Implications
Antibiotic resistance is among the top five threats to humanity, with infectious disease expected to surpass cancer as the leading cause of death by 2050.
Antibiotic resistance is a “One Health” issue with any anthropomorphic use of antimicrobials potentially promoting resistance.
There is an urgent need to identify alternatives to antibiotics for use in livestock, before further restrictions in their use occur.
Prudent use of existing antibiotics and emerging alternatives throughout the “One Health” continuum, coupled with disease prevention, are integral to sustaining human and animal health.
Introduction
By 2050, global infectious disease caused by antibiotic-resistant bacteria is projected to be responsible for 10 million human deaths per year—1.8 million more than cancer. Antibiotic resistance is a natural phenomenon, but the anthropomorphic use of antimicrobials has created heightened selective pressure that has led to an increased presence of antibiotic-resistant bacteria in agriculture, aquaculture, and hospital environments. Concerns over the emergence of antibiotic-resistant bacteria that threaten human health has prompted the retail and fast food sector to promote meat and milk produced from livestock that are raised “without the use of antibiotics.”
Antibiotics have become an integral component of intensive livestock production and are used to treat (therapeutic use) and prevent (prophylactic, metaphylactic) infectious disease and promote growth (subtherapeutic). The growing restriction of antibiotic use in livestock production has promoted research into a plethora of potential alternatives.
Antibiotic Alternatives and One Health
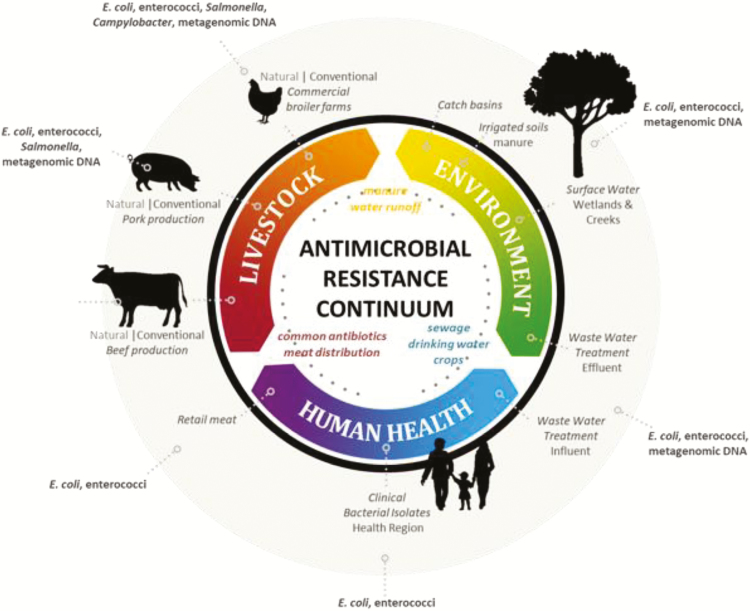
Some alternatives to antibiotics, such as plant secondary compounds (i.e., essential oils, tannins, saponins) and probiotics, have been used in human medicine for centuries, but have only recently been seriously considered for use in livestock and poultry. Over the last 70 yr, antibiotics largely replaced the use of these alternatives in most human societies and have been rapidly adopted for use in livestock and poultry production. Antibiotics are used to control bacterial infections in a range of environments and if resistance is to be reduced, antibiotic use must be curtailed and made more prudent throughout the “One Health” continuum. Such an outcome will require the integrative efforts of experts in human, livestock, and environmental health and the adoption of recommendations by stakeholders. Logically, studies on the ability of alternatives to reduce antibiotic resistance should also be structured from a “One Health” perspective using indicator bacteria, pathogens, and molecular techniques to document the extent to which they impact antibiotic resistance in humans, livestock, and the environment (Figure 1). Antimicrobial compounds in plants such as essential oils and condensed tannins (CT) as well as bacteriophages, vaccines, and probiotics are examples of a few of the alternatives that are being explored for use in livestock. However, antimicrobial activity of plant bioactives is often broad, inhibiting both commensal and pathogenic bacteria and reducing productivity. In contrast, the activity of bacteriophages is often too narrow, targeting only a subset of pathogenic bacteria. Compared with antibiotics, the mechanisms whereby these alternatives inhibit or kill bacteria are poorly defined, making therapeutic outcomes difficult to predict. Understanding the mode of actions of these alternatives is integral to using them in a manner that offsets the present reliance on the use of antibiotics to control infectious disease in livestock.
