Proteomics increasingly contributes to our understanding of the roles that proteins play in biology and a wide range of applications including the microbiome, bioremediation,1 and diseases such as cancer,2 Alzheimer’s,3 diabetes,4 cardiovascular disease,5 obesity,6 and many aspects of human health.7,8 The size of the human genome, ~20300 protein coding genes, results in an estimated three billion proteoforms9 that potentially exist in biological samples subject to proteomic analysis. The traditional approach of bottom-up proteomics requires digestion of proteins into peptides which further increases sample complexity. Despite this added complexity, however, peptides that “fly” well into the mass spectrometer are easily fragmented and detected and can be sequenced routinely using numerous data acquisition and analysis pipelines. To provide proteome depth across a dynamic range of 10–12 orders of magnitude requires sophisticated analytical instrumentation and extensive sample fractionation techniques. Quite impressively, this level of analyte multiplexing in single experiments has been taken advantage of in numerous studies over the last 15 years.
Many biological questions of interest, however, seek to determine differences in protein concentrations across two or more conditions, multiple time points, and in various tissues. This leads to a desire to increase the overall sample throughput in bottom-up proteomics. Mass spectrometry (MS)-based proteomics and protein microarray technology have made high throughput protein quantification possible. Microarray-based technology for proteomics includes full-length protein, peptide, antibody, reverse-phase, and tissue arrays10 that detect tens to thousands of proteins with fluorescent detection. Array-based approaches have advantages of multiplexing samples to simultaneously screen interactions among several biomolecules with linearity.11 Despite the advantages array-based approaches offer, they also suffer from intense experimental design, chip customization, protein immobilization in native state, normalization, nonspecific binding, cross reactivity,12 lack of highly specific antibodies, background corrections, and high level user expertise to handle the generated data.11–16
MS-based approaches can detect proteins at very low concentration with high quantitative accuracy. MS analyzers such as the quadrupole ion trap, time-of-flight MS, and FTICR MS instruments including the Orbitrap have high sensitivity, linearity, and dynamic range which are requirements for obtaining accurate protein quantitation. The dynamic range of detection for MS-based techniques ranges from 4 to 6 orders of magnitude, while plasma samples exhibit 12 orders of magnitude.17,18 These merits allow MS-based approaches to identify thousands of proteins while a smaller fraction of those proteins are quantifiable.19,20 Additionally, the incorporation of automation into the MS platform with autosamplers make large studies with numerous samples more feasible.
Sample multiplexing is a technique in proteomics that has been introduced in 1999, enabling scientists to compare and analyze two different sample preparations simultaneously within a single MS injection.21,22 Sample multiplexing can be performed by introducing an isotopic variant (light/heavy) at the peptide or protein level such that resulting samples are pooled to a single mixture and subject to LC-MS analysis. Quantification of the peptides/proteins is performed by comparing the intensities of the isotopic variants in the MS in a MS, MS/MS, or MS3 scan. Sample multiplexing in proteomics increases the confidence of results due to the ability to compare and analyze high numbers of biological samples in tandem while also including an internal standard or quality control for normalization.
In this review, a focus on approaches that increase sample throughput in quantitative proteomics with sample multiplexing is provided. Sample multiplexing strategies for proteins23 or peptides are such that multiple samples are metabolically or chemically “barcoded” with tags containing heavy isotope atoms.22,24–26 These tags introduce minor mass differences between nonisotopic and heavy isotopic versions of the tags which generally do not introduce differences in the chromatographic profile.27,28 Such coelution is desirable to enable capture of the information from all multiplexed samples simultaneously. Proteomics applications have advanced tremendously with the ability to sample multiplex two to 54 samples in a single run.29 While a number of reviews have been published on quantitative proteomics strategies to multiplex up to eight samples19,30–35 herein, we highlight strategies that combine eight or more samples in a single experiment. Once the level of multiplexing reaches more than 12 samples, we term these as enhanced multiplexing36 or hyperplexing approaches.37
MS-based quantitative proteomics includes a growing set of ancillary technologies that provide a means for high-throughput characterization and quantification of proteins in a biological sample or system.1,38 Stable isotope labeling of peptides or proteins prior to analysis is the most widely used quantification strategy other than label-free quantification.39 Labeling strategies for sample multiplexing fall under three main categories: chemical, enzymatic, and metabolic labeling. Chemical labeling is the most widely used due to its ability to label specific residues or peptide termini and to have highly efficient labeling reactions. Chemical or metabolic tags introduced to complex peptide mixtures should have the following criteria: (1) maximum specificity and efficiency, (2) wide dynamic range, and (3) stable physiochemical properties of the peptide.
SAMPLE MULTIPLEXING STRATEGIES
One of the main advantages of sample multiplexing is the ability to combine multiple samples and analyze them in a single MS run, reducing the instrument time and cost. There are multiple methods used for relative quantification, which are a consistent way to analyze protein expression patterns and the effects of biological perturbation in disease states. Quantitative proteomics is performed either by label-free methods or by isotopically labeling proteins or peptides prior to MS analysis. Isotope incorporation can be performed at the protein or peptide level using, for example, 2H, 13C, 15N, or 18O, as heavy isotopes.40 The use of deuterated tags has been reduced due to the fact that they elute quicker than the nondeuterated isotopomer.41 Hence, most of the chemical tagging strategies have focused on using 13C, 15N, or 18O isotopes which do not alter the chromatographic interaction of the analyte when combined. While the samples are pooled and analyzed, similar peptides from different samples elute at the same m/z in the MS scan with similar retention times.30 This enables the user to directly compare the relative abundances of the peptides in the MS scan or depending on the tag, in the MS/MS or MS3 scan.
Chemical Labeling.
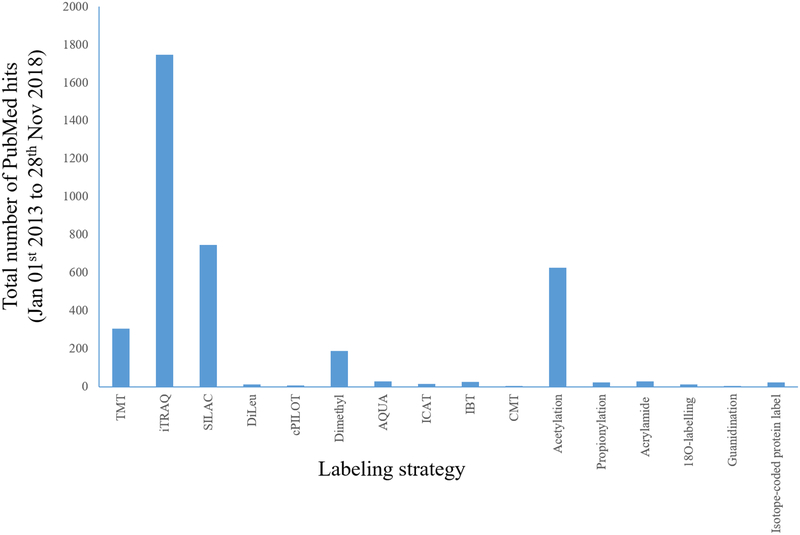
Chemical derivatization-based stable isotope labeling includes techniques such as isotope-coded affinity tag (ICAT),22,42–45 isotope-coded protein label (ICPL),46,47 isobaric tags for relative and absolute quantification (iTRAQ),48 tandem mass tags (TMT),49 isotope encoded dimethylated leucine or isobaric N,N-dimethyl leucine (DiLeu),50 isobaric tag (IBT),51 and combinatorial isobaric mass tags (CMTs).52 Other labeling approaches like dimethylation,35 acetylation,53,54 propionylation,55 acrylamide labeling,56 and guanidination of lysine residues57–59 have also been widely used. IBT 10-plex reagents (similar to the DiLeu with a difference in 13C and 15N heavy atoms) have been recently introduced with an extension of the 6-plex version of deuterium isobaric amine reactive tag (DiART) that has a minor mass difference of 6.3 mDa between isotopic pairs of 13C/12C and 15N/14N.60,61 Figure 1 shows a histogram of the total number of PubMed hits with search criteria for the labeling strategies (keywords: Proteomic + “Labeling strategy”) in quantitative proteomics from 1 Jan 2013 to 28 Nov 2018. The figure shows that iTRAQ, TMT, and SILAC have the most applications in the last five years, with the number of studies using dimethylation and acetylation following. In recent years, the use of TMT for isobaric peptide quantification has masked other strategies due to its popularity and commercial availability, however, DiLeu has emerged as a cost-effective alternative. In most cases, these strategies are used for quantitation, however, sometimes they are also used to track endogenous modifications.
Figure 1.
Histogram showing the total number of publications in Pubmed with the key word search (Proteomics and “Labeling strategy”) from 1 Jan 2013 to 28 Nov 2018 (last five years).
