Abstract
Permanent neonatal diabetes mellitus is a rare disorder known to be caused by activating mutations in KCNJ11 or ABCC8, inactivating mutations in INS, or very rarely in GCK or IPF-1 genes. We report a patient with permanent neonatal diabetes mellitus and severe exocrine pancreatic insufficiency. Ultrasound examination revealed pancreatic agenesis with a suggestion of a small amount of tissue in the head of the pancreas. Genetic testing revealed that the neonate had a homozygous Pro63fsX60 IPF-1 mutation. This is the second reported case of neonatal diabetes mellitus secondary to a homozygous mutation in the IPF-1 gene and supports the previously proposed biological role of IPF-1 in the pancreatic development in human.
Keywords: neonatal diabetes, IPF-1, pancreatic agenesis
Neonatal diabetes mellitus is a rare disease with an estimated incidence of 1 in 450,000 (1). It is defined as insulin-requiring hyperglycemia occurring within the first few months of life and lasting more than 2 weeks (2). The cases appear to be almost evenly divided between permanent and transient neonatal diabetes with similar presentations of growth retardation, dehydration and hyperglycemia. While transient neonatal diabetes usually resolves within 6 months to one year with potential recurrence of diabetes during childhood or adolescence, permanent neonatal diabetes does not resolve or remit (2). Permanent neonatal diabetes can have different etiologies, which can include abnormalities in the genes coding for pancreatic β-cell ATP-sensitive potassium channels, enzymes such as glucokinase or the proteins involved in the development of the pancreas (3–5). In 1993 a neonate was described with permanent neonatal diabetes and exocrine pancreatic insufficiency due to congenital pancreatic agenesis (6) who in 1997 was then found to harbor a homozygous IPF-1 gene mutation (4). A second infant was described with pancreatic agenesis, neonatal diabetes and pancreatic insufficiency due to a compound heterozygous mutation of the IPF-1 gene (7). We are now presenting a third neonate with permanent neonatal diabetes and severe exocrine pancreatic insufficiency due to an IPF-1 gene mutation and the second case with a homozygous IPF-1 mutation, who on ultrasound examination has pancreatic agenesis and a suggestion of a small amount of tissue in the head of the pancreas.
Case report
The propositus, a male infant with a birth weight of 1.56 kilograms, was born at 37 weeks gestation to a 21 year old, Caucasian G2P1 mother. Pregnancy was complicated by gestational diabetes that was treated with insulin. At 37 weeks, he was delivered by caesarean section because of oligohydramnios and intrauterine growth retardation. APGAR scores were 8 at one minute and 9 at 5 minutes. Since he was an infant of a mother who had gestational diabetes, blood glucoses were monitored carefully. His initial blood glucose was 110 mg/dL (6.05 mmol/L). Within twenty-four hours of life, his blood glucose was as high as 378 mg/dL (20.79 mmol/L). A sepsis evaluation was initiated which was eventually reported as negative. He was started on an intravenous insulin drip and received between ½ to 1 unit of regular insulin a day. Urine ketones were negative. However, he was prone to quick changes in blood glucose and experienced hypoglycemia with small changes in the insulin dosage. At eight days of life, when it was evident that the hyperglycemia was not a transient problem, he was transferred to our facility.
On arrival to our Neonatal Intensive Care Unit, the following measures were recorded when blood glucose was 346 mg/dL (19.03 mmol/L): GAD-65 and insulin autoantibodies were negative, C-peptide <0.5 ng/mL (<0.166 nmol/L) with a reference range of 1–5.2ng/mL (0.33–1.72 nmol/L) and insulin <2 μU/mL (<0.08 μg/L) with a normal range of 5–15 μU/mL (0.2–0.62 μg/L). Physical exam was unremarkable and did not reveal dysmorphic features or physical abnormalities. He was continued on the insulin drip and fed continuously with Neosure 22kcal/oz. Target blood glucose was set at 120–300 mg/dL (6.6 – 16.5 mmol/L).
