Abstract
Background
Daptomycin and ceftaroline (DAP-CPT) have been used for persistent methicillin-resistant Staphylococcus aureus bacteremia (MRSAB), but have rarely been compared with other therapies. This study provides an exploratory analysis of patients placed on DAP-CPT vs standard of care (SOC) for MRSAB.
Methods
This is a retrospective, matched cohort study MRSAB patients at 4 hospitals in the United States. Patients receiving DAP-CPT for ≥72 hours at any point in therapy were matched 2:1 when possible, 1:1 otherwise, to SOC, first by infection source, then age and renal function. SOC was empiric treatment with vancomycin or daptomycin and any subsequent combination antibiotic(s), except for DAP-CPT.
Results
Fifty-eight patients received DAP-CPT with 113 matched SOC. Ninety-six percent of SOC received vancomycin, and 56% (63/113) escalated therapy at least once in the treatment course. Twenty-four patients received DAP-CPT within 72 hours of index culture; 2 (8.3%) died within 30 days vs 14.2% (16/113) with SOC (P > .05). Subgroup analysis identified numerically lower mortality in DAP-CPT patients with a Charlson comorbidity index ≥3, endovascular source, and receipt of DAP-CPT within 72 hours of index culture. The median MRSAB duration was 9.3 vs 4.8 days for DAP-CPT and SOC, respectively. DAP-CPT was initiated on day 6 on average; after receipt of DAP-CPT, MRSAB duration was 3.3 days.
Conclusions
DAP-CPT treatment is often delayed in MRSAB. Combination therapy may be more beneficial if initiated earlier, particularly in patients at higher risk for mortality. Blinded, randomized, prospective studies are needed to eliminate selection bias inherent in retrospective analyses when examining DAP-CPT vs SOC.
Keywords: antimicrobial resistance, combination, gram-positive, salvage therapy, vancomycin
This multicenter, retrospective, exploratory, matched study compares demographics and clinical outcomes of patients receiving daptomycin plus ceftaroline at any time in treatment course versus other therapy for MRSA bacteremia. This combination used within 72 hours of index culture may be more beneficial for survival than as salvage therapy.
Methicillin-resistant Staphylococcus aureus bacteremia (MRSAB) is associated with significant morbidity and mortality despite widely available treatment options. Guideline-recommended firstline therapy for MRSAB consists of vancomycin or daptomycin monotherapy, both of which have been associated with clinical failure [1]. Options for salvage therapy after initial treatment failure include escalating daptomycin dose, switching to an alternative agent, or using a combination of 2 or more antibiotics [2]. The synergy of β-lactam antibiotics combined with vancomycin (VAN) or daptomycin (DAP) against S. aureus has been consistently shown in vitro [3–7]. Combination therapy has been used to successfully salvage cases of MRSAB, but clearance of bacteremia in these cases may not improve outcomes such as reducing mortality or infection relapse [8–10]. Consequently, optimal positioning of combination antimicrobial therapy in the algorithm of MRSAB management remains unknown.
Ceftaroline fosamil (CPT) was approved by the Food and Drug Administration in October 2010 for the treatment of community-acquired pneumonia and skin and skin structure infections, but its use has been explored beyond these indications [11–13]. Studies have demonstrated the clinical success of CPT alone or in combination for treatment-refractory MRSAB [10, 14, 15]. Ceftaroline is a particularly appealing β-lactam option to use in combination with other agents due to its inherent activity against MRSA mediated by PBP2a binding. It also demonstrates the “seesaw effect” when used in combination with VAN and DAP, wherein reduced glycopeptide and lipopeptide susceptibility confers increased susceptibility to antistaphylococcal β-lactams [16–18]. Additionally, ceftaroline has been shown to enhance DAP binding and cell membrane depolarization, resulting in rapid, sustained bactericidal activity against MRSA [19, 20]. As stated, this has translated to clearance of persistent bacteremia when DAP and CPT (DAP-CPT) are used as salvage therapy [10].
Controlled clinical data comparing DAP-CPT combination therapy vs standard of care (SOC) for MRSAB are limited to a pilot study of 40 patients wherein 17 patients treated with DAP-CPT within 72 hours of index culture were compared with 23 patients treated with standard monotherapy [21]. Treatment with DAP-CPT resulted in a significant reduction of in-hospital mortality. There are limited real-world data comparing demographics and clinical outcomes in patients receiving DAP-CPT vs SOC treatment for MRSAB. The purpose of this study was to provide a descriptive exploratory analysis of patients placed on DAP-CPT at any point in the treatment course vs matched patients receiving SOC for MRSAB.