Figure 1.
Proposed approach to evaluating the impact of alternatives to antibiotics in livestock and poultry production on antibiotic resistance throughout the One Health Continuum. Indicator bacteria and samples for metagenomic analysis are collected from conventional and natural livestock and poultry production systems. Natural production systems limit the use of antibiotics and rely heavily on alternatives. Similar samples are collected from the surrounding environment, surface waters, sewage, hospital settings, and retail meat. Such a system should provide insight as to the degree that a reduction in the use of antibiotics in natural livestock production systems contributes to a reduction in antibiotic resistance throughout the One Health continuum.
Essential Oils and Plant Bioactives
There are about 4,500-individual essential oils and numerous other bioactives that have been extracted from various plant components, including flowers, buds, seeds, leaves, twigs, bark, herbs, wood, fruits, and roots, with some sources used in human medicine since antiquity (Baby and George, 2009). The major constituents of essential oils include carvacrol, citronellol, geraniol, eugenol, and thymol (Perricone et al., 2015). Several essential oils have been shown to have antibacterial, antifungal, antiviral, and anti-inflammatory properties (Baby and George, 2009; de Cássia da Silveira e Sá et al., 2014; Chouhan et al., 2017). Constituents of essential oils seem to interact with bacterial cell membrane lipids and cause cell lysis. For example, terpinen-4-ol, α-terpineol, 8-cineole in tea tree oil caused the autolysis of Staphylococcus aureus and the formation of mesosomes (Carson et al., 2002), whereas cinnamaldehyde and carvacrol readily lysed Mycobacterium avium (Nowotarska et al., 2017).
In addition to controlling pathogens, essentials oils have been investigated for their potential to improve the growth performance of livestock by increasing feed palatability. However, their antibacterial activity is often less pronounced in vivo than in vitro, possibly due to the inactivation by other feed ingredients and to their poor absorption (Si et al., 2006). Some have proposed that essential oils be protected from degradation in the upper digestive tract in order to promote their passage to the lower tract. Diets supplemented with encapsulated carvacrol or citral at either 250 or 650 μg/g reduced the prevalence and severity of necrotic enteritis associated with Clostridium perfringens in chickens (Liu et al., 2016; Yanga et al., 2016).
Adverse effects have also been observed when essential oils are included in poultry diets. For example, even though cinnamaldehyde and eugenol decreased colonization of the broiler gut by Salmonella enteritidis, birds exhibited reduced growth (Kollanoor-Johny et al., 2012). Similarly, Patraa and Yu (2012) reported that essential oils from clove, eucalyptus, garlic, oregano, and peppermint decreased methane and ammonia production as well as the abundance and diversity of archaea in ruminal laboratory batch cultures, but others found adverse effects on feed digestion and fermentation in cattle. In general, these additives have shown promise in laboratory studies, but have frequently failed to deliver similar responses when included in the diets of livestock.
Bioactives from Berries
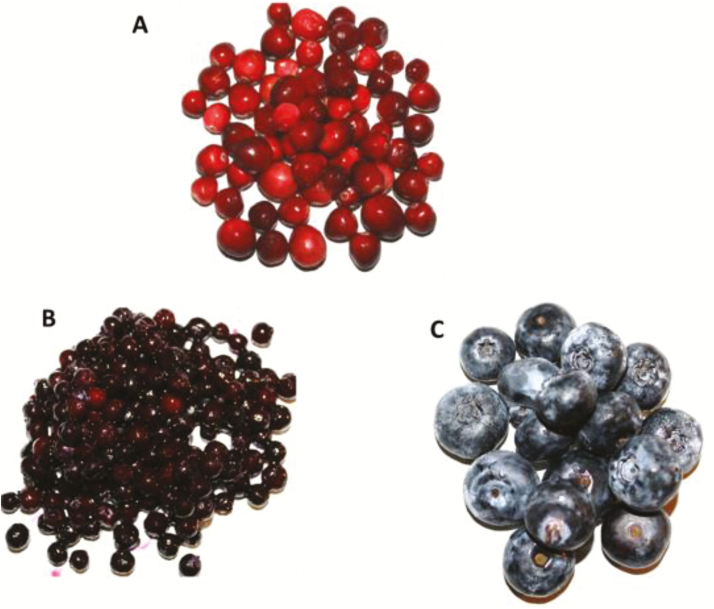
American cranberry (Vaccinium macrocarpon), wild-blueberry (Vaccinium angustifolium), and highbush blueberry (Vaccinium corymbosum) are important commercial fruits in the United States and Canada (Figure 2). They are rich sources of bioactives including polyphenolics (Harrison et al., 2013). The ability of polyphenolics to alter the diversity of the intestinal microbiome has been well documented (Cardona et al., 2013). The commensal bacteria, Bifidobacterium and Lactobacillus have been shown to ferment cranberry xyloglucans to produce formate which may aide these bacteria in colonizing the human gut (Özcan et al., 2017). Cranberry extracts were found to be nearly as effective as trimethoprim, with less adverse effects when used to treat urinary tract infections in women (McMurdo et al., 2009).
