Abstract
Translational multidisciplinary research is important for the Center for Devices and Radiological Health's efforts for utilizing real‐world data (RWD) to enhance predictive evaluation of medical device performance in patient subpopulations. As part of our efforts for developing new RWD‐based evidentiary approaches, including in silico discovery of device‐related risk predictors and biomarkers, this study aims to characterize the sex/race‐related trends in hip replacement outcomes and identify corresponding candidate single nucleotide polymorphisms (SNPs). Adverse outcomes were assessed by deriving RWD from a retrospective analysis of hip replacement hospital discharge data from the National Inpatient Sample (NIS). Candidate SNPs were explored using pre‐existing data from the Personalized Medicine Research Project (PMRP). High‐Performance Integrated Virtual Environment was used for analyzing and visualizing putative associations between SNPs and adverse outcomes. Ingenuity Pathway Analysis (IPA) was used for exploring plausibility of the sex‐related candidate SNPs and characterizing gene networks associated with the variants of interest. The NIS‐based epidemiologic evidence showed that periprosthetic osteolysis (PO) was most prevalent among white men. The PMRP‐based genetic evidence associated the PO‐related male predominance with rs7121 (odds ratio = 4.89; 95% confidence interval = 1.41−17.05) and other candidate SNPs. SNP‐based IPA analysis of the expected gene expression alterations and corresponding signaling pathways suggested possible role of sex‐related metabolic factors in development of PO, which was substantiated by ad hoc epidemiologic analysis identifying the sex‐related differences in metabolic comorbidities in men vs. women with hip replacement‐related PO. Thus, our in silico study illustrates RWD‐based evidentiary approaches that may facilitate cost/time‐efficient discovery of biomarkers for informing use of medical products.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Despite the widely recognized need for biomarkers and risk predictors for joint replacement‐related adverse outcomes, there are presently no reliable study end points that can inform evaluation of real‐world performance in patient subpopulations and enable pre‐implantation testing for identifying individuals with enhanced susceptibility to implant‐related adverse outcomes.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This study addresses the role of sex in joint replacement‐related outcome heterogeneity and identifies candidate single nucleotide polymorphisms (SNPs) for predicting the risk of periprosthetic osteolysis (PO) as a major adverse outcome with sexual dimorphism.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ This study elucidates the patient factors (sex and SNPs) underlying susceptibility to joint replacement‐related adverse outcomes (PO). This study also illustrates the overall potential of in silico methodology for identifying biomarkers that are indicative of real‐world performance and thereby can inform use of medical products and improve treatment outcomes.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ In silico biomarker discovery that reutilizes pre‐existing genetic data and is guided by epidemiologic real‐world evidence can pave the way for development of cost/time‐efficient precision medicine applications.
Translational research is important for achieving the Center for Devices and Radiological Health (CDRH)'s goals and priorities and providing access to safe and effective medical devices.1 As part of the Center's regulatory research efforts to enhance predictive evaluation of real‐world device performance, we are developing an in silico framework for integrating multidisciplinary (e.g., epidemiologic and genetic) real‐world evidence (RWE) that can be used for identifying biomarkers and study end points indicative of device performance in different patient subpopulations.2
The current study is focused on new evidentiary approaches to evaluation of the hip replacement‐related adverse outcomes. As a main treatment of end‐stage osteoarthritis, hip replacement (also known as hip arthroplasty) has a staggering societal impact, which is projected to rise due to the growing aging population and, therefore, entails the demand for reliable long‐term implant performance.3, 4 Both periprosthetic osteolysis (PO) and resultant aseptic loosening are among the leading causes of implant failure and revision.5 Although the occurrence of PO seems to be decreasing with the advanced implant designs and increased knowledge about the implant's wear and biological responses to the resultant wear/corrosion debris, the continuing increase of arthroplasties necessitates better ways of managing PO and other implant‐related adverse outcomes.6
Despite possible sex‐related disparities in adverse outcomes pertaining to orthopedic implants7 and overall importance of demographics,8 its role in the implant‐related outcome heterogeneity remains elusive due to the lack of studies reporting sex/race‐stratified data. As a result, mechanistic understanding of patients' demographics as a risk modifier in implant‐related outcomes is lacking. Although some studies addressed the obvious need for biomarkers predicting the risk of implant‐related complications,9, 10 none of the published studies, to our knowledge, provided comprehensive assessment of patient‐related (genetic and nongenetic) factors that may impact adverse outcomes in hip replacement. To address the paucity of such studies, we attempted to characterize adverse outcomes in the sex/race‐stratified hip replacement subpopulations and identify candidate single nucleotide polymorphisms (SNPs) that may predict the risk of adverse outcomes and indicate their molecular underpinnings in relation to demographic‐related modifying effects.
In addition to discovery of actual candidate SNPs for PO, our study was focused on development of in silico methodology for reutilizing pre‐existing real‐world data (RWD) and integrating multidisciplinary—epidemiologic and genetic—evidence with healthcare applications. As a starting point, we analyzed putative sex/race‐related trends associated with hip replacement. Following the derived epidemiologic RWE on higher risk of PO among white men, a subsequent biomarker discovery was focused on the sex/race‐related biomarkers for PO and PO‐related wear. In silico plausibility analysis of the candidate SNPs and corresponding gene expression correlations suggested that PO risk may be modified by the sex‐related metabolic factors. Our ad hoc epidemiologic queries, which were prompted by the SNP‐based evidence, further indicated the role of sex‐related metabolic modification of PO risk. Thus, our study suggested the sex‐related modifying effects on PO risk and subsequently identified candidate SNPs, which seem to be associated with the predominance of PO in white men. Most importantly, our study illustrated transferable approaches to in silico discovery of biomarkers for informing the use of medical products.
Methods
Epidemiologic RWE on hip replacement outcomes was derived from a retrospective analysis using RWD from the National (Nationwide) Inpatient Sample (NIS; 2006–2012) from the Agency for Healthcare Research and Quality (AHRQ; a healthcare database accessible at https://www.hcup-us.ahrq.gov/) and the Personalized Medicine Research Project (PMRP)11 of Marshfield Clinic Research Institute (MCRI). Joint replacement‐related procedures and diagnoses were identified using the following International Classification of Diseases, Clinical Modification (ICD‐9‐CM) codes:
V43.64: Hip joint replacement
81.51: Total hip replacement
81.52: Partial hip replacement
81.53: Revision of hip replacement not otherwise specified
00.70: Revision of hip replacement, both acetabular and femoral components
00.71: Revision of hip replacement, acetabular component
00.72: Revision of hip replacement, femoral component
00.73: Revision of hip replacement, acetabular liner, and/or femoral head only
996.41: Mechanical loosening of prosthetic joint
996.42: Dislocation of prosthetic joint
996.43: Broken prosthetic joint implant
996.44: Periprosthetic fracture around prosthetic joint
996.45: Periprosthetic osteolysis
996.46: Articular bearing surface wear of prosthetic joint
Adverse outcomes identified by 996.41−996.46 ICD‐9‐CM codes were primary outcomes of interest. Sex and race were the main covariates of interest. Total NIS records with hip replacement comprised 615,251 discharges. Hip replacement discharges were first encompassed by using V43.64. Discharge records with V43.64 but without 996.41−996.46 codes were considered as hip replacement with no adverse outcomes. Discharge records with V43.64 in combination with any of 996.41−996.46 codes were considered as hip replacement complicated by one or more adverse outcomes (e.g., V43.64 + 996.45 + 996.46 as discharges reflecting hip replacement complicated by PO and wear). Cases with revised hip replacement were identified by code combinations for both a revision procedure and an adverse outcome as possible underlying causes (e.g., 00.70 + 996.45 as hip replacement subpopulation with total revision due to PO). Additional ICD‐9‐CM and Clinical Classifications Software (CCS) codes for diabetes, obesity, and lipid disorders were used for epidemiologic analysis on metabolic comorbidities (more details can be found in the corresponding tables).
