Abstract
CD19-targeted chimeric antigen receptor modified T-cell immunotherapy (CAR-T cell therapy) is a novel treatment with promising results in patients with relapsed/refractory lymphoid malignancies. CAR-T cell therapy has known early toxicities of cytokine release syndrome (CRS) and neurotoxicity, but little is known about long-term neuropsychiatric adverse effects. We have utilized patient-reported outcomes (PROs), including PROMIS® measures, to assess neuropsychiatric and other patient-reported outcomes of 40 patients with relapse/refractory chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), and acute lymphoblastic leukemia (ALL), one to five years after treatment with CD19-targeted CAR-T cells. Mean T scores of PROMIS domains of Global Mental health, Global Physical Health, Social Function, anxiety, depression, fatigue, pain and sleep disturbance were not clinically meaningfully different from the mean in the general US population. However, 19 patients (47.5%) reported at least one cognitive difficulty and/or clinically meaningful depression and/or anxiety, and 7 patients (17.5%) scored ≤ 40 in Global Mental Health, indicating at least one standard deviation worse than the general population mean. Younger age was associated with worse long-term Global Mental Health (p=0.02), anxiety (p=0.001) and depression (p=0.01). Anxiety prior to CAR-T cell therapy was associated with increased likelihood of anxiety after CAR-T cell therapy (p=0.001). 15 patients (37.5%) reported cognitive difficulties post CAR-T cell therapy. Depression prior to CAR-T cell therapy was statistically significantly associated with higher likelihood of self-reported post CAR-T cognitive difficulties (p=0.02) and there was a trend for association between acute neurotoxicity and self-reported post-CAR-T cognitive difficulties (p=0.08). Having more post-CAR-T cognitive difficulties was associated with worse Global Mental Health and Global Physical Health. Our study demonstrates overall good neuropsychiatric outcomes in 40 long-term survivors after CAR-T cell therapy. However, nearly50% of patients in the cohort reported at least one clinically meaningful negative neuropsychiatric outcome (anxiety, depression or cognitive difficulty), indicating that there is a significant number of patients who would likely benefit from mental health services following CAR-T cell therapy. Younger age, pre-CAR-T anxiety or depression, and acute neurotoxicity may be risk factors for long-term neuropsychiatric problems in this patient population. Larger studies are needed to confirm these findings.
Keywords: CD19-targeted CAR-T cells, late events, patient reported outcomes
Introduction
CD19-targeted chimeric antigen receptor modified T-cell immunotherapy (CD19 CAR-T cells) has shown excellent anti-tumor activity in patients with relapsed/refractory acute lymphoblastic leukemia (ALL) (1) and non-Hodgkin lymphoma (NHL) (2, 3), which led to the approval of tisagenlecleucel (Kymriah®) and axicabtagene ciloleucel (Yescarta®) by regulatory agencies in the United States, Europe, Canada, Japan and Australia. This approval has transformed the care of patients with relapsed/refractory ALL and aggressive B cell NHL (4–6).
At Fred Hutchinson Cancer Research Center (FHCRC), a phase I/II clinical trial using CD19 CAR-T cells demonstrated high response rates in patients with relapsed/refractory ALL (85% of patients achieved minimal residual disease (MRD)-negative complete remission, NHL (51% overall response rate (ORR); 40% complete response (CR)) and chronic lymphocytic leukemia (CLL) (74% ORR; 21% CR) (7–9).
CD19 CAR-T cells cause unique early toxicities, such as cytokine release syndrome (CRS) (10–14) and acute neurotoxicity (15, 16). CRS is a systemic inflammatory response that arises within several days of the CAR-T infusion and may manifest as fever, hypotension, capillary leak syndrome and multiorgan failure. Severe CRS may affect 10–40% of patients (17). Acute neurotoxicity can manifest as a multitude of different neurological adverse events including but not limited to confusion, expressive aphasia, obtundation, myoclonus and seizure. Severe neurotoxicity may affect 10–40% of patients (17). It is important to note that the CAR-T cell product infused, CAR-T cell dose and patients’ characteristics (such as disease treated, disease burden or prior neurological conditions) may affect rates and manifestations of CRS and neurotoxicity. CRS and neurotoxicity can be fatal but with appropriate monitoring and treatment they typically resolve within days to a couple of weeks after CAR-T cell infusion (17). However, potential long-term neuropsychiatric adverse effects of CAR-T cells as well as CRS and acute neurotoxicity are still not fully elucidated (18).
Until recently the only cellular therapy modality for treatment of hematological malignancies had been allogeneic hematopoietic cell transplantation (HCT), which provides immune response against tumor cells derived by alloreactive donor cells, but with the risk of acute and chronic graft versus host disease (GVHD) as well as other late effects that adversely affect morbidity, mortality, and quality of life of transplant survivors, including significant neuropsychiatric effects (19). At this time only limited data are available regarding the long-term effects of CAR-T cell therapy (20). Thus, it is important to evaluate the late neuropsychiatric effects of CAR-T and evaluate their effect on survivors’ quality of life.
Patient-reported outcomes (PROs), a method of measuring health status directly from the patient without clinician interpretation, has been demonstrated to be a reliable tool for evaluation of treatment-related toxicities (21–23). A number of studies have used PROs to evaluate symptoms in cancer patients (24–26), and functional deficits and late effects after HCT (27, 28). Additionally, PROs have been used to evaluate outcomes and quality of life (QOL) after treatment with immune checkpoint inhibitors (ICIs) (29, 30). These studies reveal that patient-reported global health status and QOL were better among patients treated with ICIs compared to patients treated with standard chemotherapy (30–34). Recently there has been interest in incorporating PROs for evaluation of patients after CAR-T cell therapy (35). Standardized PRO measurement systems, such as the Patient-Reported Outcomes Measurement Information System (PROMIS®) allow comparisons across patients with different diseases and treatment histories. PROMIS uses modern psychometric theory to standardize PRO assessment for use in both clinical research and health care delivery settings, including oncology settings (25, 36–38). A key feature of PROMIS is that scores are not disease- or treatment-specific and thus are applicable for use in settings where multiple different diseases and treatment histories may be represented in the patient population. Thus, we have incorporated PROs, including PROMIS measures for evaluation of symptoms and function of long-term survivors after CAR-T cell therapy.
The aim of this study was to use PROs to evaluate the frequency and potential risk factors for adverse neuropsychiatric and other patient-reported outcomes in long-term survivors of CAR-T cell therapy.
