic nutritional index (PNI); (d) red cell distribution width (RDW); and (e) lymphocyte/monocyte ratio (LMR) for overall survival (OS) in patients with glioma. Studies were ordered according to the authors. Heterogeneity among studies was determined using I 2 statistics at a significance level at P < 0.05. If P ≥ 0.05, a fixed‐effects model was used to calculate forest plots. If P < 0.05, the random‐effects models were used. A higher NLR or RDW, or a lower PNI is significantly associated with shorter OS in patients with gliomas. There was no significant difference in the pooled HR of PLR and LMR for OS in patients with glioma.
Subgroup analysis
Significant heterogeneities were observed for the prognostic significance of NLR (I 2 = 87%; P < 0.001) and PLR (I 2 = 79%; P < 0.001). Thus, subgroup analyses were performed by categorizing subgroups by region (Asian vs. non‐Asian), cutoff value (NLR: ≤ 4 vs. > 4; and PLR: ≤ 150 vs. > 150), adjust HR (multivariate vs. univariate), grade (GBM vs. various grades), and treatment (surgery vs. surgery + adjuvant therapy).
In the subgroup analyses of NLR, there were no significant associations among region, cutoff value, adjusted HR, grade, treatment, and the prognostic significance of NLR in gliomas, respectively (test for subgroup differences: P > 0.05; Figure S1 ). However, in the subgroup analyses of PLR, there were significant subgroup difference between the patients with GBM and the glioma patients (various grades; test for subgroup differences: I 2 = 80.9%; P = 0.02). There were no significant associations among region, cutoff value, adjusted HR, treatment, and the prognostic significance of PLR in gliomas, respectively (test for subgroup differences: P ≥ 0.05; Figure S2 ).
Sensitivity analysis and quality assessment
To test the robustness of the results, we removed one study each time in the pooled analysis and found that no single study substantially influenced the pooled association of interest. The 18 retrospective studies were scored a 6 or higher on the Newcastle–Ottawa Scale (Table 2 ). On the basis of the funnel plots, it could be concluded that studies that evaluated prognostic significance of pre‐operative NLR might have publication bias with the symmetric figure (Figure 3 a), however, studies that evaluated prognostic significance of pre‐operative PLR rarely have publication bias (Figure 3 b).
Table 2.
Qualities of included studies based on modified Newcastle‐Ottawa Scale
| Study | Case definition | Representativeness | Selection of controls | Definition of controls | Comparable for therapy | Comparable for etiology | Assessment of outcomes | Integrity of follow‐up | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Auezova et al.11, Lebanon | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Bambury et al.18, Ireland | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Bao et al.19, China | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Gandhi et al.20, India | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Han et al.21, China | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| He et al.22, China | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Kaya et al.23, Turkey | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Liang et al.24, China | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Lopes et al.25, Portugal | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Mason et al.26, Canada | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| McNamara et al.27, Canada | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Wang et al.28, China | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Wang et al.9, China | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Wang et al.29, China | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 5 |
| Weng et al.30, China | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Xu et al.31, China | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Yersal et al.32, Turkey | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Zhou et al.33, China | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
Figure 3.
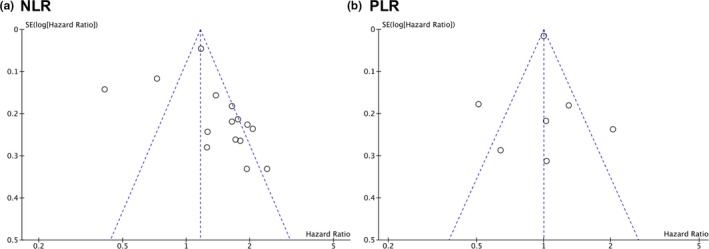
Funnel plot for (a) neutrophil/lymphocyte ratio (NLR) and (b) platelet/lymphocyte ratio (PLR). The funnel plot demonstrates whether there is evidence of publication bias in the studies that evaluated prognostic significance of preoperative NLR and PLR. Dotted lines, pseudo 95% confidence limits.
