Implications
Heat stress is a global issue constraining animal agriculture productivity, negatively affects welfare, and reduces production efficiency in many countries.
The effects of heat stress on pig production will intensify, if climate change continues as predicted.
To date, modifying the environment is the most effective way to mitigate the effects of heat stress.
Identifying additional strategies (nutritional and genetics) to maximize pork production during the warm summer months is necessary to satiate a growing demand for high quality meat for human consumption.
Introduction
Heat stress is a major environmental issue negatively affecting animal welfare and production efficiency in almost every livestock sector (Baumgard and Rhoads, 2013). When animals are exposed to environmental conditions that exceed their thermoneutral zone, production efficiency is compromised because the hierarchy of nutrient utilization is reprioritized to maintain euthermia, and consequently productivity is deemphasized. Heat stress is not an issue limited to tropical regions, as temperate countries are also affected during warm summer months (Renaudeau et al., 2012a). In fact, estimated annual losses due to heat stress in the U.S. livestock industry alone is US$1.5 billion for dairy and nearly US$1 billion for swine (Pollmann, 2010; Key and Sneeringer, 2014). Furthermore, increased genetic selection for production traits (i.e., lean tissue accretion, milk yield, and fecundity) leads to reduced heat stress tolerance as these phenotypes are associated with increased metabolic heat production (Renaudeau et al., 2012a; Baumgard and Rhoads, 2013).
In the swine industry, economic losses associated with heat stress are mainly explained by reduced and inconsistent growth, decreased feed efficiency, decreased carcass quality (increased lipid deposition and decreased protein accretion), poor sow performance, increased mortality (especially in sows and market hogs) and morbidity, and decreased facility efficiency (Baumgard and Rhoads, 2013; Ross et al., 2015). Reduced reproductive performance is characterized by anestrus, increased wean-to-estrus interval, decreased farrowing rate, and reduced litter size (Ross et al., 2015). Similarly, poor semen production and quality occur in boars exposed to heat stress. Thus, heat stress compromises almost every economically important phenotype within the industry.
Although the aforementioned postnatal effects of heat stress are easily recognized and well-defined, the effects of in utero heat stress experienced by the developing piglet on future postnatal production traits are more inconspicuous. Specifically, piglets derived from heat-stressed dams have increased body temperature and accumulate adipose tissue more efficiently during later growth stages at the expense of lean tissue (Johnson et al., 2015; Ross et al., 2017). Both increased body temperature and altered body composition have profound implications on maintenance costs, feed efficiency, ration formulation, and facility efficiency. However, these inefficient phenotypes would be expressed during the following year’s winter and spring and would thus be less remarkable. The prenatal effects of heat stress on future production phenotypes (which are currently not considered in the economic estimates) may ultimately be a larger constraint to efficient pig production than the more distinguishable effects of postnatal heat stress.
General Context of the Evolution of the Global Pork Production
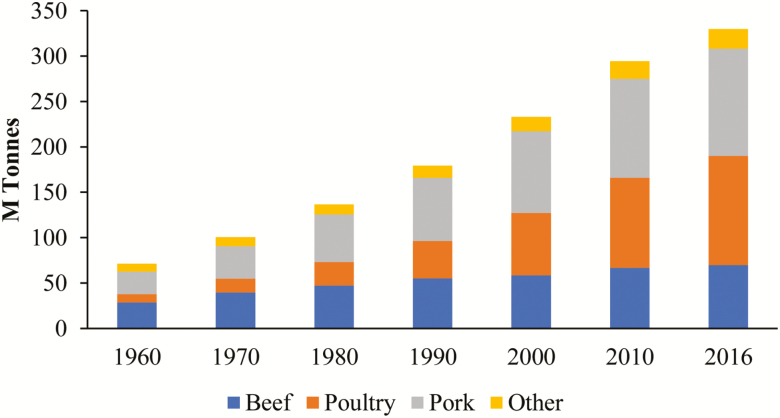
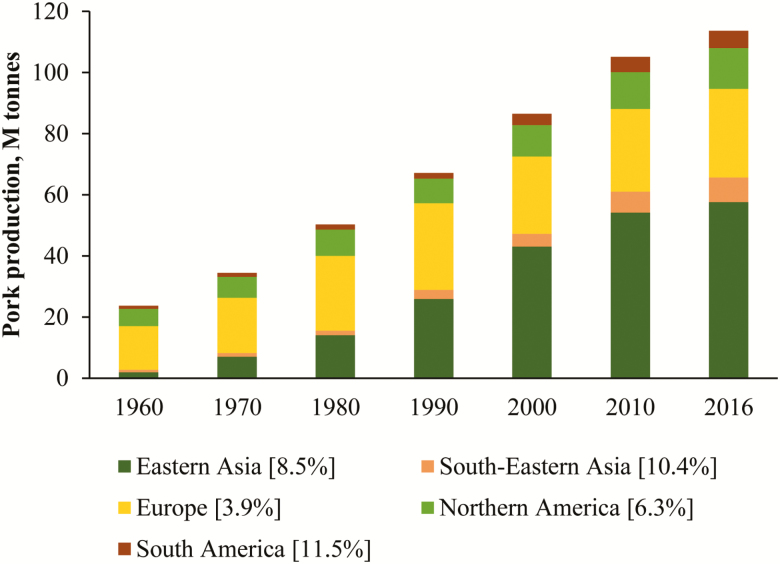
Globally, pork is one of the most consumed farm animals (Figure 1). China has about half of the pork production (49%), European countries are the second largest in pig production (25%), and North American countries are third with 11% (Figure 2). When considering growth rates between 1990 and 2016, emerging countries (e.g., South America and South Eastern Asia) are expected to contribute more significantly to global pork production with lower growth expected in Europe and North America. Incidentally, many of the aforementioned regions of expected growth in pig production are characterized by long periods of warm and humid conditions. Coupled with a rapidly expanding human population is an expected decrease in poverty rates. In this context, worldwide appetite for pork is expected to increase by 50% by 2050, especially in emerging tropical and subtropical countries. In most cases, the expansion in pig production will be achieved through intensification based on modern management practices and on animals of high genetic merit.