STATA 14 (Stata Corp, College Station, TX) was applied for comparative analysis of the frequencies of adverse outcomes in sex/race‐stratified NIS discharges, using a custom‐written algorithm adapted to filter NIS discharges with relevant ICD‐9 codes. Frequency analysis, along with descriptive statistics, was performed using the two‐tailed Student t‐test for comparing continuous variables and the χ2 test for categorical data; statistical significance was assessed at P < 0.05.
Odds ratios (ORs) with 95% confidence intervals (CIs) were used to identify potential differences in the odds of adverse outcomes in different subgroups (e.g., white women vs. white men) and assess potential correlations between adverse outcomes and patient‐related factors (e.g., sex/race and SNP alleles).
Cytel Studio software was used for statistical assessment of the main candidate SNP (rs7121); sensitivity, specificity, and positive and negative likelihood ratios (PLR and NLR, respectively) were calculated per ratio of two independent binomial proportions.
The PMRP11 of the MCRI provided a cohort of patients with hip replacements (total = 871 patients) with pre‐existing epidemiologic (demographics and ICD‐9 codes, as shown above) and genetic (a total of 34 disease‐related SNPs; Table S1 ) data for genotype‐phenotype analysis. High‐Performance Integrated Virtual Environment (HIVE), an in‐house computing platform available at the US Food and Drug Administration (FDA; public HIVE domain at the George Washington University is accessible at https://hive.biochemistry.gwu.edu/dna.cgi?cmd=main), was used for hierarchical clustering, which was applied for analyzing and visualizing the sex‐stratified associations between SNP alleles and adverse outcomes in hip replacements (additional details are provided in the legends of corresponding figures).
Open‐access National Center for Biotechnology Information (NCBI) ( https://www.ncbi.nlm.nih.gov) databases, such as Gene and Gene Expression Omnibus, were used to explore SNPs of interest and corresponding genes. Open‐access genomic browser Ensembl ( https://www.ensembl.org) and Genotype‐Tissue Expression portal data were used for characterizing SNPs, identifying expression quantitative trait loci (eQTLs), and corresponding gene expression alterations associated with the variants of interest. The population genetics data from 1000 Genomes accessible through Ensembl were used for comparing baseline SNP allele frequencies in different racial subgroups. The biomedical knowledge bases and causal analytics tools from Correlation Engine/BaseSpace (Illumina) and Ingenuity Pathway Analysis (IPA; Qiagen Bioinformatics) were used to explore possible gene/protein interactions, signaling/metabolic pathways, and upstream/downstream effects pertaining to candidate SNPs and corresponding eQTLs/genes. More details on in silico data sources and methods used are provided in the legends of corresponding figures.
Results
Epidemiologic evidence on sex/race‐related trends pertaining to adverse outcomes in hip replacement
Based on the analysis of annual hospital discharges with V43.64 as the main procedure of interest (NIS, 2006–2012), women with hip joint replacement consistently outnumbered their male counterparts (as shown by the yearly discharge numbers in Figure S1 ). Subsequently, almost all adverse outcomes, including the most frequent mechanical loosening and dislocation, showed significant female predominance in both white and black subpopulations (Figure S2 ). The single adverse outcome that did not follow this pattern was PO, which was more frequent in black women (P = 0.0017), but not white women (P = 0.1192; Figure 1). A more detailed analysis in sex/race‐stratified subpopulations (Table 1; more detailed demographic characterization can be found in Table S2 ) confirmed a substantially lower risk of PO in white women compared with white men (OR = 0.54; 95% CI = 0.50−0.58), whereas the same trend in the black subpopulation failed to reach significance levels. Women, however, were more likely to have dislocation and periprosthetic fracture.
Figure 1.
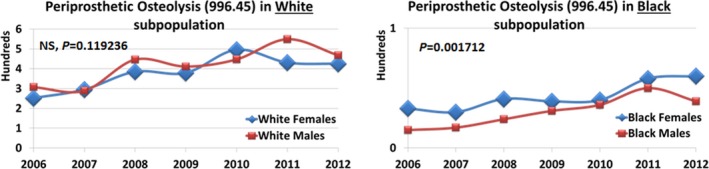
Sex/race‐related trends in periprosthetic osteolysis, per annual discharge numbers (National Inpatient Sample/Agency for Healthcare Research and Quality (NIS/AHRQ)). The curves representing annual discharges in sex‐stratified subpopulations are derived from STATA analysis of NIS/AHRQ discharges (2006–2012) pertaining to joint prostheses (including but not limited to hip replacement); P values are derived using two‐tailed t‐test (P < 0.05). NS, not significant.
Table 1.
Sex/race‐related trends in adverse outcomes pertaining to hip replacement
| ORs for adverse outcomes in sex/race‐stratified subpopulations with hip replacement (OR [95% CI]) | ||||||
|---|---|---|---|---|---|---|
| Mechanical loosening | Dislocation | Broken implant | Wear | Periprosthetic fracture | Periprosthetic osteolysis | |
| All women vs. all men [n = 380,601 and n = 234,650, respectively) | 0.80 [0.77−0.83] | 1.17 [1.14−1.20] | 0.85 [0.80−0.90] | 0.71 [0.68−0.76] | 1.31 [1.25−1.37] | 0.56 [0.53−0.60] |
| White women vs. white men (n = 280,046 and n = 171,815, respectively) | 0.80 [0.77−0.84] | 1.16 [1.12−1.20] | 0.86 [0.80−0.92] | 0.70 [0.65−0.76] | 1.32 [1.25−1.40] | 0.54 [0.50−0.58] |
| Black women vs. black men (n = 25,833 and n = 17,524, respectively) | 0.85 [0.75−0.98] | 1.12 [0.99−1.26] | 0.78* [0.61−1.00] | 0.96* [0.71−1.30] | 1.20* [0.94−1.52] | 0.85* [0.67−1.08] |
The presented trends are based on National Inpatient Sample/Agency for Healthcare Research and Quality discharges with hip replacement. “All women vs. all men” category includes all available race/ethnicity subcategories, some of which are not shown individually due to mostly nonsignificant ORs. ORs representing the most pronounced changes are highlighted in bold; 95% CIs including 1 are designated by an asterisk. This table represents numbers of discharge records (not numbers of patients).
CI, confidence interval; OR, odds ratio.