Discussion
Complex interactions between tumor cells and inflammatory cytokines regulate tumor growth and inflammatory progression. Systemic cellular inflammatory markers can be a noninvasive biomarker with relatively high sensitivity and specificity for glioma diagnosis.8 Moreover, the pre‐operative inflammatory response is correlated with glioma grading, which can be quantified as scores for predicting glioma survival after different therapeutic methods are used.30, 35 In this study, we performed a meta‐analysis to evaluate the prognostic role of systemic cellular inflammatory markers (NLR, PLR, LMR, RDW, and PNI) in 3,261 patients with glioma from 18 studies. Our findings indicated that NLR, RDW, and PNI are new prognostic markers for predicting the prognosis of gliomas. An increased NLR or RDW was found to be an independent predictor of worse survival in patients with gliomas. The pooled HR was considered significant if the 95% CI did not overlap 1 and the P value was < 0.05. The patient with a high NLR or RDW has a poorer prognosis compared with the patient with a low NLR or RDW (HR: 1.38; 95% CI: 1.09–1.74; P = 0.008; and HR: 1.40; 95% CI: 1.13–1.74; P = 0.002, respectively). Furthermore, a higher PNI indicates a better OS (HR: 0.57; 95% CI: 0.42–0.77; P = 0.0002). We also evaluated the PLR and LMR, but none of these variables correlated with OS (P = 0.91 and P = 0.21, respectively). Thus, NLR, RDW, and PNI may be served as the prognosticator. In the subgroup analysis of PLR, there were significant subgroup difference between the patients with GBM and the patients with glioma (various grades; test for subgroup differences: I 2 = 80.9%; P = 0.02), which indicated that the grade of glioma might lead to the significant heterogeneity in the overall analysis. There were no significant differences in all comparisons within other subgroups.
The molecular mechanisms through which the NLR, RDW, and PNI are associated with poor outcome of glioma remain unknown, but several hypotheses can be proposed. First, gliomas are complex tumors composed of neoplastic and non‐neoplastic cells, which cause a large accumulation of immune cells in the tumor microenvironment and systemic immune response during growth.36 Neutrophils are the most abundant white blood cells and are the first to be recruited to inflammatory sites.37 Furthermore, inflammatory cytokines or reactive oxygen species secreted by tumor cells cause the neutrophil count to increase, both in tumor stroma and in peripheral blood.23 The blood‐brain barrier is easily impaired in many neurologic tumors, resulting in an easy infiltration of monocytes. Growing evidence indicates that the neutrophil count is positively related with glioma grade, and is an early predictor of tumor progression in patients with GBM.29 NLR is positively correlated with the proliferation potency of gliomas.38 An elevated number of circulating and infiltrating neutrophils is influenced by glioma‐derived factors that can directly promote the proliferation of GBM cells by upregulating S100A4.37, 39 The increase in the neutrophil count may cause a decrease in lymphocytes and lymphocyte apoptosis.23 This interaction between neutrophils and lymphocytes in response to inflammation is of significance in immunosuppression.20, 23, 40 Secretion of Arginase‐1 by neutrophil supports immunosuppression and VEGF‐A production induces angiogenesis.29, 37 Neutrophils also promote angiogenesis by inducing a shift of gliomas from the proneural to the mesenchymal subtype, contributing to anti‐angiogenic therapy resistance.
RDW is a parameter reflecting the heterogeneity of red blood cell (RBC) volume. Higher RDW level indicates greater variation in the size of RBCs, which is associated with worse outcomes in patients with heart failure, stroke, head and neck cancer, and lung cancer.41 In accordance with previous studies,12, 24 we found that higher RDW was associated with a poorer OS of patients with glioma (HR: 1.40; 95% CI: 1.13–1.74; P = 0.002). It is reported that RDW correlated with IL‐6, tumor necrosis factor‐alpha, and other circulating cytokines that can affect the tumor cell biological behavior.7 Patients with the higher RDW are more likely to have a poorer prognosis than those with the lower RDW, an increased RDW can increase reactive oxygen species and induce tissue hypoxia, associated with an increased risk of postoperative complications.42 Additionally, a high RDW due to inflammatory cytokines results in increased hepcidin levels, which then reduce RBC production, causing anemia.43 Higher RDW in patients with glioma may be attributed to a variety of underlying metabolic abnormalities, such as inflammation, oxidative stress, and poor nutritional status.
