Abstract
Adherence with antiretroviral therapy is important for preventing disease progression and HIV transmission. The co‐encapsulated pill sensor system sends a signal through a cutaneous patch and allows real‐time monitoring of pill ingestion. A 16‐week pilot study used a sensor system in 15 HIV‐infected individuals with real‐time monitoring of pill‐taking with a personalized short message system text. System acceptability was assessed by survey at weeks 4, 8, 12, and 16. Follow‐up occurred in 80% of subjects through 8 weeks. The system effectively collected measures of pill ingestion, which triggered text message reminders. Only 2 of 14 participants stated that co‐encapsulated pills were “unable to take” or “poorly tolerated.” At least 75% of respondents stated at each visit that the patch was very or somewhat comfortable. With regard to text message reminders, only 10–15% of the participants at any visit did not find the messages to be helpful. Larger studies will define the utility of this system to assess antiretroviral adherence relative to standard measures.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ There is growing body of data using novel co‐encapsulation of ingestion sensor to track adherence. This technology has not yet been applied to those chronically infected with HIV on antiretroviral (ARV) therapy.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ The implementation, participant acceptability, and satisfaction of the co‐encapsulation of ingestion sensor and ARVs with real‐time monitoring and short message system reminders in HIV‐infected individuals.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The study demonstrates that the ingestible sensor system using real‐time monitoring with personalized short message system can be implemented with commonly used ARV agents. The study further provides an assessment of the acceptability of taking co‐encapsulated ARV/sensor, using a dermal patch with a paired mobile device and an overview as to how study subjects felt about using the overall system.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ This pilot study sets the stage for implementing novel monitoring technology for HIV‐infected patients, particularly those who are poorly adherent to ARVs, to further define utility as well as ability to assess adherence in comparison to standard measures.
The availability of potent antiretroviral (ARV) therapy has transitioned HIV infection into a chronic disease. Maximizing the benefits of ARV therapy requires optimal adherence with pill‐taking and clinic visits to reach viral suppression, which reduces likelihood of disease progression, emergence of drug‐resistant virus, and HIV transmission.1, 2, 3, 4 Adherence can be challenging to quantify and has traditionally been done with self‐report questionnaires and in clinical trials, often incorporating pill counts and pharmacy refill records.5 Additional methods have been developed using electronic monitoring, such as Medication Event Monitoring Systems that records when pill bottles are opened.6 Although this and related technologies advance the ability to measure adherence, they are limited to inferred estimates of adherence because they depend upon the pill‐taking individuals' cooperation, do not directly record medication taking, and may not report adherence in real‐time. Innovative monitoring systems have been created that are able to detect real‐time opening through Bluetooth and Wi‐Fi technologies vs. historical pill bottle opening, as with Medication Event Monitoring Systems.7 However, many of these only detect when the pill bottle is opened but not when the pill is actually ingested. It also does not allow use of other simple technology to assist with adherence, such as pill boxes.
A novel technology called the Proteus Digital Health Feedback (PDHF) system (Proteus Digital Health, Redwood, CA) was approved by the US Food and Drug Administration in 2012 and given clearance in 2015 for real‐time monitoring of medication ingestion, with additional provision for automated short message system (text) reminders.8 The PDHF system involves use of an ingestible sensor made of an inert edible material that can be co‐encapsulated (Capsugel, Greenwood, SC) with prescribed medications. The ingestible sensor is activated when stomach liquid dissolves the encapsulating material, and the sensor is then detected by a monitor‐patch worn over patient's upper abdomen. The patch contains a microchip and a tiny battery. The microchip sends a Bluetooth signal to the individual's mobile device (e.g., tablet or cell phone), which, in turn, sends an encrypted message to a central server, thus allowing for a real‐time signal that is day and time stamped for when a dose has been taken (Figure 1). A short message system can be programmed in response to the receipt (or not) of the ingestion signal, for example, a reinforcing message if a dose is taken and a reminder after a scheduled dose is missed. This technology has been used in those treated for a variety of disease states, including hypertension, mental health conditions, tuberculosis, and organ transplantation.9, 10, 11, 12, 13 Studies have reported at least 95% detection sensitivity and 99.7% specificity.8, 12 However, this device has not yet been tested for ARV therapy use in HIV‐infected individuals. We now report the results of a pilot study to introduce this system, along with personalized text reminders to HIV‐infected individuals to assess functionality and participant acceptability and satisfaction with the system.
