Abstract
During 2013, the New York City Department of Health and Mental Hygiene (DOHMH) received reports of 6 hepatitis A cases among food handlers. We describe our decision-making process for public notification, type of postexposure prophylaxis (PEP) offered, and lessons learned. For 3 cases, public notification was issued and DOHMH offered only hepatitis A vaccine as PEP Subsequent outbreaks resulted from 1 case for which no public notification was issued or PEP offered, and 1 for which public notification was issued and PEP was offered too late. DOHMH continues to use environmental assessments to guide public notification decisions and offer only hepatitis A vaccine as PEP after public notification but recognizes the need to evaluate each situation individually. The PEP strategy employed by DOHMH should be considered because hepatitis A vaccine is immunogenic in all age groups, can be obtained by local jurisdictions more quickly, and is logistically easier to administer in mass clinics than immunoglobulin.
Keywords: food handlers, hepatitis A, New York City, outbreak
Hepatitis A, caused by the hepatitis A virus (HAV), is acquired through fecal-oral person-to-person transmission or through contaminated foods. Persons are most infectious during the 2 weeks before onset of jaundice and infectivity declines during the week after onset.1 Foodborne hepatitis A outbreaks are recognized infrequently in the United States and are usually associated with contamination of food by an HAV-infected food handler.1 Proper handwashing is the most effective way to prevent transmission.
Postexposure prophylaxis (PEP) is recommended for contacts of HAV-infected persons and is effective (immunoglobulin [IG] 80%-90%, hepatitis A vaccine 73%-86%)2,3 if received within 2 weeks after exposure.4 The Advisory Committee on Immunization Practices recommends healthy persons aged 12 months to 40 years receive the hepatitis A vaccine for PEP and recommends persons older than 40 years or younger than 12 months, immunocompromised persons, persons with chronic liver disease, and persons for whom vaccine is contraindicated receive IG.5 Although safety of hepatitis A vaccine during pregnancy has not been determined, it is unlikely to harm a fetus because it is inactivated.5 Most food handlers with hepatitis A do not transmit HAV to patrons, and routine vaccination of all food handlers is not currently recommended by the Advisory Committee on Immunization Practices.5
During 2005-2012, an average of 2.3 cases of hepatitis A (range, 1-3 cases) among New York City (NYC) food handlers were reported yearly to the NYC Department of Health and Mental Hygiene (DOHMH). Only once was the public notified and PEP offered to exposed patrons. During 2013, a total of 6 cases of hepatitis A among food handlers was reported, which required substantial health department resources.6,7
We describe the decision-making process for public notification, including hand hygiene assessments; the type of PEP offered by DOHMH; and lessons learned after cases among food handlers during 2013.
Methods
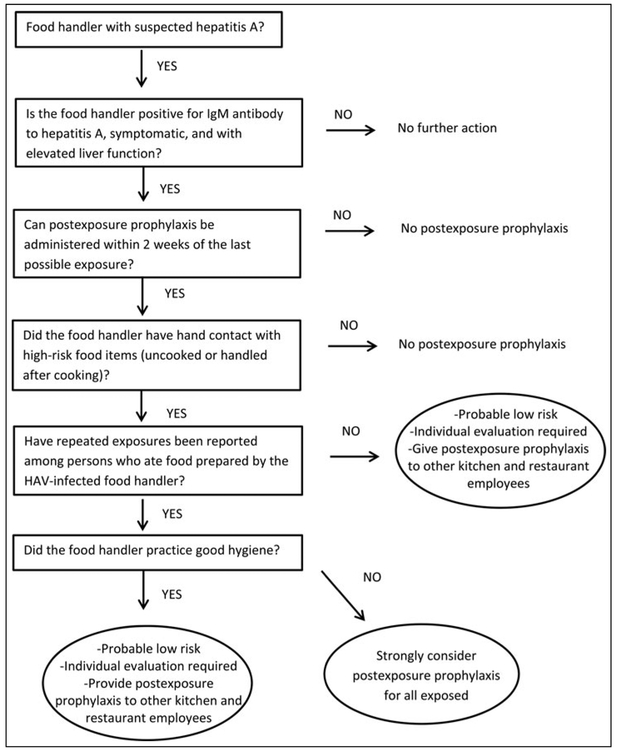