Patients and Methods
Study Population
The study cohort included 40 patients with relapsed/refractory ALL, NHL or CLL treated with CD19-targeted CAR-T cells on a phase I/II clinical trial () between December 2013 and February 2018 and survived at least 12 months after treatment (in remission or with disease progression). The study was approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board, and all patients provided informed consent for treatment and for long-term follow-up.
Clinical Data
Clinical and sociodemographic variables were abstracted from each patient’s chart. These included age, sex, race, employment status, marital status, past medical and psychiatric history, date of CAR-T cell infusion, CRS grading based on the Lee criteria (10) and acute neurotoxicity grading based on the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) version 4.03.
All patients underwent evaluation prior to CAR-T cell therapy, including social and psychiatric history. Most patients also underwent standardized mental health screening by a clinical social worker using validated instruments (e.g., the 2- or 9-item Patient Health Questionnaire [PHQ-2/9], the 2- or 7-item Generalized Anxiety Disorder scale [GAD-2/7]) (39–42). Several patients underwent additional evaluations by a consulting psychiatrist or psychologist.
Pre CAR-T (current or history of) depression was defined as having any of the following: (1) PHQ-2 ≥ 3, (2) PHQ-9 ≥ 10, (3) a charted diagnosis of a depressive disorder (major depressive disorder, mixed depression and anxiety, adjustment disorder with depression), or (4) prescribed antidepressant medication prior to CAR-T cell therapy. Pre CAR-T anxiety was defined by having any of the following: (1) GAD-2 ≥ 3, (2) GAD-7 ≥ 10, (3) charted diagnosis of an anxiety disorder, or (4) prescribed anxiolytic medications that was clearly listed for treatment of anxiety. All patients on an antidepressant had a charted diagnosis of anxiety, depression or both and so were categorized under the appropriate diagnosis. Baseline sleep disturbance was determined by either a charted diagnosis of insomnia or a charted prescription for a medication that was being used to treat sleep disturbance.
Assessments and Measures
A link to an online questionnaire was emailed to patients who were at least one year after CAR-T cell therapy and at +/− two months of their yearly anniversary of T cell infusion between October 2018 through February 2019, if requested by patient a paper form questionnaire was sent.
The questionnaire included the PROMIS Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, as well as 30 additional questions, including four questions pertaining to cognitive function. PROMIS-Global Health is an overall evaluation of physical and mental health consisting of 10 items and scored as a Global Physical Health component and a Global Mental Health component. PROMIS-29 contains 7 domains, each 4-item short forms, assessing depression, anxiety, physical function, pain interference, fatigue, sleep disturbance, and ability to participate in social roles and activities, plus 1 item assessing pain intensity. Symptom domains (depression, anxiety, fatigue, sleep disturbance, pain) were assessed in the past 7 days. There were no time restriction for function domains. Additional questions included demographic data, cancer disease status, additional diagnoses and symptoms which the patients may have developed since CAR-T cell therapy, current medications, and evaluation of cognitive functioning. Cognitive functioning was assessed by questions we developed that asked if patients had experienced difficulties with concentration, finding words, memory, or solving problems since their CAR-T cell therapy; answer “yes” to each of those four questions received “1” point to determine the total cognitive difficulty score (0–4).
The PROMIS measures were scored using the HealthMeasures Scoring Service (https://www.assessmentcenter.net/ac_scoringservice). Raw scores were converted to standardized T-scores that can be compared to normative data from the US general population. T-Score distributions are standardized such that a score of 50 points represents the mean for the US general population, and the standard deviation (SD) around that mean is 10 points. A higher PROMIS T-score indicates more of the concept being measured. For symptom measures such as including anxiety, depression, sleep disturbance, pain, and fatigue, a higher score indicates worse symptoms (for example, a T-score of 60 indicates one standard deviation increased anxiety compared to the mean for the US general population and a score of 40 indicates one standard deviation less anxiety than the general population). For functional domains, including Global Mental and Physical Health, Social Function, and physical Function, a higher score indicates better function (for example, a T-score of 60 indicates one SD better Mental Health Functioning than the average for the general US population, and a T score of 40 indicates one SD worse Mental Health Function compared to the average for the general US population) (PROMIS, 2015: https://www.assessmentcenter.net/documents/PROMIS Profile Scoring Manual.pdf). Clinically meaningful difference was defined as one-half of a standard deviation (a 5-point difference in T score) (24, 27, 43).
Statistical Methods
Baseline demographics, clinical characteristics, and PROMIS measures were summarized by medians or means with standard deviation and range for continuous variables, and numbers and proportions for categorical variables. The strength of association between PROMIS scores was evaluated by Spearman correlation coefficients (r). To show the trends in PROMIS scores by the number of cognitive difficulties, we used box-plots and calculated p-values using linear regression, treating cognitive difficulties as a continuous measure. Histograms were generated for each of the PROMIS T scores to show the sample distribution, and 2-sided p-values were calculated by one-sample t-test to compare our sample to the US general population’s T score mean of 50. Univariate analyses were conducted on the neuropsychiatric outcomes of particular interest. Linear regression was used to analyze continuous scores (PROMIS Global Mental Health, PROMIS Global Physical Health, PROMIS Anxiety, PROMIS Depression), and logistic regression was used to analyze cognitive difficulties (any vs none). Depression or anxiety that were diagnosed prior to CAR-T cell therapy (pre-CAR-T depression or pre-CAR-T anxiety) were included in the models to adjust for a baseline effect if appropriate. Multivariate analyses were performed on selected outcomes using stepwise selection, with a p-value threshold of less than 0.10 for model entry and exit. All variables in the univariate analyses were included in the multivariable selection process, and the threshold of 0.10 guaranteed inclusion of all variables with p-value < 0.10 in the model (7, 44). Due to the limited sample size and the nature of exploratory analysis, multiplicity adjustments were not considered. All analyses were conducted using R, version 3.4.1.
Results
Questionnaire Response Rate
Between October 2018 to February 2019, 38 patients were emailed a link to the electronic questionnaire and 14 patients were provided a hard copy of the questionnaire (per their request or due to inability to contact patients via email). As of February 28, 2019, 40 questionnaires were returned and included in the analysis (29 of 38 electronic questionnaires and 11 of 14 hard copy questionnaires, for a total response rate of 76.9%).