Enzymatic Labeling.
18O-labeling during proteolysis is a method for quantitative proteomics which involves digestion of one pool of proteins in H218O to isotopically label each C-terminus with two 18O atoms and the second pool of proteins in H2O. Peptides after labeling have a 4 Da mass increase and can be combined and fractionated prior to MS analysis.62,63 Enzymatic labeling has been also applied to study post-translational modifications including phosphorylation64 and glycosylation.65 These enzymatic approaches, however, are limited to only two samples in a multiplex experiment.
Metabolic Labeling.
Metabolic labeling approaches include stable isotope labeling by amino acids in cell culture (SILAC),66 stable isotope labeling in mammals (SILAM),67 super-SILAC,68 neutron encoding (NeuCode) SILAC,69,70 and absolute quantification (AQUA).71 Metabolic labeling using stable isotopes is an efficient method and has been shown to be successfully applied to cultured cells, human tissues, and biofluids.30,72 Traditional SILAC has limitations in multiplexing capability compared to isobaric tagging, whereas as many as three samples have been multiplexed. The degree of multiplexing depends on amino acids with heavy isotope versions being available. Generally, beyond three samples, the MS scan can become rather congested due to the triplet peaks for each peptide. SILAC in combination with TMT or iTRAQ, however, allows the number of sample channels to be increased to 54.73–75
Isobaric Tagging Strategies.
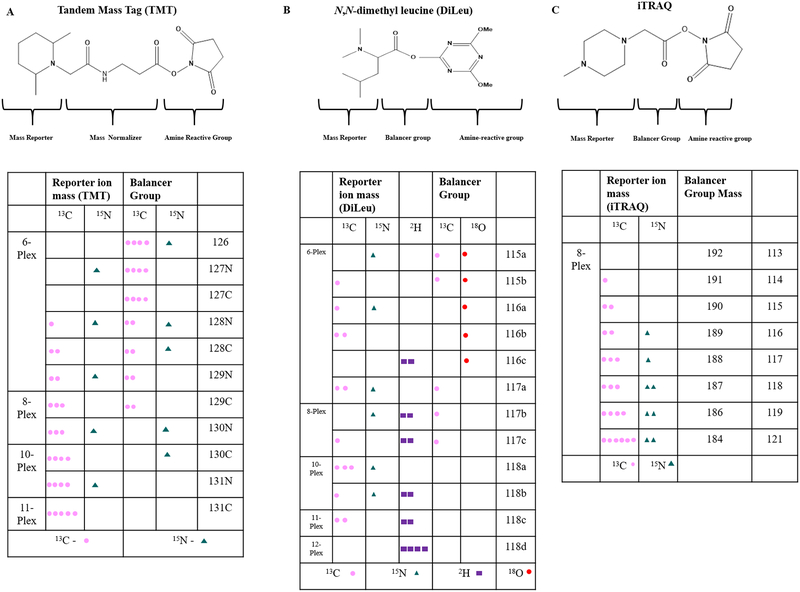
Isobaric tags improve the accuracy for peptide and protein quantification by simultaneous identification and relative quantification of peptides from many samples.49 Isobaric tagging reagents attach labels to free amino groups of proteolytic peptides and consist of three primary groups (Figure 2). The first group can be of different molecular weights and generates MS/MS spectra that can be used for quantification (reporter ion), while the second group provides mass balance and ensures that the molecular weight remains the same with different reporter ions. The third group provides the reactive group to attach the mass balancer and reporter ion groups to the primary amino group or residue (e.g., cysteine) in all peptides in a quantitative manner. Isobaric mass tags such as TMT, iTRAQ,76 and DiLeu77 have different versions with identical overall mass while they vary in the distribution and location of the heavy isotopes in the reporter ion and mass balancer groups (Figure 2). Isobaric tags currently available were developed sequentially from 2-plex to 11-plex for TMT. The TMT 11-plex currently available was expanded from 6-plex reagents utilizing neutron encoded tags with reporter ion mass differences of 6 mDa.74,78 This small mass difference requires a resolution of 30–60 K to differentiate each tag. Among the available isobaric mass tags, TMT and DiLeu have a multiplexing capacity of combining 11 and 12 samples in a single run, respectively, while iTRAQ can multiplex up to eight samples. The iTRAQ 4-plex reagent, however, has been shown to be more efficient compared to the 8-plex reagent based on the number of proteins identified.79 The reporter ions across different isobaric tags have m/z values 115–131 (Figure 2) and are accessible on modern MS instruments (Orbitrap Fusion or Lumos) with CID, HCD, or ECD/ETD.79–81 The isobaric tags such as TMT and iTRAQ are commercially available from Thermo Scientific and AB Sciex, which makes it readily and widely available. The tags cost ~$170 per sample run, which is expensive for many academic researchers. Alternatively, DiLeu reagents are readily synthesizable in house and cost $5 per sample run.82 This reagent represents a more affordable option without any sacrifice in multiplexing capability or data quality as discussed below.
Figure 2.
Multiplexing capabilities for combined analysis of more than eight proteomes. (A) Structure of tandem mass tags with the table showing the number of possible isotopes for 6-plex, 8-plex, 10-plex, and 11-plex reagents. (B) Structure of DiLeu and its isotopomers. (C) Structure of iTRAQ with its isotopomers.
Tandem Mass Tags.
TMT-based peptide quantification studies are globally increasing in recent years. TMT-based quantification has enabled users to achieve deep coverage in global and phosphoproteomics with more than 10000 proteins quantified in a single study.83–85 Density gradient-based subcellular fractionation using localization of organelle proteins by isotope tagging (LOPIT) method has led to deep proteome coverage at the subcellular level.86,87 LOPIT when combined with TMT allows one to fractionate cells and pool them into a single run. The protein dynamics of single cell development of Xenopus laevis embryo from egg to hatching at 10 different time points has been measured in a single experiment using TMT-10 plex barcoding reagents.88 Also, TMT has been shown to detect single amino acid variations related to cancer from nine cells with 6000 proteins identified using a TMT 11-plex reagent.89
Increasing the multiplexing capacity of the stable isotopes and isobaric tags helps us to analyze similar peptides from different samples in a single experiment. As mentioned, this increases the sample complexity while the presence of coeluting ions of similar charge can affect the accuracy and precision of the quantitative label using isobaric tags.90 Various technical improvements in liquid chromatography, MS resolution, and bioinformatics platforms have allowed us to maximize peptide/protein identification without losing quantitative information. TMT reagents suffer from quantification accuracies due to ion interference, which has been shown to produce inaccuracies in reporter ion signals. Generally, less than 10% of the total peptide ions are fragmented to generate TMT reporter ions for quantification, which is a major limiting factor for sample multiplexing strategies.91 The distorted reporter ion ratios of the coeluting fragments can be resolved by using multiple frequency notches or synchronous precursor selection (SPS) and conducting quantification in a MS3 spectrum instead of the MS/MS spectrum.90,92 A disadvantage of the MS3 isolation, however, is that it results in fewer proteins quantified.20 Extensive fractionation, narrowing the precursor ion isolation width, and delaying peptide fragmentation to occur close to the apex of chromatographic peaks have been helpful in reducing the cofragmentation and ratio compression issues.93,94 Low signal peptides are mostly affected by interference, which leads to ratio compression more so than high signal peptides.94
Sample complexity in proteomics has been tackled by various approaches including two-dimensional liquid chromatography,95 improved mass resolution, and even gas-phase ion fractionation techniques such as ion mobility. The incorporation of ion mobility can increase the number of quantifiable peptides by 2.5 fold.96–98 TMT has been shown to increase the number of quantifiable proteins in plasma after depletion followed by basic reverse phase fractionation or high pH fractionation.99 In phosphoproteomics, the complexity of the sample is reduced prior to tagging by enrichment which also reduces the interference. Additionally, the SPS-MS3 method has been shown to increase phosphopetide quantification accuracy.100 TMT can also be used for parallel quantitative analysis of proteomics and metabolomics measurements with a similar LC-MS setup with internal standards for amino acids.101
Isobaric Tags for Relative and Absolute Quantification (iTRAQ).