Investigation of the nutritional and immunologic statuses using the PNI could be a useful clinical approach for managing malignancy patients. Patients with higher grade gliomas probably have a poorer PNI,33, 44 supporting the results of our meta‐analysis that a lower PNI is significantly associated with shorter OS in patients with gliomas. PNI is calculated based on the serum albumin concentration and total lymphocyte count, which is a significant indicator of postoperative complications.45 In general, patients with glioma who have poorer nutritional and immune conditions have a higher risk of postoperative complications, especially for severe complications that could significantly shorten survival time of patients.46 Previous reports have shown that the incidence of severe postoperative complications in patients with cancer with low‐PNI is twice that of patients with high‐PNI.45 In addition, low albumin and low total lymphocyte count are closely related to the development of an inflammatory response. Moreover, during cancer treatment and in patients with advanced disease, inadequate food intake and physical inactivity may lead to malnutrition, which may lead to chronic systemic inflammatory, chronic catabolism, and cachexia.47
PLR and LMR have been implicated in multiple tumor development and progression, such as gastric cancer, lung cancer, and gastrointestinal stromal tumors,48, 49 whereas the prognostic role of those in gliomas remains controversial. A high pre‐operative PLR and a low LMR is a predictor of poor prognosis for patients with glioma.19 On the contrary, some studies have demonstrated that pretreatment PLR or LMR is not associated with improved OS of patients with GBM.13, 32 This difference may be due to the difference in sample size and cutoff value. In a study of GBM, Han et al.21 has reported that the prognostic significance of PLR is far less than that of NLR. The level of PLR activation occurs prior to surgery in many intracranial neoplasms, PLR may not have the ability to differentiate glioma from meningioma or acoustic neuroma.8, 10 Although relatively high levels of PLR were found in patients with glioma, the underlying mechanism for the elevation still needs further investigation. For the LMR, there was no significant difference in the prognosis between the patients with low LMR and those with high LMR. This might be caused by limited studies. Only two studies were included. It is reported that LMR is not superior to the NLR for predicting the long‐term survival of patients with cancer, whereas it has significant diagnostic value for GBM compared with healthy controls.8, 49
There are several limitations to this study. First, this meta‐analysis was based on a limited number of retrospective studies. More well‐designed studies are needed to validate the result. Second, only summarized data and not individual patient data were available to analyze. Inflammatory and nutritional statuses are easily influenced by accompanying systemic diseases or medications taken.50 In addition, several factors, such as the glioma grade, cutoff values, and the adjustment of HR, were not coherent, which might partly account for the heterogeneity.
Conclusion
Our findings revealed that the NLR, RDW, and PNI rather than PLR and LMR are an independent index for predicting the prognosis of gliomas. A higher NLR or RDW, or a lower PNI is significantly associated with shorter OS in patients with gliomas. In spite of the limited number of studies, this meta‐analysis provides incremental understanding on the importance of systemic cellular inflammatory markers and nutritional index on predicting OS and improving further treatment in gliomas.
Funding
The study was supported by the National Nature Science Foundation of China (No. 81771410) and Priority of Shanghai Key Discipline of Medicine (No. 2017ZZ02020).
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
D.‐P.W. and K.K. wrote the manuscript. J.H. and D.‐P.W. designed the study. D.‐P.W. and K.K. performed the research. K.K. and Q.L. analyzed the data. Q.L. and J.H. contributed new reagents/analytical tools.
Supporting information
Table S1. Search algorithm.
Figure S1. Subgroup analysis of NLR.
Figure S2. Subgroup analysis of PLR.
Contributor Information
Da‐Peng Wang, Email: wdpboj@126.cm.
Jian Hai, Email: haijiandoct@zoho.com.cn.