Patient Characteristics
The median age of the patients in the study cohort at time of CAR-T cell therapy was 54 years (SD, 11.9; range, 22–74), and at time of questionnaire completion 57 years (SD, 11.7, range 26–76). The majority was male (62.5%), white (82.5%) and married (77.5%). Patients received CAR-T cell therapy for NHL (35%), CLL (37.5%) and ALL (27.5%). Median lines of treatment prior to CAR-T was 5 (range, 1–10). Six patients (15%) had a history of CNS involvement at any time during the disease course prior to CAR-T cell therapy, and 14 patients (35%) received directed CNS chemotherapy as treatment or prophylaxis. The majority of patients had maximum CRS grade 1 (30%) or 2 (40%) and the majority of patients did not have acute neurotoxicity (62.5%). Four patients (10%) had maximum grade 3 CRS and four patients (10%) had maximum grade 3 neurotoxicity. Two patients (5%) had grade maximum 4 CRS and 2 patients (5%) had maximum grade 4 neurotoxicity (one of those patients had both grade 4 CRS and grade 4 neurotoxicity). The median time from CAR-T cell therapy to questionnaire completion was 3 years (range, 1–5). Twenty-three patients (57.5%) received additional treatment(s) after CAR-T cell therapy (including 12 allogeneic hematopoietic cell transplantation (allo HCT), given as consolidation or salvage), and 33 patients (82.5%) were in remission at time of questionnaire completion. Characteristics of the cohort are shown in Table 1.
Table 1.
Demographic and clinical characteristics (N=40)
| Variable | n (%) |
|---|---|
| Demographic | |
| Age at time of CAR-T cell therapy | |
| Median | 54 (11.9) |
| Range | 22.0–74.0 |
| Age at questionnaire completion | |
| median (SD) | 57 (11.7) |
| Range | 26.0–76.0 |
| Year after CAR-T cell therapy – median (range) | 3 (1–5) |
| Sex | |
| Male | 25 (62.5%) |
| Female | 15 (37.5%) |
| Race | |
| American Indian/Alaska Native | 1 (2.5%) |
| Asian | 3 (7.5%) |
| Asian, White | 1 (2.5%) |
| White | 33 (82.5%) |
| Unknown | 2 (5%) |
| Marital status | |
| Married | 31 (77.5%) |
| Single/Divorced/Widowed | 9 (22.5%) |
| Employment status at time of questionnaire completion | |
| Working | 17 (42.55) |
| Unable to work due to health | 11 (27.5%) |
| Not working but not due to health | 12 (30.0%) |
| Medical | |
| Diagnosis | |
| ALL | 11 (27.5%) |
| CLL | 15 (37.5%) |
| NHL | 14 (35%) |
| Number of prior lines of therapy | |
| Median (SD) | 5 (1.9) |
| Range | 1–10 |
| Prior HCT before CAR-T | |
| Autologous (auto) | 4 (10%) |
| Allogeneic (allo) | 11 (27.5%) |
| Auto and allo | 2 (5%) |
| No HCT | 19 (47.5%) |
| Prior Radiation before CAR-T | |
| Local radiation (no cranial) | 7 (17.5%) |
| Total body irradiation (low dose; ≤ 4 Gy) | 7 (17.5%) |
| Total body irradiation (high dose; ≥ 12 Gy) | 3 (7.5%) |
| CNS disease involvement at anytime before CAR-T cell therapy | |
| No | 34 (85.0%) |
| Yes | 6 (15.0%) |
| CNS directed chemotherapy (as treatment for CNS involvement or prophylaxis) | |
| No | 26 (65%) |
| Yes | 14 (35%) |
| Maximum Cytokine Release Syndrome grade | |
| 0 | 6 (15.0%) |
| 1 | 12 (30.0%) |
| 2 | 16 (40.0%) |
| 3 | 4 (10.0%) |
| 4 | 2 (5.0%) |
| Maximum Neurotoxicity grade | |
| 0 | 25 (62.5%) |
| 1 | 1 (2.5%) |
| 2 | 8 (20.0%) |
| 3 | 4 (10.0) |
| 4 | 2 (5.0%) |
| Additional treatment after CAR-T cell therapy* | |
| No | 17 (42.5%) |
| Yes: | |
| - Allo HCT | 12 (30.0%) |
| - Targeted therapy | 12 (30.0%) |
| - Local radiation (no cranial) | 2 (5.0%) |
| - CAR-T cell therapy | 2 (5.0%) |
| - Checkpoint inhibitors | 1 (2.5%) |
| Remission status at time of questionnaire completion | |
| In remission | 33 (82.5%) |
| Not in remission | 7 (17.5%) |
| Pre-CAR-T Psychiatric Problems | |
| Depression | |
| No | 28 (70.0%) |
| Yes | 12 (30.0%) |
| Anxiety | |
| No | 23 (57.5%) |
| Yes | 17 (42.5%) |
| Sleep disturbance | |
| No | 22 (55.0%) |
| Yes | 18 (45.0%) |
Abbreviations: ALL = acute lymphocytic leukemia, CLL = chronic lymphocytic leukemia, CNS = central nervous system, HCT = hematopoietic cell transplantation, NHL= non-Hodgkin lymphoma. NHL includes: Diffuse large B cell lymphoma (N=7), high grade B cell lymphoma (N=1), Burkitt lymphoma (N=1), mantle cell lymphoma (N= 3) and follicular lymphoma (N=2).
some patients re.ceived more than one treatment
Psychiatric History Prior to CAR-T Cell Therapy
Twelve (30%) patients met our criteria for pre-CAR-T depression, 17 (42.5%) met our criteria for pre-CAR-T anxiety, and 18 (45%) met criteria for pre-CAR-T sleep disturbance. Immediately prior to CAR-T therapy 8 (20%) of the patients were on antidepressant medications, 12 (30%) were on anxiolytic medications and 18 (45%) were prescribed medications for sleep disturbance.
Psychiatric and Cognitive Outcomes after CAR-T Cell Therapy
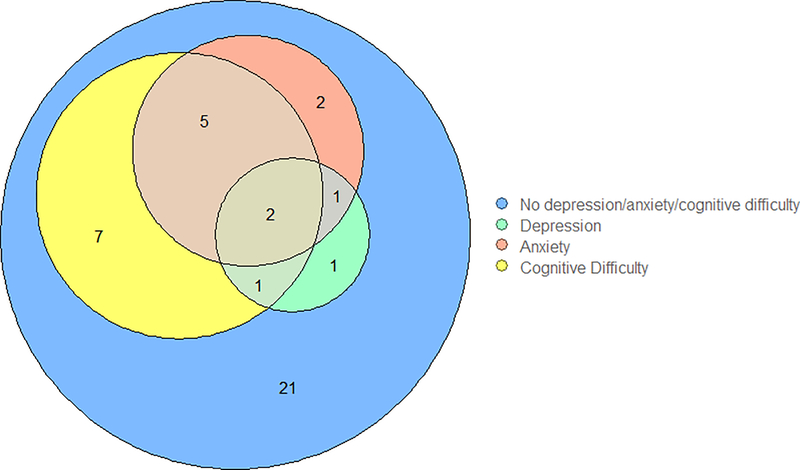
As demonstrated in Figure 1, at the time of questionnaire completion, 8 (20%) patients reported clinically meaningful depression or anxiety (PROMIS T-score > 55) and at least one cognitive difficulty, four (10%) reported depression or anxiety but no cognitive difficulties, and 7 (17.5%) reported cognitive difficulties with no depression or anxiety. Twenty-one (52.5%) patients reported no neuropsychiatric symptoms.