Figure 1.
Evolution of the world market demand for meat production between 1960 and 2016 (FAO Statistics).
Figure 2.
Evolution of pig meat production per country across 60 yr (FAO Statistics). Data enclosed in square brackets are the yearly variation in pork production from 1990 to 2016.
However, the environment where pork is actually produced is often markedly different from the conditions where the genetic selection occurred. Consequently, climate change combined with the migration of pig production and a suboptimal genetics by environment interaction creates a significant barrier of sustainably meeting the global requirement for animal protein.
Biological Adaptation/Acclimation
Thermoregulation
Animals lose heat in the form of sensible and latent (evaporative) heat. Conduction, convection, and radiation are primary mechanisms sensible heat loss occurs, and each requires a temperature gradient between the animal and its environment (Collier and Gebremedhin, 2015). Therefore, as ambient temperature increases, animals redistribute blood towards the skin in an attempt to increase radiant heat loss. With a further increase in ambient temperature (the temperature gradient between the environment and animal becomes smaller or even negative), the transfer of heat by conductive, convective, and radiative modes decreases. In fact, when ambient temperature increases above the upper critical temperature, evaporation is the only route of heat loss. Swine have few functional sweat glands and their thermoregulatory ability is further complicated by a thick subcutaneous adipose tissue layer, which impedes sensible heat loss; thus, pigs depend more on the respiratory route (i.e., panting) for heat dissipation (Collier and Gebremedhin, 2015). If the efforts of increasing heat loss to maintain euthermia are inadequate, the pig will initiate a variety of strategies to minimize heat production (behavior, etc., discussed below).
Feed Intake and Growth Performance
Normally, adjusting voluntary feed intake is one of the main adaptations employed to modify metabolic heat production in response to ambient temperature changes. Therefore, when ambient temperature increases, euthermia is maintained mainly by increasing heat loss and reducing heat production (Collin et al., 2001). Strategies to reduce heat production include decreasing feed intake and its associated thermic effect of feeding (Quiniou et al., 2000), along with decreased physical activity and reducing basal metabolic rate (Collin et al., 2001). Reduced feed intake is a highly conserved response to heat stress across species (Baumgard and Rhoads, 2013), and in pigs it can be represented as a curvilinear decrease with increasing ambient temperature, but varies depending on genotype, diet composition, body weight, and ambient temperature (Renaudeau et al., 2011).
Average daily gain during heat stress is usually reduced, and this is partly a consequence of decreased nutrient intake. Similar to feed intake, average daily gain has a curvilinear response during a thermal load and is affected by the animal’s body weight with heavier pigs more susceptible to heat stress than lighter ones (Renaudeau et al., 2011). As reviewed by Renaudeau et al. (2012b), the effect of heat stress on feed efficiency depends on both the temperature level and pig body weight. For a mild heat stress, feed efficiency generally increases because of the effect of feed restriction on the composition of body weight gain (more lean/less fat). Reduced feed efficiency is reported in finishing pigs kept at a temperature higher than 30 °C. This decrease in feed efficiency is related to a reduced proportion of energy intake available for tissue growth, which is mainly explained by a strong reduction in feed intake. However, regardless of the nuances within the feed efficiency equation, there is no question that heat stress reduces facility and operational efficiency (amount of carcass weight produced per barn per year) as it markedly decreases the time it takes to reach market weight.