Isobaric tags for relative and absolute quantification has three distinct regions similar to TMT: the reporter ion for peptide quantification with a mass difference of 1 Da starting from 113–121 Da, a balancer group, and an amine reactive group that binds the tags to the lysine side chains and peptide N-termini with an overall total mass of 304 Da (Figure 2B). iTRAQ reagents are commercially available as 4-plex and 8-plex reagents. iTRAQ 4-plex has been reported to perform better than iTRAQ 8-plex and TMT 6-plex, with increased numbers of proteins identified and differentially expressed proteins with lesser intersample variations.102 Several studies have been published in the last five years (Figure 1) utilizing iTRAQ technology to compare and analyze bacterial,103 mouse,104 human,105 and plant proteomes.106 iTRAQ (8-plex) in combination with triplex dimethyl tags (light, medium, and heavy) has been reported to process 24 samples in parallel using high sample throughput multiple reaction monitoring (HST-MRM).107 The triplex dimethyl tags generate peptides with mass increases of 28, 32, and 36 Da, respectively, for light, medium, and heavy labeled peptides in the MS scan. The peptides are dimethylated at the N-terminus with triplex dimethyl tags and labeled at the C-termini of the lys-C terminated peptides with iTRAQ tags.
N,N-Dimethyl Leucine (DiLeu) Labeling.
DiLeu provides relative quantification of peptides108 and amine containing metabolites.109 DiLeu provides increased protein coverage, high labeling, quantification efficiency, inexpensive costs, easy synthesis, and high multiplexing capability, making it an attractive alternative to commercially available isobaric tags. DiLeu can be synthesized in-house in 1–2 days in a two-step process with high yield (~80%) and can be stored at −20 °C with a long shelf life (several years) prior to activation.50 After activating, the tag should be used immediately to yield optimal labeling efficiency. The synthesis of the DiLeu tag starts with the 18O exchange of leucine for the 115 and 116 variants, followed by dimethylation of leucine for all of the remaining tags (117–118) and purification via flash column chromatography.110 DiLeu resembles TMT and iTRAQ and has a triazine ester (amine reactive group) that binds the N-terminal and primary amino group of the lysine side chain, a balancer group, and a reporter group (Figure 2C). Tagged peptides exhibit a mass shift of 145 Da, and 12 different samples can be tagged and analyzed simultaneously. DiLeu labeled peptide offers improved confidence in peptide identification and quantification due to enhanced collision-induced fragmentation, leading to greater reporter ion intensities than iTRAQ.30,111 Ion mobility helps to reduce the coisolation and cofragmentation of DiLeu labeled peptides.112 The multiplexing capacity of the first-generation 4-plex DiLeu reagent was increased to 12-plex by adding mass defect-based isotopologues with a difference of ~6 mDa. The 12-plex DiLeu consists of two variants of 115, three variants of 116, three variants of 117, and four variants of 118, which also can be extended in the future. DiLeu tags can also be used for absolute quantification of peptides in addition to the relative quantification using a 5-plex isotopic DiLeu reagent.113,114 In addition to the global proteome approach, these tags have been used for post-translational modification analysis.80,115 The tags have been used for relative quantification of peptides and proteins in yeast,36 animal,116 and human studies.117 In addition, DiLeu can also be used to simultaneously analyze proteomes and amine metabolomes using the mass defect-based DiLeu.109,118 On the basis of the comparable efficiencies, cheaper costs, and overall quantification performance of these tags, wide implementation by the proteomics research community is encouraged.
HYPERPLEXING
The ability to multiplex utilizing isobaric tags either stand-alone or in combination with metabolic or chemical tags provides an opportunity for unbiased biological discovery. Approaches which multiplex up to 54 samples in a single MS injection represent a drastic increase in throughput, reproducibility, and robustness of quantitative proteomics. Multiplexing approaches currently available have developed tremendously in recent years, with a capability to process and compare up to 12 samples in a single injection. Proof-of-concept studies have been reported to combine 24–54 samples29,36,107 using a combination of two different labeling strategies. To develop and validate a biomarker, the candidate must be identified and quantified in samples in a statistically significant manner. To obtain significance, the number of samples generally has to be very high (10–100 samples). Additionally, the samples must be run in parallel with similar conditions to reduce the intersample variability. To reduce the variability between samples and to compare the expression patterns of proteins between diseased and healthy, treated and untreated, and so on, it can be advantageous to obtain peptide analyte concentrations in a single experiment. For example, the expression of proteins of different samples in a Western blot can be followed visually by the expression of the particular protein across different experimental conditions, and internal standards are available. For Western blot, up to 18 (or more) samples can be multiplexed in a single gel.
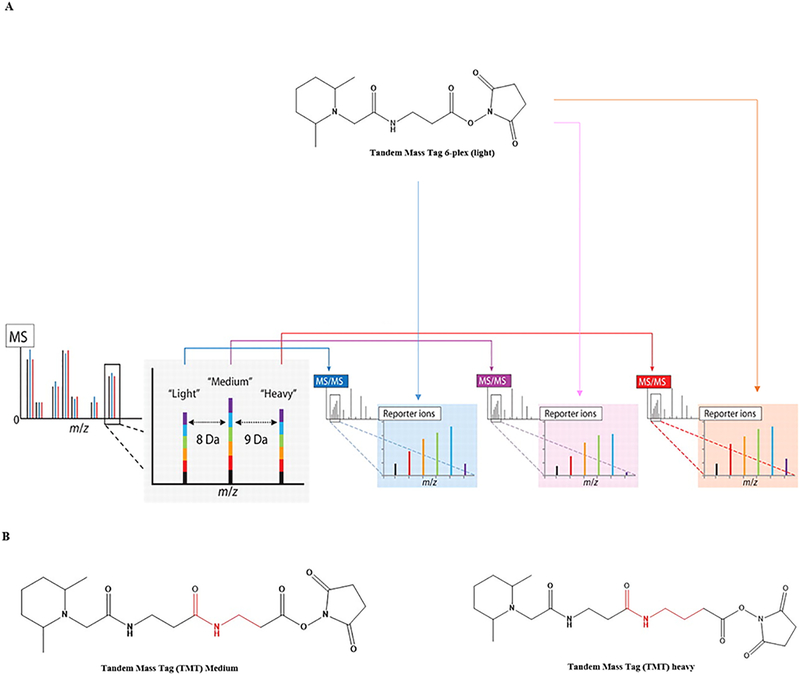
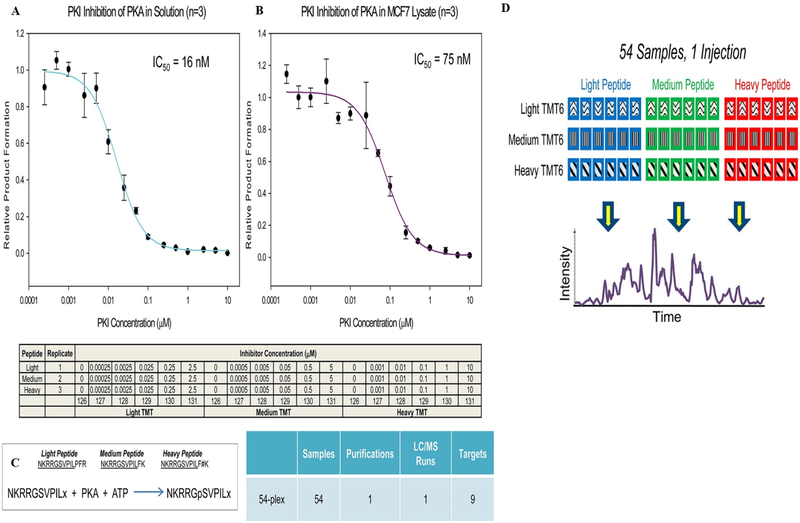
In 2012, Noah and Gygi showed that multiplexing can be increased up to 18 samples in a single experiment. Multiplexing was demonstrated by combining triplex metabolic labeling and 6-plex isobaric tags using three separable MS SILAC metabolic labels and corresponding TMT 6-plex isobaric tags (3 × 6) (Figure 3A).37 Later, this approach has been shown to combine 54 samples in an experiment by using light, medium, and heavy metabolic labeling (triplex SILAC) with three variants of 6-plex TMT using a targeted approach.29 The 54-plex study has been made possible by introducing two novel TMT tags (with insertion of aminopropanoic for medium and aminobutyric acid for heavy) in addition to the commercially available TMT (light) (Figure 3B). The novel medium tag is 71.02 Da (C3H5NO) heavier than the light tag, while the heavy tag is 14.02 Da (CH2) heavier than the medium tag. The caveat is that the three TMT tags elute at different times from the column. The proof-of-concept experiment showed the multiplexing ability by combining 54 experimental conditions to one sample using a kinase activity assay (Figure 4). The figure shows the inhibition of protein kinase A (PKA) in solution (Figure 4A) and breast cancer cell line MCF7 lysate by a peptide inhibitor (PKI) at 18 different conditions analyzed with triplicate injections (Figure 4B). A single peptide (residue 243–252) from the PKA substrate (EPB42) was serine phosphorylated upon inhibition (Figure 4C). The study further compares three different peptide transitions of the PKA substrate peptides by SILAC labeling and six variations from TMT, resulting in nine peptide transitions in a single study. The TMT and SILAC labeled peptides elute at different times, with the light eluting earlier than medium and heavy labeled peptides (Figure 4D). The peptides were quantified throughout the three different transitions with ultimately low standard error (8.75%). The increase in sample multiplexing helps researchers to reduce the instrument time and sample handling, while the novel TMT tags are not commercially available. This makes it difficult to access by the global research community.
