The microgravity environment results in a multitude of physiological changes associated with aging and altered organ function. Deployment of microphysiological systems, also known as “tissue chips” to the International Space Station United States National Laboratory, will allow the study of these changes at the cellular/molecular level. The goal of these studies is to expand understanding of age‐related conditions to improve human health on earth.
Tissue Chips
In 2012, the National Center for Advancing Translational Sciences (NCATS) sponsored several projects to develop three‐dimensional (3D) microphysiological system (MPS) technologies to model human organ functions as a more representative alternative to two‐dimensional (2D) cell culture or animal testing for prediction of drug‐induced toxicities. The program was intended to address the high failure rate once drugs reach in‐human clinical trials; with failure rates in excess of 30% in phases I and III, and 15% in phase II due to safety issues.1 This high failure rate is a major factor in the cost of new drug development, with recent cost estimates in excess of US $2 billion.2
Our team at the University of Washington was selected to create a kidney MPS based on primary cells derived from pathologically normal surgical nephrectomy tissue remnants.3 Our proximal tubule MPS platform recapitulates the biochemical, synthetic, and physiological characteristics of the proximal tubule segment of the nephron, in particular the polarized epithelium.4 In addition, the kidney MPS has met the NCATS mandate of the Tissue Chips Program for predictive toxicity testing of drugs and xenobiotics in our laboratory.5, 6 The next phase of the Tissue Chips program has focused on disease modeling and efficacy testing, which includes the Tissue Chips in Space initiative. In partnership with the International Space Station United States National Laboratory (ISSNL), NCATS issued five awards in June 2017 to conduct MPS‐based research on board the ISSNL.
Tissue Chips in Space
The environment of microgravity exerts a unique range of stresses and pathophysiological perturbations on the human body resulting in muscle wasting, immunosuppression, cardiovascular deconditioning, and decreased bone density.7 Numerous studies over the 50+ years of manned space flights have been conducted to investigate these changes, including the recent National Aeronautics and Space Administration (NASA) Twins study of astronauts Mark and Scott Kelly. That study, as well as others conducted in Russian cosmonauts and crewmembers on the Space Shuttle missions, have been limited to analysis of easily accessible biosamples (blood or urine), precluding any examination of organs or tissue at the cellular or molecular level. As a surrogate, studies in mice have been conducted that included transcriptomic analysis on the effects in the liver and kidneys after 12 days in microgravity8 and biochemical/proteomic analysis of mouse retina following a 35‐day mission on the International Space Station.9 However, whether these findings translate to human health effects is unknown. The Tissue Chips in Space initiative was designed to bridge this gap in much the same manner that MPS technology has been used to elucidate mechanisms of disease progression and drug‐induced toxicity.
The Challenges
The MPS system at the University of Washington is based on the Nortis Bio (Woodinville, WA) microfluidic culture platform. Each chip contains three independently perfused channels (triplex) with identical structural dimensions as previous single channel versions.4 Our original proposal was to deploy 24 single‐channel MPS to the ISSNL for ~ 14 days of experimentation. In order to address interindividual variability and sexual dimorphism, we increased capacity to 24 triplex chips to allow testing of 72 independent kidney MPS tubules. This increase in capacity presented a significant challenge due to extreme physical space limitations on launch and installation in the ISSNL.
The footprint required to maintain chips in the conventional laboratory is significant, requiring four syringe pumps, two incubators, and > 30.5 m of tubing (Figure 1 b) with a total volume of ~ 1,350 L. To meet spaceflight limitations, that had to be compressed into 55 L for launch in a powered locker on the SpaceX Dragon capsule, with a final installation volume on the ISSNL of 45 L (Figure 1 c). This required a complete re‐engineering of the perfusion and environmental control system. The final platform design (Figure 2 a) is composed of four modules, each containing six triplex MPS. These modules are completely self‐contained, housing motors to drive syringe pump pistons, temperature/CO2/humidity control, and cassettes containing cell media and Teflon‐coated effluent collection bags. The design eliminated all flexible tubing. The hands‐on involvement of crewmembers Anne McClain and Christina Koch (Figure 2 b) was limited to replacing media and fixative cassettes at defined time points in the Life Sciences Glovebox (the ISSNL biosafety cabinet).