Since 2005, DOHMH has investigated all acute hepatitis A cases, including confirming diagnosis; interviewing patients to review symptoms, onset date, risk factors, and job duties; and identifying close contacts requiring PEP. When a hepatitis A case is identified in a food handler, we review previous restaurant evaluation reports and perform enhanced surveillance, reinterviewing every patient with acute hepatitis A diagnosed in the previous 6 weeks asking whether he or she ate at the respective establishment during his or her exposure period. DOHMH excludes food handlers from work until the end of the infectious period,8 defined as 1 week after jaundice onset. An algorithm adapted from published literature is used to determine need for PEP of restaurant patrons (Figure).1,9 Hand hygiene practices are assessed through interviews about handwashing frequency with the ill worker, coworkers, and supervisors. Restaurant hand hygiene practices are assessed by observing worker handwashing practices and glove use when handling high-risk foods and availability of functioning handwashing stations with soap and water. All investigations were deemed public health practice not meeting criteria for human subjects research. This work was assessed for human subject protection by the Centers for Disease Control and Prevention and determined to be public health practice not research.
FIGURE.
Algorithm Used by New York City Department of Health and Mental Hygiene to Determine the Need for Hepatitis A Virus Postexposure Prophylaxis of Restaurant Patronsa
Abbreviations: HAV, hepatitis A virus; IgM, immunoglobulin M.
When HAV PEP is required for a relatively small defined population, DOHMH typically refers potentially exposed persons to established providers for IG or hepatitis A vaccine. When public notification of a potential hepatitis A exposure is issued and a large number of individuals is affected, DOHMH offers PEP through points of dispensing (PODs); PODs are temporary sites operated by DOHMH, designed to rapidly provide prophylaxis to large populations.
In response to hepatitis A cases among food handlers during 2013, 1 dose of hepatitis A vaccine was offered to exposed persons at PODs; IG was not provided. Vaccinated persons were informed that a second dose from their health care provider would be needed for long-term immunity. Infants younger than 1 year and pregnant women were not administered vaccine and were referred to nearby NYC public hospitals for IG administration when indicated. Screening for high-risk conditions was not performed at the 2013 PODs.
To ensure proper treatment of affected children, we examined the NYC Citywide Immunization Registry, a centralized database of all immunizations administered to persons 18 years or younger.10 For children who had received only 1 dose of vaccine, we provided the second dose to complete the hepatitis A series. Vaccine doses administered at the POD were subsequently entered in the Citywide Immunization Registry.
Results
During 2013, a total of 6 cases of hepatitis A occurred among food handlers (Table 1); 3 resulted in public notification (cases 2, 4, and 5), and 2 had additional cases or clusters identified (cases 3 and 5). DOHMH did not offer PEP in case 3, as investigation revealed excellent hand hygiene practices among all workers and workers denied eating at the deli; no public notification was issued. Throughout the next 30 days, DOHMH identified 4 likely secondary cases of hepatitis A, all patrons who consumed deli meats from the grocery store during the workers’ infectious period. All other food handlers at the store tested negative for acute infection at that time. After identification of secondary cases, public notification was not issued because the recommended period for PEP had elapsed and there was no evidence of ongoing transmission or risk of HAV infection.
TABLE 1.