Figure 2.
(A) American cranberry (Vaccinium macrocarpon), (B) wild-blueberry (Vaccinium angustifolium), and (C) highbush blueberry (Vaccinium corymbosum) are all sources of bioactives with the potential to be used as alternatives to antibiotics. Photo taken by Dr. M. S. Diarra.
Few studies have assessed the potential of these berry polyphenolics to serve as alternatives to antibiotics in livestock. Inclusion of a commercial cranberry juice powder in the feed of broilers did not improve feed intake or gain (Leusink et al., 2010). Cranberry juice has been found to contain at least 19 phenolic compounds, with quercetin, vanillic acid, and protocatechuic acid shown to enhance immune function in poultry (Islam et al., 2017). Considering that the pomace remaining after juice extraction also contains these polyphenolics, the use of this by-product is likely a more economical alternative to antibiotics.
Berry pomaces can be a good source of nutrients and other functionally important molecules including vitamins, minerals, and polyphenolics (Ross et al., 2017). A polyphenol rich bioactive fraction from cranberry pomace exhibited high antibacterial activity against a number of multidrug-resistant bacteria including methicillin resistant Staphylococcus aureus (Diarra et al., 2013).
Feeding turkeys a diet containing 5% pomace from strawberries resulted in no differences in the final body weights or meat yield as compared to birds fed a conventional diet (Juskiewicz et al., 2017). However, the strawberry pomace did increase the α-linolenic acid level in breast meat. Coddensa et al. (2017) showed that spray-dried cranberry powder reduced the intensity and duration of shedding in piglets challenged with F18+ E. coli. The potential and modes of action whereby berry products may modulate gut microbiota in food animals has recently been reviewed (Das et al., 2017). Key research gaps include the effects of processing on the stability and bioavailability of bioactives in these sources (Das et al., 2017). Despite the lack of information on the toxicity of these sources for livestock, cranberry extract powder has been approved for human use by the European Food Safety Authority (EFSA, 2017).
Condensed Tannins
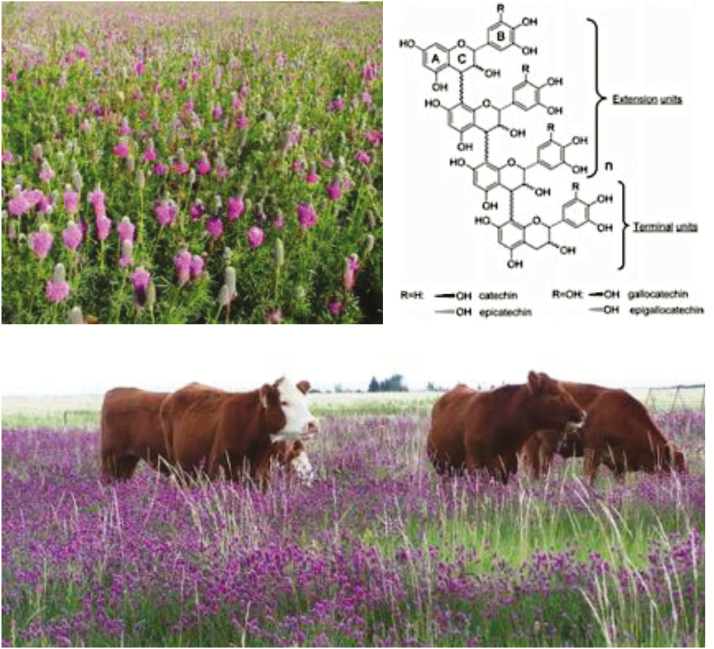
Condensed tannin (CT) are naturally occurring plant secondary compounds that are oligomeric or polymeric flavonoids consisting of flavan-3-ol units that include catechin, epicatechin, gallocatechin, and epigallocatechin with molecular weights ranging from 1,000 to 20,000 Da (Figure 3). They protect the plant against invasion by pathogens and attack by insects and herbivory, mainly through their ability to form complexes with proteins, polysaccharides, and minerals. CT possess anti-bacterial, anti-parasitic, anti-oxidant, and immunomodulation activities and have been used as herbal medicines for centuries. In the last few decades, researchers have demonstrated that CT could potentially be used as natural alternatives to in-feed antibiotics (Huang et al., 2017a). Generally, CT exhibit greater activity against gram positive than gram negative bacteria (Smith and Mackie, 2004). However, some CT also exhibit activity against gram negative bacteria such as Escherichia coli O157:H7 (Wang et al., 2013). Inhibition of extracellular microbial enzymes, deprivation of nutrients, inhibition of oxidative phosophorylation, sequestration of metal ions, and the formation of complexes with cell membrane proteins are a few of the mechanisms whereby CT inhibit or kill bacteria. These polyphenolics can also enhance the hosts’ antioxidant status and immunity. Observed effects of feeding CT-containing forages or supplementing CT directly to livestock on gut health and productivity vary greatly and have been extensively reviewed (Mueller-Harvey, 2006; Wang et al., 2015; Huang et al., 2017a). Inconsistencies in the effects of CT are related to their variation in chemical composition and concentration in various plants and plant tissues (Peng et al., 2017).