METHODS
Study Design and Patient Population
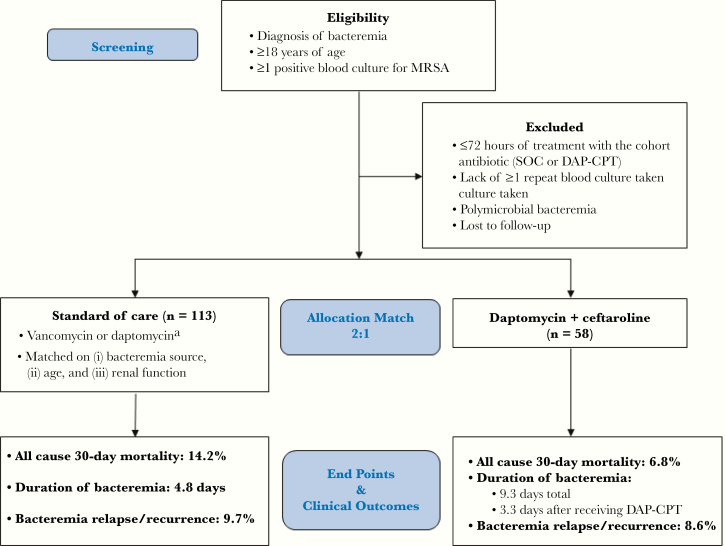
This was a retrospective, multicenter, matched cohort study of hospitalized patients describing treatment for MRSAB at 4 urban medical centers (2 academic) in the United States from January 2013 to October 2017 (Madison, WI, USA; Detroit, MI, USA; San Diego, CA, USA). Figure 1 describes the patient screening and inclusion and exclusion criteria. This study was approved by the institutional review board at each institution.
Figure 1.
Study population and analysis cohort with study outcome results. aIncludes empiric treatment with vancomycin or daptomycin; any subsequent non-DAP-CPT combination allowed. Abbreviations: DAP-CPT, daptomycin and ceftaroline; MRSA, methicillin-resistant Staphylococcus aureus; SOC, standard of care.
The electronic health record (EHR) was queried at each center to identify all patients with MRSAB and treated with DAP-CPT for ≥72 hours at any point in therapy during the study period for inclusion in the combination therapy group. As displayed in Figure 1, Patients were matched (2 SOC for every DAP-CPT patient) according to bacteremia source (primary, endovascular; secondary, nonendovascular; or catheter-related) [22, 23] then by age (±10 years), and finally by renal function (creatinine clearance ≥50 mL/min, <50 mL/min, dialysis-dependent). SOC patients had to match all 3 criteria for inclusion in the study population. Investigators classified patients by source according to documentation in the infectious diseases (ID) consult note. If the patient did not have ID consultation, the source documented in the primary team’s progress note was used for classification. Additionally, all patients had diagnostic imaging performed to corroborate primary source diagnosis (eg, transesophageal echocardiogram if endocarditis), along with clinical and microbiological data.
Additional data extracted from the EHR included demographic information, microbiological data, diagnostic imaging, antibiotic therapy, surgical source control, and length of stay in intensive and general care units. Antimicrobial susceptibility testing was performed by MicroScan WalkAway (UW Health, Sharp Healthcare Hospitals), BD Phoenix (Detroit Medical Center), and Vitek-2 (Henry Ford Hospital). Charlson Comorbidity Index and Pitt Bacteremia Score were calculated for every patient included in the study population to quantify the degree of comorbidity and severity of illness, respectively, at the time of index culture [24–26]. If a patient was transferred from a referring facility, Pitt Bacteremia Score was calculated using the worst set of clinical data recorded within the first 24 hours of study center admission.
Definitions
The study end points are displayed in Figure 1. The Cockcroft-Gault equation was used to calculate creatinine clearance using variables provided at the time of index culture [27]. Duration of bacteremia was calculated as the number of days between the first positive blood culture and the first negative blood culture without subsequent positive cultures within 72 hours of the negative result. The negative result was also considered time of microbiological cure. Bacteremia recurrence was defined as at least 1 positive blood culture for MRSA 7 or more days after initial microbiological cure. Source control was defined as catheter removal (if line-associated), amputation (if bone/joint source), drainage and/or debridement (if abscess or skin source), valve replacement (if endocarditis), abdominal surgery or graft removal (if abdominal source), laminectomy (if spinal osteomyelitis).