Figure 1.
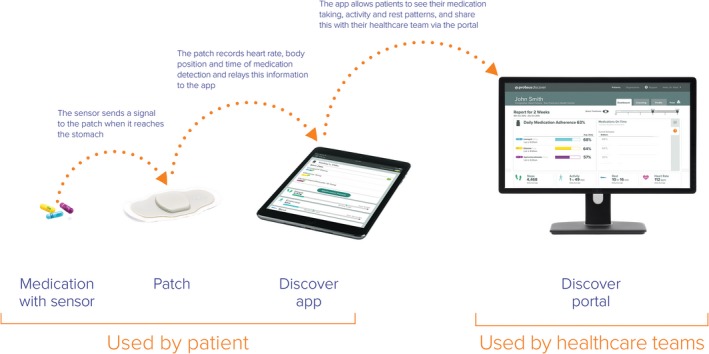
Monitoring system (courtesy of Proteus Digital Health, Redwood, CA).
Materials and Methods
The current study was designed to pilot the PDHF system in HIV‐infected individuals on ARV therapy and to determine approaches to maximize utilization and assess acceptability in 15 HIV‐infected individuals with evidence of poor adherence. This was designed to address issues related to real‐time data handling, co‐encapsulation, patch use, and medication delivery along with personalized text messaging feedback. Co‐encapsulation was performed by investigational drug pharmacists per manufacturer guidelines and as summarized by Browne et al.,14 using the same size gelatin cap for all medications, regardless of their size. Bioavailability of utilized co‐encapsulated ARVs was previously presented.15 The personalized text message was developed with input from each study participant. For the pilot study, the system was programed to send a message if a dose was not detected to be taken within 1 hour of when it was scheduled. If a dose was missed, the system also sent a customized predose reminder for future doses with each participant being able to request that reminders be stopped at any time.
We recruited HIV‐infected individuals in HIV care who were receiving stable ARV therapy with evidence of suboptimal adherence as defined by < 90% adherence over the last 28 days by self‐report, or over the last 6 months as perceived by the treating clinician based on missed appointments, reported missed doses, or viral load elevations. In order to be enrolled, participants needed to indicate that they tolerated a single co‐encapsulated medication at the time of screening and be able to provide informed consent. Subjects were excluded if they were unable to follow study procedures, had evidence of cognitive deficits or substance use that were thought by the investigator to render them unable to follow study procedures, or were pregnant. The study was reviewed and approved by the Institutional Review Board at the Los Angeles Biomedical Research Institute at Harbor‐University of California, Los Angeles Medical Center and the David Geffen School of Medicine at University of California, Los Angeles.
Enrolled individuals had one of the ARV pills they were routinely taking co‐encapsulated during the 16 weeks of study, a process that we performed per manufacturer guidelines by an investigational drug pharmacist.14 Participants were provided instructions on how to use the PDHF system, including patch use and synchronization with the patch and mobile devices. Participants were contacted over the phone on days 3 and 14 to assess early problems and then scheduled for in‐person study visits at weeks 4, 8, 12, and 16 at which they completed surveys related to satisfaction with different aspects of the system and perceived benefits. Results were tabulated and presented in a descriptive fashion for the purposes of this pilot study.