Figure 3.
Combination of metabolically and chemically labeled stable isotopes to increase the sample multiplexing capabilities using a triplex SILAC and 6-plex TMT simultaneously. (A) Metabolic labels provide intact mass differences distinguishable in an MS1 scan of intact peptide ions. Upon isolation and fragmentation of the light, medium, and heavy versions of a peptide, the isobaric labels provide separate multiplexed quantitative measurements for each in the MS/MS spectra. (B) Structure of the novel TMT tags. Reproduced with permission from ref 37. Copyright 2012 American Association for the Advancement of Science.
Figure 4.
Increasing the multiplexing capabilities of tandem mass tags for combined analysis. Two 54-plex analyses consisting of an 18-point IC50 curve performed in triplicate. Inhibition of PKA using the peptide inhibitor PKI was conducted in solution (A) using 2 ng of commercially available PKA and in 5 μg of lysate from the breast cancer cell line MCF7. (B) Three variants of the substrate peptide based on two novel TMT reagents (C), resulting in nine target peptides. The resulting three variants eluted at different retention times (D). Reproduced with permission from ref 29. Copyright 2013 American Chemical Society.
ENHANCED MULTIPLEXING
Isobaric tags in combination with dimethyl tags or metabolic tags like SILAC help enhance the multiplexing ability of the isobaric tags. Various studies have successfully enhanced the multiplexing ability using dimethyl tags and SILAC in combination with TMT, iTRAQ, and DiLeu. Global measurement of protein turnover in primary human dermal fibroblasts has been shown as a proof-of-concept for enhanced multiplexing using SILAC and TMT labeling.119 SILAC strategies however are only relevant for cell cultures. Our laboratory has developed “combined precursor isotopic labeling and isobaric tagging” (cPILOT), which combines isobaric tags with reductive dimethylation for enhanced multiplexing.
Combined Precursor Isotopic Labeling and Isobaric Tagging.
Enhanced multiplexing of cPILOT is achieved by combining precursor MS labeling with isobaric tags (TMT, DiLeu) or iTRAQ. cPILOT uses stable isotope dimethylation of peptide N-termini with light [−(CH3)2] and heavy [−(13C2H3)2] isotopes at low pH (~2.5), which keeps the lysine residue available for subsequent high pH (8.5) TMT tagging.120 Addition of dimethyl tags in light and heavy versions help to increase the sample multiplexing ability of available isobaric tags by a factor of 2×. Also dimethylation reduces the amount of isobaric tag needed because the N-termini of half the peptides are occupied. Stable isotope dimethylation is inexpensive,121 has high capability (5-plex),122 versatility,123 high labeling efficiencies,124 and is pH dependent.125 Because cPILOT is a chemical derivatization strategy at the peptide level, it can be used for any sample type like cells, tissue, and body fluids. We have recently used cPILOT to combine up to 24 samples in a single experiment with DiLeu reagents.36 cPILOT has been shown to enhance sample multiplexing of global proteomes and oxidative post-translational modifications such as protein nitration.126 cPILOT was originally developed to study nitrated peptides,126 and later it has been extended to global,120 cysteine selective (cyscPILOT),127 and S-nitrosylated (OxycyscPILOT) approaches.128 These selective methods also have sample tagging steps conducted on-resin, which further increases labeling efficiencies. The global nature of cPILOT has been demonstrated using peptide mixtures from brains of wild-type C-57BL/6 and APP/PS-1 mice (an Alzheimer’s disease model). Also, reductive dimethylation alone results in quantification of less proteins compared to TMT labeling.129
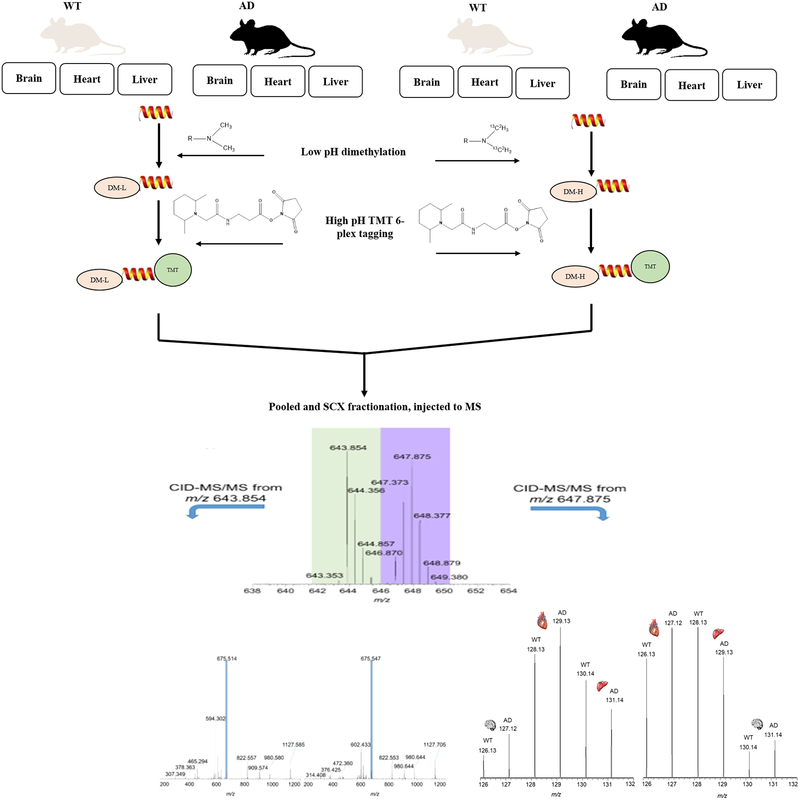
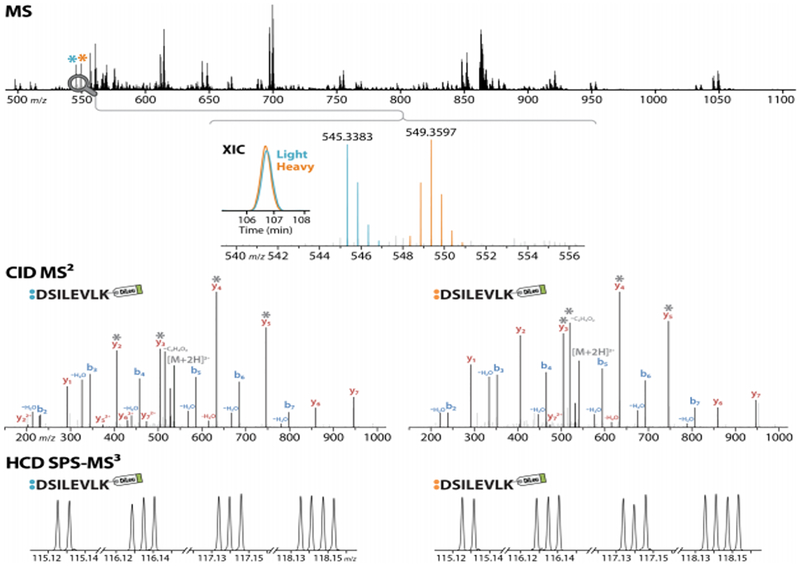
cPILOT has been used to compare proteomes in 12-plex130,131 and recently 24-plex36 applications. Light and heavy dimethylated peptides, when pooled and separated using nanoflow LC and electro sprayed into a LTQ Orbitrap MS, result in precursor spectra with peak pairs separated by 8 Da (Figure 5). King et al. demonstrated a 12-plex cPILOT analysis approach of protein from brain, heart, and liver tissues across biological replicates from APP/PS-1 mice compared with wild-type (Figure 5).130 In this study, protein expression changes across different tissues were compared in a single LC-MS run by combining peptides from three different tissue types after labeling by cPILOT. This is a powerful example of using enhanced nultiplexing to study changes in disease, across tissue types, and with biological replication in a single analysis. More recently, an enhanced sample multiplexing strategy using cPILOT and DiLeu isobaric tags has been demonstrated as a proof-of-concept in yeast.36 This approach achieved 24-plex quantification in a single LC-MS analysis using yeast tryptic digest subjected to light and heavy stable isotope dimethyl tags (Figure 6). The versatility of cPILOT is high and with the utilization of DiLeu, makes the approach very cost-effective. These approaches are available for all interested researchers to use and can be implemented easily,130 with consideration of a few important caveats.
Figure 5.