Summary of Hepatitis A Cases Among Food Handlers and Response of DOHMH—New York City, 2013
| Case | Case Report Month |
Occupation | Concerning Investigation Results |
Postexposure Prophylaxis Recommended |
DOHMH Public Notification |
DOHMH PODa Established (Only Hepatitis A Vaccine Offered) |
Number Who Received Postexposure Prophylaxis (Type) |
Associated Cases or Clusters |
|---|---|---|---|---|---|---|---|---|
| 1 | Feb | Cook, adult residential treatment facility | HR; RE; BHC | Yes | No | No | 16 (vaccine) 20 (IG) | No |
| 2 | Apr | Pastry chef, restaurant | HR; BHC | Yes | Yes | Yes | 489 (vaccine) | No |
| 3 | May | Deli worker, grocery store | HR | No | No | No | Yesb | |
| 4 | Aug | Fruit preparer, grocery store | HR; BHC | Yes | Yes | Yes | 2068 (vaccine) | No |
| 5 | Sep | Host or manager, restaurant | HR; BHC | No | Yes | Yes | 3053 (vaccine) | Yesc |
| 6 | Dec | Busboy, restaurant | None | No | No | No | No |
Abbreviations: BHC, bare hand contact with high-risk foods observed; DOHMH, Department of Health and Mental Hygiene; HR, ill worker handled high-risk foods; IG, immunoglobulin; POD, point of dispensing; RE, risk of repeated exposure.
Temporary emergency sites set up rapidly and operated by New York City DOHMH. These sites are designed to distribute or administer prophylaxis to substantial populations after a biological attack or the emergence of an infectious disease. Only hepatitis A vaccine was offered as postexposure prophylaxis at the PODs in 2013.
Four likely secondary cases identified among deli patrons.
Eight additional cases identified with same exposure period as case 5. A secondary case and a tertiary case were identified; both were family members of an original patron. The first was a household contact who refused vaccination during the initial case investigation, and the tertiary was not identified as a contact in the secondary case investigation.
In case 5, although inspectors observed employees not wearing gloves handling high-risk foods, because the investigation confirmed the infected worker did not handle food, DOHMH initially decided not to offer hepatitis A vaccine PEP to exposed patrons. The next day, enhanced surveillance identified 8 persons with hepatitis A who dined at the restaurant in August; none had reported dining at the restaurant on initial interview. All infected persons reported illness onset dates that coincided with the onset date of the infected worker. Because it was unclear whether there was an ongoing risk for transmission, DOHMH closed the restaurant until all staff members were tested and vaccinated for HAV. Because evidence of HAV exposure associated with dining at the restaurant existed and because employee test results would not be available within the PEP window, DOHMH notified the public, advising all patrons who ate at this restaurant during the exposure period to receive HAV PEP.
Discussion
The six 2013 cases highlight the challenges a health department encounters in deciding how to verify adequate hand hygiene practices, whether to notify the public of hepatitis A in a food handler and offer PEP, and which PEP to offer.11-13
As indicated in case 3, excellent hand hygiene practices among employees during an environmental assessment might offer false assurance. Hand hygiene assessments, the final decision point for public notifications (Figure),1,9 are usually conducted when the infected person is excluded from work; this prevents direct observation. Also, response to questions about hand hygiene and actual hand hygiene behavior among all employees might be modified when asked by DOHMH staff. Despite this, DOHMH continues to use information collected during restaurant environmental assessments, including hand hygiene indicators, to determine whether to notify the public. While each situation needs to be evaluated individually, public notification might be warranted when high-risk foods are involved, regardless of hand hygiene indicators.
The difficult decision to notify the public and offer PEP when hepatitis A cases are identified in food must be made quickly because PEP is only indicated up to 2 weeks postexposure. Much of the window for prophylaxis has usually elapsed before the investigation is complete.
Jurisdictions in other US states and certain countries endorse different recommendations for HAV PEP, specifically regarding the age cutoff and combination of PEP administered.14-17 Canada and Australia recommend hepatitis A vaccine for everyone older than 1 year, although IG is recommended additionally for persons with certain conditions.14,16 The United Kingdom recommends hepatitis A vaccine for persons aged 1 to 50 years and vaccine plus IG for those older than 50 years.15 DOHMH decided to offer only hepatitis A vaccine in hepatitis A PEP POD, rather than IG, because vaccine is easier to acquire and administer, is cheaper, and is associated with fewer side effects and longer-term protection. DOHMH plans to continue to offer only hepatitis A vaccine at PODs. Certain studies have reported high rates of seroconversion after hepatitis A vaccination among adults older than 40 years at 2 weeks after the first dose, suggesting vaccination might be adequate as prophylaxis for adults older than 40 years; however, sample sizes were small or the age distributions were not discrete.18-20 No subsequent cases of hepatitis A were identified in persons who received PEP with hepatitis A vaccine after any of the 2013 DOHMH vaccination operations; the possibility exists that no additional persons were exposed.