Results
All 15 participants screened met inclusion criteria and were enrolled. The baseline characteristics of the enrolled individuals are summarized in Table 1. Nine of the subjects were on single‐tablet ARV regimens that included elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (n = 1), tenofovir alafenamide/emtricitabine/rilpivirine (n = 3), and abacavir/lamivudine/dolutegravir (n = 5), with the remaining being on the fixed‐dose combination of tenofovir alafenamide/emtricitabine given daily as the co‐encapsulated drug along with an unencapsulated pharmacologically boosted protease inhibitor. Although all met criteria for concerns for poor adherence, 10 had plasma HIV‐1 RNA levels < 20 copies/mL at the time of their most recent testing. Key aspects of the approved system functioned throughout study, including ability for ingestion information to be received by patch and relayed to the server, as well as automatic personalized text messages sent after 1 hour of a missed dose, and again as predose reminders for those who previously missed a dose.
Table 1.
Baseline characteristics
| Characteristics (n = 15) | Mean (SD) or n (%) |
|---|---|
| Age, years | 48 (6.9) |
| Sex | |
| Male | 13 (86.7) |
| Race and ethnicity | |
| Black | 7 (46.7) |
| Hispanic white | 6 (40.0) |
| Non‐Hispanic white | 2 (13.3) |
| Self‐identified major risk factor for HIV infection | |
| MSM | 9 (60.0) |
| Homosexual | 5 (33.3) |
| i.v. drug use | 1 (6.7) |
| Co‐encapsulated ARV | |
| TAF/FTC | 6 (40.0) |
| EVG/COBI/FTC/TAF | 1 (6.7) |
| TAF/FTC/RPV | 3 (20.0) |
| ABC/3TC/DTG | 5 (33.3) |
| Most recent CD4 count, cells/uL [min, max] | 774.2 [275, 1375] |
| Most recent plasma HIV RNA | |
| < 20 copies/mL | 10 (66.7) |
| ≥ 20 copies/mL | 4 (26.7) |
| Unknown | 1 (6.7) |
ABC/3TC/DTG, abacavir/lamivudine/dolutegravir; ARV, antiretroviral; EVG/COBI/FTC/TAF, elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine; MSM, Men who have sex with men; TAF/FTC, tenofovir alafenamide/emtricitabine; TAF/FTC/RPV, tenofovir alafenamide/emtricitabine/rilpivirine.
Of the 15 participants, 1 stopped wearing the patch before week 4 due to persistent skin reaction, but remained in the study follow‐up. Two withdrew consent, 1 after week 4 and the other after week 8 visits. The former stated he stopped due to personal medical issues unrelated to the study and the latter stating the PDHF system was too complicated. One participant was lost to follow‐up after week 8. Therefore, 12 subjects remained on the study throughout, although there were several that intermittently missed select study visits. The acceptability and satisfaction of different aspects of the system were collected in those that presented for each of the follow‐up visits. Key survey responses were assessed regarding taking co‐encapsulated pills, using the cutaneous patch, receiving text messages, and overall utilization of the system.
As shown in Tables 2, 3, 4, attrition and missed visits resulted in 8–10 of the 15 original participants having data available at the final week 16 assessments. Participants were queried regarding tolerability of co‐encapsulated medications. More than 70% stated during the 4‐week and 8‐week visits, when there was the highest rate of follow‐up, that it was well‐tolerated (Table 2). Additional responses related to tolerability of co‐encapsulated pills were consistent with this (Table S1 ). Participants were also asked about the use of the patch. Although one participant had significant skin reaction resulting in it being stopped early, overall responses were good, including during week 4 and 8 visits when there was the highest rate of responses. For example, when asked how comfortable they were with the patch, > 70% responded that it was either very comfortable or somewhat comfortable. Approximately 15–25% at each visit stated the patch was somewhat uncomfortable with zero to one participant at each visit stating it was very uncomfortable. Additional responses related to patch use are summarized in Table S2 .
Table 2.