Statistical Analysis
Descriptive statistics and analyses were used to compare patient demographics and outcomes. Characteristics potentially associated with clinical or microbiological outcomes were compared using the χ 2 test for categorical variables. Continuous variables were compared by Student t test or the Mann-Whitney U test. Correlation was evaluated by Spearman r analysis for nonparametric data. A P value ≤.05 was considered statistically significant in the final analysis. STATA, version 15 (Stata Corp LLC, College Station, Texas, USA), and GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) were used for statistical analysis.
RESULTS
A total of 171 patients were included in the study. Fifty-eight patients receiving combination therapy were matched to 113 patients receiving SOC (Figure 1). These patients have not been reported previously, with the exception 7 patients receiving DAP-CPT, which included 5 from Sakoulas et al. [21] and 2 from Jorgensen et al. [28]. Patient demographics and clinical characteristics compared between the 2 groups are provided in Table 1. There were no statistically significant differences between groups except longer length of hospitalization (median, 23.3 ± 2.2 days vs 15.6 ± 0.9 days in SOC; P < .001) and more patients with inadequate source control (29% vs 40% in SOC; P < .001) for the DAP-CPT patients. The average DAP dose in patients receiving combination therapy was 8.2 mg/kg actual body weight. Ceftaroline was administered every 8 hours in 45/58 patients (77.6%). One patient in the combination therapy group received DAP-CPT for the entire treatment course. Of the remaining 57 patients, 29 (51%) received DAP-CPT as second-line therapy, 26 (46%) as third-line therapy, and 2 (3%) as fourth-line therapy. Thirty-two patients (55%) completed treatment on DAP-CPT, 15 (26%) were deescalated to DAP monotherapy, 6 (10%) to CPT monotherapy, and 5 (9%) to VAN monotherapy.
Table 1.
Study Population Baseline Characteristics
| Cohort | |||
|---|---|---|---|
| Variable | DAP-CPTa (n = 58) | Standard of Carea (n = 113) | P Value |
| Age, y | 58.0 ± 2.2 | 57.7 ± 1.5 | Matched |
| Female | 20 (34) | 46 (41) | .958 |
| Weight, kg | 89.0 ± 3.9 | 82.3 ± 2.4 | .129 |
| Source | Matched | ||
| Endovascular | 31 (53) | 60 (53) | |
| Secondary | 24 (42) | 47 (42) | |
| Catheter | 3 (5) | 6 (5) | |
| Creatinine clearance | Matched | ||
| Hemodialysis | 33 (22) | 25 (22) | |
| <50 mL/min | 15 (26) | 27 (24) | |
| ≥50 mL/min | 30 (52) | 61 (54) | |
| Platelet count, 103cells/µL | 240.9 ± 142.9 | 248.6 ± 48.9 | .875 |
| VAN MIC | .599 | ||
| ≤1.0 mg/L | 33 (57) | 62 (55) | |
| 1.5–2.0 mg/L | 25 (43) | 51 (45) | |
| Pitt Score, median [range] | 2 [0–9] | 1 [0–10] | .782 |
| Comorbidities | |||
| Diabetes | 20 (34) | 48 (42) | .517 |
| Liver dysfunction | 13 (22) | 18 (16) | .462 |
| Cancer | 2 (3) | 4 (4) | .138 |
| Immune supp. | 5 (9) | 9 (8) | .666 |
| Charlson Index ≥3 | 33 (57) | 55 (49) | .844 |
| Concomitant statin | 12 (21) | 28 (25) | .355 |
| Source control | 17 (29) | 45 (40) | <.001 |
| Patient location at index culture | |||
| ICU | 9 (16) | 18 (16) | .958 |
| General care | 49 (84) | 95 (84) | |
| ID consult | 58 (100) | 104 (92) | .275 |
| Hospital length of stay, d | 23.3 ± 2.2 | 15.6 ± 0.9 | <.001 |
Abbreviations: DAP-CPT, daptomycin and ceftaroline; ICU, intensive care unit; ID, infectious diseases; VAN MIC, vancomycin minimum inhibitory concentration.
aAll data are presented as No. (%) or mean ± SD depending on type of data.