Figure 1.
Distribution of depression, anxiety and neurocognitive difficulties at time of questionnaire complete ion.
Calculation based on PROMIS T score ≥ 55
At the time of questionnaire completion ten (25%) patients reported being on medications for depression, eight (20%) reported being on anxiolytics, and six (15%) reported being on medications for sleep (Table 2). The use of medications for depression and sleep (as per patients’ report) was statistically significantly lower at time of questionnaire completion compared to immediately prior to CAR-T cell therapy (p = 0.03, p= 0.007 respectively), while the use of anxiolytic medications was not statistically significantly different (p= 0.44).
Table 2.
Self-reported outcomes 1–5 years after CAR-T cell therapy
| Patient-reported Outcomes | n (%) |
|---|---|
| Neurocognitive difficulties since CAR-T cell therapy | |
| Difficulty concentrating | 9 (22.5%) |
| Difficulty with word finding | 12 (30.0%) |
| Difficulty with memory | 14 (35.0%) |
| Difficulty solving problems | 5 (12.5%) |
| Number of cognitive complaints | |
| 0 | 25 (62.5%) |
| 1 | 1 (2.5%) |
| 2 | 7 (17.5%) |
| 3 | 3 (7.5%) |
| 4 | 4 (10.0%) |
| Taking medications for: | Yes |
| Depression | 1 (2.5%) |
| Anxiety | 8 (20.0%) |
| Sleep disturbance | 6 (15.0%) |
| Fatigue | 1 (2.5%) |
| PROMIS measures (T-scores) | Mean (SD) |
| Global Mental Health | 48.9 (9.9) |
| Global Physical Health | 48.8 (10.4) |
| Ability to Participate in Social Roles and Activities | 50.9 (10.4) |
| Anxiety | 48.9 (9.0) |
| Depression | 46.3 (8.0) |
| Sleep Disturbance | 49.5 (8.3) |
| Fatigue | 48.4 (10.1) |
| Pain | 48.8 (9.3) |
| Physical Function | 48.0 (9.6) |
Fifteen (37.5%) patients reported one or more cognitive difficulties. 14 (35%) of the patients reported having difficulty with memory, 12 (30%) reported difficulty finding words, 9 (22.5%) reported difficulty with concentration and 5 (12.5%) reported difficulty solving problems. Four (10%) patients reported experiencing all four cognitive symptoms.
PROMIS Outcomes
PROMIS outcome data (Table 2; Supplemental Figure 1) demonstrate that mean Global Mental Health, Global Physical Health, Social Function, anxiety, fatigue, pain and sleep disturbance of the study cohort did not clinically meaningfully differ from that of the general population. However, five patients (12.5%) scored ≥ 60 in anxiety and/or depression and seven patients (17.5%) scored ≤ 40 in Global Mental Health, indicating at least one standard deviation worse than the general population mean.
Risk Factors for Neuropsychiatric Outcomes
Univariate analyses of potential risk factors for neuropsychiatric outcomes after CAR-T cell therapy are shown in Tables 3.A and 4.A, and Supplemental Tables 1, 2. Younger age was found to be associated with worse PROMIS global mental score (p=0.02), and worse PROMIS anxiety (p=0.001) and depression scores (p=0.01). Anxiety pre-CAR-T was associated with PROMIS anxiety post-CAR-T (p=0.001), but depression pre-CAR-T was not associated with PROMIS depression post-CAR-T (p=0.60). There was a statistically significant association between pre-CAR-T depression and cognitive difficulties (any vs none) (OR = 6.00, p=0.02), and a suggestive association between neurotoxicity grade 2–4 and cognitive difficulties (OR = 3.62, p=0.07). Longer time since CAR-T cell therapy was associated with worse global mental score (p=0.04). Inability to work due to health issues was associated with worse PROMIS Global Physical Health score (p=0.001) (Supplemental Table 3), while unemployment not due to health issues was associated with better PROMIS anxiety score (p=0.04) (Supplemental Table 1). Race (white versus others) was not found to be significantly associated with PROMIS global mental score (Table 3.A), PROMIS anxiety score (Supplementary Table 1), or PROMIS depression score (Supplementary Table 2). However, race might have been associated with reported cognitive status, as 15 of 33 white patients (45%) reported at least one cognitive difficulty while none of the 7 non-white patients reported cognitive difficulties (p=0.03) (Table 4.A). There was no association between disease status at time of questionnaire completion and patient-reported neuropsychiatric outcomes, and there was no association between allogeneic HCT before or after CAR-T cell therapy and reported neuropsychiatric outcomes (Tables 3 and 4).
Table 3.
Risk Factor Regression Analyses for PROMIS Global Mental Health T-score
| A. Univariate Analysis | |||
| Univariate* | |||
| Variable | Coefficient | Standard Error | P value |
| Demographics | |||
| Age at time of CAR-T therapy | 0.30 | 0.12 | 0.02 |
| Sex (Male vs Female) | −0.01 | 3.57 | 1.00 |
| Race (white vs other) | 1.59 | 3.91 | 0.69 |
| Marital status (non-married vs married) | −1.38 | 3.61 | 0.70 |
| Employment status (not working, but not due to health vs working) | 3.50 | 3.62 | 0.34 |
| Employment status (unable to work due to health issues vs working) | −1.70 | 3.67 | 0.65 |
| Medical Factors | |||
| Diagnosis (CLL vs ALL) | 6.51 | 3.63 | 0.08 |
| Diagnosis (NHL vs ALL) | −0.33 | 3.63 | 0.93 |
| # of Prior lines of therapy | −0.41 | 0.84 | 0.63 |
| CNS disease involvement (yes vs no) | −3.82 | −4.19 | 0.37 |
| CNS directed chemotherapy before CAR-T (yes vs no) | −3.73 | 3.17 | 0.25 |
| CRS (2–4 vs 0–1) | −3.59 | 2.94 | 0.23 |
| Neurotoxicity (2–4 vs 0–1) | −1.86 | 3.12 | 0.55 |
| Allo HCT before CAR-T (versus no Allo HCT at any time) | −4.12 | 3.60 | 0.26 |
| Allo HCT after CAR-T (versus no Allo HCT at any time) | −5.14 | 3.57 | 0.16 |
| Remission status (remission vs not in remission at time of questionnaire completion) | 0.14 | 4.11 | 0.97 |
| Assessment year after CAR-T cells | −2.65 | 1.28 | 0.04 |
| Psychiatric Problems before CAR-T (yes vs no) | |||
| Depression† | −6.25 | 3.20 | 0.06 |
| Anxiety | −3.09 | 3.14 | 0.33 |
| Insomnia | −0.28 | 3.09 | 0.93 |
| Substance Use Disorder | 6.21 | 5.76 | 0.29 |
| B. Multivariate Analysis^ | |||
| Multivariate* | |||
| Variable | Coefficient | Standard Error | P value |
| Age at time of CAR-T therapy | 0.27 | 0.12 | 0.03 |
| Assessment year after CAR-T cells | −2.23 | 1.22 | 0.08 |
Adjusted for depression prior to CAR-T therapy
Not adjusted for depression prior to CAR-T therapy
Multivariate analyses were performed on selected outcomes using stepwise selection, with a p-value threshold of less than 0.10 for model entry and exit. All variables in the univariate analyses were included in the multivariable selection process.