Enhanced multiplexing capabilities of 12-plex cPILOT for global proteome approach using a 6-plex TMT. Proteins from three different tissues of wild-type (WT) and Alzheimer’s disease (AD) mice were extracted, reduced, and alkylated and randomly grouped into two groups, dimethylated with light and heavy isotopes, tagged with TMT 6-plex reagents, and injected to an LC-MS/MS system. Precursor data shows light and heavy dimethylated peptides, represented by the peaks at m/z 643.854 and 647.875. These peptides were selected, isolated, and fragmented, thus generating CID-MS/MS spectra, which provided peptide identification. An additional isolation and fragmentation of the most intense fragment ion of the light and heavy dimethylated peptides generated HCD-MS3 spectra, respectively. The peptide sequence is T(dimethyl)ELNYFAK(TMT6) and belongs to phosphoglycerate kinase 1. Reproduced with permission from ref 130. Copyright 2017 JoVE.
Figure 6.
Enhancing the multiplexing capabilities of sample preparation using isobaric tags in combination with cPILOT. Spectra acquired on the Orbitrap Fusion Lumos of a DiLeu cPILOT-labeled peptide with sequence DSILEVLK. The coeluting light and heavy peptide peak pair, with overlapping extracted ion chromatograms (XIC), is detected in the MS scan at m/z 545.3383 and 549.3597, and each is acquired by CID MS/MS in the linear ion trap. Following each MS/MS scan, HCD SPS-MS3 acquisition in the Orbitrap (RP 60K) of the top four ions (marked by gray asterisks) generates two sets of abundant 12-plex DiLeu reporter ions for 24-plex quantification. Reproduced with permission from ref 36. Copyright 2018 American Chemical Society.
Challenges of cPILOT.
The key issues to be taken care of while performing a cPILOT analysis include careful handling of samples, exposure of similar reaction times to all samples, and maintaining low pH for dimethylation. Sample handling is very important in cPILOT to make the mixing of light and heavy samples similar in each analysis. For large numbers of samples (10s-100s), this is best facilitated by using a multichannel pipet, processing in batches, or using a robotic platform. The dimethylation reaction of cPILOT is pH specific and depends on maintaining low pH for dimethylation to prevent labeling of lysine residues. While processing large numbers of samples is desirable, the major requirement is to quantify dimethylated pairs from all of the reporter ion channels (11–12 plex). This can be achieved by using current state-of-the-art mass spectrometers (Orbitrap Fusion or Lumos) and nano flow LC systems. Additionally, it is advisable to optimize the parameters such as LC gradient time, m/z isolation window, dynamic exclusion time, targeted analysis nodes, selective y1- fragmentation,120 and SPS-MS3 for obtaining maximum multiplexing capability and analytical performance.
OTHER MULTIPLEXING APPROACHES
Targeted proteomics has emerged as a powerful quantification tool for proteins in systems biology, biomedical research, and increasingly in clinical studies.132,133 Another approach shows a 24-plex experiment using hepatocellular carcinoma to evaluate serum biomarker with triplex dimethyl tags combined with 8-plex iTRAQ reagent.107 Targeted proteomics approaches like selected reaction monitoring (SRM), also known as multiple reaction monitoring (MRM), parallel reaction monitoring (PRM), and data-independent acquisition (DIA) combined with targeted data extraction of the MS/MS spectra [e.g., sequential windowed acquisition of all theoretical product ion mass spectra (SWATH)], are developing rapidly.134 Reproducible and accurate quantification of target peptides or proteins across many samples have been made possible by SRM- and PRM-based analysis. Although both SRM and PRM have not been demonstrated with sample multiplexing to date, it is possible to ensure reliable quantification for ~500 peptides/125 proteins in a single analysis. Increasing the number of target peptides can be performed by narrowing the peptide elution time to accommodate more peptide transitions in a narrow window. Additionally, sensitivity could decrease significantly with an increase of targets and is inversely proportional to the degree of multiplexing. DIA-based targeted quantification overcomes these limitations by allowing wide precursor acquisition windows to be selected. In DIA-based quantification, the peptides within a defined m/z window are fragmented and the MS records a high accuracy product ion spectrum for each detectable peptide. Also, SRM-based quantification has been shown to be at least 10-fold higher in sensitivity than DIA-based targeted quantification.134
BENEFITS OF MULTIPLEXING
Sample multiplexing helps researchers to study proteome changes throughout multiple cohorts so as to study the effects in healthy and disease conditions. With the ability to combine up to 54 samples in a single study, it will be easier to compare and study the interaction, modification, and pharmacokinetics of a protein/molecule at one glance. Sample multiplexing drastically reduces the time required for sample analysis.
Samples for proteomics studies are mostly tissue/clinical materials from patients for discovery and validation of biomarkers. Samples are biological fluids such as plasma, cerebrospinal fluid, urine, saliva, and tissues from human, animals, cell lines, and plants. There are two main limitations which have to be overcome to make use of enhanced multiplexing strategies at its full efficiency for such samples. The first limiting factor is sample multiplexing reagents and the other factor is analytical instruments used to prepare and analyze the samples.135 Introduction of new isotopomers will only increase the complexity of the analysis by eluting all the peptides in a narrow window. Sample preparation is the critical step in a proteomics experiment, which is time-consuming, laborious, and expensive. A reliable sample preparation pipeline should be able to isolate the complete proteins from a sample in a reproducible manner and benefit from various levels of automation. Multiplexing requires extensive pipetting skills and accuracy, which heavily influence the reproducibility of the experiment. For instance, with increased sample tagging of more samples, the number of pipetting steps increases and there is a high likelihood of introducing error.
Chemical tagging, especially isobaric tagging reagents, undergo ratio distortion, signal interference, and chemical noise, leading to less quantitative accuracy at the MS2 level. This later issue is overcome by the introduction of sophisticated hybrid-Orbitrap instruments. There has been extensive use of the isobaric tag at the MS2 level, which has less quantitative accuracy than what is possible with SPS precursor selection, MS3.136 In a MS2 level identification, the measured intensity is the combination of the reporter ion intensities of the peptides identified and other coeluting peptides termed as interference.137 Advanced peak determination (APD) is a new peak-picking algorithm which increases the number of precursors selected and identified for MS2. Ratio compression is supposed to decrease with the use of APD in label free quantification, while the interference from coeluting peptides has been reported to increase while using TMT 11-plex reagents at the MS2 level.138 While some studies have reported less numbers of quantifiable peptides in TMT 10-plex approaches compared to label free approaches, isobaric tagging still has the advantage of combining multiple samples in a single run which reduces intersample variations.139
CONCLUDING REMARKS
Molecular medicine is moving beyond genomics to clinical proteomics which will require robust methodologies for biomarker profiling. Proteomics plays a crucial role in early diagnosis, prognosis, and disease monitoring, and there is a need to analyze hundreds to thousands of samples to compare the expression of proteins in multiple experimental conditions. Protocols that are amendable to automated sample preparation will be necessary because handling 1000s of samples manually will require weeks to months to prepare samples, while analysis is possible within a single or a few days. The main problem faced by academic researchers is the expensive costs of the multiplexing reagents and automated platforms. Use of multiplexing strategies that offer >8–54 sample multiplexing, increase the potential of achieving large-scale initiatives in a high-throughput manner.
ACKNOWLEDGMENTS
We acknowledge Vanderbilt University Institutional Funds and NIH (R01GM117191) to RASR.
Biographies
Albert B. Arul studied Biochemistry at the University of Madras (India), where he received his Masters and Ph.D. degrees in 2002 and 2009, respectively. After a short stay at Vimta Laboratories Ltd for a year at Hyderabad, India, as a Scientist B at a preclinical drug discovery group, he moved to the college of Applied Medical Sciences, King Saud University, Kingdom of Saudi Arabia (2010–2012). Later, he moved to South Korea as a Research Assistant Professor to work on automated sample preparation for biomarker discovery using proteomics from 2012 to 2016 at Gachon University and Seoul National University. After completing postdoctoral training at South Korea, he moved to George Washington University, Washington, DC, as a Postdoctoral scientist to work on identification of MoA of drug molecules using quantitative proteomics. Currently, he is working as a Research Assistant Professor at the Department of Chemistry, Vanderbilt University. His current research focuses on developing sample multiplexing strategies and automation of the sample preparation for proteomics studies.