Figure 3.
Condensed tannins (CT) are oligomeric or polymeric flavonoids consisting of flavan-3-ol units that include catechin, epicatechin, gallocatechin, and epigallocatechin. Purple prairie clover contains high levels of CT with unique biochemical structure and high antimicrobial activity. Photo taken by Dr. Alan Iwaasa.
Currently CT are delivered in the diet either as CT-containing forages or browse or as semi-purified extracts such as quebracho. Although feeding CT-containing forage is the easiest way to administer CT to livestock, variations in concentration and biological activity can influence their impact on microbial populations. CT extracts have not only been assessed in ruminants, but also in swine and poultry. Responses to these extracts vary considerably, in part due to alteration in their biological activity during extraction, as well as interactions with other components in the diet during storage and feeding (Huang et al., 2017a). These complexities make it difficult to conduct systematic and comprehensive evaluations of the efficacy and safety of CT and their value as alternatives to antibiotics.
Probably the greatest challenge to using CT as antibiotic alternatives is the anti-nutritional properties that they confer through nonselective binding to dietary proteins, carbohydrates, minerals, and digestive enzymes. These interactions not only reduce the digestibility of nutrients (Mueller-Harvey, 2006; Redondo et al., 2014), but also the antimicrobial activity of CT within the lower digestive tract (Huang et al., 2017b). These negative responses are even more accentuated in swine and poultry than ruminants. New technologies are needed to reduce the negative effects of CT on digestibility and to enable them to remain biologically active towards pathogens throughout the digestive tract.
Plant bioactives express their antimicrobial activity via several mechanisms, making it difficult for bacteria to develop resistance to these complex compounds. In whole plants, several different types of bioactives may act synergistically to inhibit or kill bacteria through multiple mechanisms. However, prolonged use of purified bioactives can promote resistance, most commonly through alterations in cell membrane permeability or selection for members within the microbiome that are capable of catabolizing these compounds. For example, Qinghao (Artemisia annua L, Asteraceae) has been used as an effective anti-malarial herb in Chinese medicine for centuries, without evidence of any resistance to artemisinin, the active anti-malarial compound in Qinghao (Miller and Su, 2011). However, the use of purified artemisinin to control malaria resulted in the plasmodium parasite becoming resistant to this bioactive within a matter of decades. If resistance to plant bioactives involves changes in bacterial cell membrane permeability, the use of these additives can result in resistance to other antimicrobials, including antibiotics that rely on contact with intracellular components for bactericidal activity.
Bacteriophages
Bacteriophages (phages) are viral predators of bacteria and are estimated to destroy half of the world’s population of bacteria every 48 h (Hendrix, 2002). Bacteriophages were first considered for use in human therapy as early as 1917, but with the advent of antibiotics in the 1940s, research into their therapeutic value was largely sidelined. With the rise in antibiotic resistance, phages are being re-evaluated for therapeutic use in human medicine and agriculture. Most known phages are able to interact only with bacteria expressing specific binding sites recognized by the tail fibers of the phages. This specificity is both an advantage and a challenge to the use of phages in therapy. On the plus side, phage specificity allows the specific targeting of pathogenic bacteria, leaving beneficial microflora untouched (Joerger, 2003). However, the specificity of phages to their hosts makes it unlikely that a single phage will have activity against multiple species of pathogenic bacteria or in some instances even all strains of a single pathogenic species. To increase host range and to guard against the development of bacterial resistance to phages, mixtures of multiple species of phages, termed cocktails, are generally used in phage therapy.