Participant satisfaction with co‐encapsulated pill/sensor and patch
| Week 4 | Week 8 | Week 12 | Week 16 | |
|---|---|---|---|---|
| N = 14, n (%) | N = 13, n (%) | N = 9, n (%) | N = 10, n (%) | |
| How well do you tolerate the co‐encapsulated antiretroviral medications?a | ||||
| Well tolerated | 10 (71.4) | 10 (76.9) | 9 (100) | 8 (80.0) |
| Satisfactory | 1 (7.1) | 1 (7.7) | 0 (0) | 2 (20.0) |
| Can be tolerated | 2 (14.3) | 1 (7.7) | 0 (0) | 0 (0) |
| Poorly tolerated | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) |
| Unable to take | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) |
| Week 4 | Week 8 | Week 12 | Week 16 | |
|---|---|---|---|---|
| N = 14, n (%) | N = 13, n (%) | N = 8, n (%) | N = 8, n (%) | |
| How comfortable are you with the patch? | ||||
| Very comfortable | 3 (21.4) | 6 (46.2) | 2 (25.0) | 4 (50.0) |
| Somewhat comfortable | 8 (57.1) | 4 (30.8) | 4 (50.0) | 3 (37.5) |
| Somewhat uncomfortable | 2 (14.3) | 2 (15.4) | 2 (25.0) | 1 (12.5) |
| Very uncomfortable | 1 (7.1) | 1 (7.7) | 0 (0) | 0 (0) |
All medications, regardless of size, were co‐encapsulated with the same size gelatin capsule.
Table 3.
Participant satisfaction and perceived utility of short text messaging system
| Week 4 | Week 8 | Week 12 | Week 16 | |
|---|---|---|---|---|
| N = 14, n (%) | N = 13, n (%) | N = 8, n (%) | N = 8, n (%) | |
| How comfortable are you with receiving text messages through the system? | ||||
| Very comfortable | 7 (50.0) | 7 (53.9) | 5 (62.5) | 5 (62.5) |
| Somewhat comfortable | 5 (35.7) | 5 (38.5) | 1 (12.5) | 2 (25.0) |
| Somewhat uncomfortable | 2 (14.3) | 0 (0) | 1 (12.5) | 1 (12.5) |
| Not applicablea | 0 (0) | 1 (7.7) | 1 (12.5) | 0 (0) |
| Week 4 | Week 8 | Week 12 | Week 16 | |
|---|---|---|---|---|
| N = 14, n (%) | N = 12, n (%) | N = 9, n (%) | N = 9, n (%) | |
| The text messages are helpful | ||||
| Strongly agree | 5 (35.7) | 3 (25.0) | 5 (55.6) | 3 (33.3) |
| Somewhat agree | 5 (35.7) | 3 (25.0) | 0 (0) | 0 (0) |
| Neutral | 1 (7.1) | 3 (25.0) | 2 (22.2) | 3 (33.3) |
| Somewhat disagree | 0 (0) | 0 (0) | 0 (0) | 1 (11.1) |
| Strongly disagree | 2 (14.3) | 0 (0) | 1 (11.1) | 0 (0) |
| Not applicablea | 1 (7.1) | 3 (25.0) | 1 (11.1) | 2 (22.2) |
Text messages were only sent if a dose was missed, so they were not received by everyone.
Table 4.
Participant perceived utility and satisfaction with the Proteus Digital Health Feedback System
| Week 4 | Week 8 | Week 12 | Week 16 | |
|---|---|---|---|---|
| N = 14, n (%) | N = 12, n (%) | N = 9, n (%) | N = 9, n (%) | |
| The sensor system is helpful | ||||
| Strongly agree | 8 (57.1) | 4 (33.3) | 5 (55.6) | 5 (55.6) |
| Somewhat agree | 4 (28.6) | 5 (41.7) | 1 (11.1) | 2 (22.2) |
| Neutral | 2 (14.3) | 2 (16.7) | 1 (11.1) | 0 (0) |
| Somewhat disagree | 0 (0) | 0 (0) | 2 (22.2) | 1 (11.1) |
| Strongly disagree | 0 (0) | 1 (8.3) | 0 (0) | 1 (11.1) |
| Overall I am satisfied with the sensor system | ||||
| Strongly agree | 2 (14.3) | 6 (50.0) | 5 (55.6) | 4 (44.4) |
| Somewhat agree | 6 (42.9) | 4 (33.3) | 0 (0) | 2 (22.2) |
| Neutral | 6 (42.9) | 0 (0) | 2 (22.2) | 1 (11.1) |
| Somewhat disagree | 0 (0) | 2 (16.7) | 2 (22.2) | 1 (11.1) |
| Strongly disagree | 0 (0) | 0 (0) | 0 (0) | 1 (11.1) |
Text messaging was only utilized for those who missed a dose, after which it was sent prior to subsequent scheduled doses unless the participant requested it to be stopped. All participants missed at least one dose and received a text message during the course of the study. It is noteworthy that only 2 of the 15 requested that the predose text reminder be stopped. Based upon those who responded, well over 70% stated that they were very comfortable receiving text messages through the system (Table 3). In response to whether participants thought the text messages were helpful, the results were more variable, but most either strongly or somewhat agreed that it was helpful (Table 3). Additional responses to the text messaging aspect of the system are summarized in Table S3 .