Ninety-six percent of patients in the SOC group received VAN at index culture. The remaining 4% received empiric DAP therapy. Sixty-three (56%) patients in the SOC group proceeded to a second directed therapy regimen, most commonly DAP monotherapy (n = 46, 73%). Twelve (11%) patients were exposed to 3 or more treatment regimens throughout their infection course. In the SOC group, 52 (46%) patients completed treatment on vancomycin monotherapy, 47 (42%) on DAP monotherapy, 4 (3%) on CPT monotherapy, and 10 (9%) on other anti-MRSA antibiotics.
The source was matched between patients in each group. Endovascular source was most common (53%), followed by nonendovascular (42%) and catheter-related (5%). Of patients with an endovascular source, 74% and 78% had echocardiography-proven endocarditis in the DAP-CPT and SOC groups, respectively. Three (5%) patients in the DAP-CPT group and 1 (2%) patient in the SOC group had a left ventricular assist device source. Secondary, nonendovascular sources included osteomyelitis (15% DAP-CPT and 21% SOC), skin and soft tissue infections (12% and 10%), and pneumonia (12% and 7%). Minimum inhibitory concentrations (MICs) for vancomycin were similar between groups, with 61/178 (34%) of the total population possessing an MIC of 2 mg/L. Median Pitt Bacteremia Score (P = .782) and Charlson Comorbidity Index were similar, but numerically more patients in the combination therapy group had a Charlson Index ≥3 (57% vs 49%; P = .844). Patient disposition at index culture was the same for both groups (16% intensive care unit, 84% general care).
Figure 1 displays the clinical outcomes in the cohorts. The 30-day mortality rate was 6.8% (4/58 patients) in the DAP + CPT group vs 14.2% (16/113 patients) in the SOC group. Twenty-four patients received DAP-CPT within 72 hours of index culture; 2 (8.3%) died within 30 days (P > .05 vs 14.2% in the SOC group). There was no statistical difference in 30-day mortality in patients receiving DAP-CPT within 72 hours of index culture vs SOC. However, mortality was numerically reduced by 80% for patients with a primary endovascular source receiving DAP-CPT within 72 hours of index culture vs SOC (4.3% vs 20.8%; P = .162).
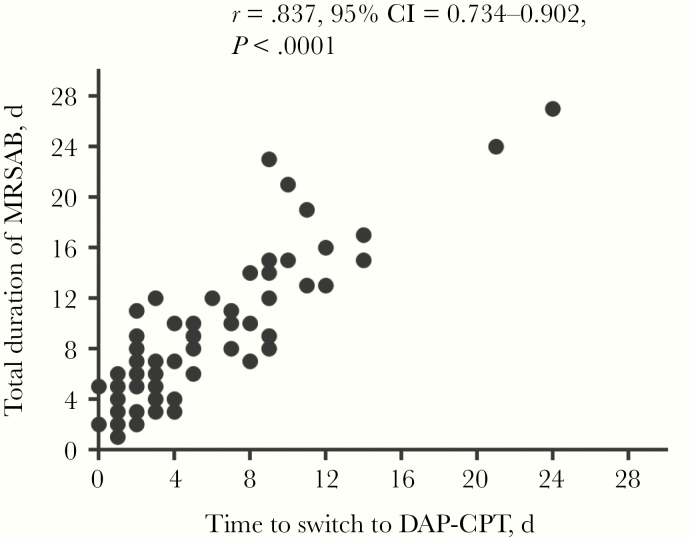
The mean duration of bacteremia was 4.8 days in the SOC group vs 9.3 days in the DAP-CPT group (P < .001); following switch to DAP-CPT, the mean duration of continued bacteremia was 3.3 days for the entire combination cohort. The duration of bacteremia for patients who had switched to combination therapy within 24, within 72, and after 72 hours was 3.6, 5.2, and 12.2 days, respectively. The median duration of bacteremia for patients switched to DAP-CPT within 72 hours vs after 72 hours was 5 and 11.5 days, respectively (P < .001), with an overall linear association between time to switch and duration (r = .84; P < .001) (Figure 2). Patients in the combination group were escalated to DAP-CPT after a mean (range) of 6 (0–24) days of bacteremia. Overall, there was no difference in bacteremia relapse or recurrence at 90 days after initial microbiological clearance between DAP-CPT and SOC.
Figure 2.