Within three weeks of birth, despite significant intake of formula, he had persistent weight loss and foul-smelling stools. Reducing substances were present in the stool. Stool elastase levels were <50 μg elastase/g stool, indicating severe exocrine pancreatic insufficiency and treatment was initiated with pancreatic enzymes.
He was eventually transitioned to bolus feeding. Initially, diluted NPH (Neutral Protamine Hagedorn) and Glargine were given. Diluted Regular insulin and Glargine were then tried. Finally, the Lilly diluent was acquired enabling the use of pre-diluted regular insulin that was diluted from the U-100 vial (100 units/ml) once every two weeks. However Glargine still had to be diluted immediately prior to administration.
Because of the difficulty of giving small amounts of diluted insulin and difficulty of maintaining his blood glucose without wide fluctuations, it was decided that the baby would benefit from a continuous subcutaneous insulin infusion (CSII) regimen using diluted insulin. Diluted Lispro (U-10, 10 units/ml) was administered via an Animas pump. This pump was chosen since it could deliver the smallest basal and boluses possible among all the commercially available insulin pumps. Soon after, the baby’s blood glucoses did improve into the 200–300 mg/dL (11–16.5 mmol/L) range, with very few hypoglycemic events. Insulin to carbohydrate coverage charts and correction charts for high blood glucoses were given to the family for them to calculate the amount of insulin based on volume of formula and blood glucose measurements done approximately every 3 hours. A low basal rate was calculated to protect him from significant hypoglycemia in case of inadequate oral intake.
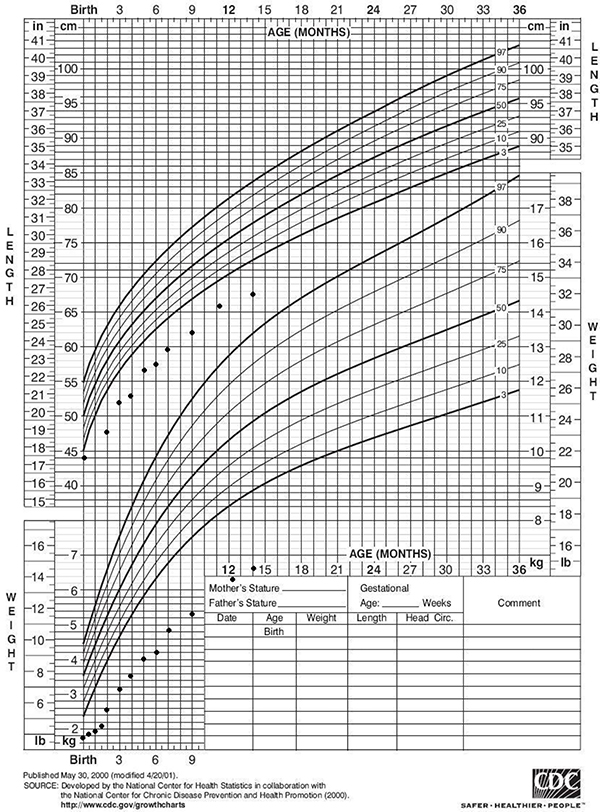
His first HbA1c (done by Bayer DCA 2000+ HbA1c analyzer) at 2 months was 7.2% but the reliability of the measurement was uncertain because of the fetal hemoglobin expected to be present based on his age (8). His three-month HbA1c (Bayer DCA 2000+) was 9%. His HbA1c (done by HPLC) at 4 months of age was 12.1%. The amount of fetal hemoglobin present was then calculated to be approximately 8–10%. His HbA1c ((Bayer DCA 2000+) at 17 months of age was 9%. He was receiving approximately 0.8 units /kg/day of Lispro insulin via CSII. This insulin requirement is approximately 75th percentile for a 2 year old child with type 1 diabetes (9). It has been difficult to manage this infant because of wide fluctuations in blood glucose concentrations from 50–400 mg/dL (2.75 – 22 mmol/L) in quick succession, inconsistency of appetite and feeding schedule, malabsorption, subcutaneous infections at the pump site insertion and frequent illnesses. This experience appears to be similar to others who have also managed infants with neonatal diabetes (10, 11). We have recently discontinued CSII therapy at his mother’s request. On basal bolus therapy with Glargine and diluted Lispro, his blood glucose concentrations now range between 100–250 mg/dL (5.5 – 11 mmol/L). His growth was poor initially, but once he was started on insulin and a pancreatic enzyme replacement, he has demonstrated steady weight gain. However, he has not yet experienced catch-up growth. (Growth chart is included in Figure 1.)