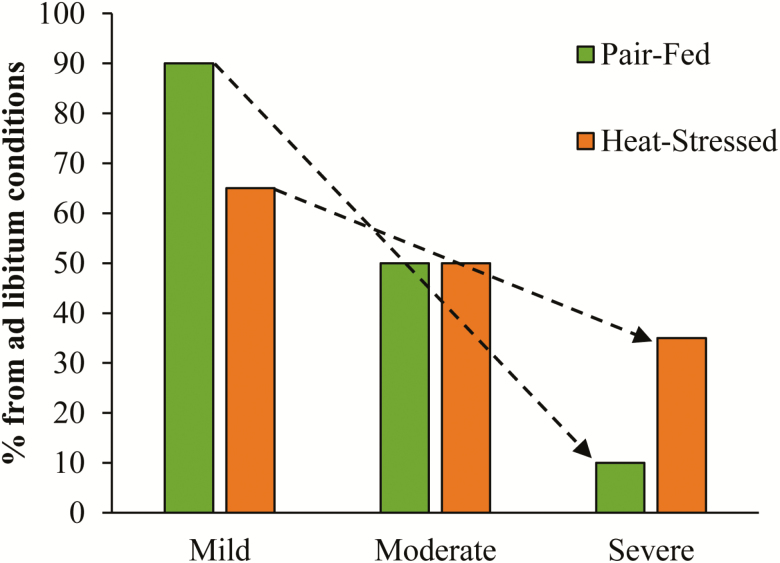
Interestingly, variations in growth performance during heat stress may also depend on the severity of the heat load and this is especially true when compared with pair-fed thermal neutral controls (Figure 3; Pearce et al., 2013; Sanz Fernandez et al., 2015). During mild heat stress (determined by small increases in body temperature variables and only mild reductions in feed intake), pigs grow slower than the pair-fed controls. However, as the severity of heat stress intensifies (determined by large increases in body temperature variables and severe reductions in feed intake), the heat stress pigs perform better (from a growth perspective) than pair-fed thermal neutral controls. This is energetically perplexing but is likely due to the fact that severe heat stress actually decreases maintenance costs rather than increasing it as reported previously (reviewed in Baumgard and Rhoads (2013) and Johnson et al. (2015)).
Figure 3.
The effects of increasing severity of heat stress on growth rates when compared with ad libitum feeding in thermal neutral conditions.
What Will Be the Main Impact of Future Climate Change on the Swine Industry?
Despite uncertainties in climate variability, the IPCC Fifth Assessment Report Climate (2009) concluded that the increase in global average surface temperature during the 21st century will likely be almost 5 °C, depending on the greenhouse gas emission scenario. Additionally, it reports that it is “virtually certain” that heat waves will occur more often and last longer, and that extreme precipitation events will become more intense and frequent. The deleterious impact this will have on the pig industry is obvious, but given the large uncertainty in the evolution of greenhouse gas emissions (within the global and local socioeconomic context), attempts to accurately evaluate future economic consequences for pork production in response to the global climate change are difficult. Despite the complexity of the assessment, this information is a prerequisite for developing adaptation strategies and implementing decisions.
Regarding the effect of global warming on crop production, most models predict a slight to moderate negative effect on simulated yield, even when beneficial effects of CO2, farm-level adaptations, and future technological yield improvements are accounted for (Parry et al., 2004). However, these simulations do not account for uncertainties related to water availability for irrigation and to the potential impacts of pests, weeds, and others stressors (Tubiello et al., 2007). An often overlooked consequence (likely because it is not well-understood) of heat stress and future climate change is the negative effects it may have on the plants eventually consumed by farm animals (Baumgard et al., 2012). For example, climate variability will likely increase instances of mycotoxin production, especially in temperate climate regions (Magan et al., 2011) and pigs have limited ability to detoxify mycotoxins (Wu et al., 2010). Additionally, the crop’s nutrient composition will likely change as protein content is expected to decrease and digestibility may be negatively affected (Hristov et al., 2018). Regardless, despite the fact that the effects of climate change on composition and digestibility of cereals and protein sources remain relatively vague, the potential for altered nutrient feeding value to negatively affect pig production is real and needs to be incorporated into future predictions.