Adjusted for depression prior to CAR-T therapy
Adjusted of the multivariate analysis for allo HCT before or after CAR-T cell therapy did not change the results.
Table 4.
Risk Factor Regression Analyses for Cognitive Difficulties (any vs none)
| A. Univariate Analysis | |||
| Univariate | |||
| Variable | OR | 95% Confidence Interval | P value |
| Demographics | |||
| Age at time of CAR-T therapy | 0.97 | 0.92–1.03 | 0.34 |
| Sex (male vs female) | 0.34 | 0.09–1.28 | 0.11 |
| Race (white vs other) | “inf” | “inf” | 0.03* |
| Marital status (non-married vs married) | 2.62 | 0.58 – 12.76 | 0.21 |
| Employment status (not working, but not due to health vs working) | 0.32 | 0.04 – 1.74 | 0.21 |
| Employment status (unable to work due to health issues vs working) | 1.43 | 0.32 – 6.52 | 0.64 |
| Medical Factors | |||
| Diagnosis (CLL vs ALL) | 0.44 | 0.08 – 2.25 | 0.32 |
| Diagnosis (NHL vs ALL) | 0.90 | 0.18 – 4.52 | 0.90 |
| # of Prior lines of therapy | −0.91 | 0.63–1.29 | 0.60 |
| CNS disease involvement (yes vs no) | 4.18 | 0.71–33.66 | 0.13 |
| CNS directed chemotherapy before CAR-T (yes vs no) | 2.25 | 0.59–8.86 | 0.23 |
| CRS (2–4 vs 0–1) | 2.17 | 0.59 – 8.72 | 0.25 |
| Neurotoxicity (2–4 vs 0–1) | 3.62 | 0.94 – 15.02 | 0.07 |
| Allo HCT before CAR-T (versus no Allo HCT at any time) | 2.00 | 0.41–10.16 | 0.39 |
| Allo HCT after CAR-T (versus no Allo HCT at any time) | 1.71 | 0.36–8.4 | 0.50 |
| Remission status (remission vs not in remission at time of questionnaire completion) | 0.23 | 0.01 – 1.53 | 0.19 |
| Assessment year after CAR-T cells | 0.97 | 0.54 – 1.74 | 0.91 |
| Psychiatric Problems before CAR-T (yes vs no) | |||
| Depression | 6.00 | 1.45 – 28.93 | 0.02 |
| Anxiety | 2.03 | 0.56 – 7.72 | 0.29 |
| Insomnia | 1.71 | 0.47 – 6.42 | 0.41 |
| B. Multivariate Analysis^ | |||
| Multivariate | |||
| Variable | OR | 95% Confidence Interval | P value |
| Neurotoxicity (2–4 vs 0–1) | 3.83 | 0.88 – 18.8 | 0.08 |
| Depression pre-CAR-T | 6.28 | 1.41 – 33.7 | 0.02 |
Due to a 0 cell (0 non-white patients with cognitive difficulties) Fisher’s exact test was used to calculate p-value.
Multivariate analyses were performed on selected outcomes using stepwise selection, with a p-value threshold of less than 0.10 for model entry and exit. All variables in the univariate analyses were included in the multivariable selection process.
Adjusted of the multivariate analysis for allo HCT before or after CAR-T cell therapy did not change the results.
Multivariate analysis suggested association between age (p=0.03) and time of assessment after treatment (p=0.08) with PROMIS Global Mental Health score (Table 3b), and suggested association between pre-CAR-T depression (p=0.02) and acute neurotoxicity (grades 2–4 vs 0–1; p = 0.08) with cognitive difficulties after treatment (any vs none) (Table 4b).
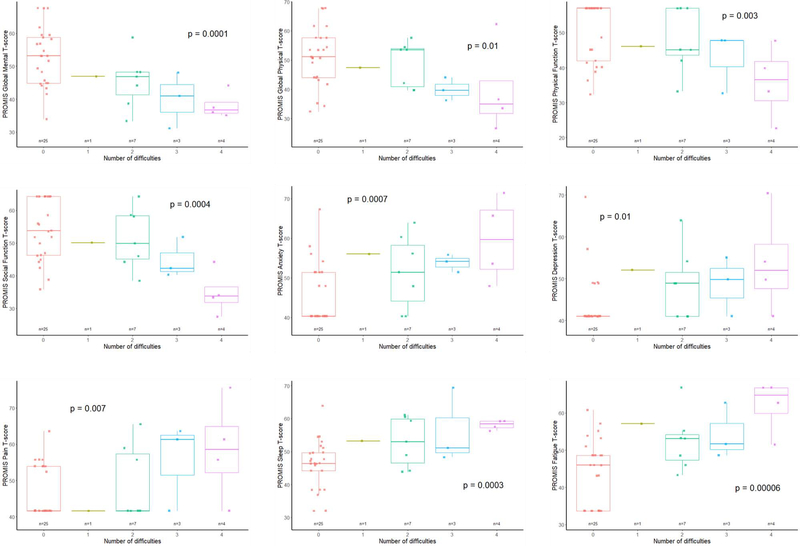
As shown in Figure 2, having more cognitive difficulties was associated with having worse Global Mental Health (p=0.0001) and Global Physical Health (p= 0.01). Similarly, worse scores for pain interference, sleep disturbance, fatigue, depression, anxiety, physical function, and Social Function were associated with more cognitive difficulties (p=0.007, p=0.0003, p=0.00006, p=0.01, p=0.0007, p=0.003, p=0.0004 respectively).