Figure 1:
Our propositus’s height and weight growth charts
The initial ultrasound (at 2 weeks of age) of his pancreas visualized portions of the mid and distal body of the pancreas that appeared structurally normal. No focal mass was identified. A subsequent ultrasound one week later was interpreted as demonstrating the presence of only the head of the pancreas. A CT of the pancreas without sedation at 7 months of age demonstrated a small amount of tissue anterior to the splenic vein that could either represent pancreas or small bowel. Ultrasound examination at one year of age revealed a small hypoechoic structure in the area of the pancreatic head, but because of his small size, a definitive conclusion was not rendered. An MRI at 5 years of age was suggested for confirmation.
Family history is notable as his mother had gestational diabetes with both of her pregnancies which were treated with insulin. The father, who is 22 years old, has had hyperglycemia by home blood glucose monitoring since age 15 years and is now treated with oral agents. Both maternal and paternal grandparents are also being treated for type 2 diabetes. The proband’s parents were later determined to have Maturity-Onset Diabetes of the Young (MODY4) (Fajans SS, Bell GI, Paz VP et al, manuscript in preparation).
Microsatellite analysis of chromosome 6 for uniparental inheritance, which has been described in transient neonatal diabetes, was reported as normal. Diagnostic genetic testing for permanent neonatal diabetes carried out by Athena Diagnostics (Worchester, MA) revealed a homozygous mutation in the IPF-1 gene (nucleotide change of c.188delC with a predicted amino acid change of p.Pro63fsX60) with normal sequences in KCNJ11 and GCK genes. Our patient was also tested for mutations in the cystic fibrosis gene since he exhibited clinical evidence of malabsorption due to exocrine pancreatic insufficiency. Interestingly, he was found to be a carrier of the mutated CFTR gene (4006-46delTATTT).
Discussion
In our infant, the combination of severe exocrine pancreatic insufficiency and permanent neonatal diabetes suggested the possibility of pancreatic agenesis and by association the presence of an IPF-1 mutation. Indeed, our patient was found to have a homozygous IPF-1 mutation Pro63fsX60. The same homozygous Pro63fsdelC mutation was present in the first infant with IPF-1 mutation previously described (4). This suggests, but does not prove, that the families of the two probands may be related. Homozygosity implies that consanguinity may have existed in the parents’ families, as was reported in the previous family (4).
There are other causes for permanent neonatal diabetes. Heterozygous activating mutations in the KCNJ11 gene, which encodes the Kir6.2 subunit of the KATP channel, are thought to be the most common cause of permanent neonatal diabetes (3, 12). In a recent study by Edghill et al, KCNJ11 mutations were found in 31% of individuals with neonatal diabetes (12). Mutations in the ABCC8 and insulin gene were respectively found in 10 and 12% of the individuals (12). With these causes, exocrine pancreatic function is preserved.
Baumeister et al compiled 14 cases of pancreatic agenesis associated with neonatal diabetes (13). Many of these neonates had associated malformations such as biliary system or heart abnormalities that increased their morbidity (13). Several other studies have also noted that pancreatic hypoplasia can be associated with congenital heart disease (14), intestinal atresia and gall bladder hypoplasia (15). Another possibility was the X-linked IPEX syndrome (immunodysregulation, polyendocrinopathy, and enteropathy) secondary to a mutation in the FOXP3 gene (16). However, since our proband did not develop any further endocrine abnormalities, this possibility was not pursued.