Renã A. S. Robinson is an Associate Professor of Chemistry at Vanderbilt University and the inaugural Dorothy J. Wingfield Phillips Chancellor’s Faculty Fellow. Dr. Robinson received her B.S. in chemistry with a concentration in business from the University of Louisville in 2000 and her Ph.D. in Analytical Chemistry from Indiana University. During her graduate studies, she developed proteomics methods to study aging in fruit flies and moved into the fields of redox proteomics and Alzheimer’s disease as a Lyman T. Johnson Postdoctoral Fellow and later UNCF/Merck Postdoctoral Fellow. Dr. Robinson joined the Department of Chemistry at the University of Pittsburgh as an Assistant Professor in 2009 and was promoted to Associate Professor in 2017. Dr. Robinson has a nationally and internationally recognized research program, and she is as an emerging leader the field of proteomics for her work in proteomics technology development, aging, Alzheimer’s disease, and applications relevant to human health.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Angel TE; Aryal UK; Hengel SM; Baker ES; Kelly RT; Robinson EW; Smith RD Chem. Soc. Rev 2012, 41 (10), 3912–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Panis C; Pizzatti L; Souza GF; Abdelhay E Cancer Lett 2016, 382 (2), 231–239. [DOI] [PubMed] [Google Scholar]
- (3).Robinson RA; Amin B; Guest PC Adv. Exp. Med. Biol 2017, 974, 21–48. [DOI] [PubMed] [Google Scholar]
- (4).Kraniotou C; Karadima V; Bellos G; Tsangaris GT J. Proteomics 2018, 188, 59–62. [DOI] [PubMed] [Google Scholar]
- (5).Smith JG; Gerszten RE Circulation 2017, 135 (17), 1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wang X; Xu S; Chen L; Shen D; Cao Y; Tang R; Wang X; Ji C; Li Y; Cui X; Guo X Proteomics: Clin. Appl 2018, 12 (6), No. 1700172. [DOI] [PubMed] [Google Scholar]
- (7).Kushner IK; Clair G; Purvine SO; Lee J-Y; Adkins JN; Payne SH J. Proteome Res 2018, 17 (11), 3914–3922. [DOI] [PubMed] [Google Scholar]
- (8).Norman KC; Moore BB; Arnold KB; O’Dwyer DN Respirology (Carlton, Vic.) 2018, 23 (11), 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Smith LM; Kelleher NL Science (Washington, DC, U. S.) 2018, 359 (6380), 1106–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Duarte JG; Blackburn JM Expert Rev. Proteomics 2017, 14 (7), 627–641. [DOI] [PubMed] [Google Scholar]
- (11).Gahoi N; Ray S; Srivastava S Proteomics 2015, 15 (2–3), 218–31. [DOI] [PubMed] [Google Scholar]
- (12).Sevecka M; MacBeath G Nat. Methods 2006, 3 (10), 825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Cha T; Guo A; Zhu XY Proteomics 2005, 5 (2), 416–9. [DOI] [PubMed] [Google Scholar]
- (14).Sokolik CW; Walker AS; Nishioka GM Anal. Chem. Insights 2011, 6, ACI.S7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gundisch S; Grundner-Culemann K; Wolff C; Schott C; Reischauer B; Machatti M; Groelz D; Schaab C; Tebbe A; Becker KF J. Proteome Res 2013, 12 (10), 4424–34. [DOI] [PubMed] [Google Scholar]
- (16).Dziembowski A; Seraphin B FEBS Lett 2004, 556 (1–3), 1–6. [DOI] [PubMed] [Google Scholar]
- (17).Anderson NL; Anderson NG Mol. Cell. Proteomics 2002, 1 (11), 845–867. [DOI] [PubMed] [Google Scholar]
- (18).Yates JR; Ruse CI; Nakorchevsky A Annu. Rev. Biomed. Eng 2009, 11, 49–79. [DOI] [PubMed] [Google Scholar]
- (19).Bantscheff M; Schirle M; Sweetman G; Rick J; Kuster B Anal. Bioanal. Chem 2007, 389 (4), 1017–1031. [DOI] [PubMed] [Google Scholar]
- (20).Altelaar AFM; Frese CK; Preisinger C; Hennrich ML; Schram AW; Timmers HTM; Heck AJR; Mohammed S J. Proteomics 2013, 88, 14–26. [DOI] [PubMed] [Google Scholar]
- (21).Shiio Y; Aebersold R Nat. Protoc 2006, 1 (1), 139–45. [DOI] [PubMed] [Google Scholar]
- (22).Gygi SP; Rist B; Gerber SA; Turecek F; Gelb MH; Aebersold R Nat. Biotechnol 1999, 17 (10), 994–9. [DOI] [PubMed] [Google Scholar]
- (23).Wu S; Lourette NM; Tolic N; Zhao R; Robinson EW; Tolmachev AV; Smith RD; Pasa-Tolic L J. Proteome Res 2009, 8 (3), 1347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tan Z; Yi X; Carruthers NJ; Stemmer PM; Lubman DM, Single Amino Acid Variant Discovery in Small Numbers of Cells. J. Proteome Res 2018. DOI: 10.1021/acs.jproteome.8b00694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Johnson ECB; Dammer EB; Duong DM; Yin L; Thambisetty M; Troncoso JC; Lah JJ; Levey AI; Seyfried NT Mol. Neurodegener 2018, 13, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Xiao H; Zhang Y; Kim Y; Kim S; Kim JJ; Kim KM; Yoshizawa J; Fan L-Y; Cao C-X; Wong DTW Sci. Rep 2016, 6, 22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zimmer JSD; Monroe ME; Qian W-J; Smith RD Mass Spectrom. Rev 2006, 25 (3), 450–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Aebersold R; Mann M Nature 2003, 422 (6928), 198–207. [DOI] [PubMed] [Google Scholar]
- (29).Everley RA; Kunz RC; McAllister FE; Gygi SP Anal. Chem 2013, 85 (11), 5340–5346. [DOI] [PubMed] [Google Scholar]
- (30).Rauniyar N; Yates JR J. Proteome Res 2014, 13 (12), 5293–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Treumann A; Thiede B Expert Rev. Proteomics 2010, 7 (5), 647–653. [DOI] [PubMed] [Google Scholar]
- (32).Chahrour O; Cobice D; Malone J J. Pharm. Biomed. Anal 2015, 113, 2–20. [DOI] [PubMed] [Google Scholar]
- (33).Tao WA; Aebersold R Curr. Opin. Biotechnol 2003, 14 (1), 110–118. [DOI] [PubMed] [Google Scholar]
- (34).Yao X Anal. Chem 2011, 83 (12), 4427–4439. [DOI] [PubMed] [Google Scholar]
- (35).Hsu J-L; Chen S-H Philos. Trans. R. Soc., A 2016, 374 (2079), 20150364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Frost DC; Rust CJ; Robinson RAS; Li L Anal. Chem 2018, 90 (18), 10664–10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Dephoure N; Gygi SP Sci. Signaling 2012, 5 (217), rs2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Domon B; Aebersold R Science (Washington, DC, U. S.) 2006, 312 (5771), 212–217. [DOI] [PubMed] [Google Scholar]
- (39).Megger DA; Pott LL; Ahrens M; Padden J; Bracht T; Kuhlmann K; Eisenacher M; Meyer HE; Sitek B Biochim. Biophys. Acta, Proteins Proteomics 2014, 1844 (5), 967–76. [DOI] [PubMed] [Google Scholar]
- (40).Heck AJR; Krijgsveld J Expert Rev. Proteomics 2004, 1 (3), 317–326. [DOI] [PubMed] [Google Scholar]
- (41).Van Damme P; Van Damme J; Demol H; Staes A; Vandekerckhove J; Gevaert K BMC Proc 2009, 3, S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Smolka MB; Zhou H; Purkayastha S; Aebersold R Anal. Biochem 2001, 297 (1), 25–31. [DOI] [PubMed] [Google Scholar]
- (43).Parker KC; Patterson D; Williamson B; Marchese J; Graber A; He F; Jacobson A; Juhasz P; Martin S Mol. Cell. Proteomics 2004, 3 (7), 625–659. [DOI] [PubMed] [Google Scholar]
- (44).Hansen KC; Schmitt-Ulms G; Chalkley RJ; Hirsch J; Baldwin MA; Burlingame AL Mol. Cell. Proteomics 2003, 2 (5), 299–314. [DOI] [PubMed] [Google Scholar]
- (45).Yu LR; Conrads TP; Uo T; Issaq HJ; Morrison RS; Veenstra TD J. Proteome Res 2004, 3 (3), 469–77. [DOI] [PubMed] [Google Scholar]
- (46).Schmidt A; Kellermann J; Lottspeich F Proteomics 2005, 5 (1), 4–15. [DOI] [PubMed] [Google Scholar]