Intervention Strategies
There is already a dire need to develop effective and sustainable management approaches to mitigate the negative effects of heat stress and this is even more important within the context of climate change. Undoubtedly, the primary priority is to modify the animal’s microenvironment and these heat stress abatement strategies are presented below. However, the input cost for optimal cooling technology is often too expensive, and this is particularly true for small stakeholders and farmers in developing countries. Genetic selection for thermal tolerance is one potential strategy to mitigate the effects of heat stress, but this is a long-term solution, and typically accompanied by reduced productivity during thermal–neutral conditions. Identifying flexible management approaches to immediately decrease heat stress susceptibility without negatively influencing traditional production traits would be of great value to global animal agriculture. Dietary supplementation and modifications (discussed below) are easily adjustable tactics that could be utilized by a variety of animal industries and are amenable to diverse production systems.
Heat Stress Abatement Strategies: Environment Modification
There are multiple engineering solutions and management strategies that can be used to mitigate heat stress, with physical environment modification the most effective. Foremost, facility design, construction, and operation are the initial mechanisms for 1) limiting amplification of ambient conditions and 2) minimizing energy required to remove heat from the system. A facility engineered with factors such as shape and orientation, thermal characteristics of construction materials, and ventilation system in careful consideration creates the foundation in which productivity can be minimally disrupted during heat stress.
Characterizing the Thermal Environment
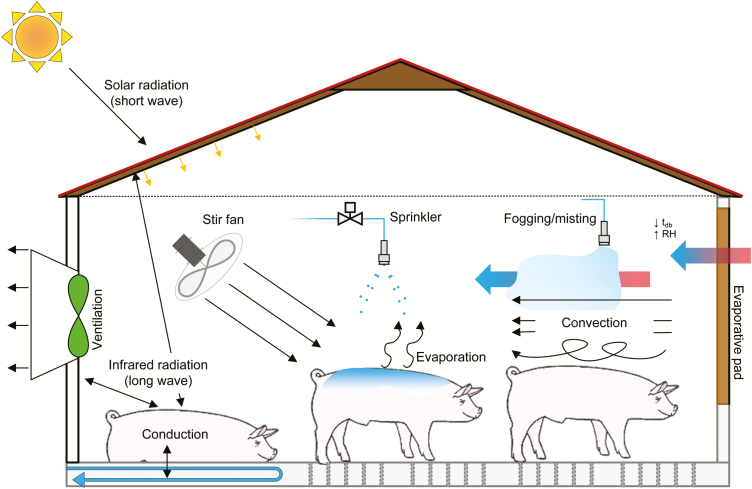
As mentioned above, the thermal environment describes the parameters that influence thermal (heat) exchange between an animal and its surroundings. As previously explained, sensible heat loss modes (conduction, convection, and radiation) are driven by a temperature gradient and latent heat loss modes (evaporation) by a water vapor pressure gradient between an animal’s outer surface (skin or pelage) and its surroundings. Animal characteristics (i.e., configuration, surface area, and surface temperature) affect all sensible modes (surface emissivity only affects radiation). Environmental characteristics each uniquely affect the different heat loss modes, such as surrounding surface temperatures (conduction and radiation), dry-bulb temperature (convection), air velocity (convection and evaporation), vapor pressure (evaporation), emissivity and orientation of surrounding objects (radiation), and lastly, heat capacity and thermal resistance of contact object (conduction). Therefore, these are the environmental parameters that can be physically modified to reduce heat stress (Figure 4).
Figure 4.
Thermal (heat) exchange between a pig and its surroundings with prediction of different cooling strategies as they relate to heat exchange.
The need to predict and support informed management decisions related to animal performance, health, and well-being has resulted in the development of thermal indices or equivalent (effective) temperatures that represent the effects produced by the heat exchange process. Although these indices substantially simplify complex physical and biological interactions, they serve as useful tools for guiding thermal environment management. For swine, the thermoneutral zone range changes predominately as a function of body mass. This is attributed to the increasing metabolic heat production and the decreasing surface area to mass ratio as a pig grows, albeit body mass is rarely ever used as an input to a thermal index, it is required to accurately assess the thermal environment. Consequently, the exact environmental conditions inducing heat stress in pigs remain ill-defined and this limits the effectiveness of heat stress abatement management.
Environmental Modification
Solar Radiation
For outdoor animals, reducing the solar radiation load by providing shade is presumably a cost-effective and simple method. Trees or artificial barriers (such as galvanized sheeting, shade cloth, and plastic snow fence) can minimize exposure to direct solar radiation, reduce the surrounding surface temperatures (radiated/reflected heat back to the animal), and does not modify the local thermal environment. Design considerations for shade structures include orientation, pitch, height, and material (da Silva and Maia, 2012). Conversely, for housed animals, although they are rarely exposed to direct solar radiation, it can substantially heat a facility’s roof, attics, and ceilings leading to facility heat accumulation inducing a higher infrared radiative load (long-wave radiation; Hoff, 2013). Thus, ceiling and attic insulation are effective methods at reducing the indoor surface temperature and heat accumulation.