Correlation of time to switch to DAP-CPT combination and total duration of bacteremia using Spearman correlation analysis. Abbreviations: CI, confidence interval; DAP-CPT, daptomycin and ceftaroline; MRSAB, methicillin-resistant Staphylococcus aureus bacteremia.
Discussion
Standard-of-care therapy for MRSAB is associated with high failure rates and substantial morbidity and mortality, necessitating exploration of alternative treatment strategies including the use of 2 or more antibiotics in combination [29]. Updated treatment guidelines incorporating CPT are lacking, and there are limited clinical data for use of salvage combinations earlier in infection courses, leaving many questions unanswered in our current treatment paradigm. Questions such as which agents (if any) to use in combination, the appropriate duration of combination therapy, and antibiotic doses when agents are used in combination are increasingly critical for clinician–researchers to answer.
Our data support previous studies that the combination of DAP-CPT results in clearance of persistent MRSAB, but the presumed clinical benefits of blood culture sterilization are mitigated if this occurs late in the treatment course [10]. These data also support the potentially DAP-sparing effect of CPT synergy and opportunity for combination de-escalation, as our combination group received a mean 8.2-mg/kg/d DAP, as opposed to the guideline-recommended option of 10 mg/kg/d [6]. Although the majority (77.6%) of patients in our cohort received CPT every 8 hours, the optimal dose of CPT when used in combination with DAP remains unknown. Furthermore, almost half of the DAP-CPT patients successfully completed their treatment course on a single agent; the role of continued combination therapy is also unclear.
To date, this is the first study evaluating the treatment course of patients with MRSAB in a matched cohort of those receiving DAP-CPT at any time in therapy vs SOC. There was a numerically lower 30-day mortality rate in those patients switched to DAP-CPT within 72 hours of index culture (8.3%) vs SOC (14.2%). These findings are further supported by a recent small, prospective, unblinded, randomized study where treatment with DAP-CPT within 72 hours of index culture was associated with reduced in-hospital mortality compared with SOC, particularly in patients with high-risk endovascular sources and/or IL-10 >5 pg/mL [21]. Additionally, even in patients switched to DAP-CPT after 72 hours and with persistent bacteremia (a well-established marker of mortality in S. aureus), 30-day mortality was the same as for the patients on SOC in the present study, revealing that patients with a high predictor of mortality still did the same as patients on SOC. Patient variables including infection source, age, and renal function are additional clinical predictors of mortality in S. aureus bacteremia [15, 30–32]. By matching combination cases to SOC using these 3 important characteristics, we avoid the limitations of previous DAP-CPT clinical studies and present evidence toward a possible survival benefit conferred by DAP-CPT combination therapy over conventional standard monotherapy for the treatment of MRSAB, particularly in patients with an endovascular infection source.
Our study has several limitations inherent to retrospective analyses, the most significant of which is inherent bias. As treatment was not randomized and was instead chosen by the clinicians managing these infections, combination therapy patients are expected to be higher risk, frequently failing firstline therapy, and combination is used as salvage treatment. Also, although the multicenter cohort is a strength, this may present issues for interpreting treatment-related outcomes, as the processes of diagnosis and managing patients at each site may vary. Future prospective, randomized studies may address this limitation and compare salvage therapies by standardizing second-line treatment for each group. The finding of statistically similar and numerically lower mortality rates in the combination therapy group compared with SOC, despite statistically significantly longer duration of bacteremia, longer hospital stay, and less source control in the DAP-CPT group, supports an advantage of DAP-CPT, had a comparison been done on equal footing, as seen in the small prospective study [21]. This is particularly important in select patient populations, such as those with left ventricular assist devices in our study. Patients treated with DAP-CPT had a higher rate of ID consultation, which has demonstrated decreased mortality in patients with MRSAB [33, 34]. However, the rate of ID consultation in the SOC group (92%) exceeded the rate of ID consultation in previous studies.
Data regarding the use of antibiotics lacking in vitro MRSA activity for the index infection were not collected. Although this is unlikely to confound the comparison between combination therapy and SOC, as both groups were equally as likely to receive empiric, concomitant antibiotics targeting gram-negative pathogens, there is literature to support that the empiric combination of vancomycin and other β-lactam antibiotics can shorten the duration of MRSA bacteremia [8, 35]. Therapeutic drug monitoring for patients receiving vancomycin therapy varied at each institution and was not included in the study analysis. However, each institution follows the general approaches recommended by the vancomycin use and monitoring guidelines and has pharmacist-driven pharmacokinetic services for the management of vancomycin therapy [36]. Susceptibility testing and MIC distribution for DAP or CPT was not documented for all cases in this analysis.