Suggesting their causative role in device failure, almost all adverse outcomes (except broken implant) were more frequent among discharges with revision. Dislocation and periprosthetic fracture among white women with revision of both acetabular and femoral components increased by fourfold to fivefold compared with their counterparts with unrevised hip replacement (from 4.3% to 22.9% and from 1.4% to 5.4%, respectively). Sex/race‐related modification of the revision‐associated adverse outcomes was shown by ORs comparing the highest and the lowest frequencies for each outcome in white and black subpopulations (Figure 2). Dislocation and mechanical loosening remained the most frequent adverse outcomes among the revised hip replacements. Similar to their counterparts with no revision, the revision‐associated dislocation and periprosthetic fracture maintained predominance of white women (ORWFvsBM = 2.04; 95% CI = 1.76−2.34 and ORWFvsBM = 1.68; 95% CI = 1.31−2.17, respectively). The revision‐associated mechanical loosening was more frequent among black men (ORBMvsWF = 1.40; 95% CI = 1.24−1.58). Although aseptic loosening is considered the end point of PO,5 the ICD‐diagnosed PO showed a lower frequency compared with mechanical loosening, which may include other types of loosening. However, PO remained most frequent in white men who had revisions (ORWMvsWF = 1.64; 95% CI = 1.53−1.77). Indicating possible associations with PO, wear was also more frequent in the white men with revisions (ORWMvsBM = 1.33; 95% CI = 1.05−1.70).
Figure 2.
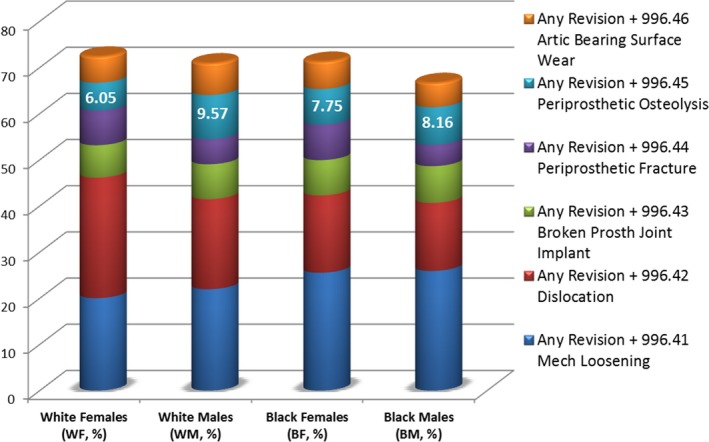
Periprosthetic osteolysis (PO) and other adverse outcomes in sex/race‐stratified patient subpopulations with revised hip replacement. “Any Revision” category combines discharges with different revisions pertaining to hip replacement (International Classification of Disease 9th revision: 81.53; 00.70−00.73). Percentages of PO (6.05–9.57%, as shown) and other adverse outcomes (not shown) are based on denominators representing total numbers of subjects with revised hip replacements in each sex/race‐stratified subgroup. The y‐axis represents cumulative percentages of revision‐related adverse outcomes in sex/race‐stratified subgroups.
Similar to the NIS‐derived sex distribution, the PMRP subcohorts with hip replacement, especially with no adverse outcomes, showed female predominance, with the male predominance limited to the PMRP subcohort with PO (M/F ratio = 1.29).
Thus, higher risk of PO in white men represented the single trend that overcame overall female predominance in hip replacement.
Genetic evidence on candidate SNPs associated with adverse outcomes in the sex/race‐stratified subpopulations with hip replacements
The presence of sex/race‐related trends indicates possible genetic factors underlying adverse outcomes in hip replacements. Osteolysis and loosening were previously associated with SNPs affecting inflammatory pathways, bone turnover, and tissue remodeling.9, 12, 13, 14, 15 GNAS‐rs7121 in particular was initially suggested as a risk factor for aseptic loosening in males.16 Although the subsequent evidence linking rs7121 to aseptic loosening seems contradictory,17, 18 GNAS‐rs7121, to our knowledge, remains the only SNP that has been thus far associated with the sex‐related differences in hip replacement outcomes. Because GNAS‐rs7121 and some other SNPs linked to adverse outcomes in hip replacement were included into the panel of disease‐associated SNPs used in the PMRP,11, 19, 20 we utilized pre‐existing PMRP SNP data (Table S1 ) to test the putative sex‐dependent role of GNAS‐rs7121 and other available SNPs in the hip replacement‐related adverse outcomes.
Similar to the original PMRP cohort,20 the PMRP subcohort with hip replacement was predominantly white (97.4%), and GNAS‐rs7121 genotypes were compatible with Hardy‐Weinberg equilibrium in both men and women. However, the PMRP subcohort with hip replacement‐related PO showed most of white men carrying rs7121‐C‐allele (Figure 3 a).
Figure 3.
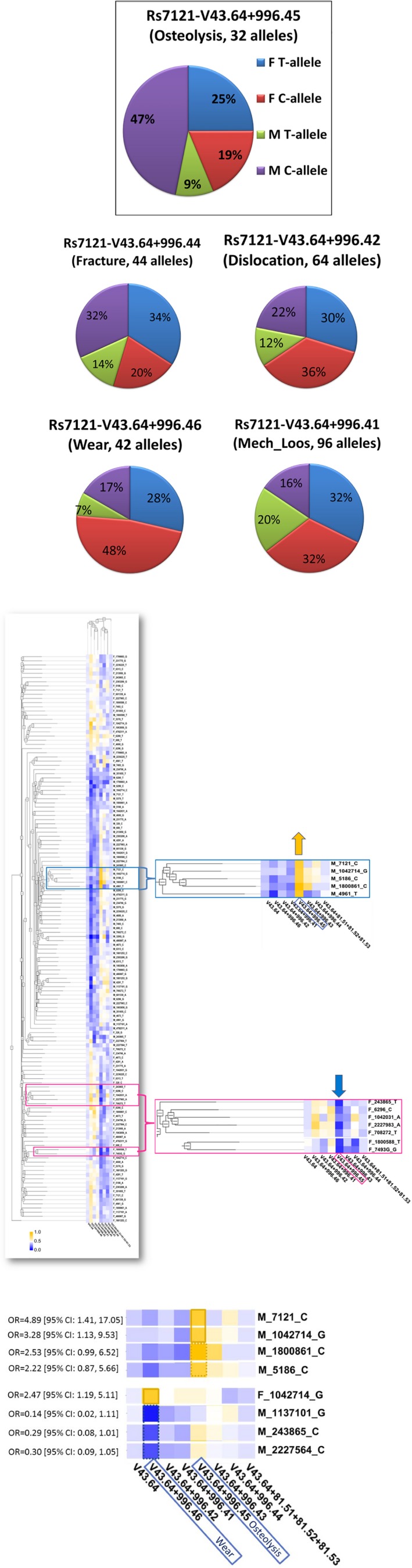
Sex‐related associations between single nucleotide polymorphisms (SNPs) and adverse outcomes in hip replacement. (a) GNAS_rs7121 allele distribution in periprosthetic osteolysis (PO) and other adverse outcomes in sex‐stratified Marshfield Clinic Research Institute (MCRI) subgroups with hip replacement. Note: Some subjects with hip replacement had more than one adverse outcome (e.g., osteolysis and wear) and, therefore, were included into more than one MCRI subgroup. (b) High‐Performance Integrated Virtual Environment (HIVE)‐based clustering heatmap presenting correlations between adverse outcomes and SNP alleles in sex‐stratified MCRI subgroups with hip replacement. The heatmap was created using HIVE unsupervised clustering algorithm. Rows show stratification by SNP alleles in male and female subgroups with certain SNP allele (e.g., M_7121_C refers to the C‐allele of rs7121 in male patients). Columns show stratification by International Classification of Diseases revision 9 (ICD‐9) codes. Figure legend represents a relative scale of allele frequencies: from 0 as lowest (dark blue) to 1 as highest (dark yellow). Two enlarged heatmap fragments represent the most noticeable allele changes in male and female subclusters (blue and pink boxes, respectively) with hip replacement‐related PO (e.g., V43.64 + 996.45). (c) HIVE‐based clustering heatmap fragments with the most significant correlations between PO or wear and certain SNP alleles in MCRI male subjects with hip replacement. The top section shows SNP alleles (×4) with highest frequencies (as designated by dark yellow) among men with PO. The bottom section represents SNP alleles (×3) with lowest frequencies (as designated by dark blue) among men with wear as well as a single SNP allele with highest frequency (as designated by dark yellow) among females with wear. Corresponding odds ratios (ORs) are shown on the left; solid and dash borders around the squares represent 95% confidence intervals (CIs) not including 1 and close to 1, respectively. F, female; M, male.