Figure 1.
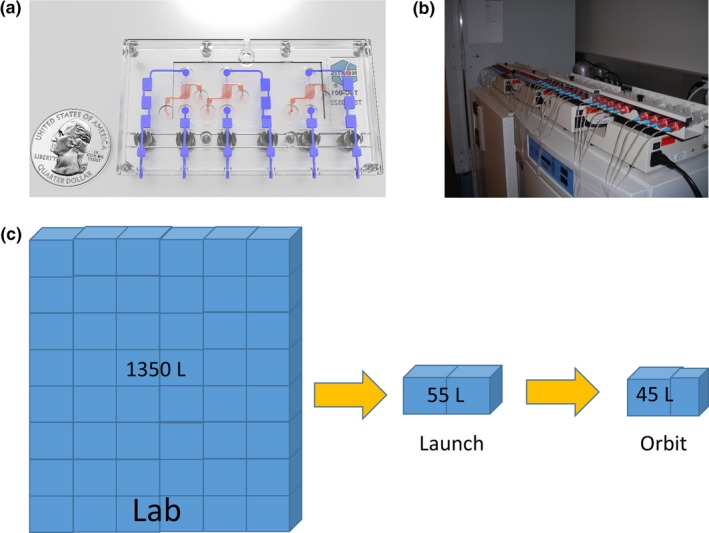
MPS system adaptation required for deployment to the International Space Station National Laboratories. (a) Kidney MPS platform designed and marketed by Nortis Bio. (b) Conventional laboratory incubator‐based and syringe pump‐based perfusion system for 40 single channel chips. (c) Perfusion system and environmental control miniaturization requirements. MPS, microphysiological systems.
Figure 2.

RE‐engineered self‐contained modules.(a) Each module contains two housings each with 3 triplex chips, two media cassettes, piston‐driven pumps and environmental control (temperature, humidity, CO2). All four modules pictured here were deployed to the International Space Station United States National Laboratory. Four identical modules, not shown, were utilized for the time‐synchronous ground control experiment. (b) On‐station manipulation was performed by Astronauts Anne McClain and Christina Koch (pictured; photograph courtesy of the National Aeronautics and Space Administration).
Due to the closed environment of the ISSNL, safety assessments for all materials (plastics, adhesives, and metal components), potential biohazards (cell culture reagents and primary cells), and powered architecture of the pumps had to be fully described, evaluated, and approved. Materials determined to have hazard potential (e.g., formalin for cell fixation) or that may carry pathogens (e.g., primary cell lines), required multiple levels of containment (sealed housing + double plastic bags). Containment was particularly important during manipulation within the ISSNL glove box when “breaking” these required levels of containment for media cassette swap‐out or when removing the chip housing from the units in order to place in temperature control post‐fixation. Engineering controls were required to contain friable materials (e.g., by bonding glass components to a flexible substrate) in the event of fragmentation to prevent small particles from damaging ISSNL infrastructure and inhalation/ingestion by crewmembers. Addressing necessary safety and crew procedure regulations added significant time‐burden and workload‐burden to the payload preparation process, as each component had to be individually documented, labeled, and organized for ready access by the on‐station crew. It is noteworthy that administrative documentation must be completed several months prior to launch, which can be difficult under challenging timelines.
The kidney MPS (24 for on‐station experiments with 24 matched ground controls) were prepared and seeded with cells in a temporary laboratory at the Kennedy Space Center based on timelines established for launch on April 26. However, the launch was delayed three times, including a scrubbed launch 15 minutes before liftoff on May 3. The SpaceX rocket finally launched at 2:48 am EST on May 4, a full 8 days after the originally scheduled launch window. Fortunately, the kidney MPS is a robust system, capable of supporting cell viability/function for at least 4 weeks, and the originally seeded chips were usable for launch. Following successful launch, unloading, and installation, the equipment performed as planned. The main lesson learned by the team was to plan for administrative and regulatory requirements and multiple and unpredictable launch delays.
