Abstract
Background:
Epidemiological reports indicate that mood-related disorders are common in the adolescent population. The prevalence of juvenile major depressive disorder has resulted in a parallel increase in the prescription rates of fluoxetine (FLX) within this age group. Although such treatment can last for years, little is known about the enduring consequences of adolescent antidepressant exposure on memory-related performance.
Methods:
We exposed separate groups of adolescent (postnatal day [PD] 35) male and female C57BL/6 mice to FLX (20 mg/kg) for 15 consecutive days (PD35–49). Three weeks after FLX exposure (PD70), we assessed learning and memory performance on a single-day training object novelty recognition test, or a spatial memory task on the Morris water maze (MWM).
Results:
We found that FLX pretreatment did not influence performance on either the object novelty recognition task or the MWM, 24 hr after training. Conversely, 48 hr post spatial-training on the MWM, FLX pretreated male mice spent significantly less time on the quadrant of the missing platform during a standard probe trial. No differences in MWM performance were observed in the adult female mice pretreated with FLX.
Limitations:
A limitation of this study is that normal adolescent mice (i.e., non-stressed) were evaluated for memory-related behavior three weeks after antidepressant exposure. Thus, it is possibility that FLX pre-exposure in combination with animal models for the study of depression may yield different results.
Conclusion:
Together, these results demonstrate enduring spatial memory-related deficiencies after pre-exposure to FLX during adolescence in male, but not female, C57BL/6 mice.
INTRODUCTION
Adolescence is a stage of development in which the incidence of mood-related disorders emerges. As a result, antidepressant medications, primarily selective serotonin reuptake inhibitors (SSRIs), are frequently administered to the juvenile population (Emslie and Judge, 2000; Schroder et al., 2017a). In particular, the prescription rate of fluoxetine (FLX), when compared to other antidepressants, has been consistently high in populations younger than 20 years of age – given that it is the only pharmacotherapeutic agent approved by the Food and Drug Administration for the treatment of pediatric major depressive disorder. Despite the heightened rates of antidepressant drug exposure, the long-lasting impact of FLX treatment during developmental periods prior to adulthood have not been thoroughly investigated (Olivier et al., 2011).
Accumulating preclinical investigations on the enduring consequences of early-life drug treatment, specifically during adolescence, have started to question the safety of exposure to antidepressant medications. While results are not always conclusive (Izquierdo et al., 2016; Norcross et al., 2008), generally, juvenile exposure to FLX results in neurobiological and behavioral alterations in adulthood (Olivier et al., 2011; Shrestha et al., 2014). For example, adolescent exposure to FLX modifies the reward valence of both natural (Iñiguez et al., 2010) and drug (Flores-Ramirez et al., 2018; Iñiguez et al., 2015) rewards later in life; per the sucrose and cocaine place conditioning behavioral paradigms. Thus, implicating potential long-lasting alterations in drug seeking behavior (Bardo and Bevins, 2000). Furthermore, juvenile FLX exposure mediates a prolonged anxiogenic-like behavioral phenotype (Homberg et al., 2011; Sass and Wortwein, 2012). These enduring FLX-induced changes in reward- and stress-related stimuli may be indicative of a generalized depressive phenotype (Flores-Ramirez et al., 2018; Popa et al., 2008), particularly because FLX re-exposure in adulthood normalizes some of these behavioral adaptations (Iñiguez et al., 2010; Karpova et al., 2009).
Responses to stress and drug-associated stimuli are correlated with learning and memory performance (Kennedy et al., 2016); as such, it is possible that adolescent antidepressant exposure may result in prolonged changes in memory function. Indeed, a study in male rats indicates that adolescent FLX exposure impairs spatial memory performance on a Morris water maze (MWM) task in adulthood (Sass and Wortwein, 2012). Yet, how such treatment influences memory performance, in females specifically, is currently not known. This is surprising, given that females are more likely than males to be diagnosed with mood related disorders, and thus, be exposed to SSRIs (Schroder et al., 2017b). Furthermore, ontogenic animal studies assessing drug-induced changes in behavior often report differential outcomes as a function of sex (Bevins and Charntikov, 2015; Mateos-Garcia et al., 2015); hence, stressing the need for investigations evaluating prolonged SSRI-induced changes in behavior between males and females. To address this issue, the purpose of this study is to evaluate how adolescent FLX exposure influences episodic (object recognition) and spatial (MWM) memory performance in adulthood, in male and female C57BL/6 mice.
METHODS
Animals
Male and female C57BL/6 mice were bred from individuals originally purchased from Charles River Laboratory (Hollister, CA). Mice were housed in standard polypropylene cages containing wood shavings and placed on a 12 hr light/dark cycle (lights on at 7 a.m.) under unrestricted access to food and water. Experiments were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with approval of the Institutional Animal Care and Use Committee at California State University San Bernardino and The University of Texas at El Paso.
Antidepressant Treatment and Experimental Design
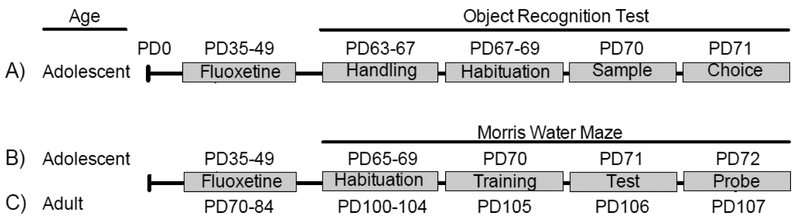
Fluoxetine hydrochloride (FLX) was obtained from Spectrum Chemicals (Gardena, CA), dissolved in sterile distilled water (VEH), and administered via intraperitoneal (IP) injections, in a volume of 2 mL/kg. Separate groups of mice (Table 1) were treated with FLX (20 mg/kg) or VEH for 15 consecutive days (postnatal day [PD] 35–49). The age at the start and duration of antidepressant exposure (PD35–49) was selected because it roughly approximates adolescence in humans (Abreu-Villaca et al., 2010; Andersen, 2003). The FLX dose/regimen (20 mg/kg/day) was selected because it yields significant effects on depression-related behavior and gene expression in rodents (Englander et al., 2005; Iñiguez et al., 2010; LaPlant et al., 2010; Surget et al., 2011). After FLX treatment, performance on an episodic or spatial memory-related task (described below) was assessed 21 days after the last day of treatment (i.e., PD70+). Specifically, we conducted three separate sets of experiments to examine how juvenile exposure influenced responses to episodic (Fig. 1A; object recognition) and spatial memory (Fig. 1B; MWM) in adulthood. Since we observed enduring SSRI-induced alterations in spatial memory performance in male mice only (Fig. 3), we conducted an additional experiment to assess whether the age of antidepressant exposure (i.e., adolescence vs. adulthood) was responsible for the behavioral changes observed (Fig. 5). To do this, we conducted a similar experiment in adult male mice, as a positive control group (Fig. 1C). Here, adult male mice (PD70) were exposed to FLX for 15 consecutive days (PD70–84). Twenty-one days post antidepressant exposure (PD105), they were assessed for memory performance in the MWM test.