Finally, our study did not assess safety outcomes of combination therapy compared with monotherapy, such as incidence of Clostridioides difficile and acute kidney injury (AKI). Recent abstract data suggest that combination therapy with VAN and flucloxacillin or cefazolin for MRSAB shortens the duration of bacteremia compared with monotherapy (11% vs 20% with positive blood cultures on day 5). However, this had no impact on 90-day mortality, presumably due to an increase in AKI in patients receiving VAN and flucloxacillin (35% vs 9%). Patients receiving VAN and cefazolin had an AKI rate of 7%, comparable to VAN monotherapy [37]. Existing data for DAP-CPT do not suggest a safety signal; however, it is imperative that larger, blinded, prospective randomized controlled trials are performed to evaluate the safety and efficacy of empiric treatment regimens for MRSAB, including, but not limited to, DAP-CPT combination therapy, VAN plus cefazolin combination therapy, and CPT monotherapy.
Conclusions
This study is the only matched cohort to our knowledge to compare DAP-CPT combination therapy used as both initial (within 72 hours of index culture) and salvage therapy with SOC for MRSAB. Combination DAP-CPT resulted in clearance of persistent MRSA bacteremia. Patients placed on DAP-CPT had a high inherent persistent bacteremia of >4 days, which has been shown to be a well-established marker of mortality in S. aureus. Despite the DAP-CPT group having a higher baseline mortality risk and statistically less source control, the overall mortality rates were still similar to SOC.
Receiving DAP-CPT early in the infection course may be more beneficial for survival, as opposed to its use as salvage therapy, especially in patients with endovascular sources or those at high risk of death. Blinded, randomized prospective studies are needed to eliminate the treatment selection bias inherent in retrospective analyses when examining SOC vs aggressive combination regimens in the treatment of MRSA bacteremia.
Acknowledgments
Financial support. G.S. receives funding from National Institutes of Health 1U54HD090259 and 1U01AI124316. W.E.R. receives funding from National Institutes of Health R01AI132627. M.J.R. receives funding from National Institutes of Health R01AI121400.
Potential conflicts of interest. G.S. has received speaking honoraria from Allergan, Theravance, and Melinta; consulting fees from Allergan and Paratek Pharmaceuticals; and is on the Scientific Advisory Board of Cidara Therapeutics and Arsanis Pharmaceuticals. M.J.R. has received research support and was part of an advisory board or speaking bureau for Allergan and Melinta. W.E.R. has received speaking honoraria from Melinta. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Liu C, Bayer A, Cosgrove SE, et al. ; Infectious Diseases Society of America Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 2. Kullar R, Sakoulas G, Deresinski S, van Hal SJ. When sepsis persists: a review of MRSA bacteraemia salvage therapy. J Antimicrob Chemother 2016; 71:576–86. [DOI] [PubMed] [Google Scholar]
- 3. Mehta S, Singh C, Plata KB, et al. β-lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 2012; 56:6192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berti AD, Wergin JE, Girdaukas GG, et al. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob Agents Chemother 2012; 56:5046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barber KE, Rybak MJ, Sakoulas G. Vancomycin plus ceftaroline shows potent in vitro synergy and was successfully utilized to clear persistent daptomycin-non-susceptible MRSA bacteraemia. J Antimicrob Chemother 2015; 70:311–3. [DOI] [PubMed] [Google Scholar]
- 6. Barber KE, Werth BJ, Rybak MJ. The combination of ceftaroline plus daptomycin allows for therapeutic de-escalation and daptomycin sparing against MRSA. J Antimicrob Chemother 2015; 70:505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barber KE, Smith JR, Ireland CE, et al. Evaluation of ceftaroline alone and in combination against biofilm-producing methicillin-resistant Staphylococcus aureus with reduced susceptibility to daptomycin and vancomycin in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 2015; 59:4497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casapao AM, Jacobs DM, Bowers DR, et al. ; REACH-ID Study Group Early administration of adjuvant β-lactam therapy in combination with vancomycin among patients with methicillin-resistant Staphylococcus aureus bloodstream infection: a retrospective, multicenter analysis. Pharmacotherapy 2017; 37:1347–56. [DOI] [PubMed] [Google Scholar]
- 9. Davis JS, Sud A, O’Sullivan MVN, et al. Combination of vancomycin and beta-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis 2016; 62(2):173–80. [DOI] [PubMed] [Google Scholar]