Air Conditioning
Air temperature can be reduced using a direct expansion air conditioning unit, which consists of a mechanical system and refrigerant circulation. However, the capital, operational, and material longevity typically make it economically unviable for swine applications.
Evaporative Pads
When water changes phase from liquid to vapor (i.e., evaporation), energy is needed (about 2425.5 kJ kgH2O−1 at 32 °C). As outside air enters an evaporative pad, energy is removed from both the wet pad and the air as the water evaporates, thereby decreasing air temperature. Hence, the air temperature entering the facility is lower (since heat was removed for evaporation) and the relative humidity as well as the water vapor content is greater. Application of evaporative pads is most commonly used in breeding herd facilities (sows/boars) where cooling demand is greater and the need for dry surroundings are desired.
Fogging/Misting
Fogging (pressure > 5 MPa) and misting systems (pressure ≤ 5 MPa) reduce air temperature via water evaporation. The portion of airborne-atomized water that evaporates increases with decreasing droplet sizes (Haeussermann et al., 2007). Fogging systems create very fine droplets usually achieved by high-pressure, atomizing nozzles placed at fresh air inlets and wetting of surrounding surfaces is generally avoided, since evaporation can occur in relatively high humidity. Conversely, misting systems generate larger droplets (low pressure) that do not fully evaporate while airborne and can wet surrounding surfaces and animals. With all cooling systems utilizing water evaporation, the evaporation rate, and subsequently, heat removal, is limited by the amount of air moisture.
Direct Cooling
As opposed to physical modifying the environment to provide a cooler effective temperature, direct cooling involves the increase in heat loss from the body surface. Different strategies are discussed below.
Elevated Airspeed
Air movement over the animal affects both convective and evaporative heat loss. Convective heat loss increases at approximately the square root of air velocity; hence, as airspeed increases, the convective heat loss benefit diminishes. Furthermore, a temperature gradient between the animal’s surface temperature and the air temperature must exist for heat loss to occur. Typical swine skin temperature fluctuates between 32 and 36 °C. Thus, if ambient temperature approaches skin temperature, the effectiveness of elevated airspeed/“wind” (without utilizing evaporative cooling) is minimized. Elevated airspeeds can be accomplished by increasing airflow per unit of cross-sectional area (principle behind tunnel ventilation), stir (mixing) fans, or the wind in naturally ventilated facilities.
Wetted Skin
Adding water to the pig’s skin can increase heat loss and if combined with elevated airspeeds becomes a powerful tool for heat stress alleviation. Heat directly from the animal (and to some extent the surrounding air) is transferred to the evaporating water (the phase change requires energy). The transfer of thermal energy from the pig into the evaporating water thereby decreases the pig’s body temperature. Large water droplets needed to wet the skin can be distributed by low-pressure sprinklers for covering larger areas or “drippers” for localized cooling (i.e., sows in a snake). Coupling intermittent water application with elevated airspeed can markedly improve the efficiency of both routes of heat loss.
Floor (Conductive) Cooling
Pigs can lose sensible heat to a solid material of a lower temperature through contact. This is predominately achieved by circulating cool water through the floor the pigs lie on. Lactating sows spend a majority of their day lying, and this behavior allows for the effectiveness of chilled floor plates. For finishing pigs, concrete slats have been casted with piping to allow for water circulation. Economic viability is limited as capital and operational costs can be substantial, in conjunction with the technical feasibility of establishing a chilled water source and designing a pipe distribution network.
Monitoring
There are several technologies that exist for monitoring the thermal environment and animal physiological responses to heat stress. Effective and real-time monitoring is necessary to make appropriate investment decisions regarding heat stress abatement.
Environment
Air temperature is often the only parameter used to manage and describe the thermal environment and this results in multiple sensing options. The main characteristic of a good sensor for swine housing is 1) ability to quickly respond to changing conditions (i.e., low thermal mass) and 2) minimal radiative load (shielded). Long-wave infrared radiation can increase the temperature of the sensing element and cause the false indication of a high air temperature; however, this also most likely indicates a high radiative load and the need for additional cooling. Accurately measuring relative humidity was once challenging, given the dust and gaseous concentrations found in swine housing, but filters and newer sensing technologies have reduced sensor cost and extended longevity. Airspeed is virtually unmeasured due to sensor cost and ability to monitor from near still air conditions (~0.12 m s−1) to elevated airspeed for convective cooling (>2 m s−1). Lastly, long-wave infrared radiation is often neglected due to a lack of practical data interpretations. Nevertheless, the ISO 7726 (2001) states that a 15.24-cm diameter, copper sphere painted flat black with an air temperature sensor at the center is the standard.