HIVE unsupervised clustering revealed a heatmap with the sex‐specific subclusters presenting SNP associations with adverse outcomes (blue and pink rectangles for men and women, respectively), where PO was associated with different SNP alleles in men vs. women (Figure 3 b). The increase of rs7121‐C allele in white men with PO was accompanied by higher frequencies (as indicated by dark yellow squares) of additional four SNP alleles, none of which were found in the subcluster of white women with PO (which was characterized by lower frequencies of other SNP alleles, as indicated by dark blue squares).
As shown in Figure 3 c, white men with GNAS_rs7121‐C allele had a fivefold higher risk of hip replacement‐related PO (OR = 4.89; 95% CI = 1.41−17.05), compared with control male subjects with no PO. The risk of PO among white men was also significantly associated with β2‐adrenergic receptor 2 (ADRB2)_rs1042714‐G (OR = 3.28; 95% CI = 1.13−9.53).
Only two candidate SNPs were associated with the sex‐independent risk for adverse outcomes. ADRB2_rs1042714‐C was significantly decreased in subgroups with wear and PO (OR = 0.38; 95% CI = 0.20−0.71 and OR = 0.34; 95% CI = 0.16−0.73, respectively). MMP2_rs243865‐C was borderline increased in the subgroup with PO (OR = 3.21; 95% CI = 0.97−10.58).
Our further statistical assessment focused on rs7121 as the main sex‐associated candidate SNP. Sensitivity, or true‐positive rate, of the rs7121‐C allele among white men with PO was assessed as 83.3% (15/18), whereas the corresponding false‐positive rate among white men with no PO reached only 48.8% (164/336). Subsequently, PLR and NLR for the rs7121‐C allele as a predictor of PO in white men were estimated as 1.71 and 0.33, respectively, suggesting a stronger risk modification by the absence rather than presence of the C‐allele (i.e., 3‐fold reduction vs. 1.7‐fold increase, respectively). The subsequent dominant protective effect suggested for T‐allele was consistent with the Ensembl‐derived predictions, which limited rs7121 deleterious effects to non‐T alleles. Further indicating the male sex‐dependent risk modification, rs7121‐C allele did not reach meaningful PLR and NLR values (0.77 and 1.30, respectively) as a predictor of PO among white women. As expected, PLR and NLR estimates for rs7121‐C allele in cases with no PO remained close to unity.
IPA‐based functional plausibility results on the PO‐associated SNP candidates
Next, we applied IPA for exploring possible functional consequences from SNPs that correlated with PO and wear in men (Figure 3 c). IPA knowledge base was queried to create the list of osteolysis‐related genes (x43), which was then compared with our list of candidate SNP‐harboring genes (x7). The rs243865‐harboring MMP2 was the only gene shared by the IPA list of osteolysis‐related genes (Figure S3 ), with rs243865 being implicated in bisphosphonate‐induced osteonecrosis21 and other MMP2 SNPs—in multicentric osteolysis (SNPedia).
The rs7121‐harboring GNAS locus (20q13.32) is known for its associations with bone diseases, including osteolytic changes (NCBI/Gene). According to our in silico research using NCBI and Correlation Engine databases, some GNAS SNPs were implicated in hip geometry (Framingham study) and GNAS gene expression was significantly higher in aseptic (wear particle‐induced) vs. septic loosening (GSE7103 study accessible at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7103, NCBI).
As a next step, we used Ensembl/Genotype‐Tissue Expression portal data to derive the lists of eQTLs (Table S3 ) pertaining to candidate SNPs from male‐PO and female‐PO subclusters (as highlighted by blue and pink rectangles, respectively, in Figure 3 b). IPA comparison of the eQTL‐associated genes revealed two distinct top pathways: cAMP/G‐protein‐coupled receptor signaling in the male‐PO and farnesoid X receptor/ retinoid X receptor activation in the female‐PO (Figure S4 ), both of which are involved in metabolic regulation.
As the key element of cAMP/G‐protein‐mediated signal transduction, GNAS is involved in energy production and lipid metabolism. Although the role of GNAS‐rs7121 in obesity is not fully elucidated,22 rs7121‐CC genotype was associated with obesity and insulin resistance.23 The rs1042714‐harboring ADRB2 is associated with regulation of energy expenditure and lipid mobilization, and the sex‐dependent obesity associations of ADRB2_rs1042714 were shown to involve triglyceride levels in Asian men, but not women.24, 25, 26 The ADRB2_rs1042714‐G allele, in particular, was decreased in obese men but increased in obese women.27 In a study on nondiabetic Japanese subjects,25 rs1042714 in ADRB2 and rs1137101 in leptin receptor (LEPR) conferred a synergistically increased obesity risk, especially in men. The LEPR_rs1137101_G allele was suggested to increase risk for diabetes,28 whereas ADRB2_rs1042714_GG genotype was associated with a twofold reduced risk for diabetes.29
Thus, the IPA‐derived evidence on candidate SNPs and eQTLs from male‐PO and female‐PO clusters highlighted distinct mechanisms potentially responsible for the sex/metabolism‐dependent modulation of PO in hip replacement.
Cross‐substantiation of genetic and epidemiologic evidence on the sex‐related risk of PO in hip replacement
To test the sex‐related metabolic associations inferred by SNP‐based genetic evidence, we conducted an ad hoc NIS‐based epidemiologic analysis comparing the odds of developing PO among the sex‐stratified hip replacement subpopulations with concurrent metabolic disorders. To match the white origin of PMRP subcohort, which provided candidate SNPs, this NIS data set was limited to hospital discharges with reported white race/ethnicity.
White women with obesity (CCS: 3.11.2) had a 24% increase in odds of having PO (Table 2; more detailed demographic characterization of subjects with metabolic disorders can be found in Table S4 ). Mixed hyperlipidemia (ICD‐9: 272.2) increased PO risk in white women by almost threefold (OR = 2.85; 95% CI = 1.27−6.39), whereas other/unspecified hyperlipidemia (ICD‐9: 272.4) decreased PO risk in white women by 17%. Pure hypercholesterolemia (ICD‐9: 272.0) moderately increased PO risk in both sex subgroups. Remarkably, diabetes (CCS: 49‐50) seemed to reduce the likelihood of PO regardless of sex (by −33% and −22% in women and men, respectively).