- 10. Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 2014; 36:1317–33. [DOI] [PubMed] [Google Scholar]
- 11. Casapao AM, Davis SL, Barr VO, et al. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob Agents Chemother 2014; 58:2541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin JC, Aung G, Thomas A, et al. The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother 2013; 19:42–9. [DOI] [PubMed] [Google Scholar]
- 13. Destache CJ, Guervil DJ, Kaye KS. Ceftaroline fosamil for the treatment of Gram-positive endocarditis: CAPTURE study experience. Int J Antimicrob Agents 2019; 53:644–9. [DOI] [PubMed] [Google Scholar]
- 14. Rose WE, Schulz LT, Andes D, et al. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother 2012; 56:5296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zasowski EJ, Trinh TD, Claeys KC, et al. Multicenter observational study of ceftaroline fosamil for methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2017; 61:e02015–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ortwine JK, Werth BJ, Sakoulas G, Rybak MJ. Reduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the “seesaw effect”: taking advantage of the back door left open? Drug Resist Updat 2013; 16:73–9. [DOI] [PubMed] [Google Scholar]
- 17. Barber KE, Ireland CE, Bukavyn N, Rybak MJ. Observation of “seesaw effect” with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infect Dis Ther 2014; 3:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Werth BJ, Steed ME, Kaatz GW, Rybak MJ. Evaluation of ceftaroline activity against heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-intermediate methicillin-resistant S. aureus strains in an in vitro pharmacokinetic/pharmacodynamic model: exploring the “seesaw effect”. Antimicrob Agents Chemother 2013; 57:2664–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhand A, Sakoulas G. Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin Ther 2014; 36:1303–16. [DOI] [PubMed] [Google Scholar]
- 20. Werth BJ, Sakoulas G, Rose WE, et al. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 2013; 57:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geriak M, Haddad F, Rizvi K, et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2019; 63:e02483–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rose WE, Shukla SK, Berti AD, et al. Increased endovascular Staphylococcus aureus inoculum is the link between elevated serum interleukin 10 concentrations and mortality in patients with bacteremia. Clin Infect Dis 2017; 64:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012; 206:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 25. Korvick JA, Bryan CS, Farber B, et al. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother 1992; 36:2639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J 2001; 31:97–103. [PubMed] [Google Scholar]
- 27. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 28. Jorgensen SCJ, Zasowski EJ, Trinh TD, et al. Daptomycin plus beta-lactam combination therapy for methicillin-resistant Staphylococcus aureus bloodstream infections: a retrospective, comparative cohort study. Clin Infect Dis. In press. [DOI] [PubMed] [Google Scholar]
- 29. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52:285–92. [DOI] [PubMed] [Google Scholar]
- 30. van Hal SJ, Jensen SO, Vaska VL, et al. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25:362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bassetti M, Peghin M, Trecarichi EM, et al. Characteristics of Staphylococcus aureus bacteraemia and predictors of early and late mortality. PLoS One 2017; 12:e0170236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ayau P, Bardossy AC, Sanchez G, et al. Risk factors for 30-day mortality in patients with methicillin-resistant Staphylococcus aureus bloodstream infections. Int J Infect Dis 2017; 61:3–6. [DOI] [PubMed] [Google Scholar]
- 33. Robinson JO, Pozzi-Langhi S, Phillips M, et al. Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 2012; 31:2421–8. [DOI] [PubMed] [Google Scholar]
- 34. Tissot F, Calandra T, Prod’hom G, et al. Mandatory infectious diseases consultation for MRSA bacteremia is associated with reduced mortality. J Infect 2014; 69:226–34. [DOI] [PubMed] [Google Scholar]
- 35. Dilworth TJ, Ibrahim O, Hall P, et al. β-lactams enhance vancomycin activity against methicillin-resistant Staphylococcus aureus bacteremia compared to vancomycin alone. Antimicrob Agents Chemother 2014; 58:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98. [DOI] [PubMed] [Google Scholar]
- 37. Davis J, Foo H, Lye D, et al. Combination antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia: the CAMERA2 randomised controlled trial. Paper presented at: European Congress on Clinical Microbiology and Infectious Diseases; 13–16 April 2019; Amsterdam, the Netherlands: Abstract L0014. [Google Scholar]