Table 1.
Experimental groups.
| Sex | Drug | n | Age | Interval | Procedure | Data |
|---|---|---|---|---|---|---|
| Male | VEH FLX |
10 11 |
PD35–49 PD35–49 |
21 d | Novel object recognition (PD70+) |
Fig. 2A–C |
| Female | VEH FLX |
10 10 |
PD35–49 PD35–49 |
21 d | Novel object recognition (PD70+) |
Fig. 2D–F |
| Male | VEH FLX |
11 11 |
PD35–49 PD35–49 |
21 d | Morris water maze (PD70+) |
Fig. 3 |
| Female | VEH FLX |
10 10 |
PD35–49 PD35–49 |
21 d | Morris water maze (PD70+) |
Fig. 4 |
| Male | VEH FLX |
8 12 |
PD70–84 PD70–84 |
21 d | Morris water maze (PD105+) |
Fig. 5 |
d, day; FLX, fluoxetine; PD, postnatal day; VEH, vehicle-control
Figure 1.
Experimental timeline. Adolescent (postnatal day [PD] 35) male and female C57BL/6 mice received fluoxetine (FLX; 20 mg/kg; IP) or water/control (VEH) for 15 consecutive days. Twenty-one days later (PD70+), separate groups of male and female mice were evaluated on memory performance on the (A) object recognition (B) or Morris water maze tests. (C) To evaluate whether the observed FLX-induced alterations on the Morris water maze in male mice were the result of age of FLX exposure, we conducted a similar experiment where adult male mice were exposed to FLX for 15 days (PD70–84). Three weeks post FLX exposure (PD105) mice were evaluated on the MWM task.
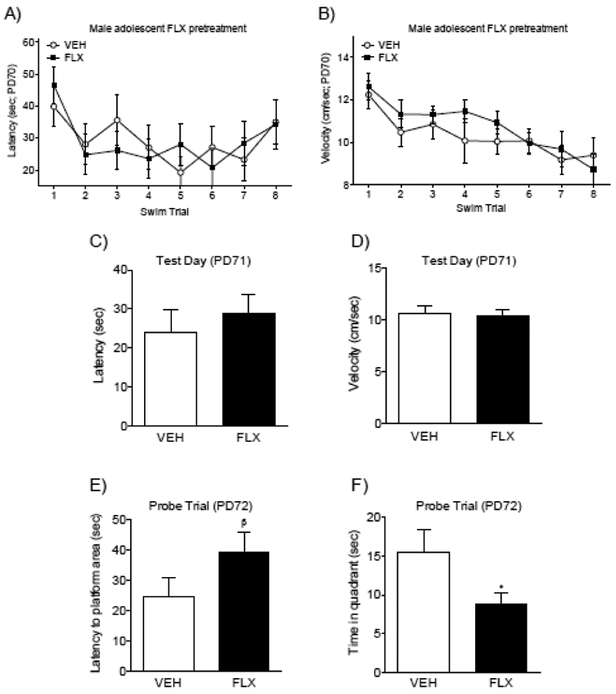
Figure 3.
Fluoxetine (FLX; 20 mg/kg) exposure during adolescence did not influence acquisition performance during the spatial training phase (8 trials) of the Morris water maze task (PD70+; n= 10–11/group). Latency (A) and swim velocity (B) decreased across the training trials, indicating that mice acquired the memory task, independent of FLX history. On test day (24 hr post spatial training), no differences between the FLX and VEH pretreated mice were noted in latency to locate the escape platform (C) or swim velocity (D). Twenty-four hr later, during the probe trial (PD72), FLX pretreated animals took longer to reach the quadrant that had previously contained the escape platform (E) and spent significantly less time in it (F). Data are presented as mean + SEM. *Indicates p<0.05 when compared to VEH; β indicates p=.057 when compared to VEH.
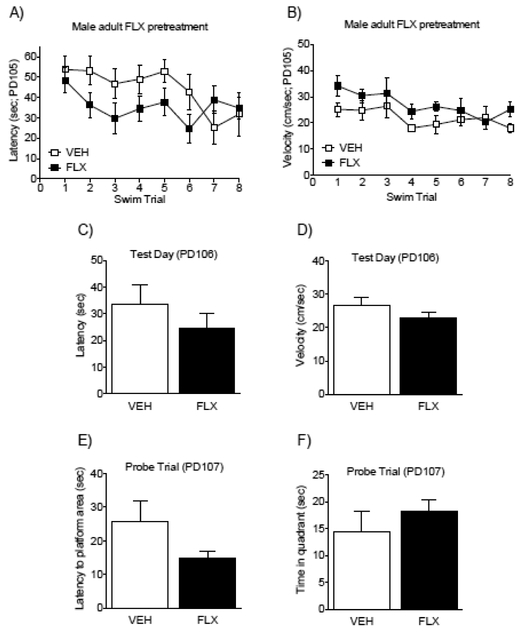
Figure 5.
Fluoxetine (FLX; 20 mg/kg) exposure in adult male mice (PD70–84) does not influence spatial memory performance later in life (PD103–105). Latency (A) and swim velocity (B) decreased across the training trials, indicating acquisition of the memory task. No differences on latency to locate the escape platform (C) or swim velocity (D) were noted between the groups during the test day (PD106). Twenty-four hr later (PD107), during a standard probe trial, no differences in latency to reach the area that previously contained the escape platform (E) were noted between the groups. Similarly, no differences in the time spent in that quadrant that previously contained the platform (F) were apparent. Data are presented as mean + SEM.