Animal
The predominate physiological responses measured as an indicator of heat stress are respiration rate, skin temperature, rectal temperature, tympanic temperature, and vaginal temperature. Accurately measuring core-body temperature would be an ideal metric but this is accompanied with obvious hurdles. Good proxies for core-body temperature are rectal, vaginal, and tympanic membrane temperature, but obtaining these requires restraint and proper training and each has potential negative side effects. Respiration can be simply counted via human observation as the chest cavity expands and contracts, while automatic monitoring respiration rate in swine has been achieved. Skin temperature reflects the balance between metabolic heat production and the heat loss to the surroundings. With regards to skin temperature, both sides of the thermal balance must be known; that is, heat produced from the animal (core body temperature, tissue resistance, peripheral blood flow, respiration, passive skin diffusion, and pelage temperature) and energy removed from the animal (sensible and latent modes of heat transfer requiring surface area, shape and orientation, and all the environmental measurements). Thus, although frequently measured during environmental physiology experiments, skin temperature has little utility in determining the severity of heat stress.
Nutritional Considerations for Heat Stress
Nutritional interventions represent a practical, adaptable, and cost-effective opportunity to ameliorate the negative effects of heat stress and improve animal productivity. Typical dietary management practices include formulating low thermic effect of feeding diets and this is primarily accomplished by increasing dietary fat and reducing the amount of crude protein or crude fiber. Digesting, absorbing, and assimilating dietary fat generate the least amount of heat compared with other nutrients. Fermenting fiber in the large intestine generates heat and metabolizing excess dietary protein is associated with increased heat production, so minimizing fermentative diets and accurately predicting protein and amino acid requirements during the warm summer months should help pigs cope with a heat strain (Patience et al., 2015). It should be emphasized that these dietary recommendations are in large part theoretical and evidence supporting them are not as abundant and overwhelming as expected. In fact, Rauw et al. (2017) recently reported that performance in growing pigs exposed to repeated episodes of heat stress was not affected by a high-fiber diet. Consequently, the applied nutrition field needs systematic research that challenges long-held dogmas regarding diet formulation during the warm summer months.
Other dietary strategies involve supplementing bio-active compounds that have utility beyond their requirement (Rhoads et al., 2013). Many of the negative consequences that heat stress has on animal health and productivity are mediated by reduced intestinal barrier integrity (Baumgard et al., 2012; Baumgard and Rhoads, 2013). As already mentioned, during heat stress there is a redistribution of blood to the periphery in an attempt to increase heat loss. Consequently, the gastrointestinal tract vasoconstricts in an effort to support the altered blood distribution and the reduced splanchnic blood and nutrient flow creates intestinal barrier dysfunction. Intestinal infiltrating antigens stimulate a local immune reaction and, if severe enough, cause systemic endotoxemia associated with inflammation and an acute phase protein response. Consequently, heat stress is in large part an immune response caused by “leaky gut.” Thus, dietary strategies to prevent or minimize intestinal hyper-permeability are of particular interest and include antioxidants (selenium, vitamin E, vitamin C, etc.), specific amino acids (i.e., glutamine, betaine), and minerals (i.e., zinc). Additionally, functional molecules that have immunomodulatory effects could potentially ameliorate production loses during heat stress and these include chromium and vitamin C.
Genetic Opportunities
As stated above, heat stress susceptibility will worsen if genetic selection continues to emphasize traditional production traits, as these are associated with increased heat production. Fortunately, heat susceptibility appears to be a heritable trait in finishing pigs, and therefore, genetics may offer a viable strategy to improve production during the warm summer months. The biological and phenotypic responses to heat stress represent an extremely complex trait for which genetic information is insufficient. In recent work, many significant genomic regions in relation with heat tolerance were identified in pigs (Riquet et al., 2017). This new genomic information could be used in the future to identify pigs capable of maintaining high levels of productivity during heat stress. However, there remains a considerable knowledge gap and a critical need to improve our understanding of the genetic contributions to the variation in response to heat stress.
Summary
In summary, heat stress compromises a variety of production parameters in the swine industry including growth, carcass composition, and reproduction. Evidence suggests that maternal exposure to heat stress has long-lasting effects on postnatal offspring performance. The combination of climate change forecasts increased pork production in tropical and subtropical regions of the globe and improved genetic capacity for lean tissue accretion and fecundity, all point to increasingly negative impacts of heat stress on pork production efficiency and quality in the future. Physically modifying the environment is currently the primary abatement strategy that should be utilized to mitigate the negative effects of heat stress, but other approaches include dietary modifications and genetic improvement.