Questions also probed the level of satisfaction and perceived usefulness of the overall system. In response to the question, “The sensor system is helpful,” a majority, including 85% and 75% of those responding at weeks 4 and 8, respectively, noted that they somewhat or strongly agreed. In response to the question “Overall I am satisfied with the sensor system,” ~ 55% of respondents at week 4 and 83% at week 8 stated that they somewhat or strongly agreed (Table 4). Additional responses to the acceptability of the overall system are included in Table S4 .
Discussion
This pilot study assessed the functionality, participant satisfaction with and potential utility of the novel PDHF system that incorporates the use of co‐encapsulated medication with ingestible sensor, application of a patch, real‐time monitoring of adherence through a mobile device, and personalized text message reminders. There was reasonable follow‐up during the first 8 weeks, which fell off after that due to two withdrawals of consents, one of which was due to complexity of the system. There was an additional person lost to follow‐up with others intermittently missing scheduled visits. The text message system worked as programed through the course of follow‐up. For those with follow‐up, all but one subject per visit stated they were able to tolerate taking the co‐encapsulated pill. One participant stopped the patch early due to a skin reaction, with one to three participants per visit stating that it was somewhat or very uncomfortable. Only one to two per visit did not find the text messaging to be helpful, with similar numbers not finding the overall system to be helpful.
The PDHF system has been used in a variety of patient populations in whom treatment adherence is important, such as those with hypertension, mental health conditions, tuberculosis, and transplantation.9, 10, 11, 12 The PDHF system has proven to be highly accurate for detecting ingestions and without serious adverse events.8 The satisfaction responses in our pilot were similar to those previously reported in patients with diseases other than HIV. For example, Belknap et al.12 studied 30 individuals treated for tuberculosis with 20 of them completing a post‐treatment survey. Of those surveyed, 83% said they were comfortable using the system with only three device‐related adverse events, all of which were skin irritation. Satisfaction was similar in other studies, such as one that included 37 individuals monitored for 16 weeks of their hypertension treatment. In this case, 40% complained of mild and transient skin patch‐associated irritation with 90% responding that they were satisfied with the co‐encapsulated pill, and 75% stating that the overall experience was positive.11 Eisenberger et al.9 reported a study of 20 patients who underwent kidney transplantation using the PDHF system with seven experiencing a rash or erythema from the patch, all occurring in the first 4 weeks of follow‐up with two stopping because of this adverse event.
Our study explored the acceptability and satisfaction of the PDHF system using co‐encapsulated ARVs. Although there were concerns about the size of the co‐encapsulated pills, all screened patients were able to take them and most found them to be acceptable for use. This issue should become even less of a concern as HIV treatments continue to evolve toward smaller pills with many commonly used regimens. Future studies will assess acceptability of the system in select groups, including those with the greatest adherence problems. Larger studies will also need to consider the benefits relative to the costs of this novel intervention to improve treatment adherence.