- (47).Lottspeich F; Kellermann J, ICPL Labeling Strategies for Proteome Research In Gel-Free Proteomics: Methods and Protocols; Gevaert K, Vandekerckhove J, Eds.; Humana Press: Totowa, NJ, 2011; pp 55–64. [DOI] [PubMed] [Google Scholar]
- (48).Ross PL; Huang YN; Marchese JN; Williamson B; Parker K; Hattan S; Khainovski N; Pillai S; Dey S; Daniels S; Purkayastha S; Juhasz P; Martin S; Bartlet-Jones M; He F; Jacobson A; Pappin DJ Mol. Cell. Proteomics 2004, 3 (12), 1154–69. [DOI] [PubMed] [Google Scholar]
- (49).Thompson A; Schafer J; Kuhn K; Kienle S; Schwarz J; Schmidt G; Neumann T; Hamon C Anal. Chem 2003, 75 (8), 1895–1904. [DOI] [PubMed] [Google Scholar]
- (50).Frost DC; Greer T; Li L Anal. Chem 2015, 87 (3), 1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Xing L; Sun L; Liu S; Li X; Zhang L; Yang H Comp. Biochem. Physiol., Part D: Genomics Proteomics 2017, 23, 17–26. [DOI] [PubMed] [Google Scholar]
- (52).Braun CR; Bird GH; Wühr M; Erickson BK; Rad R; Walensky LD; Gygi SP; Haas W Anal. Chem 2015, 87 (19), 9855–9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Mischerikow N; Heck AJ Proteomics 2011, 11 (4), 571–89. [DOI] [PubMed] [Google Scholar]
- (54).Polevoda B; Sherman F Genome Biology 2002, 3, reviews0006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Wu Z; Cheng Z; Sun M; Wan X; Liu P; He T; Tan M; Zhao Y Mol. Cell. Proteomics 2015, 14 (2), 329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Cahill MA; Wozny W; Schwall G; Schroer K; Holzer K; Poznanovic S; Hunzinger C; Vogt JA; Stegmann W; Matthies H; Schrattenholz A Rapid Commun. Mass Spectrom 2003, 17 (12), 1283–90. [DOI] [PubMed] [Google Scholar]
- (57).Beardsley RL; Reilly JP Anal. Chem 2002, 74 (8), 1884–90. [DOI] [PubMed] [Google Scholar]
- (58).Thevis M; Ogorzalek Loo RR; Loo JA J. Proteome Res 2003, 2 (2), 163–72. [DOI] [PubMed] [Google Scholar]
- (59).Brancia FL; Butt A; Beynon RJ; Hubbard SJ; Gaskell SJ; Oliver SG Electrophoresis 2001, 22 (3), 552–9. [DOI] [PubMed] [Google Scholar]
- (60).Ren Y; He Y; Lin Z; Zi J; Yang H; Zhang S; Lou X; Wang Q; Li S; Liu S Anal. Chem 2018, 90, 12366–12371. [DOI] [PubMed] [Google Scholar]
- (61).Chen Z; Wang Q; Lin L; Tang Q; Edwards JL; Li S; Liu S Anal. Chem 2012, 84 (6), 2908–2915. [DOI] [PubMed] [Google Scholar]
- (62).Yao X; Freas A; Ramirez J; Demirev PA; Fenselau C Anal. Chem 2001, 73 (13), 2836–42. [DOI] [PubMed] [Google Scholar]
- (63).Reynolds KJ; Yao X; Fenselau C J. Proteome Res 2002, 1 (1), 27–33. [DOI] [PubMed] [Google Scholar]
- (64).Winter D; Seidler J; Ziv-Lehrman S; Shiloh Y; Lehmann WD Anticancer Res 2009, 29 (12), 4949–4958. [PubMed] [Google Scholar]
- (65).Shakey Q; Bates B; Wu J Anal. Chem 2010, 82 (18), 7722–8. [DOI] [PubMed] [Google Scholar]
- (66).Ong SE; Blagoev B; Kratchmarova I; Kristensen DB; Steen H; Pandey A; Mann M Mol. Cell. Proteomics 2002, 1 (5), 376–86. [DOI] [PubMed] [Google Scholar]
- (67).Wu CC; MacCoss MJ; Howell KE; Matthews DE; Yates JR Anal. Chem 2004, 76 (17), 4951–9. [DOI] [PubMed] [Google Scholar]
- (68).Geiger T; Cox J; Ostasiewicz P; Wisniewski JR; Mann M Nat. Methods 2010, 7, 383. [DOI] [PubMed] [Google Scholar]
- (69).Rose CM; Merrill AE; Bailey DJ; Hebert AS; Westphall MS; Coon JJ Anal. Chem 2013, 85 (10), 5129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Hebert AS; Merrill AE; Bailey DJ; Still AJ; Westphall MS; Strieter ER; Pagliarini DJ; Coon JJ Nat. Methods 2013, 10 (4), 332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Kirkpatrick DS; Gerber SA; Gygi SP Methods (Amsterdam, Neth.) 2005, 35 (3), 265–73. [DOI] [PubMed] [Google Scholar]
- (72).Beynon RJ; Pratt JM Mol. Cell. Proteomics 2005, 4 (7), 857–872. [DOI] [PubMed] [Google Scholar]
- (73).Merrill AE; Hebert AS; MacGilvray ME; Rose CM; Bailey DJ; Bradley JC; Wood WW; El Masri M; Westphall MS; Gasch AP; Coon JJ Mol. Cell. Proteomics 2014, 13 (9), 2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).McAlister GC; Huttlin EL; Haas W; Ting L; Jedrychowski MP; Rogers JC; Kuhn K; Pike I; Grothe RA; Blethrow JD; Gygi SP Anal. Chem 2012, 84 (17), 7469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Werner T; Becher I; Sweetman G; Doce C; Savitski MM; Bantscheff M Anal. Chem 2012, 84 (16), 7188–94. [DOI] [PubMed] [Google Scholar]
- (76).Choe L; D’Ascenzo M; Relkin NR; Pappin D; Ross P; Williamson B; Guertin S; Pribil P; Lee KH Proteomics 2007, 7 (20), 3651–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Frost DC; Greer T; Li L Anal. Chem 2015, 87 (3), 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Werner T; Sweetman G; Savitski MF; Mathieson T; Bantscheff M; Savitski MM Anal. Chem 2014, 86 (7), 3594–601. [DOI] [PubMed] [Google Scholar]
- (79).Pichler P; Köcher T; Holzmann J; Mazanek M; Taus T; Ammerer G; Mechtler K Anal. Chem 2010, 82 (15), 6549–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Yu Q; Shi X; Feng Y; Kent KC; Li L Anal. Chim. Acta 2017, 968, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Yu Q; Shi X; Greer T; Lietz CB; Kent KC; Li L J. Proteome Res 2016, 15 (9), 3420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Frost DC; Greer T; Xiang F; Liang Z; Li L Rapid Commun. Mass Spectrom 2015, 29 (12), 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Bai B; Tan H; Pagala VR; High AA; Ichhaporia VP; Hendershot L; Peng J Methods Enzymol 2017, 585, 377–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Huang FK; Zhang G; Lawlor K; Nazarian A; Philip J; Tempst P; Dephoure N; Neubert TA J. Proteome Res 2017, 16 (3), 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Huttlin EL; Jedrychowski MP; Elias JE; Goswami T; Rad R; Beausoleil SA; Villen J; Haas W; Sowa ME; Gygi SP Cell 2010, 143 (7), 1174–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Christoforou A; Mulvey CM; Breckels LM; Geladaki A; Hurrell T; Hayward PC; Naake T; Gatto L; Viner R; Martinez Arias A; Lilley KS Nat. Commun 2016, 7, 9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Mulvey CM; Breckels LM; Geladaki A; Britovsek NK; Nightingale DJH; Christoforou A; Elzek M; Deery MJ; Gatto L; Lilley KS Nat. Protoc 2017, 12 (6), 1110–1135. [DOI] [PubMed] [Google Scholar]
- (88).Gupta M; Sonnett M; Ryazanova L; Presler M; Wühr M, Quantitative Proteomics of Xenopus Embryos I, Sample Preparation In Xenopus: Methods and Protocols; Vleminckx K, Ed.; Springer: New York, 2018; pp 175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Tan Z; Yi X; Carruthers NJ; Stemmer PM; Lubman DM, Single Amino Acid Variant Discovery in Small Numbers of Cells. J. Proteome Res 2018. DOI: 10.1021/acs.jproteome.8b00694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Ting L; Rad R; Gygi SP; Haas W Nat. Methods 2011, 8 (11), 937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Chen Y; Wang S; Jia J; Tian X; Xu H; Ning M; Bai B Proteomics 2018, 18 (13), No. 1700336. [DOI] [PubMed] [Google Scholar]
- (92).McAlister GC; Nusinow DP; Jedrychowski MP; Wühr M; Huttlin EL; Erickson BK; Rad R; Haas W; Gygi SP Anal. Chem 2014, 86 (14), 7150–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Savitski MM; Sweetman G; Askenazi M; Marto JA; Lang M; Zinn N; Bantscheff M Anal. Chem 2011, 83 (23), 8959–67. [DOI] [PubMed] [Google Scholar]