About the Author
Edith J. Mayorga is a PhD student in the Department of Animal Science at Iowa State University. Her research focuses on studying the effects of environmental hyperthermia on post-absorptive metabolism and growth performance in pigs. Edith is originally from Colombia, where she attended the National University of Colombia for her Bachelor’s in Animal Science. She earned a M.S degree in Animal Science at Iowa State University.

Dr. David Renaudeau is a senior research scientist in the PEGASE (Physiology, Environment, and Genetics for the Animal and Livestock Systems) joint research unit between INRA and AGROCAMPUS OUEST. He is leading the Feeding and Nutrition team. The main objective of his team is to acquire fundamental and applicable knowledge about animal nutrition (pigs, dairy cattle) with special emphasis on protein, lipid, energy, and mineral metabolism. He has more than 20 yr of experience on the adaptation of the pigs to thermal heat stress.

Dr. Brett C. Ramirez is an assistant professor in the Department of Agricultural and Biosystems Engineering at Iowa State University. His research and extension program encompass swine and poultry production systems with a focus on housing, natural resource and energy efficiency, animal energetics, environmental control, and precision livestock farming. His expertise is in instrumentation and analysis of the key thermal environment and indoor air quality parameters that affect animal performance. He holds a BS and MS from the University of Illinois at Urbana-Champaign, and his PhD is from Iowa State University.

Dr. Jason W. Ross is the Lloyd L. Anderson Endowed Professor in Physiology in the Department of Animal Science at Iowa State University and Director of the Iowa Pork Industry Center. His research program utilizes basic and applied research approaches to test hypotheses from which the results will help enable livestock producers and associated industries improve swine production efficiency. His research efforts include genetic modification of pigs to improve understanding the genetic control of phenotypes associated with production efficiency and to improve the use of pigs as biomedical models. Dr. Ross earned his BS at Iowa State University, his MS and PhD at Oklahoma State University and was a postdoctoral research fellow at the University of Missouri.

Dr. Lance Baumgard is the Jacobson Professor in the Department of Animal Science at Iowa State University. His research efforts are primarily in Environmental and Nutritional Physiology. His scientific focus is on learning how heat stress reduces farm animal activity and to develop nutritional/management strategies to ameliorate heat stress.
Literature Cited
- Baumgard L. H. and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., Rhoads R. P., Rhoads M. L., Gabler N. K., Ross J. W., Keating A. F., Boddicker R. L., Lenka S., and Sejian V.. 2012. Impact of climate change on livestock production. In: Sejian V., Naqvi S. M. K., Ezeji T., Lakritz J., and Lal R., editors. Environmental stress and amelioration in livestock production. Berlin: Springer-Verlag; p. 413–468. [Google Scholar]
- Collier R. J. and Gebremedhin K. G.. 2015. Thermal biology of domestic animals. Annu. Rev. Anim. Biosci. 3:513–532. doi: 10.1146/annurev-animal-022114-110659 [DOI] [PubMed] [Google Scholar]
- Collin A., van Milgen J., Dubois S., and Noblet J.. 2001. Effect of high temperature on feeding behaviour and heat production in group-housed young pigs. Br. J. Nutr. 86:63–70. [DOI] [PubMed] [Google Scholar]
- Haeussermann A., Hartung E., Jungbluth T., Vranken E., Aerts J. M., and Berckmans D.. 2007. Cooling effects and evaporation characteristics of fogging systems in an experimental piggery. Biosyst. Enginer. 97:395–405. doi:10.1016/j.biosystemseng.2007.03.019 [Google Scholar]
- Hoff S. J. 2013. The impact of ventilation and thermal environment on animal health, welfare and performance. In: Aland A., and T. Banhazi, editors. Livestock Housing: Modern management to ensure optimal health and welfare of farm animals. Wageningen, NL: Wageningen Academic; p. 209–236. [Google Scholar]
- Hristov A. N., Degaetano A. T., Rotz C. A., Hoberg E., Skinner R. H., Felix T., Li H., Patterson P. H., Roth G., Hall M., T.L, et al. 2018. Climate change effects on livestock in the Northeast US and strategies for adaptation. Climatic Change. 146:33–45. doi: 10.1007/s10584-017-2023-z [Google Scholar]
- IPCC 2009. Intergovernmental panel on climate change, fifth assessment report. Climate change: the physical science basis. Bali: Intergov. Panel Clim. Change. [Google Scholar]