In summary, this pilot study demonstrated that the PDHF system was able to collect real‐time ingestion data and automatically transmit reminder text messages to HIV‐infected individuals taking ARV therapy. In addition, data were collected on the overall acceptability of different aspects of the system in this patient population. The information and experience gained from this pilot study set the stage for future studies to assess whether this novel system is capable of monitoring real‐time ingestion of ARVs, which would allow for immediate interventions in response to pill‐taking lapses. The goal of real‐time monitoring is to identify early problems with adherence and intervene to enhance viral suppression for the good of the HIV‐infected individuals and to minimize their risk of transmitting HIV to others.
Funding
This work was supported by grant R01‐MH110056 from the National Institute of Mental Health/National Institute of Health.
Conflicts of interest
T.Y. is currently an employee of Proteus Digital Health. All other authors declared no competing interests for this work.
Author Contributions
E.S.D. and H.L. wrote the manuscript. E.S.D., M.R., C.V.F., and H.L. designed the research. E.S.D., M.R., L.S., J.S., M.G., D.X., K.C., and H.L. performed the research. E.S.D., Y.W., J.S., D.X., and H.L. analyzed the data. J.D. and T.Y. contributed new reagents/analytical tools.
Supporting information
Tables S1–S4.
Acknowledgment
We thank all patients who participated in the pilot study to assess function and acceptability of co‐encapsulated ARV monitoring by the PDHF system.
References
- 1. Chesney, M.A. The elusive gold standard: future perspectives for HIV adherence assessment and intervention. J. Acquir. Immun. Defic. Syndr. 43, S149–S155 (2006). [DOI] [PubMed] [Google Scholar]
- 2. Vrijens, B. et al Modelling the association between adherence and viral load in HIV‐infected patients. Stat. Med. 24, 2719–2731 (2006). [DOI] [PubMed] [Google Scholar]
- 3. Cohen, M.S. et al Prevention of HIV‐1 infection with early antiretroviral therapy. N. Engl. J. Med. 365, 493–505 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodger, A.J. et al Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV‐positive partner is using suppressive antiretroviral therapy. JAMA 316, 171–181 (2016). [DOI] [PubMed] [Google Scholar]
- 5. Osterberg, L. & Blaschke, T. Adherence to medication. N. Engl. J. Med. 353, 487–497 (2005). [DOI] [PubMed] [Google Scholar]
- 6. Blaschke, T.F. , Osterberg, L. , Vrijes, B. & Urquhart, J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu. Rev. Pharmacol. Toxicol. 52, 275–301 (2012). [DOI] [PubMed] [Google Scholar]
- 7. Moore, B.A. et al A remotely‐delivered CBT and contingency management therapy for substance using people with HIV. AIDS Behav. 2, 156–162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hafezi, H. et al An ingestible sensor for measuring medication adherence. IEEE Trans. Biomed. Eng. 62, 99–109 (2015). [DOI] [PubMed] [Google Scholar]
- 9. Eisenberger, U. , Wuthrich, R.P. & Bock, A. Medication adherence assessment: high accuracy of the new ingestible sensor system in kidney transplants. Transplantation 96, 245–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters‐Strickland, T. , Pestreich, L. & Hatch, A. Usability of a novel digital medicine system in adults with schizophrenia treated with sensor‐embedded tablets of aripiprazole. Neuropsychiatr. Dis. Treat. 12, 2587–2594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiCarlo, L.A. et al Patient‐centered home care using digital medicine and telemetric data for hypertension: feasibility and acceptability of objective ambulatory assessment. J. Clin. Hyperten. 18, 901–906 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belknap, R. , Weis, S. & Brookens, A. Feasibility of an ingestible sensor‐based system for monitoring adherence to tuberculosis therapy. PLoS One 8, e53373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vallejos, X. & Wu, C. Digital medicine: innovative drug‐device combination as new measure of medication adherence. J. Pharm. Technol. 33, 137–139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Browne, S.H. et al Digitizing medicines for remote capture of oral medication adherence using co‐encapsulation. Clin. Pharmacol. Ther. 103, 502–510 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu, H. et al Bioavailability of co‐encapsulated antiretrovirals with ingestible sensor for measuring adherence. 12th International Conference on HIV Treatment and Prevention Adherence, Miami, FL, June 4−6, 2017, Abstract #298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4.