Figure 2.
Association between cognitive difficulties and PROMIS outcomes
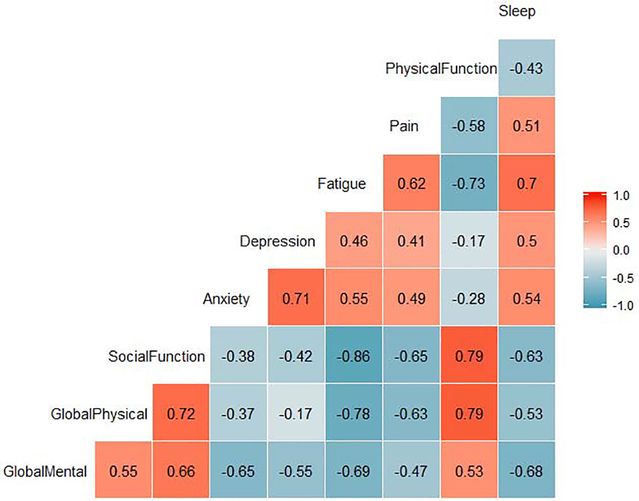
Correlation analysis of PROMIS outcomes (Figure 3) show strong positive correlations (r ≥0.6) between physical and Social Function, global mental and Social Function, global physical function and physical function, anxiety and depression, sleep disturbance and fatigue, and fatigue and pain. There was a very strong inverse correlation (r ≤−0.8) between fatigue and Social Function and strong inverse correlations (r ≤−0.6) between fatigue and physical function, pain and global physical function, pain and Social Function, anxiety and global mental function, fatigue and global mental function and sleep disturbance and global mental function.
Figure 3.
Correlation Analysis of PROMIS scores
Discussion
CD19-targeted CAR-T cell therapy has revolutionized the treatment of relapsed/refractory B cell malignancies, with an unprecedented response rate in this heavily pre-treated population. However, data regarding long term effects after this novel therapy are still scarce. In this study, we examine self-reported long-term neuropsychiatric status and other patient-reported outcomes of patients after CAR-T cell therapy.
Patients in our study received a median of 5 lines of therapy prior to CAR-T cells, including HCT in 11 patients (27.5%), and 12 patients (30%) received allo HCT after their CAR-T cell therapy. The high rate of prior or subsequent treatment and lack of baseline PRO data prior to CAR-T cell therapy prevents us from determining the direct impact of CAR-T cell therapy on outcomes. However, overall, our data demonstrate that the mean self-reported neuropsychiatric status of long-term survivors after CD-19 CAR-T cell therapy was not clinically meaningfully different than the general US.
Cella et al. suggested PROMIS T score thresholds to differentiate severity levels for pain, fatigue, anxiety and depression in cancer patients (26) and our outcomes fell within the designated “normal” range for cancer patients. Jensen et al. proposed reference mean T scores for PROMIS measures in cancer patients 6 to13 months after initial diagnosis (25), and our study cohort’s mean T scores are equivalent or slightly better than those values, for example: fatigue 48.4 vs 52.2, pain 48.8 vs 52.4 and Physical Function 48.8 vs 44.8. The authors’ included a subset of NHL patients, and compared to that group our study cohort demonstrated slightly better mean T scores in depression (46.3 vs 49.3), fatigue (48.4 vs 52.2), and pain (48.8 vs 51.9), however the differences are below the 5 point threshold we consider as clinically meaningful difference. Despite these overall mean scores, nearly one in five patients in our cohort scored at least one standard deviation lower than the general and cancer populations’ means in Global Mental Health, indicating that there is still a significant number of patients who would likely benefit from additional mental health services in the years following CAR-T therapy.
Our findings demonstrate similar PROMIS Global Physical Health among patients at 1 to 5 years after CAR-T cell therapy (mean T score 48.8) and the general population. This is consistent with prior studies demonstrating similar PROMIS Global Physical Health scores in survivors of hematologic cancers (mean T score 48.5) (45), older adults with cancer (mean T score 48) (46) and long term survivors after allogeneic or autologous HCT (median T scores 50.8 and 47.7 respectively) (27). Similarly, PROMIS Global Mental Health in our cohort (mean T score 48.9) was similar to the general population or other cancer patient population. In comparison, mean PROMIS Global Mental Health T scores of 51.7 and 51 were found in hematologic cancer survivors and in older adults with cancer, respectively (45, 46), and Shaw et al. found a median PROMIS Global Mental T score of 50.8 among long-term transplant survivors (27). Furthermore, allo HCT before or after CAR-T cell therapy did not seem to affect the PROMIS Global Mental score of patients in our cohort.
Older age was found to be statistically significantly associated with better Global Mental Health and less anxiety and depression, which is consistent with prior studies that demonstrated younger age as a risk factor for worse mental health in cancer patients (25, 27, 45, 47). Additionally, we found that having more cognitive difficulties was associated with worse Global mental and physical health, as well as more pain, fatigue, sleep disturbance, depression, and anxiety. This finding is consistent with Reid-Arndt et al. who demonstrated association between worse cognitive function and poorer quality of life in breast cancer patients (48).
Although no intra-patient longitudinal data are currently available in our cohort, our data suggest worse Global Mental Health later after treatment. A potential explanation to this finding is that early after CAR-T cell therapy patients may be relieved to be alive given their previously refractory cancer. However, over time, survival may hold less psychological weight as patients adjust back to life and need to cope with ongoing symptoms and other psychosocial stressors. In the future, it will be important to examine longitudinal data for individual patients.
37.5% of patients in our cohort reported at least one cognitive difficulty. Similar cognitive difficulties (memory, attention, concentration) are known to arise after chemotherapy and HCT, with rates of impairment varying between 16–50% among cancer survivors assessed between 6 months and 10 years after treatment (48–51). In an HCT survivor population 5 years after transplant, 41.5% had at least mild impairment on neuropsychological function (50). It is notable that in our cohort neither age, number of prior therapies, allo HCT before or after CAR-T cell therapy, nor presence or severity of CRS, were associated with self-reported cognitive difficulties. However, there was a trend towards association between early neurotoxicity and reported cognitive difficulties. Similarly, delirium immediately following hematopoietic stem cell transplant has been shown to be associated with subjective reports of worse memory and executive functioning six months and one year after HCT (52). Larger studies of patients receiving CAR-T cell therapy are necessary to determine if there is significant association between acute neurotoxicity after CAR-T cell therapy and long-term cognitive impairment. Although acute neurotoxicity is a well-documented adverse event of CAR-T cell therapy, only limited data are available regarding its risk factors and pathophysiology (15, 53, 54). Better understanding of acute neurotoxicity after CAR-T cell administration is needed in order to understand the potential association between acute neurotoxicity and long-term neurocognitive outcomes after CAR-T cell therapy.