Our patient may have a small portion of the head of the pancreas as suggested by ultrasound examination at 12 months of age. Multiple transcription factors are involved in the development, maintenance and function of the pancreas (17). Our patient has a homozygous mutation in the Insulin promoter factor (IPF-1) gene, also known as Pancreas Duodenum Homeobox -1 (PDX-1). IPF-1 is a transcription factor necessary for development and growth of the pancreas (18), but may not be involved in the primary initiation of pancreatic development (17). In mice, IPF-1 expression can be first identified while the exocrine pancreas is forming embryonically (18). In the pancreatic endoderm of Pdx1−/− mice, small rudimentary buds with glucagon+ cells have been detected (17, 19), but further growth of the pancreas did not occur, which suggested that PDX1/IPF-1 may be necessary for sustained differentiation for both the endocrine and exocrine components of the pancreas (17).
A heterozygous IPF-1 mutation results in a MODY4 phenotype. Homozygous mutations lead to neonatal diabetes, which has been attributed to pancreatic agenesis. Both parents carry the heterozygous mutation in IPF-1 gene (Fajans SS, Bell GI, Paz VP et al, manuscript in preparation). To date there has been only one other case with the same homozygous mutation as our proband. (4). That child presented at 18 days of life and was confirmed by ultrasound in the neonatal period to have pancreatic agenesis (4). Another female who developed hyperglycemia at 12 days of life was found to have a compound heterozygous mutation of the IPF-1 gene that also resulted in pancreatic agenesis (7). Marsh’s group reported four more children with neonatal diabetes and pancreatic agenesis or hypoplasia have been described (20). Several genes that encode for transcription factors other than IPF-1 (Ptf1a, Sox9, Sox17, Hnf6, H1xB9) were screened. None carried an IPF-1 mutation, but new polymorphisms were found that were not thought to be disease-causing since similar changes were also found in unaffected individuals (20).
In summary we report what we believe to be the second case of neonatal diabetes, exocrine pancreatic insufficiency and pancreatic agenesis caused by a homozygous IPF-1 mutation. In mice, IPF-1 is known to be involved in the continuing development and maintenance of the pancreas but not in its induction. The possible presence of a small amount of tissue in the head of the pancreas on ultrasound examination could provide clues as to biological role of IPF-1 in the pancreatic development in humans.
Acknowledgements
We thank Dr. Michael A. DiPietro for the expertise with the interpretation and review of the ultrasound images, Jennifer Schwab, RN, CDE and Catherine L. Martin, MS, APRN, BC-ADM, CDE for their assistance.
This work was supported in part by grants from the National Institutes of Health (T32-DK071212 [NKS and AA], DK082386 [JML], DK49845 [RKM] and DK-20572 [SSF]). JML was also supported by the Clinical Sciences Scholars Program at the University of Michigan.