References
- 1. Sutera, P. , Kalash, R. , Flickinger, J. , Engh, J. & Heron, D.E. Clinical and molecular recursive partitioning analysis of high‐grade glioma treated with IMRT. Am. J. Clin. Oncol. 42, 27–35 (2019). [DOI] [PubMed] [Google Scholar]
- 2. Wesseling, P. & Capper, D. WHO 2016 classification of gliomas. Neuropathol. Appl. Neurobiol. 44, 139–150 (2018). [DOI] [PubMed] [Google Scholar]
- 3. Lapointe, S. , Perry, A. & Butowski, N.A. Primary brain tumours in adults. Lancet 392, 432–446 (2018). [DOI] [PubMed] [Google Scholar]
- 4. Lieberman, N.A.P. et al Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol. 21, 83–94 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marshall, H.T. & Djamgoz, M.B.A. Immuno‐oncology: emerging targets and combination therapies. Front. Oncol. 8, 315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charrier, M. et al Circulating innate immune markers and outcomes in treatment‐naive advanced non‐small cell lung cancer patients. Eur. J. Cancer 108, 88–96 (2019). [DOI] [PubMed] [Google Scholar]
- 7. Hu, L. et al Prognostic value of RDW in cancers: a systematic review and meta‐analysis. Oncotarget 8, 16027–16035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng, S.H. et al Diagnostic value of preoperative inflammatory markers in patients with glioma: a multicenter cohort study. J. Neurosurg. 129, 583–592 (2018). [DOI] [PubMed] [Google Scholar]
- 9. Wang, P.F. et al Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival. Oncotarget 8, 50117–50123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kayhan, A. et al Preoperative systemic inflammatory markers in different brain pathologies: an analysis of 140 patients. Turk. Neurosurg. 29, 799–803 (2018). [DOI] [PubMed] [Google Scholar]
- 11. Auezova, R. et al Association of preoperative levels of selected blood inflammatory markers with prognosis in gliomas. Onco Targets Ther. 9, 6111–6117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu, W. , Wang, D. , Zheng, X. , Ou, Q. & Huang, L. Sex‐dependent association of preoperative hematologic markers with glioma grade and progression. J. Neurooncol. 137, 279–287 (2018). [DOI] [PubMed] [Google Scholar]
- 13. Diaz, R. , Westhuyzen, J. , Dwyer, P. & Aherne, N. Prognostic value of pre‐treatment systemic inflammatory markers in glioblastoma multiforme patients. J. Radiat. Oncol. 7, 121–122 (2018). [Google Scholar]
- 14. Zhang, J. et al Prognostic role of neutrophil lymphocyte ratio in patients with glioma. Oncotarget 8, 59217–59224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D.G. & PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patsopoulos, N.A. , Evangelou, E. & Ioannidis, J.P. Sensitivity of between‐study heterogeneity in meta‐analysis: proposed metrics and empirical evaluation. Int. J. Epidemiol. 37, 1148–1157 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyatt, G.H. et al GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J. Clin. Epidemiol. 64, 1294–1302 (2011). [DOI] [PubMed] [Google Scholar]
- 18. Bambury, R.M. et al The association of pre‐treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J. Neurooncol. 114, 149–154 (2013). [DOI] [PubMed] [Google Scholar]
- 19. Bao, Y. et al Preoperative hematologic inflammatory markers as prognostic factors in patients with glioma. World Neurosurg. 119, e710–e716 (2018). [DOI] [PubMed] [Google Scholar]
- 20. Gandhi, P. , Khare, R. , VasudevGulwani, H. & Kaur, S. Circulatory YKL‐40 & NLR: underestimated prognostic indicators in diffuse glioma. Int. J. Mol. Cell Med. 7, 111–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han, S. et al Pre‐treatment neutrophil‐to‐lymphocyte ratio is associated with neutrophil and T‐cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer 15, 617 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He, Z.Q. et al Low preoperative prognostic nutritional index predicts poor survival in patients with newly diagnosed high‐grade gliomas. J. Neurooncol. 132, 239–247 (2017). [DOI] [PubMed] [Google Scholar]
- 23. Kaya, V. et al Prognostic significance of indicators of systemic inflammatory responses in glioblastoma patients. Asian Pac. J. Cancer Prev. 18, 3287–3291 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang, R.F. , Li, M. , Yang, Y. , Mao, Q. & Liu, Y.H. Significance of pretreatment red blood cell distribution width in patients with newly diagnosed glioblastoma. Med. Sci. Monit. 23, 3217–3223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopes, M. , Carvalho, B. , Vaz, R. & Linhares, P. Influence of neutrophil‐lymphocyte ratio in prognosis of glioblastoma multiforme. J. Neurooncol. 136, 173–180 (2018). [DOI] [PubMed] [Google Scholar]