Object Recognition Test
The object-recognition test is commonly used to assess episodic memory in rodents (Ennaceur and Delacour, 1988), given that it capitalizes on rodents’ natural affinity for novelty. Testing was performed in four phases (handling, habituation, sample, and choice; Fig. 1A), adopting a similar protocol to what has been previously described (Frick and Gresack, 2003). In the first phase (habituation), mice were handled (5 min) for five consecutive days (PD63–67). On the last day of handling, mice were also habituated (second phase) to the testing apparatus (42 × 42 × 42 cm white open field box), as well as for the following two days (PD67–69) in the absence of any object for three min each day. No data were collected during handling or habituation. We adopted this procedure to reduce levels of stress/anxiety prior to the sample phase, since juvenile FLX pre-treatment induces an anxiogenic effect in adulthood (Homberg et al., 2011; Iñiguez et al., 2010) – potentially reducing motivation to explore the objects. Twenty-four hr later (sample phase; PD70), animals were re-habituated to the testing box for 1-min, and then placed in a holding cage while two identical objects (clear plastic cups; 9 cm in diameter, 4 cm in height) were placed in the left and right corners (~1.5 cm from the wall) of the box. Mice were immediately placed back into the testing box and allowed to freely investigate the identical objects until they accumulated 30 sec exploring the objects. Subjects were excluded from the experiment if they did not reach 30 sec of cumulative exploration time within a 7-min session. Twenty-four hr later (choice phase; PD71), one copy of the familiar object (clear plastic cup) and a new object (yellow plastic square; 9 cm in length, 4.5 cm in height) were placed in the same location as in the sample trial (i.e., for object recognition memory). The location of the novel object was counterbalanced (left vs. right) across mice to control for potential side-preference bias. Dependent variables recorded were the latency (sec) to approach, as well as the total time (sec) spent with the novel object during the 5-min recognition test (choice phase).
Morris Water Maze (MWM)
The MWM consisted of a circular water tank, 97 cm in diameter and 58 cm in height. The maze was filled with water to a depth of 18 cm. The water was made opaque with white nontoxic paint, and its temperature was maintained at 24±1°C using a standard heat-lamp. Around the perimeter of the water tank, four starting points (north, south, east, west) were equally positioned, thus dividing the maze into four equal quadrants. During spatial training and testing day (described below), the escape platform (10 × 10 cm2) was submerged to a depth of 0.5 cm on the south-east quadrant. Extra-maze cues were placed throughout the walls of the testing room. MWM behavioral testing was conducted as previously described (Iñiguez et al., 2012), adopting four phases: habituation, training, test day, and probe trial (Fig. 1B).
Habituation.
All mice were handled for five days (5-min each time) in order to habituate them to the experimenter (PD65–69 for adolescent FLX pretreated mice [Fig. 1B]; PD100–104 for adult FLX pre-treated mice [Fig. 1C]). On the last day of handling (PD69 and PD104, respective of FLX pretreatment age), mice were also habituated to the testing room for 20 min. This approach was adopted to reduce stress/anxiety, given that juvenile FLX exposure results in an enduring anxiogenic phenotype (Iñiguez et al., 2014a; Iñiguez et al., 2010; Warren et al., 2011). Lastly, mice were habituated to the water immersion process (as previously described: Gresack and Frick, 2006; Iñiguez et al., 2012). Here, mice were given 4 shaping trials. On trial 1, each mouse was placed for 10 sec on the escape platform (visible above water). For the remaining trials, each mouse was placed at three distances progressively further from the platform and allowed to swim to the platform. If the mouse did not find the platform within 60 sec, it was gently led to the platform by the experimenter. No data were collected during habituation.
Spatial Training.
Water maze training was performed as previously described (Gresack and Frick, 2006; Packard and Teather, 1997). The mice received one training session of eight-trials (training day; PD70 and PD105, respective of FLX pre-treatment age). Mice were placed in the water maze at one of the four starting points and allowed 60 sec to freely swim and find the submerged escape platform. Every starting point was used twice within the eight trials in a randomized fashion. If a mouse did not locate the hidden platform within the allotted 60 sec, the experimenter gently directed it to the escape platform. Once on the escape platform, each mouse was allowed to remain on the platform for 10 sec. After every trial, each mouse was dried with a towel and placed in a holding cage for a 45 sec inter-trial. Immediately after the last training trial, mice were returned to their home-cage.
Spatial Test Day.
Twenty-four hr after the last training trial (PD71 and PD106, respective of FLX pre-treatment age), the mice were returned to the MWM for a single memory retention trial. All mice were released from the same starting point (north point). Latency (sec) and velocity (cm/sec) to find the escape platform were recorded via an automated computer tracking system (EthovisionXT, Noldus, Leesburg, VA). Lower swim latencies were interpreted as better memory performance (Leon et al., 2010), while swim velocity was used as a control for potential differences in locomotor-induced swimming ability as a function of FLX pre-exposure (Iñiguez et al., 2012).
Probe Trial.
Twenty-four hr after Test Day (i.e, 48 hr after spatial training), mice returned one last time to the MWM for a single 60 sec swim trial (PD72 and PD107, respective of adolescent and adult FLX pre-exposure). In this case, the escape platform was removed from the maze, and all mice were released from the same starting point (north point). The following measures were recorded during the probe trial: latency (sec) to reach the location where the escape platform was originally located (i.e., time to reach platform location), and total time spent within the quadrant (south-east) that contained the escape platform during spatial training and test day.
Data Analysis
Data was analyzed using two-way analysis of variance (ANOVA) for repeated measures, with drug pretreatment (between measure: VEH vs. FLX) and swim-trial (repeated measure: 8-trials) as sources of variance. Separate analyses were conducted between male and female mice, given that baseline sex differences are commonly reported for both spatial navigation and object recognition tasks (Frick and Gresack, 2003). Correspondingly, separate analyses were performed as a function of age given locomotor-induced differences between the age groups (Flores-Ramirez et al., 2018). When appropriate, two-group comparisons were conducted using two-tail Student’s t-tests. Data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was defined as p<0.05.