Our data demonstrate potential association between race and patient reported cognitive difficulties, as 45% of white patients reported at least one cognitive difficulty, while none of the non-white patients reported any cognitive difficulties. It could be that the difference in reported cognitive status is derived from cultural differences, however, larger cohort is needed to validate those findings. Additionally, as there is some evidence that self-reported cognitive impairment may not correlate with objective cognitive function (55, 56), it will be useful to include objective cognitive testing in future studies.
Correlation analysis of PROMIS outcomes (Figure 2) confirm strong associations between Social Function and physical and mental health, and strong inverse association between Social Function and fatigue, pain and sleep disturbance. Additionally, the findings highlight the inverse association between fatigue and global mental and physical health. Fatigue is one of the most common and distressing symptoms for cancer patients and these correlations further argue for the importance of providing cancer survivors access to treatments for fatigue (57–59).
While our results suggest overall no difference in mean mental health in patients after CAR-T cell therapy as compared to the mean of the general population, there was a subset of patients suffering from clinically meaningful mental (30%) issues (PROMIS T score greater than one-half of the standard deviation) or cognitive (17.5%) impairment. Our regression analyses suggest that younger age, longer time since treatment, depression pre-CAR-T, and acute neurotoxicity are potential risk factors for these negative outcomes. This demonstrates the need for longitudinal clinical monitoring of survivors’ neuropsychiatric symptoms, particularly among younger patients, and connecting them with mental health and neurologic services as well as cognitive rehabilitation services (45, 60). Drawing from our multivariate analyses, future studies should examine whether preventing or treating depression and neurotoxicity during CAR-T cell therapy can help prevent long-term neuropsychiatric problems.
Limitations of our study include sampling bias (including patients who survived at least one year after CAR-T cell therapy), small sample size, lack of standardized neuropsychiatric and cognitive assessments, lack of baseline PRO data at time of treatment, and variability in time points when data were collected post treatment (between one and five years) with no intra-patient longitudinal assessment after treatment. As pre-CAR-T data was derived from medical records and post-CAR-T data was derived from PROs we cannot directly compare neuropsychiatric symptoms before and after treatment. Future studies comparing PROMIS measures before and after CAR-T cell therapy will be necessary to better understand the direct impact of CAR-T cell therapy on neuropsychiatric outcomes. Additionally, it will be useful to collect longitudinal PRO data to understand the trajectory of neuropsychiatric outcomes over time. Our study did not include domains of spirituality and religiosity. As CAR-T cell therapy is being given worldwide, future studies may include these domains to better understand how cultural, spiritual and religious differences throughout the world potentially affect neuropsychiatric outcome after CAR-T cell therapy.
In conclusion, despite acute neurotoxicity, our findings suggest overall good neuropsychiatric outcomes in long-term survivors after CAR-T cell therapy. However, a subset of patients may experience psychiatric symptoms or cognitive impairment (which may be related to CAR-T cell therapy or other treatments patients have been exposed to), and it is important to identify those patients in order to assist with intervention strategies.
Supplementary Material
Highlights.
Overall, long-term survivors after CAR-T cell therapy report good mental health.
A subset of patients may experience psychiatric symptoms or cognitive impairment.
Allo HCT before or after CAR-T cells does not affect long-term mental health.
Older age is associated with better mental health in long-term CAR-T cell survivors.
Cognitive difficulties are associated with worse global mental and physical health.
Acknowledgement
The authors thank the patients who participated in the clinical trial and answer the questionnaire, the members of the research and clinical staff at the Fred Hutchinson Cancer Research Center (FHCRC)/Seattle Cancer Care Alliance (SCCA), and referring physicians for their care of our patients after CAR-T cell therapy.
This study was supported by the National Institutes of Health (NIH) National Cancer Institute grants R01 CA136551 and P30 CA15704, NIH National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK56465, NIH National Heart, Lung, and Blood Institute funded National Gene Vector Biorepository at Indiana University (Contract # 75N92019D00018), Life Science Discovery Fund, Bezos Family, FHCRC Immunotherapy Integrated Research Cente, and Juno Therapeutics/Celgene, Inc.
Footnotes
Financial Disclosure
J.R., E.M., N.C., A.C., E.D.B., V.W., J.V., B.E.S., K.F., S.J.L., J.R.F., M.B. declare no competing financial interests.