- 26. Mason, M. et al Neutrophil‐lymphocyte ratio dynamics during concurrent chemo‐radiotherapy for glioblastoma is an independent predictor for overall survival. J. Neurooncol. 132, 463–471 (2017). [DOI] [PubMed] [Google Scholar]
- 27. McNamara, M.G. et al Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J. Neurooncol. 117, 147–152 (2014). [DOI] [PubMed] [Google Scholar]
- 28. Wang, J.L. , Xiao, W.J. , Chen, W.Y. & Hu, Y.H. Prognostic significance of preoperative neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in patients with glioma. EXCLI J. 17, 505–512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang, P.F. et al Preoperative changes in hematological markers and predictors of glioma grade and survival. Front. Pharmacol. 9, 886 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weng, W. , Chen, X. , Gong, S. , Guo, L. & Zhang, X. Preoperative neutrophil‐lymphocyte ratio correlated with glioma grading and glioblastoma survival. Neurol. Res. 40, 917–922 (2018). [DOI] [PubMed] [Google Scholar]
- 31. Xu, W.Z. et al Preoperative albumin‐to‐globulin ratio and prognostic nutrition index predict prognosis for glioblastoma. Onco Targets Ther. 10, 725–733 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yersal, O. , Odabasi, E. , Ozdemir, O. & Kemal, Y. Prognostic significance of pre‐treatment neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in patients with glioblastoma. Mol. Clin. Oncol. 9, 453–458 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou, X.W. et al Significance of the prognostic nutritional index in patients with glioblastoma: a retrospective study. Clin. Neurol. Neurosurg. 151, 86–91 (2016). [DOI] [PubMed] [Google Scholar]
- 34. Han, S. , Huang, Y. , Li, Z. , Hou, H. & Wu, A. The prognostic role of preoperative serum albumin levels in glioblastoma patients. BMC Cancer 15, 108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuny, E. et al Association of elevated glial expression of interleukin‐1beta with improved survival in patients with glioblastomas multiforme. J. Neurosurg. 96, 294–301 (2002). [DOI] [PubMed] [Google Scholar]
- 36. Hambardzumyan, D. , Gutmann, D.H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Massara, M. et al Neutrophils in gliomas. Front. Immunol. 8, 1349 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weng, Y. et al Do selected blood inflammatory markers combined with radiological features predict proliferation index in glioma patients? World Neurosurg. 118, e137–e146 (2018). [DOI] [PubMed] [Google Scholar]
- 39. Liang, J. et al Neutrophils promote the malignant glioma phenotype through S100A4. Clin. Cancer Res. 20, 187–198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luo, Y. et al Evaluation of the clinical value of hematological parameters in patients with urothelial carcinoma of the bladder. Medicine (Baltimore) 97, e0351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kiriu, T. et al Prognostic value of red blood cell distribution width in non‐small cell lung cancer treated with anti‐programmed cell death‐1 antibody. In Vivo 33, 213–220 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xie, D.X. et al Association between red blood cell distribution width and outcomes of open airway reconstruction surgery in adults. JAMA Otolaryngol. Head Neck Surg. 145, 210–215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Topaz, G. et al The association between red cell distribution width and clinical outcomes in patients hospitalised due to chest pain. Acta Cardiol. 16, 1–6 (2019). [DOI] [PubMed] [Google Scholar]
- 44. Gaviani, P. et al Infections in neuro‐oncology. Neurol. Sci. 32 (suppl. 2), S233–S236 (2011). [DOI] [PubMed] [Google Scholar]
- 45. Tokunaga, R. et al Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis. Colon Rectum 58, 1048–1057 (2015). [DOI] [PubMed] [Google Scholar]
- 46. Marra, J.S. et al Survival after radiation therapy for high‐grade glioma. Rep. Pract. Oncol. Radiother. 24, 35–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arends, J. [Nutrition in cancer: effective in prevention and treatment?] Dtsch. Med. Wochenschr. 142, 889–895 (2017). [DOI] [PubMed] [Google Scholar]
- 48. Cananzi, F.C.M. et al Preoperative monocyte‐to‐lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J. Surg. Oncol. 119, 12–20 (2019). [DOI] [PubMed] [Google Scholar]
- 49. Dupre, A. & Malik, H.Z. Inflammation and cancer: what a surgical oncologist should know. Eur. J. Surg. Oncol. 44, 566–570 (2018). [DOI] [PubMed] [Google Scholar]
- 50. Arends, J. Struggling with nutrition in patients with advanced cancer: nutrition and nourishment‐focusing on metabolism and supportive care. Ann. Oncol. 29, ii27–ii34 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search algorithm.
Figure S1. Subgroup analysis of NLR.
Figure S2. Subgroup analysis of PLR.