RESULTS
Novel Object Recognition Test
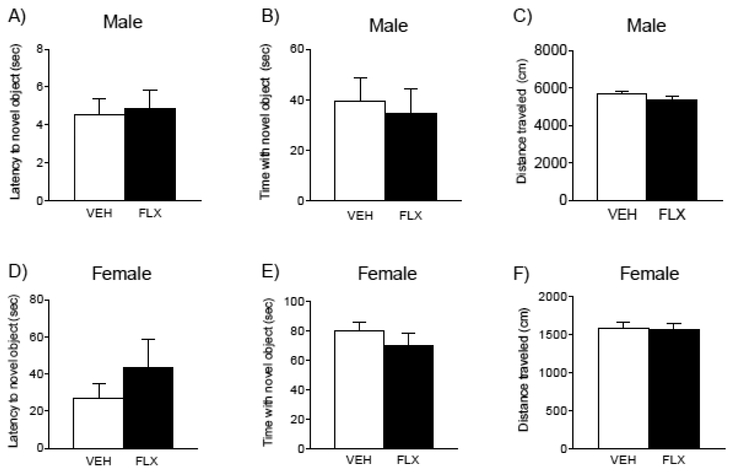
Adolescent FLX exposure (PD35–49) did not influence novel object recognition memory performance when assessed in adulthood (PD70+; Fig. 2). Specifically, FLX pre-exposed male (Fig. 2A–B) or female (Fig. 2D–E) mice did not differ in the time to initially approach the novel object, nor in the total amount of time spent exploring it, when compared to their respective VEH-pretreated controls (PD71; n= 10–11/group). Similarly, no differences in total locomotor activity (distance traveled) were observed in male (Fig. 2C) or female (Fig. 2F) mice, as a function of antidepressant pre-treatment.
Figure 2.
Fluoxetine (FLX; 20 mg/kg) exposure during adolescence (PD35–49) did not influence responses to a novel object recognition test in adulthood (n= 10–11/group), in either male (top panels) or female (bottom panels) C57BL/6 mice. Adult mice pretreated with FLX during adolescence did not differ in the latency (sec) to initially approach (males, A; females, D), or the total time spent exploring a novel object, when compared to respective (VEH) pre-treated controls (males, B; females, E). No differences in total locomotor activity were observed as a function of antidepressant pre-exposure (males, C; females, F). Data are presented as mean ± SEM.
Morris Water Maze
Male adolescent FLX exposure (PD35–49) impairs MWM performance in adulthood (PD70+).
Figure 3 shows the effects of juvenile FLX pre-treatment on a MWM spatial acquisition task in adulthood (PD70+). There were no statistical mean group differences on time (latency) to locate the platform, or swim velocity (cm/sec), as a function of adolescent FLX pretreatment (see Fig. 3A–B, respectively). Significant main effects for swim trial (repeated measure) on latency to locate the platform (F7,140= 2.65, p<0.05) and for swim velocity (F7,140= 6.04, p<0.05) indicated that mice learned to locate the platform, similarly, across the training regardless of FLX pretreatment. Specifically, there was a decrease in latency (Fig. 3A) and velocity (Fig. 3B) to reach the platform when comparing Trials 4–8 (latency) and Trials 3–8 (velocity) to Trial 1 (p<0.05, respectively). Figure 3C–D shows no differences between the groups on the time (latency), or swim velocity, to locate the escape platform on Test Day (PD71). Conversely, 48 hr after spatial training (PD72), adolescent FLX pretreatment altered spatial memory performance, during a probe trial, in adult male mice. In this case, FLX-pretreated animals displayed a trend for longer latency (time) to reach the original location of the missing platform when compared to controls (t20= 1.65, βp= 0.057; Fig. 3E). Furthermore, FLX pretreated male mice spent significantly less time in the quadrant corresponding to the missing platform (south-east), when compared to VEH pretreated male mice (t20= 2.03, p<0.05; Fig. 3F). This suggests that adolescent FLX pretreatment impaired memory performance during the probe trial, in male mice.
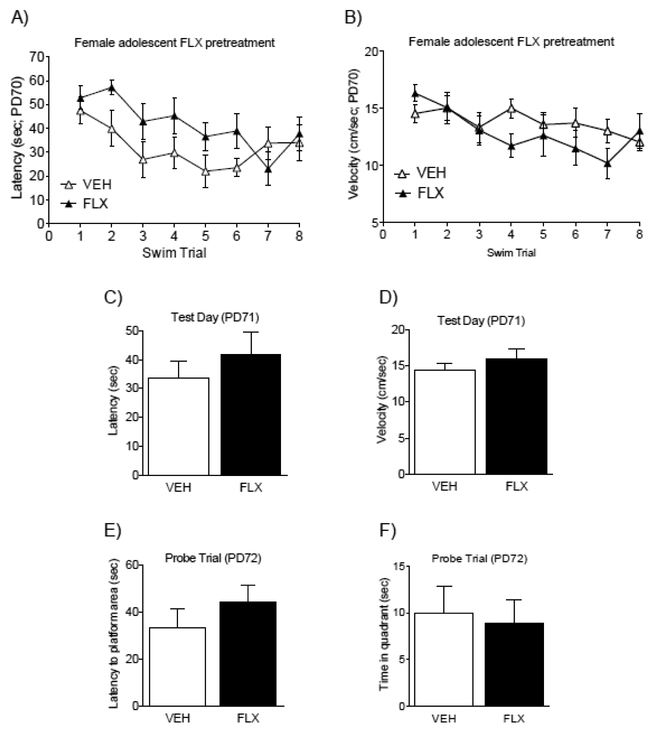
Female adolescent FLX exposure (PD35–49) does not influence MWM performance in adulthood (PD70+).