Conclusion
During 2013, NYC experienced the greatest number of hepatitis A cases among food handlers since 2005; 3 cases resulted in public notifications. Because public notification and POD activation require substantial resources, decision makers must assess the information available while recognizing that notification is not always warranted. Responses to hepatitis A cases among food handlers from other jurisdictions and a thorough assessment regarding effects of offering only hepatitis A vaccine as PEP to exposed persons might provide evidence to support this practice for large PEP campaigns. Training on hand hygiene, proper handling of uncooked food, and encouraging ill food handlers to stay at home and seek medical attention may help prevent cases in the future.
Implications for Policy & Practice.
Our experience with the 2013 hepatitis A cases provided multiple lessons learned, which DOHMH currently employs and might be valuable to other jurisdictions that have limited experience with cases among food handlers.
DOHMH continues to use the published algorithm,a including restaurant environmental assessments, as a guide to determine the need for hepatitis A virus postexposure prophylaxis of restaurant patrons but evaluates each case individually.
Enabling the NYC Public Health Laboratory to test for hepatitis A virus serology allows for improved timeliness of results and assists in rapid decision making.
DOHMH continues to offer only hepatitis A vaccine as PEP at PODs.b However, we implemented a screening process to identify persons who should receive IG (eg, immunocompromised) in addition to or instead of hepatitis A vaccine and preidentified a health care facility to refer those individuals.
DOHMH continues to use the City-wide Immunization Registryc at PODsb to determine if exposed persons younger than 19 years had previously received vaccine.
Temporary emergency sites set up rapidly and operated by New York City DOHMH. These sites are designed to distribute or administer prophylaxis to substantial populations after a biological attack or the emergence of an infectious disease. Only hepatitis A vaccine was offered as postexposure prophylaxis at the PODs in 2013.
DOHMH centralized database of all immunizations administered to persons 18 years or younger.10
Acknowledgments
This work was supported by Centers for Disease Control and Prevention (CDC) Cooperative Agreement for Public Health Emergency Preparedness (No. 5U90TP000546-02) and CDC Epidemiology and Laboratory Capacity for Infectious Diseases Cooperative Agreement (No. 3U50CI000899-02S4). The authors acknowledge all the New York City Department of Health and Mental Hygiene staff members who assisted in investigation and response efforts; and Drs Stacey A. Bosch and Melissa G. Collier at Centers for Disease Control and Prevention for assistance with manuscript preparation.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Alison Ridpath, Division of Disease Control, New York City Department of Health and Mental Hygiene, New York, New York; Epidemic Intelligence Service, Division of Scientific Education and Professional Development, Centers for Disease Control and Prevention, Atlanta, Georgia..
Vasudha Reddy, Division of Disease Control, New York City Department of Health and Mental Hygiene, New York, New York.
Marcelle Layton, Division of Disease Control, New York City Department of Health and Mental Hygiene, New York, New York.
Mark Misener, Division of Disease Control, New York City Department of Health and Mental Hygiene, New York, New York.
Allison Scaccia, Office of Emergency Preparedness and Response, New York City Department of Health and Mental Hygiene, New York, New York.
David Starr, Office of Emergency Preparedness and Response, New York City Department of Health and Mental Hygiene, New York, New York.
Faina Stavinsky, Division of Environmental Health, New York City Department of Health and Mental Hygiene, New York, New York.
Jay K. Varma, Division of Disease Control, New York City Department of Health and Mental Hygiene, New York, New York; National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
HaeNa Waechter, Division of Disease Control, New York City Department of Health and Mental Hygiene, New York, New York.
Jane R. Zucker, Division of Disease Control, New York City Department of Health and Mental Hygiene, New York, New York; National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Sharon Balter, Division of Disease Control, New York City Department of Health and Mental Hygiene, New York, New York.