Opportunities
Extended microgravity has been shown to cause accelerated rate of disease development and progression in many body systems, including rapid bone loss (1–2% loss of bone mineral density per month), cardiovascular deconditioning, and loss of skeletal muscle mass and strength. These changes mimic conditions associated with aging and chronic human diseases on Earth. Conducting experiments in microgravity may provide a unique opportunity to observe disease initiation and progression, and to test potential therapeutics with greater speed and efficiency than in conventional laboratories.
The microgravity environment might also be useful for other investigators to enhance the development of complex tissues composed of multiple cell types. Cell and tissue culture in microgravity occurs in the absence of two important factors, buoyancy and density‐driven convection, which cause cells to sink to the bottom of culture vessels and aggregate based on size and density instead of distributing in an anatomically correct manner. In contrast, cells in microgravity tend to self‐assemble slowly and aggregate based on cell‐to‐cell contact and intrinsic physiologic affinity.10
The unique combination of a 3D microfluidic tissue chip and a microgravity environment can provide unique insights into disease models and therapeutic targets. For example, the development of a biomimetic kidney organoid has been the focus of intense investigation. Due to the anatomic complexity and interplay between at least 26 cell types in the kidneys, and slow and insidious progression of many kidney diseases (occurring over years to decades), the pathophysiology of both acute and chronic kidney diseases is challenging to model in vitro. Current induced pluripotent stem cell‐derived kidney organoids are composed of multiple cell types but maintain a somewhat fetal phenotype and may not be fully useful in the study of kidney disease of the aging adult. A truly biomimetic organoid with properly polarized and physiologically self‐assembled cell types developed in microgravity could be used to identify initiating events, biomarkers of disease progression, and therapeutic targets for conditions including polycystic kidney disease, diabetic kidney disease, and chronic kidney disease of unknown etiology.
The use of 3D microphysiological systems in microgravity to study aging and altered organ function at the cellular/molecular level has only recently been explored. The combination of tissue chips in the unique microgravity environment will hopefully provide unique insights into disease development and progression, and help to identify novel therapeutic targets for the prevention and treatment of many chronic health conditions.
Funding
These studies were supported by funding from the National Center for Advancing Translational Sciences (UG3TR002178 and UG3TR002158), The International Space Station U.S. National Laboratory, and an unrestricted gift from the Northwest Kidney Centers to the Kidney Research Institute.
Conflict of Interest
The authors declared no competing interests for this work.
References
- 1. Applied Clinical Trials Editors . Reasons for clinical failures by phase. Appl. Clin. Trials 22 (2013). <http://www.appliedclinicaltrialsonline.com/reasons-clinical-failures-phase>. [Google Scholar]
- 2. DiMasi, J.A. , Grabowski, H.G. & Hansen, R.W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47, 20–33 (2016). [DOI] [PubMed] [Google Scholar]
- 3. Kelly, E.J. et al Innovations in preclinical biology: ex vivo engineering of a human kidney tissue microperfusion system. Stem Cell Res. Ther. 4(suppl. 1), S17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weber, E.J. et al Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 90, 627–637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang, S.‐Y. et al Human liver‐kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight 2, pii: 95978 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weber, E.J. et al Human kidney on a chip assessment of polymyxin antibiotic nephrotoxicity. JCI Insight 3, pii: 123673 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demontis, G.C. , Germani, M.M. , Caiani, E.G. , Barravecchia, I. , Passino, C. & Angeloni, D. Human pathophysiological adaptations to the space environment. Front. Physiol. 8, 547 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammond, T.G. , Allen, P.L. & Birdsall, H.H. Effects of space flight on mouse liver versus kidney: gene pathway analyses. Int. J. Mol. Sci. 19, 4106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mao, X.W. et al Impact of spaceflight and artificial gravity on the mouse retina: biochemical and proteomic analysis. Int. J. Mol. Sci. 19, 2546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braddock, M. From target identification to drug development in space: using the microgravity assist. Curr. Drug Discov. Technol. (2019). 10.2174/1570163816666190112150014. [DOI] [PubMed] [Google Scholar]