Figure 4 shows the effects FLX pre-treatment during adolescence on a MWM spatial acquisition task in adulthood. No statistical mean group differences on time (latency) to locate the platform, or swim velocity (cm/sec), as a function of adolescent FLX pretreatment (see Fig. 4A–B, respectively), were found between the groups. Significant main effects for swim trial (repeated measure) on latency to locate the platform (F7,126= 3.68, p<0.05) and for swim velocity (F7,126= 5.45, p<0.05) showed that mice learned to locate the platform across the training trials in a similar fashion, regardless of juvenile FLX pretreatment. Specifically, there was a decrease in latency (Fig. 4A) and velocity (Fig. 4B) to reach the platform when comparing Trials 5–8 (latency) and Trials 3–8 (velocity) to Trial 1 (p<0.05, respectively). Figure 4C–D further shows that FLX pretreatment did not influence spatial memory retention, 24 hr after spatial training (i.e., Test Day; PD71). No differences in the time (latency), or swim velocity were detected between the groups as a function of adolescent drug pre-treatment. Lastly, no differences in spatial memory retention between the groups were observed during the probe trial (48 hr post spatial training; PD72). Specifically, no differences between the groups were observed on the latency to reach the location of the missing platform (Fig. 4E), nor differences in swim velocity across the 60 sec swim trial (Fig. 4F). Collectively, these data indicate that adolescent FLX pretreatment does not influence MWM performance in adulthood, in female C57BL/6 mice.
Figure 4.
Fluoxetine (FLX; 20 mg/kg) exposure during adolescence did not influence memory performance in adulthood (PD70+; n= 10 per group) in female c57BL/6 mice. Latency (A) and swim velocity (B) decreased across the training trials, indicating acquisition of the memory task. No differences on latency to locate the escape platform (C) or swim velocity (D) between the groups were noted on test day (PD71). Twenty-four hr later (PD72), during a standard probe trial, no differences on latency to reach the area that previously contained the escape platform (E) were observed between the groups. Similarly, no differences in the time spent in that quadrant (F) were apparent on the probe trial. Data are presented as mean + SEM.
Adult male FLX exposure (PD70–84) does not influence MWM performance later in life (PD105+).
Figure 5 demonstrates the effects of FLX pre-treatment on a MWM spatial acquisition task in adulthood (PD105+). No statistical mean group differences on time (latency) to locate the platform, or swim velocity (cm/sec), as a function of adolescent FLX pretreatment (see Fig. 5A–B, respectively) were observed between the experimental groups. Significant main effects for swim trial (repeated measure) on latency to locate the platform (F7,126= 2.30, p<0.05; Fig. 5A) and for swim velocity (F7,126= 3.94, p<0.05; Fig. 5B) indicated that mice learned to locate the platform across the training trials, similarly, regardless of pre-exposure to FLX. Specifically, there was a decrease in latency (Fig. 5A) and velocity (Fig. 5B) to reach the platform when comparing Trials 6–8 (latency) and 4–8 (velocity) to Trial 1 (p<0.05, respectively). Figure 5C–D further shows that FLX pretreatment did not influence memory retention on Test day, 24 hr after spatial training (PD106). No differences in the time (latency; Fig. 5C), or swim velocity (Fig. 5D) were detected between the groups. Lastly, no differences in spatial memory retention were observed during the probe trial (48 hr post spatial training; PD107). Here, no differences between the groups were observed on latency to reach the location of the missing platform (Fig. 5E), nor differences in the time spent in the quadrant that previously contained the platform (Fig. 5F). Collectively, these data indicate that FLX exposure in adulthood does not influence MWM performance 21-days after treatment, in male C57BL/6 mice.
Effects of FLX on Body Weight
Enduring effects of FLX exposure in adolescent male mice.
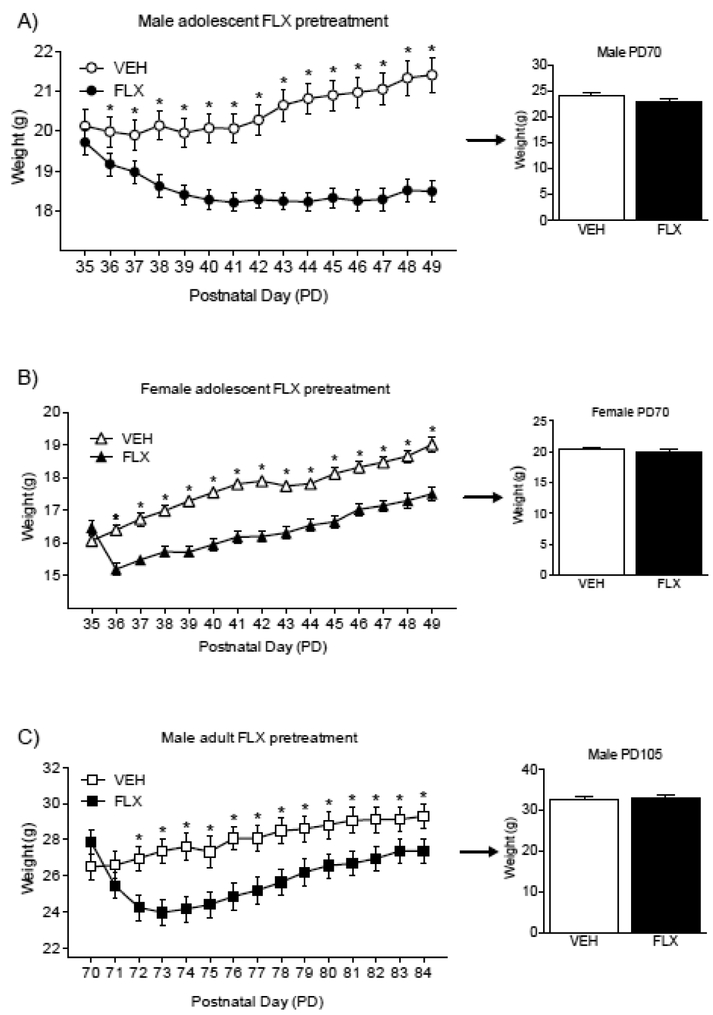
Figure 6A shows the effects of adolescent FLX exposure (PD35–49) on body weight in male C57BL/6 mice. A mixed-design repeated measures ANOVA revealed that weight was influenced by a main effect of FLX treatment (between measure: F1,574=19.93, p<0.05), a main effect of day of antidepressant exposure (repeated measure: F14,574=12.16, p<0.05), and their interaction (FLX by day of exposure; F14,574=26.96, p<0.05). Post-hoc analyses revealed that when compared to controls (n=21), FLX-exposed mice (n=22) displayed lower body-weight as of the second day of treatment (PD36), remaining lower throughout FLX exposure (PD49). No enduring differences in body weight, as a function of adolescent FLX pre-exposure, were observed at PD70 (i.e. prior to behavioral testing in adulthood; see Figure 6A, right panel).