C.J.T. receives research funding from Juno Therapeutics and Nektar Therapeutics; has served on advisory board of Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, T-CURX, Nektar Therapeutics, Allogene, Kite/Gilead, Novartis, Humanigen; and has Stock/options in Precision Biosciences, Eureka Therapeutics, Caribou Biosciences. D.G.M. has received research funding from Kite Pharma, a Gilead Company, Juno Therapeutics, a Celgene/Bristol-Myers Squibb company, and Celgene; has served on advisory boards for Kite Pharma, Gilead, Genentech, Novartis and Eureka Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 4.Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, et al. Clinical Utilization of Chimeric Antigen Receptor T Cells in B Cell Acute Lymphoblastic Leukemia: An Expert Opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25(3):e76–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Utilization of Chimeric Antigen Receptor (CAR) T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B cell non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Byrne M, Oluwole OO, Savani B, Majhail NS, Hill BT, Locke FL. Understanding and Managing Large B Cell Lymphoma Relapses after Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirayama AV, Gauthier J, Hay KA, Voutsinas JM, Wu Q, Gooley T, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133(17):1876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J Clin Oncol. 2017;35(26):3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey N, Porter D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant. 2019;25(4):e123–e7. [DOI] [PubMed] [Google Scholar]
- 13.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Hu Y, Yang S, Wei G, Zhao X, Wu W, et al. Role of Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Predicting the Adverse Effects of Chimeric Antigen Receptor T Cell Therapy in Patients with Non-Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2019;25(6):1092–8. [DOI] [PubMed] [Google Scholar]
- 15.Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7(12):1404–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018;8(8):958–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018;183(3):364–74. [DOI] [PubMed] [Google Scholar]
- 18.Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu V, Voutsinas J, et al. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica. 2017;102(4):614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu V, Voutsinas J, et al. Late events after treatment with CD19-Targeted Chimeric Antigen Receptor Modified T-cells. Biol Blood Marrow Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):319–25. [DOI] [PubMed] [Google Scholar]
- 23.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318(2):197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen RE, Potosky AL, Moinpour CM, Lobo T, Cella D, Hahn EA, et al. United States Population-Based Estimates of Patient-Reported Outcomes Measurement Information System Symptom and Functional Status Reference Values for Individuals With Cancer. J Clin Oncol. 2017;35(17):1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Choi S, Garcia S, Cook KF, Rosenbloom S, Lai JS, et al. Setting standards for severity of common symptoms in oncology using the PROMIS item banks and expert judgment. Qual Life Res. 2014;23(10):2651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw BE, Syrjala KL, Onstad LE, Chow EJ, Flowers ME, Jim H, et al. PROMIS measures can be used to assess symptoms and function in long-term hematopoietic cell transplantation survivors. Cancer. 2018;124(4):841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhotra J, Jabbour SK. Patient-related outcomes with the use of checkpoint inhibitors for the treatment of metastatic non-small cell lung cancer. Transl Lung Cancer Res. 2018;7(Suppl 2):S138–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall ET, Singhal S, Dickerson J, Gabster B, Wong HN, Aslakson RA, et al. Patient-Reported Outcomes for Cancer Patients Receiving Checkpoint Inhibitors: Opportunities for Palliative Care-A Systematic Review. J Pain Symptom Manage. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Harrington KJ, Ferris RL, Blumenschein G Jr., Colevas AD, Fayette J, Licitra L, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18(8):1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reck M, Brahmer J, Bennett B, Taylor F, Penrod JR, DeRosa M, et al. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 2018;102:23–30. [DOI] [PubMed] [Google Scholar]
- 33.Reck M, Taylor F, Penrod JR, DeRosa M, Morrissey L, Dastani H, et al. Impact of Nivolumab versus Docetaxel on Health-Related Quality of Life and Symptoms in Patients with Advanced Squamous Non-Small Cell Lung Cancer: Results from the CheckMate 017 Study. J Thorac Oncol. 2018;13(2):194–204. [DOI] [PubMed] [Google Scholar]
- 34.Tykodi SS, Schadendorf D, Cella D, Reck M, Harrington K, Wagner S, et al. Patient-reported outcomes with nivolumab in advanced solid cancers. Cancer Treat Rev. 2018;70:75–87. [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty R, Sidana S, Shah GL, Scordo M, Hamilton BK, Majhail NS. Patient-Reported Outcomes with Chimeric Antigen Receptor T Cell Therapy: Challenges and Opportunities. Biol Blood Marrow Transplant. 2019;25(5):e155–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Cella D, Gershon R, Shen J, Morales LS, Riley W, et al. Representativeness of the Patient-Reported Outcomes Measurement Information System Internet panel. J Clin Epidemiol. 2010;63(11):1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol. 2010;63(11):1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thekkumpurath P, Walker J, Butcher I, Hodges L, Kleiboer A, O’Connor M, et al. Screening for major depression in cancer outpatients: the diagnostic accuracy of the 9-item patient health questionnaire. Cancer. 2011;117(1):218–27. [DOI] [PubMed] [Google Scholar]
- 40.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–92. [DOI] [PubMed] [Google Scholar]
- 41.Esser P, Hartung TJ, Friedrich M, Johansen C, Wittchen HU, Faller H, et al. The Generalized Anxiety Disorder Screener (GAD-7) and the anxiety module of the Hospital and Depression Scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology. 2018;27(6):1509–16. [DOI] [PubMed] [Google Scholar]
- 42.Plummer F, Manea L, Trepel D, McMillan D. Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry. 2016;39:24–31. [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, Onstad L, Chow EJ, Shaw BE, Jim HSL, Syrjala KL, et al. Patient-reported outcomes and health status associated with chronic graft-versus-host disease. Haematologica. 2018;103(9):1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khera N, Hamilton BK, Pidala JA, Wood WA, Wu V, Voutsinas J, et al. Employment, Insurance, and Financial Experiences of Patients with Chronic Graft-versus-Host Disease in North America. Biol Blood Marrow Transplant. 2019;25(3):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, et al. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pergolotti M, Deal AM, Williams GR, Bryant AL, Bensen JT, Muss HB, et al. Activities, function, and health-related quality of life (HRQOL) of older adults with cancer. J Geriatr Oncol. 2017;8(4):249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun CL, Francisco L, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS). Blood. 2011;118(17):4723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reid-Arndt SA, Hsieh C, Perry MC. Neuropsychological functioning and quality of life during the first year after completing chemotherapy for breast cancer. Psychooncology. 2010;19(5):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ono M, Ogilvie JM, Wilson JS, Green HJ, Chambers SK, Ownsworth T, et al. A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol. 2015;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Syrjala KL, Artherholt SB, Kurland BF, Langer SL, Roth-Roemer S, Elrod JB, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29(17):2397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vitali M, Ripamonti CI, Roila F, Proto C, Signorelli D, Imbimbo M, et al. Cognitive impairment and chemotherapy: a brief overview. Crit Rev Oncol Hematol. 2017;118:7–14. [DOI] [PubMed] [Google Scholar]
- 52.Basinski JR, Alfano CM, Katon WJ, Syrjala KL, Fann JR. Impact of delirium on distress, health-related quality of life, and cognition 6 months and 1 year after hematopoietic cell transplant. Biol Blood Marrow Transplant. 2010;16(6):824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity Associated with CD19-Targeted CAR-T Cell Therapies. CNS Drugs. 2018;32(12):1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: Mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019. [DOI] [PubMed] [Google Scholar]
- 55.Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, D’Alonzo M, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care (Engl). 2012;21(4):485–92. [DOI] [PubMed] [Google Scholar]
- 56.Gehring K, Taphoorn MJ, Sitskoorn MM, Aaronson NK. Predictors of subjective versus objective cognitive functioning in patients with stable grades II and III glioma. Neurooncol Pract. 2015;2(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Breitbart WS, Alici Y. Psycho-oncology. Harv Rev Psychiatry. 2009;17(6):361–76. [DOI] [PubMed] [Google Scholar]
- 58.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4–10. [DOI] [PubMed] [Google Scholar]
- 59.Hjermstad MJ, Knobel H, Brinch L, Fayers PM, Loge JH, Holte H, et al. A prospective study of health-related quality of life, fatigue, anxiety and depression 3–5 years after stem cell transplantation. Bone Marrow Transplant. 2004;34(3):257–66. [DOI] [PubMed] [Google Scholar]
- 60.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.