Abbreviations
- CSII
Continuous Subcutaneous Insulin Infusion
- IPF-1
Insulin Promotor Factor-1
- MODY
Maturity-Onset Diabetes of the Young
- NPH
Neutral Protamine Hagedorn
- PDX-1
Pancreas Duodenum Homeobox -1
References
- 1.von Muhlendah KE and Herkenhoff H Long-Term Course of Neonatal Diabetes. N Engl J Med. 1995; 333:704–8. [DOI] [PubMed] [Google Scholar]
- 2.Sperling MA. Neonatal diabetes mellitus: from understudy to center stage. Current Opinion in Pediatrics. 2005; 17:512–8. [DOI] [PubMed] [Google Scholar]
- 3.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, et al. Activating Mutations in the Gene Encoding the ATP-Sensitive Potassium-Channel Subunit Kir6.2 and Permanent Neonatal Diabetes. N Engl J Med. 2004; 350:1838–49. [DOI] [PubMed] [Google Scholar]
- 4.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL and Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997; 15:106–10. [DOI] [PubMed] [Google Scholar]
- 5.Njolstad PR, Sovik O, Cuesta-Munoz A, Bjorkhaug L, Massa O, Barbetti F, et al. Neonatal Diabetes Mellitus Due to Complete Glucokinase Deficiency. N Engl J Med. 2001; 344:1588–92. [DOI] [PubMed] [Google Scholar]
- 6.Wright NM, Metzger DL, Borowitz SM and Clarke WL Permanent Neonatal Diabetes-Mellitus and Pancreatic Exocrine Insufficiency Resulting from Congenital Pancreatic Agenesis. Am J Dis Child. 1993; 147:607–9. [DOI] [PubMed] [Google Scholar]
- 7.Schwitzgebel VM, Mamin A, Brun T, Ritz-Laser B, Zaiko M, Maret A, et al. Agenesis of Human Pancreas due to Decreased Half-Life of Insulin Promoter Factor 1. J Clin Endocrinol Metab. 2003; 88:4398–406. [DOI] [PubMed] [Google Scholar]
- 8.Puukka R and Puukka M Effect of hemoglobin F on measurements of hemoglobin A1c with physicians’ office analyzers. Clin Chem. 1994; 40:342–3. [PubMed] [Google Scholar]
- 9.Wiegand S, Raile K, Reinehr T, Hofer S, Nake A, Rabl W, et al. Daily insulin requirement of children and adolescents with type 1 diabetes: effect of age, gender, body mass index and mode of therapy. Eur J Endocrinol. 2008; 158:543–9. [DOI] [PubMed] [Google Scholar]
- 10.Bharucha T, Brown J, McDonnell C, Gebert R, McDougall P, Cameron F, et al. Neonatal diabetes mellitus: Insulin pump as an alternative management strategy. Journal of Paediatrics and Child Health. 2005; 41:522–6. [DOI] [PubMed] [Google Scholar]
- 11.Wintergerst KA, Hargadon S and Hsiang HY. Continuous subcutaneous insulin infusion in neonatal diabetes mellitus. Pediatr Diabetes. 2004; 5:202–6. [DOI] [PubMed] [Google Scholar]
- 12.Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, et al. Insulin mutation screening in 1,044 patients with diabetes: Mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008; 57:1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumeister FAM, Engelsberger I and Schulze A Pancreatic agenesis as cause for neonatal diabetes mellitus. Klinische Padiatrie. 2005; 217:76–81. [DOI] [PubMed] [Google Scholar]
- 14.Yorifuji T, Matsumura M, Okuno T, Shimizu K, Sonomura T, Muroi J, et al. Hereditary pancreatic hypoplasia, diabetes mellitus, and congenital heart disease: a new syndrome? J Med Genet. 1994; 31:331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell J, Punthakee Z, Lo B, Bernard C, Chong K, Newman C, et al. Neonatal diabetes, with hypoplastic pancreas, intestinal atresia and gall bladder hypoplasia: search for the aetiology of a new autosomal recessive syndrome. Diabetologia. 2004; 47:2160–7. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi I, Shiari R, Yamada M, Kawamura N, Okano M, Yara A, et al. Novel mutations of FOXP3 in two Japanese patients with immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome (IPEX). J Med Genet. 2001; 38:874–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver-Krasinski JM and Stoffers DA On the origin of the {beta} cell. Genes Dev. 2008; 22:1998–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habener JF, Kemp DM and Thomas MK Minireview: Transcriptional Regulation in Pancreatic Development. Endocrinology. 2005; 146:1025–34. [DOI] [PubMed] [Google Scholar]
- 19.Ahlgren U, Jonsson J and Edlund H The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996; 122:1409–16. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Hussain K, Al-Ali M, Dattani MT, Hindmarsh P, Jones PM, et al. Neonatal and Late-Onset Diabetes Mellitus Caused by Failure of Pancreatic Development: Report of 4 More Cases and a Review of the Literature. Pediatrics. 2008; 121:e1541–7. [DOI] [PubMed] [Google Scholar]