Figure 6.
Enduring effects of fluoxetine (20 mg/kg) exposure on body weight. (A) When compared to controls (VEH), FLX-exposed adolescent male mice displayed lower weight gain as of the second day of antidepressant exposure (PD36), remaining lower until PD49. Twenty-one days after FLX treatment (PD70), no differences in body weight were noted between the groups. (B) Similarly, adolescent female c57BL/6 mice displayed lower body weight 24 h after the beginning of FLX treatment (PD36), when compared to controls. On PD70 (21 days after the last day of FLX exposure), no differences in body weight were apparent between the groups. (C) When compared to VEH-pretreated mice, adult FLX-exposed mice displayed lower body weight as of the third day of treatment (PD72). Twenty-one days after the last day of FLX exposure (PD105), no differences in body weight between the groups were observed. Data are presented as mean + SEM. *Significantly different when compared to VEH (p<0.05). Arrow indicates 21 days of drug washout.
Enduring effects of FLX exposure in adolescent female mice.
Figure 6B shows the effects of adolescent FLX exposure (PD35–49) on body weight in female C57BL/6 mice. A mixed design repeated measures ANOVA showed that weight decreased as a function of FLX treatment (between measure: F1,532=37.75, p<0.05), day of antidepressant exposure (repeated measure: F14,532=90.10, p<0.05), as well as their interaction (FLX by day of exposure; F14,532=10.59, p<0.05). Tukey post-hoc analyses revealed that when compared to controls (n=20), FLX-exposed adolescent female mice (n=20) displayed lower body-weight as of the second day of treatment (PD36), remaining lower until the end of FLX exposure (PD49). No enduring differences in body weight, as a function of FLX pre-exposure, were apparent at PD70 (i.e. prior to behavioral testing in adulthood (Figure 6B, right panel).
Enduring effects of FLX exposure in adult male mice.
The effects of FLX exposure (PD70–84) on body weight in adult male C57BL/6 mice can be observed in Figure 6C. A mixed-design repeated measures ANOVA revealed a main effect of FLX treatment (between measure: F1,252=4.99, p<0.05), a main effect of day of antidepressant exposure (repeated measure: F14,252=68.59, p<0.05), as well as their interaction (FLX by day of exposure; F14,252=25.25, p<0.05). Tukey post-hoc analyses showed that adult FLX-exposed mice (n=12) displayed lower body-weight as of the third day of treatment (PD72), when compared to their VEH-treated counterparts (n=8), an effect that remained throughout FLX exposure (PD84). No differences in body weight, as a function of antidepressant pretreatment were apparent at PD105 (i.e. prior to behavioral testing in adulthood; see Figure 6C, right panel)
DISCUSSION
Mounting preclinical evidence suggests that prolonged exposure to antidepressant medications, during the juvenile stage of development, alter responses to a variety of stress-and reward-related stimuli in adulthood (Flores-Ramirez et al., 2018; Karpova et al., 2009; Olivier et al., 2011). However, less is known about the potential enduring side effects of such treatment on memory function. Thus, the present investigation was designed to examine if adolescent (PD35–49) exposure to the antidepressant FLX (20 mg/kg/day) would result in long-lasting alterations on memory-related performance (PD70+), using male and female C57BL/6 mice as a model system. We report that juvenile FLX exposure impairs spatial, but not episodic memory function, in a sex specific manner, 21 days post treatment.
Episodic memory, per the object recognition task, was not altered in adulthood as a function of SSRI history. No differences in either the latency (sec) to approach the novel object, nor the total time spent exploring it, were evident in either male (Fig.2A–B), or female (Fig. 2D–E) mice, as a result of adolescent FLX pre-exposure. Likewise, no differences in the distance traveled (cm) during the 5-min memory retention trial were noted between the experimental groups (Fig. 2C and 2F, respectively). This indicates that FLX pre-exposure does not influence locomotor activity and/or body weight later in life (Fig. 6), similar to what has been previously reported in both rats (Amodeo et al., 2015; Iñiguez et al., 2010) and mice (Flores-Ramirez et al., 2018; Iñiguez et al., 2014a). Conversely, juvenile SSRI exposure resulted in a long-term spatial memory deficit in male (Fig. 3), but not female mice (Fig. 4). On the MWM task, both adult male and female mice (PD70+), pre-exposed to FLX during adolescence (PD35–49), displayed normal spatial acquisition for the location of a submerged escape platform (Fig. 3A–B and 4A–B), as well as in memory retention, 24 hr after the 8-training trials (Fig. 3C–D and 4C-D). However, 48 hr after spatial training, male (Fig. 3E–F), but not female mice (Fig. 4E–F), displayed impaired memory performance during a standard probe trial. In the absence of the escape platform, FLX-pretreated male mice displayed increases in the latency (sec) to reach the area that previously contained the escape platform, while also spending less time (sec) within the quadrant that previously contained it, when compared to respective controls. These results support previous work where adult male rats pre-exposed to FLX during adolescence displayed impaired MWM performance (Sass and Wortwein, 2012). Yet, here, we extend these findings to adult male C57BL/6 mice with juvenile FLX history. Importantly, our experimental approach uncovered that males are more vulnerable to prolonged SSRI-induced spatial memory related deficits than their female counterparts – highlighting that ontogenic antidepressant exposure mediates long-lasting effects differentially between males and females (Gemmel et al., 2019).