Table 2.
Sex/race‐related trends in PO pertaining to hip replacement
| Metabolic comorbidities | ORs for PO among white subpopulation with hip replacement (OR [95% CI]) | ||
|---|---|---|---|
| All | Women | Men | |
| Disorders of lipid metabolism (single‐level CCS 53, n = 297,273): | 1.11 [1.02, 1.19] | 0.98* [0.87, 1.10] | 1.14 [1.03, 1.27] |
| Pure hypercholesterolemia (ICD‐9 272.0, n = 92,898) | 1.28 [1.14, 1.43] | 1.28 [1.08, 1.51] | 1.23 [1.06, 1.44] |
| Pure hyperglyceridemia (ICD‐9 272.1, n = 1,735) | 0.56* [0.18, 1.75] | 0.52* [0.07, 3.69] | 0.51* [0.13, 2.51] |
| Mixed hyperlipidemia (ICD‐9 272.2, n = 1,921 | 2.11 [1.16, 3.83] | 2.85 [1.27, 6.39] | 1.46* [0.60, 3.53] |
| Other and unspecified hyperlipidemia (ICD‐9 272.4, n = 210,647) | 1.00* [0.92, 1.09] | 0.83 [0.73, 0.96] | 1.07* [0.96, 1.20] |
| Obesity (multilevel CCS 3.11.2, n = 85,932) | 1.14 [1.01, 1.29] | 1.24 [1.04, 1.47] | 1.03* [0.87, 1.22] |
| Diabetes with or without complications (single‐level CCS 49 and CCS 50, n = 163,474) | 0.76 [0.69, 0.84] | 0.67 [0.57, 0.79] | 0.78 [0.68, 0.89] |
The presented trends are based on National Inpatient Sample/Agency for Healthcare Research and Quality (AHRQ) discharges with hip replacement. ORs with 95% CIs including 1 are designated by an asterisk. Metabolic comorbidities were identified using ICD‐9 and CCS (AHRQ) codes. Discharges with ICD‐9 272.3 code were not available. This table represents numbers of discharge records (not numbers of patients).
CCS, Clinical Classifications Software; CI, confidence interval; ICD‐9, International Classification of Diseases revision 9; OR, odds ratio; PO, periprosthetic osteolysis.
Thus, our ad hoc epidemiologic RWE on metabolic comorbidities was consistent with the SNP‐based evidence on possible sex‐dependent metabolic modification of PO risk.
Our further IPA queries associated the genes harboring PO/wear SNP candidates with biofunctions and diseases (Figure 4 a) potentially relevant to PO (e.g., bone‐related traits) and implant reactivity (e.g., inflammatory/immune responses) as well as endocrine and metabolic effects (e.g., insulin resistance and susceptibility to obesity). Most genes, including GNAS, were linked to biofunctions related to bone morphology.
Figure 4.
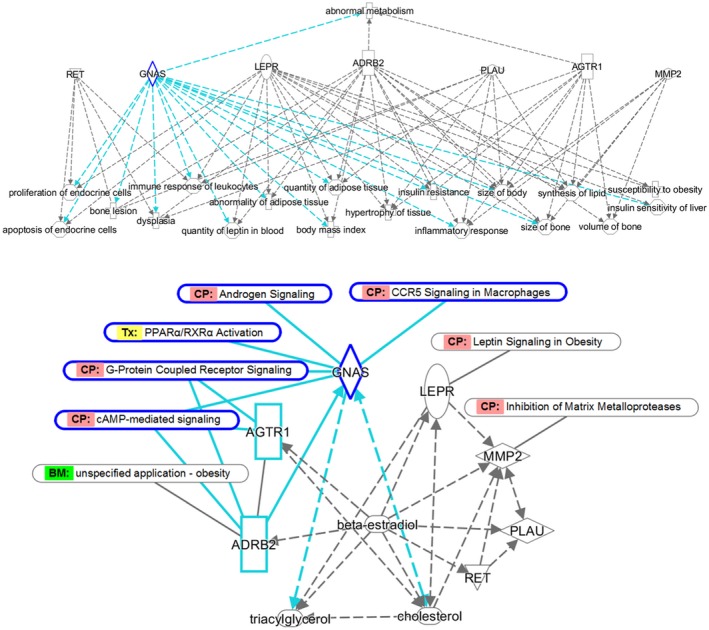
Ingenuity Pathway Analysis (IPA)‐based plausibility analysis of candidate single nucleotide polymorphisms (SNPs). (a) Gene functions potentially relevant to bone tissue remodeling and modification by hormonal and metabolic factors. The network was created using IPA knowledge base and IPA core analysis tool and was based on known gene/protein interactions of the genes harboring candidate SNPs. The demonstrated gene/protein‐related functions and conditions (with P values ranging from 8.15E‐09 to 1.03E‐05) were selected based on their possible relevance to bone tissue changes and metabolism. Different molecule shapes reflect IPA labels for different gene categories; the arrowed dash lines point at the pathways associated with individual genes/proteins. GNAS‐related functions and conditions are highlighted in blue. (b) Gene pathways potentially relevant to bone tissue remodeling and modification by hormonal and metabolic factors. The network was created using IPA knowledge base and IPA core analysis, which was based on known interactions of the genes harboring candidate SNPs. The demonstrated canonical pathways (CP), biomarker (BM) functions, or toxicological (Tx) processes were selected based on their possible relevance to bone tissue changes and metabolism. Different molecule shapes reflect IPA labels for gene/chemical categories. Solid and dash lines reflect direct and indirect interactions, respectively; arrows indicate the direction of interactions, such as activation or inhibition. As an identified upstream regulator, beta‐estradiol is capable of regulating expression of almost all network genes (except GNAS) as its downstream targets. A more detailed analysis of GNAS interactions (as highlighted in blue) shows that GNAS is interconnected with both shown lipid species and is particularly capable of affecting cholesterol levels. GNAS and ADRB2 proteins are known to interact directly, and ADRB2 is capable of regulating GNAS protein expression, activation, and localization as well as GNAS binding to GTP. In addition to being part of cAMP/G‐protein coupled receptor signaling, GNAS is connected to metabolic (PPARα/RXRα activation) and male sex hormone‐related regulation (androgen signaling) as well as to proinflammatory cytokine‐mediated pathways (signaling in macrophages). ADRB2, β2‐adrenergic receptor 2; LEPR, leptin receptor.
The genes harboring PO/wear SNP candidates were also connected to signaling pathways (Figure 4 b) that can be implicated in implant‐related adverse outcomes (e.g., MMP2 in tissue remodeling by matrix metalloproteinases). Metabolic connections were shown by LEPR as the major regulator of leptin signaling and by ADRB2 as a recognized biomarker for obesity. The PO/wear SNP‐harboring genes were linked to lipids, such as cholesterol and triglycerides (triacylglycerol). In addition to being regulated by these lipid species, some genes including GNAS were shown to be their upstream regulators. Some PO/wear SNP‐harboring genes also showed potential relationships with glucose (not shown).