- (94).Ahrne E; Glatter T; Vigano C; Schubert C; Nigg EA; Schmidt A J. Proteome Res 2016, 15 (8), 2537–47. [DOI] [PubMed] [Google Scholar]
- (95).Plubell DL; Wilmarth PA; Zhao Y; Fenton AM; Minnier J; Reddy AP; Klimek J; Yang X; David LL; Pamir N Mol. Cell. Proteomics 2017, 16 (5), 873–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Pfammatter S; Bonneil E; McManus FP; Prasad S; Bailey DJ; Belford M; Dunyach JJ; Thibault P Mol. Cell. Proteomics 2018, 17 (10), 2051–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).High AA; Tan H; Pagala VR; Niu M; Cho JH; Wang X; Bai B; Peng J J. Vis. Exp 2017, No. 129, e56474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Lim MY; O’Brien J; Paulo JA; Gygi SP J. Proteome Res 2017, 16 (11), 4217–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Keshishian H; Burgess MW; Specht H; Wallace L; Clauser KR; Gillette MA; Carr SA Nat. Protoc 2017, 12 (8), 1683–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Erickson BK; Jedrychowski MP; McAlister GC; Everley RA; Kunz R; Gygi SP Anal. Chem 2015, 87 (2), 1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Murphy JP; Everley RA; Coloff JL; Gygi SP Anal. Chem 2014, 86 (7), 3585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Casey TM; Khan JM; Bringans SD; Koudelka T; Takle PS; Downs RA; Livk A; Syme RA; Tan K-C; Lipscombe RJ J. Proteome Res 2017, 16 (2), 384–392. [DOI] [PubMed] [Google Scholar]
- (103).Thai VC; Lim TK; Le KPU; Lin Q; Nguyen TTH Journal of global antimicrobial resistance 2017, 8, 82–89. [DOI] [PubMed] [Google Scholar]
- (104).Li X; Li X; Lu J; Huang Y; Lv L; Luan Y; Liu R; Sun R BMC Complementary Altern. Med 2017, 17, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Shum AMY; Poljak A; Bentley NL; Turner N; Tan TC; Polly P Oncotarget 2018, 9, 22001–22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Martinez-Esteso MJ; Casado-Vela J; Selles-Marchart S; Pedreno MA; Bru-Martinez R Methods Mol. Biol. (N. Y., NY, U. S.) 2014, 1072, 155–69. [DOI] [PubMed] [Google Scholar]
- (107).Jiang H; Zhang L; Zhang Y; Xie L; Wang Y; Lu H, HST-MRM-MS: A Novel High-Sample-Throughput Multiple Reaction Monitoring Mass Spectrometric Method for Multiplex Absolute Quantitation of Hepatocellular Carcinoma Serum Biomarker. J. Proteome Res 2018. DOI: 10.1021/acs.jproteome.8b00790 [DOI] [PubMed] [Google Scholar]
- (108).Xiang F; Ye H; Chen R; Fu Q; Li L Anal. Chem 2010, 82 (7), 2817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Hao L; Zhong X; Greer T; Ye H; Li L Analyst 2015, 140 (2), 467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Frost DC; Li L, High-Throughput Quantitative Proteomics Enabled by Mass Defect-Based 12-Plex DiLeu Isobaric Tags In Quantitative Proteomics by Mass Spectrometry; Sechi S, Ed.; Springer: New York, 2016; pp 169–194. [DOI] [PubMed] [Google Scholar]
- (111).Hui L; Xiang F; Zhang Y; Li L Peptides 2012, 36 (2), 230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Sturm RM; Lietz CB; Li L Rapid Commun. Mass Spectrom 2014, 28 (9), 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Greer T; Lietz CB; Xiang F; Li L J. Am. Soc. Mass Spectrom 2015, 26 (1), 107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Greer T; Li L Methods Mol. Biol. (N. Y., NY, U. S.) 2016, 1410, 195–206. [DOI] [PubMed] [Google Scholar]
- (115).Chen Z; Yu Q; Hao L; Liu F; Johnson J; Tian Z; Kao WJ; Xu W; Li L Analyst 2018, 143 (11), 2508–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Jiang X; Xiang F; Jia C; Buchberger AR; Li L ACS Chem. Neurosci 2018, 9 (8), 2054–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Greer T; Hao L; Nechyporenko A; Lee S; Vezina CM; Ricke WA; Marker PC; Bjorling DE; Bushman W; Li L PLoS One 2015, 10 (8), No. e0135415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Hao L; Johnson J; Lietz CB; Buchberger A; Frost D; Kao WJ; Li L Anal. Chem 2017, 89 (2), 1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Welle KA; Zhang T; Hryhorenko JR; Shen S; Qu J; Ghaemmaghami S Mol. Cell. Proteomics 2016, 15 (12), 3551–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Evans AR; Robinson RA Proteomics 2013, 13 (22), 3267–72. [DOI] [PubMed] [Google Scholar]
- (121).Bantscheff M; Lemeer S; Savitski MM; Kuster B Anal. Bioanal. Chem 2012, 404 (4), 939–65. [DOI] [PubMed] [Google Scholar]
- (122).Wu Y; Wang F; Liu Z; Qin H; Song C; Huang J; Bian Y; Wei X; Dong J; Zou H Chem. Commun. (Cambridge, U. K.) 2014, 50 (14), 1708–10. [DOI] [PubMed] [Google Scholar]
- (123).Boersema PJ; Raijmakers R; Lemeer S; Mohammed S; Heck AJ Nat. Protoc 2009, 4 (4), 484–94. [DOI] [PubMed] [Google Scholar]
- (124).Gu L; Evans AR; Robinson RAS J. Am. Soc. Mass Spectrom 2015, 26 (4), 615–630. [DOI] [PubMed] [Google Scholar]
- (125).Qin H; Wang F; Zhang Y; Hu Z; Song C; Wu R; Ye M; Zou H Chem. Commun. (Cambridge, U. K.) 2012, 48 (50), 6265–7. [DOI] [PubMed] [Google Scholar]
- (126).Robinson RAS; Evans AR Anal. Chem 2012, 84 (11), 4677–4686. [DOI] [PubMed] [Google Scholar]
- (127).Gu L; Evans AR; Robinson RA J. Am. Soc. Mass Spectrom 2015, 26 (4), 615–30. [DOI] [PubMed] [Google Scholar]
- (128).Gu L; Robinson RA Analyst 2016, 141 (12), 3904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).King CD; Singh D; Holden K; Govan AB; Keith SA; Ghazi A; Robinson RAS J. Proteomics 2018, 181, 92–103. [DOI] [PubMed] [Google Scholar]
- (130).King CD; Dudenhoeffer JD; Gu L; Evans AR; Robinson RAS Enhanced Sample Multiplexing of Tissues Using Combined Precursor Isotopic Labeling and Isobaric Tagging (cPILOT), J. Vis. Exp 2017. 123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Evans AR; Gu L; Guerrero R Jr.; Robinson RA Proteomics: Clin. Appl 2015, 9 (9–10), 872–84. [DOI] [PubMed] [Google Scholar]
- (132).Shi T; Song E; Nie S; Rodland KD; Liu T; Qian W-J; Smith RD Proteomics 2016, 16 (15–16), 2160–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Erickson BK; Rose CM; Braun CR; Erickson AR; Knott J; McAlister GC; Wühr M; Paulo JA; Everley RA; Gygi SP Mol. Cell 2017, 65 (2), 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Gillet LC; Navarro P; Tate S; Rost H; Selevsek N; Reiter L; Bonner R; Aebersold R Mol. Cell. Proteomics 2012, 11, O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Garbis S; Lubec G; Fountoulakis M Journal of Chromatography A 2005, 1077 (1), 1–18. [DOI] [PubMed] [Google Scholar]
- (136).Sonnett M; Yeung E; Wuhr M Anal. Chem 2018, 90 (8), 5032–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Wenger CD; Lee MV; Hebert AS; McAlister GC; Phanstiel DH; Westphall MS; Coon JJ Nat. Methods 2011, 8 (11), 933–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Myers SA; Klaeger S; Satpathy S; Viner R; Choi J; Rogers J; Clauser K; Udeshi ND; Carr SA, Evaluation of Advanced Precursor Determination for Tandem Mass Tag (TMT)-Based Quantitative Proteomics across Instrument Platforms. J. Proteome Res 2018. DOI: 10.1021/acs.jproteome.8b00611 [DOI] [PubMed] [Google Scholar]
- (139).Sande CJ; Mutunga M; Muteti J; Berkley JA; Nokes DJ; Njunge J Sci. Rep 2018, 8, 13814. [DOI] [PMC free article] [PubMed] [Google Scholar]