Preclinical studies indicate that psychotropic drug and/or stress exposure alter behavior differentially, as a function of sex (Izquierdo et al., 2016; Luine et al., 2017). Similarly, under normal conditions, male and female rodents display differences in memory-related performance across numerous behavioral tasks, including object recognition and MWM performance (Frick and Gresack, 2003). Yet, less is known on how juvenile SSRI antidepressant exposure may influence specific types of memory in adulthood. Thus, our results directly highlight the importance of sex as a factor influencing behavioral alterations in adulthood, as a function of juvenile antidepressant history. To further explore if this lasting FLX-induced spatial memory deficit in male mice was dependent on the age of antidepressant exposure (i.e., adolescence vs. adulthood), we exposed adult (PD70) male mice to FLX for 15 consecutive days (PD70–84). Twenty-one days later (PD105), we tested these mice in a similar MWM task (see Fig. 1C). Not surprisingly, we found that adult FLX pre-exposure did not influence spatial memory performance later in life (Fig. 5); an age-dependent effect that has been previously reported in male rodents when assessing sensitivity to both natural and drug rewards (Iñiguez et al., 2014b; Iñiguez et al., 2010), as well as affect-related stimuli (Iñiguez et al., 2014a; Karpova et al., 2009). Collectively, our findings indicate that adolescence is a developmental window that is sensitive to lasting SSRI-induced alterations in spatial memory in male, but not female, C57BL/6 mice.
While the antidepressant-induced neurobiological factors underlying this lasting sex-dependent memory impairment have not been directly examined, recent data suggest that early-life FLX exposure mediates enduring neuroplastic alterations in brain regions associated with memory performance (Airan et al., 2007; Shrestha et al., 2014). For example, in males, juvenile FLX exposure has been found to arrest hippocampal spine densities in adulthood (Norrholm and Ouimet, 2000), thus, potentially underlying the spatial navigation impairment observed during the probe trial of the MWM task. This is likely the case, given that spatial memory performance is hippocampal-dependent under normal conditions (Jessberger et al., 2009; Snyder et al., 2005). Additionally, this lasting FLX-induced neurobiological alteration in hippocampal spine density could further explain why juvenile SSRI pre-treatment did not alter spatial navigation in female mice, since FLX alters hippocampal neurogenesis differentially between males and females (Rayen et al., 2015). Specifically, early-life FLX exposure leads to enduring increases in hippocampal cell survival in female, but not male mice (Hodes et al., 2010) – likely underlying the resilient phenotype observed in the female groups. Of course, future detailed investigations will be needed to delineate the role that lasting FLX-induced hippocampal neuroplastic changes play in MWM performance between males and females.
A limitation of the present investigation is that we administered FLX to normal adolescent mice (i.e., non-stressed), and evaluated memory-related behavior three weeks post antidepressant exposure. Therefore, it is possible that FLX pre-exposure in animal models for the study of depression (Iñiguez et al., 2018; Iñiguez et al., 2014b; Krishnan and Nestler, 2011) may yield different results. However, we must note that previous research where FLX was administered in both stressed and non-stressed animals induced similar neurobiological effects in brain regions associated with learning/memory performance (Todorovic and Filipovic, 2017). Comparably, acute FLX exposure in normal human volunteers has been shown to impair emotional-memory performance (Capitao et al., 2015). Accordingly, our data from normal animals may highlight the translational implications of our work to normal human volunteers. Another limitation is that we did not control for sex steroid hormones in our experimental design. The estrous cycle influences memory performance (Frick and Gresack, 2003; Gresack and Frick, 2006), which, in turn, may potentially underlie the resilient-like findings within the female groups (Fig. 3) – given the neurogenic effects of estrogen (Galea, 2008).
We demonstrate that juvenile exposure to FLX results in spatial memory deficits in male, but not female C57BL/6 mice, later in life. This finding underscores the need for future work aimed at delineating the enduring neurobiological factors that underlie this prolonged FLX-induced spatial memory impairment in a sex specific manner. Understanding sex differences in long-term SSRI-induced cognitive deficits will inform about the safety and/or consequences associated with juvenile antidepressant exposure.
Highlights.
Adolescent fluoxetine history impairs spatial memory in adult male mice.
Adolescent exposure to fluoxetine does not influence spatial memory in adult female mice.
Adolescent exposure to fluoxetine does not impact episodic memory in adulthood.
Acknowledgements
This work was supported by grants from the National Institutes of Health to Sergio D. Iñiguez (NIGMS-SC2GM109811 and NIGMS-SC3GM130467).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no competing financial and/or non-financial interests in relation to the work described. The funding agency had no involvement in the design of the study, data collection process, interpretation of the results, nor writing of the manuscript.