Reflecting possible influence of sex hormones, beta‐estradiol was identified as an upstream regulator of the PO/wear‐related gene network (P = 2.35E‐04, IPA), with almost all SNP‐harboring genes as potential downstream targets of beta‐estradiol (Figure 4 b). The rs7121‐harboring GNAS is known to be involved in androgen signaling (Figure S4 ).
Thus, the integrated genetic and epidemiologic evidence further suggested that the risk of PO in hip replacement may be affected by the sex‐dependent metabolic and hormonal factors.
Discussion
As a retrospective study utilizing pre‐existing RWD, the current study was subject to inherent limitations, such as the lack of device‐related, procedure‐related, or patient‐related information representing potential confounders. A small sample size and an SNP data set limited to certain disease‐related biomarkers were the main limitations of the derived genetic evidence. For example, because almost all PMRP subjects were white, the current SNP findings might not be generalizable to nonwhite subjects. However, many of these limitations are being addressed in our ongoing larger study on discovery of candidate SNPs for adverse outcomes in various joint replacements.
As determined by its focus on developing new methodological approaches to multidisciplinary RWE, our study involved deriving and cross‐substantiating two—epidemiologic and genetic—evidentiary streams (Figure 5). First, the AHRQ/NIS‐derived epidemiologic RWE revealed an elevated PO risk in white men with hip replacements. Second, the PMRP‐derived genetic RWE and subsequent IPA plausibility analysis identified PO biomarkers whose effects can be modified by metabolic factors. Third, our ad hoc AHRQ/NIS queries further corroborated the possibility of sex‐related metabolic modification of the PO risk in hip replacement and, thus, substantiated the results of biomarker discovery and subsequent SNP‐based analyses.
Figure 5.
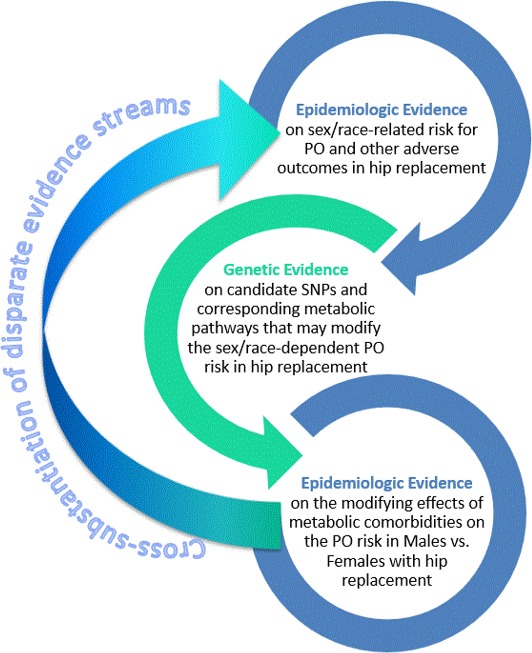
Epidemiologic and genetic evidence streams in in silico analysis on the hip replacement‐related periprosthetic osteolysis (PO) risk and corresponding candidate single nucleotide polymorphisms (SNPs).
Sex is recognized as a biological variable that is crucial for mechanistic understanding of disease risk vs. resilience.30 Epidemiologic RWE from our study underscored the need for correct interpretation of the sex‐related outcome disparities in hip replacement. Illustrating the methodological importance of sex‐based stratification in biomarker discovery, only two of seven candidate SNPs retained their associations with PO or wear in both men and women.
Male sex has been previously associated with the risk for PO,6 which was attributed to the higher levels of activity and increased wear. Our study suggested additional reasons for the sex‐related differences in PO. The rs7121‐harboring GNAS is involved in androgen signaling and is known for its sex‐dependent patterns of gene expression. Similar to the study on African American mother‐newborn pairs,31 in which only maternally transmitted GNAS alleles were associated with birth weight and that association was restricted to male offspring, the male sex‐specific association between rs7121 C‐allele and PO in our study may indicate the sex‐dependent patterns of GNAS splicing and imprinting. Providing further clues for the GNAS role in the PO risk modification by metabolic factors, alternative murine Gnas gene products were shown to have opposite effects on glucose/lipid metabolism.32 In addition, parental transmission of G‐alpha subunit in the knockout murine model was shown to produce opposite—hypermetabolic and hypometabolic—effects that were likely due to deficiency of maternal‐specific and paternal‐specific Gnas gene products.33
Obesity has been deemed a relative contraindication for hip replacement and a risk factor for complications and prosthesis failure.34 Although obesity was shown to increase the risk of early revision due to aseptic loosening and/or osteolysis by almost fivefold,35 statins were suggested to reduce femoral osteolysis in hip arthroplasty.36 However, some studies revealed obesity paradox, in which obesity or its correlates provided some protective effect in hip replacement.37 Further, the risk of femoral osteolysis in some normal‐weight patients was higher compared with overweight patients.38 Studies on genetically obese mice suggested that obesity may protect against particle‐induced bone resorption.39 Although our finding on the reduced PO risk in patients with diabetes with hip replacement seems controversial, similar protective effect was suggested in the nationwide register‐based Finnish study,40 in which patients with diabetes with hip or knee replacements had slightly decreased hazards ratios for the risk of revision. Further, both obesity and diabetes (hyperglycemia in particular) were shown to increase the risk of periprosthetic infection after hip/knee replacement,41, 42 whereas hypoglycemia was shown to increase the risk for revision in knee arthroplasty.43 Thus, further elucidation of metabolic factors in the PO risk should account for possible differences between glucose‐related and lipid‐related effects.
The role of lipid metabolism in PO has been highlighted by the previously suggested role of SNPs in low‐density lipoprotein receptor‐related proteins (LRP5 and LRP6).9 Although hyperlipidemia may promote osteoclastic potential,44 the sex‐related differences in energy storage, lipid turnover, and oxidation45 (e.g., higher insulin sensitivity in women, or triglyceride synthesis suppression by estrogens) may affect deposition of lipids, such as low‐density lipoproteins in subendothelial spaces of the bone,46 thereby shaping the sex‐dependent host responses leading to PO.
Although the nature of sex‐dependent effects on PO remains to be elucidated, it is plausible to suggest that overall hyperlipidemia may activate foreign body responses leading to inflammation and bone tissue remodeling,46 whereas the sex‐specific metabolic and hormonal differences45 may fine‐tune distinct PO risks in men and women. The female sex‐specific hormonal factors,47 such as use of contraceptives and postmenopausal hormone replacement therapy, may further modify the PO risk in women.
In summary, by demonstrating the guiding role of epidemiologic RWE and using unconventional analytics in biomarker discovery, the present study highlighted the potential of in silico methodology for optimizing use of medical products and improving treatment outcomes. The sex/race‐related trends and candidate SNPs identified in this study can be further corroborated for development of healthcare measures aimed to reduce the risk of PO as a main orthopedic implant‐related complication.
Funding
The main body of work was performed at the FDA and was supported by a grant from the Office of Women's Health. The PMRP was supported by grants from the National Institutes of Health, NHGRI UO1HG8701 and NCATS UL1TR000427, and by donated funds to the MCRI.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
Y.T. wrote the manuscript, designed and performed the research. S.D. performed the research. S.D., T.Ka., Y.A., and N.L.‐B. analyzed the data. T.Ki., M.B., and V.S. contributed new analytical tools.