References
- 1.Fiore AE. Hepatitis A transmitted by food. Clin Infect Dis. 2004;38:705–715. [DOI] [PubMed] [Google Scholar]
- 2.Winokur PL, Stapleton JT. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis. 1992;14:580–586. [DOI] [PubMed] [Google Scholar]
- 3.Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;357:1685–1694. [DOI] [PubMed] [Google Scholar]
- 4.Advisory Committee on Immunization Practices; Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 5.Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080–1084. [PubMed] [Google Scholar]
- 6.Craig AS, Watson B, Zink TK, Davis JP, Yu C, Schaffner W. Hepatitis A outbreak activity in the United States: responding to a vaccine-preventable disease. Am J Med Sci. 2007;334:180–183. [DOI] [PubMed] [Google Scholar]
- 7.Tricco AC, Pham B, Duval B, et al. A review of interventions triggered by hepatitis A infected food-handlers in Canada. BMC Health Serv Res. 2006;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.New York City Health Code 81.13 (a). https://www1.nyc.gov/site/doh/about/about-doh/health-code-and-rules.page. Accessed June 13, 2016. [Google Scholar]
- 9.Carl M, Francis DP, Maynard JE. Food-borne hepatitis A: recommendations for control. J Infect Dis. 1983;148:1133–1135. [DOI] [PubMed] [Google Scholar]
- 10.New York City Department of Health and Mental Hygiene. Citywide Immunization Registry. http://www.nyc.gov/html/doh/html/hcp/cir.shtml. Accessed May 10, 2016.
- 11.Foodborne transmission of hepatitis A—Massachusetts, 2001. MMWR Morb Mortality Wkly Rep. 2003;52:565–567. [PubMed] [Google Scholar]
- 12.Foodborne hepatitis A—Missouri, Wisconsin, and Alaska, 1990-1992. MMWR Morb Mortality Wkly Rep. 1993;42:526–534. [PubMed] [Google Scholar]
- 13.Rowe SL, Tanner K, Gregory JE. Hepatitis A outbreak epidemiologically linked to a food handler in Melbourne, Victoria. Commun Dis Intell Q Rep. 2009;33:46–48. [PubMed] [Google Scholar]
- 14.Public Health Agency of Canada. Canadian Immunization Guide. http://www.phac-aspc.gc.ca/publicat/cig-gci/p04-hepa-eng.php#a4. Accessed May 6, 2016.
- 15.Thomas LM. Guidance for the Prevention and Control of Hepatitis A Infection. London, England: Health Protection Agency; 2009. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/363023/Guidance_for_the_Prevention_and_Control_of_Hepatitis_A_Infection.pdf. Accessed May 10, 2016. [Google Scholar]
- 16.Department of Health and Ageing, Australian Government Department of Health. Hepatitis A: national guidelines for public health units. http://www.health.gov.au/internet/main/publishing.nsf/Content/FB28A405CBF6E64ECA257BF0001DAB33/$File/hepa-song.pdf. Published 2009. Accessed May 10, 2016.
- 17.California Department of Public Health. Hepatitis A postexposure prophylaxis guidance. http://www.cdph.ca.gov/programs/immunize/Documents/CDPH_HAV%20PEP%20Clinical%20Guidance.pdf. Published 2013. Accessed May 10, 2016.
- 18.Reuman PD, Kubilis P, Hurni W, Brown L, Nalin D. The effect of age and weight on the response to formalin inactivated, alum-adjuvanted hepatitis A vaccine in healthy adults. Vaccine. 1997;15(10):1157–1161. [DOI] [PubMed] [Google Scholar]
- 19.Briem H, Safary A. Immunogenicity and safety in adults of hepatitis A virus vaccine administered as a single dose with a booster 6 months later. J Med Virol. 1994;44(4):443–445. [DOI] [PubMed] [Google Scholar]
- 20.Nelson NP, Murphy TV, McMahon BJ. Hepatitis A vaccination for post-exposure prophylaxis in persons aged 40 years and older. Vaccine. 2014;32(25):2939. [DOI] [PMC free article] [PubMed] [Google Scholar]