REFERENCES
- Abreu-Villaca Y, Filgueiras CC, Guthierrez M, Medeiros AH, Mattos MA, Pereira Mdos S, Manhaes AC, Kubrusly RC. 2010. Exposure to tobacco smoke containing either high or low levels of nicotine during adolescence: differential effects on choline uptake in the cerebral cortex and hippocampus. Nicotine Tob Res 12(7):776–80. [DOI] [PubMed] [Google Scholar]
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. 2007. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317(5839):819–23. [DOI] [PubMed] [Google Scholar]
- Amodeo LR, Greenfield VY, Humphrey DE, Varela V, Pipkin JA, Eaton SE, Johnson JD, Plant CP, Harmony ZR, Wang L and others. 2015. Effects of acute or repeated paroxetine and fluoxetine treatment on affective behavior in male and female adolescent rats. Psychopharmacology (Berl) 232(19):3515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. 2003. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27(1–2):3–18. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. 2000. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153(1):31–43. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Charntikov S. 2015. We know very little about the subjective effects of drugs in females. ACS Chem Neurosci 6(3):359–61. [DOI] [PubMed] [Google Scholar]
- Capitao LP, Murphy SE, Browning M, Cowen PJ, Harmer CJ. 2015. Acute fluoxetine modulates emotional processing in young adult volunteers. Psychol Med 45(11):2295–308. [DOI] [PubMed] [Google Scholar]
- Emslie G, Judge R. 2000. Tricyclic antidepressants and selective serotonin reuptake inhibitors: use during pregnancy, in children/adolescents and in the elderly. Acta Psychiatr Scand Suppl 403:26–34. [DOI] [PubMed] [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. 2005. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci 25(3):648–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. 1988. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31(1):47–59. [DOI] [PubMed] [Google Scholar]
- Flores-Ramirez FJ, Garcia-Carachure I, Sanchez DO, Gonzalez C, Castillo SA, Arenivar MA, Themann A, Lira O, Rodriguez M, Preciado-Pina J and others. 2019. Fluoxetine exposure in adolescent and adult female mice decreases cocaine and sucrose preference later in life. J Psychopharmacol 33(1):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. 2003. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci 117(6):1283–91. [DOI] [PubMed] [Google Scholar]
- Galea LA. 2008. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev 57(2):332–41. [DOI] [PubMed] [Google Scholar]
- Gemmel M, De Lacalle S, Mort SC, Hill LA, Charlier TD, Pawluski JL. 2019. Perinatal fluoxetine has enduring sexually differentiated effects on neurobehavioral outcomes related to social behaviors. Neuropharmacology 144:70–81. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. 2006. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav 84(1):112–9. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Hill-Smith TE, Suckow RF, Cooper TB, Lucki I. 2010. Sex-specific effects of chronic fluoxetine treatment on neuroplasticity and pharmacokinetics in mice. J Pharmacol Exp Ther 332(1):266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, Blom T, Arentsen T, van Brunschot C, Schipper P, Korte-Bouws G, van Luijtelaar G, Reneman L. 2011. Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One 6(1):e16646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V, Wright KN, Dayrit G, Nieto SJ, Wilkinson MB and others. 2014a. Fluoxetine Exposure during Adolescence Alters Responses to Aversive Stimuli in Adulthood. J Neurosci 34(3):1007–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Charntikov S, Baella SA, Herbert MS, Bolaños-Guzmán CA, Crawford CA. 2012. Post-training cocaine exposure facilitates spatial memory consolidation in c57bl/6 mice. Hippocampus 22(4):802–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH and others. 2018. Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, Warren BL. 2014b. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress 17(3):247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Riggs LM, Nieto SJ, Wright KN, Zamora NN, Cruz B, Zavala AR, Robison AJ, Mazei-Robison MS. 2015. Fluoxetine exposure during adolescence increases preference for cocaine in adulthood. Sci Rep 5:15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Bolaños-Guzmán CA. 2010. Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry 67(11):1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Pozos H, Torre Ade L, DeShields S, Cevallos J, Rodriguez J, Stolyarova A. 2016. Sex differences, learning flexibility, and striatal dopamine D1 and D2 following adolescent drug exposure in rats. Behav Brain Res 308:104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD Jr., Consiglio A, Lie DC, Squire LR, Gage FH. 2009. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem 16(2):147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castren E. 2009. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol 19(2):97–108. [DOI] [PubMed] [Google Scholar]
- Kennedy BC, Kohli M, Maertens JJ, Marell PS, Gewirtz JC. 2016. Conditioned object preference: an alternative approach to measuring reward learning in rats. Learn Mem 23(11):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. 2011. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7:121–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD and others. 2010. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci 13(9):1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon WC, Bruno MA, Allard S, Nader K, Cuello AC. 2010. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn Mem 17(6):297–305. [DOI] [PubMed] [Google Scholar]
- Luine V, Gomez J, Beck K, Bowman R. 2017. Sex differences in chronic stress effects on cognition in rodents. Pharmacol Biochem Behav 152:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Garcia A, Manzanedo C, Rodriguez-Arias M, Aguilar MA, Reig-Sanchis E, Navarro-Frances CI, Valverde O, Minarro J, Arenas MC. 2015. Sex differences in the long-lasting consequences of adolescent ethanol exposure for the rewarding effects of cocaine in mice. Psychopharmacology (Berl) 232(16):2995–3007. [DOI] [PubMed] [Google Scholar]
- Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, Harvey-White J, Holmes A. 2008. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 200(3):413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. 2000. Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res 883(2):205–15. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Blom T, Arentsen T, Homberg JR. 2011. The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: A review. Prog Neuropsychopharmacol Biol Psychiatry 35(6):1400–8. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. 1997. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem 68(2):172–88. [DOI] [PubMed] [Google Scholar]
- Popa D, Lena C, Alexandre C, Adrien J. 2008. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci 28(14):3546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayen I, Gemmel M, Pauley G, Steinbusch HW, Pawluski JL. 2015. Developmental exposure to SSRIs, in addition to maternal stress, has long-term sex-dependent effects on hippocampal plasticity. Psychopharmacology (Berl) 232(7):1231–44. [DOI] [PubMed] [Google Scholar]
- Sass A, Wortwein G. 2012. The effect of subchronic fluoxetine treatment on learning and memory in adolescent rats. Behavioural brain research 228(1):169–75. [DOI] [PubMed] [Google Scholar]
- Schroder C, Dorks M, Kollhorst B, Blenk T, Dittmann RW, Garbe E, Riedel O. 2017a. Outpatient antidepressant drug use in children and adolescents in Germany between 2004 and 2011. Pharmacoepidemiol Drug Saf 26(2):170–179. [DOI] [PubMed] [Google Scholar]
- Schroder C, Dorks M, Kollhorst B, Blenk T, Dittmann RW, Garbe E, Riedel O. 2017b. Outpatient antipsychotic drug use in children and adolescents in Germany between 2004 and 2011. Eur Child Adolesc Psychiatry 26(4):413–420. [DOI] [PubMed] [Google Scholar]
- Shrestha SS, Nelson EE, Liow JS, Gladding R, Lyoo CH, Noble PL, Morse C, Henter ID, Kruger J, Zhang B and others. 2014. Fluoxetine Administered to Juvenile Monkeys: Effects on the Serotonin Transporter and Behavior. Am J Psychiatry 171(3):323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. 2005. A role for adult neurogenesis in spatial long-term memory. Neuroscience 130(4):843–52. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R and others. 2011. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16(12):1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic N, Filipovic D. 2017. The antidepressant- and anxiolytic-like effects of fluoxetine and clozapine in chronically isolated rats involve inhibition of hippocampal TNF-alpha. Pharmacol Biochem Behav 163:57–65. [DOI] [PubMed] [Google Scholar]
- Warren BL, Iñiguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, Bolaños-Guzmán CA. 2011. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci 31(28):10347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]