Supporting information
Figure S1. Sex/race‐related trends in prosthesis‐related adverse outcomes.
Figure S2. Female predominance in discharges with hip joint replacement (NIS/AHRQ, 2006−2012).
Figure S3. IPA‐based Venn diagram comparing genes harboring candidate SNPs for periprosthetic osteolysis and wear with osteolysis‐related genes identified by IPA.
Figure S4. Main pathways derived from the analysis of eQTLs associated with sex‐related subclusters of candidate SNPs for periprosthetic osteolysis.
Table S1. List of SNPs used for deriving genetic evidence on putative biomarker candidates for periprosthetic osteolysis.
Table S2. Demographic characteristics of AHRQ/NIS (2010–2014) discharges with hip replacement‐related adverse outcomes.
Table S3. eQTLs associated with SNPs from sex‐related subclusters with periprosthetic osteolysis.
Table S4. (a) Metabolic complications in the sex/age‐stratified white subpopulation with hip arthroplasty and periprosthetic osteolysis (AHRQ/NIS, 2010–2014). (b) Metabolic complications in the sex/age‐stratified white subpopulation with hip arthroplasty (AHRQ/NIS, 2010–2014).
Acknowledgment
We would like to thank Marina Kondratovich (Associate Director for Clinical Studies and Mathematical Statistician at the Office of In Vitro Diagnostics and Radiological Health, CDRH/FDA) for her comments and methodological assistance with predictive values and likelihood ratios, which greatly improved the assessment of candidate SNPs.
References
- 1. 2016–2017 Strategic Priorities Center for Devices and Radiological Health. <https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHVisionandMission/UCM481588.pdf>. Accessed May 27, 2017.
- 2. Torosyan, Y. , Hu, Y. , Hoffman, S. , Luo, Q. , Carleton, B. & Marinac‐Dabic, D. An in silico framework for integrating epidemiologic and genetic evidence with health care applications: ventilation‐related pneumothorax as a case illustration. J. Am. Med. Inform. Assoc. 23, 711–720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurtz, S.M. , Lau, E. , Ong, K. , Zhao, K. , Kelly, M. & Bozic, K.J. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin. Orthop. Relat. Res. 467, 2606–2612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurtz, S. , Ong, K. , Lau, E. , Mowat, F. & Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 89, 780–785 (2007). [DOI] [PubMed] [Google Scholar]
- 5. Harris, W.H. The problem is osteolysis. Clin. Orthop. Relat. Res. 311, 46–53 (1995). [PubMed] [Google Scholar]
- 6. Beck, R.T. , Illingworth, K.D. & Saleh, K.J. Review of periprosthetic osteolysis in total joint arthroplasty: an emphasis on host factors and future directions. J. Orthop. Res. 30, 541–546 (2012). [DOI] [PubMed] [Google Scholar]
- 7. Haughom, B.D. , Erickson, B.J. , Hellman, M.D. & Jacobs, J.J. Do complication rates differ by gender after metal‐on‐metal hip resurfacing arthroplasty? A systematic review. Clin. Orthop. Relat. Res. 473, 2521–2529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leopold, S.S. et al Editorial: The complexity of reporting race and ethnicity in orthopaedic research. Clin. Orthop. Relat. Res. 476, 917–920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacInnes, S.J. et al Genetic variation in inflammatory and bone turnover pathways and risk of osteolytic responses to prosthetic materials. J. Orthop. Res. 33, 193–198 (2015). [DOI] [PubMed] [Google Scholar]
- 10. Pearson, M.J. , Grover, L.M. , Lord, J.M. , Jones, S.W. & Davis, E.T. Bearings in hip arthroplasty: joint registries vs precision medicine: review article. HSS J. 13, 20–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCarty, C.A. , Wilke, R.A. , Giampietro, P.F. , Wesbrook, S.D. & Caldwell, M.D. Marshfield Clinic Personalized Medicine Research Project (PMRP): design, methods and recruitment for a large population‐based biobank. Per. Med. 2, 49–79 (2005). [DOI] [PubMed] [Google Scholar]
- 12. Del Buono, A. , Denaro, V. & Maffulli, N. Genetic susceptibility to aseptic loosening following total hip arthroplasty: a systematic review. Br. Med. Bull. 101, 39–55 (2012). [DOI] [PubMed] [Google Scholar]
- 13. Gallo, J. , Mrazek, F. & Petrek, M. Variation in cytokine genes can contribute to severity of acetabular osteolysis and risk for revision in patients with ABG1 total hip arthroplasty: a genetic association study. BMC Med. Genet. 10, 109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon, A. , Kiss‐Toth, E. , Stockley, I. , Eastell, R. & Wilkinson, J.M. Polymorphisms in the interleukin‐1 receptor antagonist and interleukin‐6 genes affect risk of osteolysis in patients with total hip arthroplasty. Arthritis Rheum. 58, 3157–3165 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Malik, M.H.A. , Jury, F. , Bayat, A. , Ollier, W.E.R. & Kay, P.R. Genetic susceptibility to total hip arthroplasty failure: a preliminary study on the influence of matrix metalloproteinase 1, interleukin 6 polymorphisms and vitamin D receptor. Ann. Rheum. Dis. 66, 1116–1120 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bachmann, H.S. et al Gender‐dependent association of the GNAS1 T393C polymorphism with early aseptic loosening after total hip arthroplasty. J. Orthop. Res. 26, 1562–1568 (2008). [DOI] [PubMed] [Google Scholar]
- 17. Gallo, J. , Mrazek, F. & Petrek, M. Gender‐dependent association of the GNAS1 T393C polymorphism with early aseptic loosening after total hip arthroplasty. J. Orthop. Res. 28, 1257–1258 (2010). [DOI] [PubMed] [Google Scholar]
- 18. Stelmach, P. et al Relationship between GNAS1 T393C polymorphism and aseptic loosening after total hip arthroplasty. Eur. J. Med. Res. 22, 29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cross, D.S. , Ivacic, L.C. & McCarty, C.A. Development of a fingerprinting panel using medically relevant polymorphisms. BMC Med. Genomics 2, 17 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cross, D.S. , Ivacic, L.C. , Stefanski, E.L. & McCarty, C.A. Population based allele frequencies of disease associated polymorphisms in the Personalized Medicine Research Project. BMC Genet. 11, 51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katz, J. et al Genetic polymorphisms and other risk factors associated with bisphosphonate induced osteonecrosis of the jaw. Int. J. Oral Maxillofac. Surg. 40, 605–611 (2011). [DOI] [PubMed] [Google Scholar]
- 22. Nicoletti, C.F. et al The genetic predisposition score of seven obesity‐related single nucleotide polymorphisms is associated with better metabolic outcomes after Roux‐en‐Y gastric bypass. J. Nutrigenet. Nutrigenomics 9, 222–230 (2016). [DOI] [PubMed] [Google Scholar]
- 23. Hahn, S. , Frey, U.H. , Siffert, W. , Tan, S. , Mann, K. & Janssen, O.E. The CC genotype of the GNAS T393C polymorphism is associated with obesity and insulin resistance in women with polycystic ovary syndrome. Eur. J. Endocrinol. 155, 763–770 (2006). [DOI] [PubMed] [Google Scholar]