- ISO 7726 2001. Ergonomics of the thermal environment-instruments for measuring physical quantities. Geneva: International Standardization Organization. [Google Scholar]
- Johnson J. S., Abuajamieh M., Sanz Fernadez M. V., Seibert J. T., Stoakes S. K., Nteeba J., Keating A. F., Ross J. W., and Baumgard L.. 2015. Thermal stress alters post-absorptive metabolism during pre- and postnatal development. In: Climate change impact on livestock: adaptations and mitigation. New Delhi, India: Springer; p. 61–79. [Google Scholar]
- Key N., and Sneeringer S.. 2014. Potential effects of climate change on the productivity of U.S dairies. Am. J. Agric. Econ. 96:1136–1156. doi:10.1093/ajae/aau002 [Google Scholar]
- Magan N., Medina A., and Aldred D.. 2011. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 60:150–163. doi:10.1111/j.1365-3059.2010.02412.x [Google Scholar]
- Parry M. L., Rosenzweig C., Iglesias A., Livermore M., and Fischer G.. 2004. Effects of climate change on global food production under SRES emissions and socio-economic scenarios. Global Environ. Change. 14:53–67. doi:10.1016/j.gloenvcha.2003.10.008 [Google Scholar]
- Patience J. F., Rossoni-Serão M. C., and Gutiérrez N. A.. 2015. A review of feed efficiency in swine: biology and application. J. Anim. Sci. Biotechnol. 6:33. doi: 10.1186/s40104-015-0031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L. H.. 2013. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91:2108–2118. doi: 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Pollmann D. S. 2010. Seasonal effects on sow herds: industry experience and management strategies. J. Anim. Sci. 88 (Suppl. 3):9 (Abstr). [Google Scholar]
- Quiniou N., Dubois S., and Noblet J.. 2000. Voluntary feed intake and feeding behavior of group housed growing pigs are affected by ambient temperature and body weight. Livest. Prod. Sci. 3: 245–253. [Google Scholar]
- Rauw W. M., Mayorga E. J., Lei S. M., Dekkers J. C. M., Patience J. F., Gabler N. K., Lonergan S. M., and Baumgard L. H.. 2017. Effects of diet and genetics on growth performance of pigs in response to repeated exposure to heat stress. Front. Genet. 8:155. doi: 10.3389/fgene.2017.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. 2012a. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Gilbert H., and Noblet J.. 2012b. Effect of climatic environment on feed efficiency in swine. In: Patience J. F., editor, Feed efficiency in swine. Wageningen, NL: Wageningen Academic; p. 183–210. [Google Scholar]
- Renaudeau D., Gourdine J. L., and St-Pierre N. R.. 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi: 10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- Rhoads R. P., Baumgard L. H., Suagee J. K., and Sanders S. R.. 2013. Nutritional interventions to alleviate the negative consequences of heat stress. Adv. Nutr. 4:267–276. doi: 10.3945/an.112.003376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquet J., Gilbert H., Feve K., Labrune Y., Rose R., Billon Y., Giorgi M., Loyau T., Gourdine J. L., and Renaudeau D.. 2017. Genetic dissection of mechanisms underlying heat adaptation in pigs. In: 36th Conference of the International Society for Animal Genetics (ISAG), Illinois, USA p.133. [Google Scholar]
- Ross J. W., Hale B. J., Gabler N. K., Rhoads R. P., Keating A. F., and Baumgard L. H.. 2015. Physiological consequences of heat stress in pigs. Anim. Prod. Sci. 55:1381–1390. doi:10.1071/AN15267 [Google Scholar]
- Ross J. W., Hale B. J., Seibert J. T., Romoser M. R., Adur M. K., Keating A. F., and Baumgard L. H.. 2017. Physiological mechanisms through which heat stress compromises reproduction in pigs. Mol. Reprod. Dev. 84:934–945. doi: 10.1002/mrd.22859 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Stoakes S. K., Abuajamieh M., Seibert J. T., Johnson J. S., Horst E. A., Rhoads R. P., and Baumgard L. H.. 2015. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 3:e12478. doi:10.14814/phy2.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva R. G., and Campos Maia A. S.. 2012. Shade and shelter. In: da Silva R. G., and Campos Maia A. S., editors. Principles of animal biometeorology. New York: Springer; p. 181–206. [Google Scholar]
- Tubiello F. N., Soussana J. F., and Howden S. M.. 2007. Crop and pasture response to climate change. Proc. Natl. Acad. Sci. U. S. A. 104:19686–19690. doi: 10.1073/pnas.0701728104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. H., Dohnal V., Huang L. L., Kuca K., Yuan Z. H.. 2010. Metabolic pathways of trichothecenes. Drug Metabol. Rev. 12:1–10. doi:10.1080/03602530903125807 [DOI] [PubMed] [Google Scholar]