- 24. Apalasamy, Y.D. , Ming, M.F. , Rampal, S. , Bulgiba, A. & Mohamed, Z. Gender‐dependent association of a β(2)‐adrenergic gene variant with obesity parameters in Malaysian Malays. Asia Pac. J. Public Health 27, NP154‐N165 (2015). [DOI] [PubMed] [Google Scholar]
- 25. Pereira, T.V. , Mingroni‐Netto, R.C. & Yamada, Y. ADRB2 and LEPR gene polymorphisms: synergistic effects on the risk of obesity in Japanese. Obesity (Silver Spring) 19, 1523–1527 (2011). [DOI] [PubMed] [Google Scholar]
- 26. Zhang, H. , Wu, J. & Yu, L. Association of Gln27Glu and Arg16Gly polymorphisms in Beta2‐adrenergic receptor gene with obesity susceptibility: a meta‐analysis. PLoS One 9, e100489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hellstrom, L. , Large, V. , Reynisdottir, S. , Wahrenberg, H. & Arner, P. The different effects of a Gln27Glu beta 2‐adrenoceptor gene polymorphism on obesity in males and in females. J. Intern. Med. 245, 253–259 (1999). [DOI] [PubMed] [Google Scholar]
- 28. Yang, M.M. et al Variations in the obesity gene “LEPR” contribute to risk of type 2 diabetes mellitus: evidence from a meta‐analysis. J. Diabetes Res. 2016, 5412084 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinelli, M.A. et al Beta2‐adrenergic receptor and UCP3 variants modulate the relationship between age and type 2 diabetes mellitus. BMC Med. Genet. 7, 85 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bale, T.L. & Epperson, C.N. Sex as a biological variable: who, what, when, why, and how. Neuropsychopharmacology 42, 386–396 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adkins, R.M. et al Association of maternally inherited GNAS alleles with African‐American male birth weight. Int. J. Pediatr. Obes. 5, 177–184 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen, M. et al Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc. Natl. Acad. Sci. USA 102, 7386–7391 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu, S. et al Paternal versus maternal transmission of a stimulatory G‐protein alpha subunit knockout produces opposite effects on energy metabolism. J. Clin. Invest. 105, 615–623 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MacInnes, S.J. , Gordon, A. & Wilkinson, M.J. Risk factors for aseptic loosening following total hip arthroplasty In Recent Advances in Arthroplasty (ed. Fokter S.K.) 275–294 (In Tech, 2012) <https://www.intechopen.com/books/recent-advances-in-arthroplasty/risk-factors-for-aseptic-loosening-following-total-hip-arthroplasty>. Accessed May 27, 2017. [Google Scholar]
- 35. Electricwala, A.J. , Narkbunnam, R. , Huddleston, J.I. 3rd , Maloney, W.J. , Goodman, S.B. & Amanatullah, D.F. Obesity is associated with early total hip revision for aseptic loosening. J. Arthroplasty 31, 217–220 (2016). [DOI] [PubMed] [Google Scholar]
- 36. Lübbeke, A. et al Statins may reduce femoral osteolysis in patients with total hip arthroplasty. J. Orthop. Res. 31, 814–820 (2013). [DOI] [PubMed] [Google Scholar]
- 37. Shaparin, N. , Widyn, J. , Nair, S. , Kho, I. , Geller, D. & Delphin, E. Does the obesity paradox apply to early postoperative complications after hip surgery? A retrospective chart review. J. Clin. Anesth. 32, 84–91 (2016). [DOI] [PubMed] [Google Scholar]
- 38. Lübbeke, A. , Garavaglia, G. , Barea, C. , Roussos, C. , Stern, R. & Hoffmeyer, P. Influence of obesity on femoral osteolysis five and ten years following total hip arthroplasty. J. Bone Joint Surg. Am. 92, 1964–1972 (2010). [DOI] [PubMed] [Google Scholar]
- 39. von Knoch, M. et al Decrease in particle‐induced osteolysis in obese (ob/ob) mice. Biomaterials 25, 4675–4681 (2004). [DOI] [PubMed] [Google Scholar]
- 40. Jämsen, E. , Nevalainen, P. , Eskelinen, A. , Huotari, K. , Kalliovalkama, J. & Moilanen, T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single‐center analysis of 7181 primary hip and knee replacements for osteoarthritis. J. Bone Joint Surg. Am. 94, e101 (2012). [DOI] [PubMed] [Google Scholar]
- 41. Chun, Y.S. , Lee, S.H. , Lee, S.H. , Cho, Y.J. & Rhyu, K.H. Clinical implication of diabetes mellitus in primary total hip arthroplasty. Hip Pelvis. 26, 136–142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jämsen, E. , Peltola, M. , Eskelinen, A. & Lehto, M.U. Comorbid diseases as predictors of survival of primary total hip and knee replacements: a nationwide register‐based study of 96 754 operations on patients with primary osteoarthritis. Ann. Rheum. Dis. 72, 1975–1982 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roche, M.W. , Law, T.Y. , Triplet, J.J. , Hubbard, Z.S. , Kurowicki, J. & Rosas, S. Effect of hypoglycemia on the incidence of revision in total knee arthroplasty. J. Arthroplasty 32, 499–502 (2017). [DOI] [PubMed] [Google Scholar]
- 44. Tintut, Y. , Morony, S. & Demer, L.L. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler. Thromb. Vasc. Biol. 24, e6–e10 (2004). [DOI] [PubMed] [Google Scholar]
- 45. Varlamov, O. , Bethea, C.L. & Roberts, C.T. Jr Sex‐specific differences in lipid and glucose metabolism. Front. Endocrinol. (Lausanne) 5, 241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Demer, L.L. & Tintut, Y. Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award lecture. Arterioscler. Thromb. Vasc. Biol. 23, 1739–1743 (2003). [DOI] [PubMed] [Google Scholar]
- 47. Dragoman, M. , Curtis, K.M. & Gaffield, M.E. Combined hormonal contraceptive use among women with known dyslipidemias: a systematic review of critical safety outcomes. Contraception 94, 280–287 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sex/race‐related trends in prosthesis‐related adverse outcomes.
Figure S2. Female predominance in discharges with hip joint replacement (NIS/AHRQ, 2006−2012).
Figure S3. IPA‐based Venn diagram comparing genes harboring candidate SNPs for periprosthetic osteolysis and wear with osteolysis‐related genes identified by IPA.
Figure S4. Main pathways derived from the analysis of eQTLs associated with sex‐related subclusters of candidate SNPs for periprosthetic osteolysis.
Table S1. List of SNPs used for deriving genetic evidence on putative biomarker candidates for periprosthetic osteolysis.
Table S2. Demographic characteristics of AHRQ/NIS (2010–2014) discharges with hip replacement‐related adverse outcomes.
Table S3. eQTLs associated with SNPs from sex‐related subclusters with periprosthetic osteolysis.
Table S4. (a) Metabolic complications in the sex/age‐stratified white subpopulation with hip arthroplasty and periprosthetic osteolysis (AHRQ/NIS, 2010–2014). (b) Metabolic complications in the sex/age‐stratified white subpopulation with hip arthroplasty (AHRQ/NIS, 2010–2014).