To be useful in phage therapy, phages should be strictly lytic, as temperate phages can integrate into the host DNA and increase the risk of transferring virulence factors including antibiotic resistance genes to targeted pathogens. However, finding lytic phages that specifically target bacterial pathogens of interest to the livestock industry can be difficult. For example, despite an exhaustive search for lytic phages, only temperate phages have been isolated with activity against Mannheimia haemolytica, one of the principal agents of bovine respiratory disease (BRD; Hsu et al., 2013; Urban-Chmiel et al., 2015). To avoid possible transference of virulence elements within phages, an alternative is to expose pathogenic bacteria directly to the lytic enzymes produced by phage. Such an approach may be suitable for topical infections or those associated with mucous membranes, but is impractical for more complex environments such as the respiratory or digestive tract (Joerger, 2003).
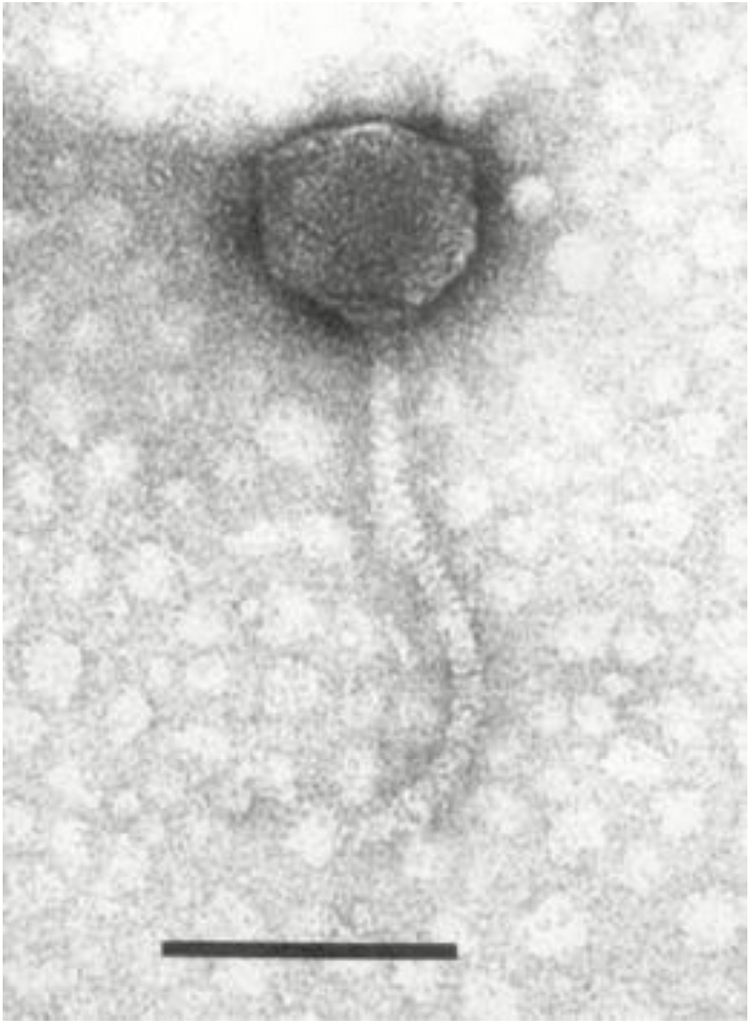
Care must also be taken in selecting phages for use in therapeutic cocktails. It is not possible to predict the efficacy of a cocktail against a targeted pathogen, solely on the activity of individual phages. We have found that the phage in our collection with the most virulent activity against E. coli O157:H7, the T5-like phage AKFV33 (Figure 4; Niu et al., 2012) exhibited reduced lytic capability when used in a cocktail with three other phages. The three other phages in the cocktail acted synergistically as their overall lytic capacity was improved compared with when they were applied individually.
Figure 4.
An electron micrograph of the T5-like phage, AKFV33, isolated from the environment of feedlot cattle. This phage exhibits the highest activity against E. coli O157:H7 within our collection (Niu et al., 2012).
Phages lack flagella and only randomly contact host bacteria, a requirement to activate adherence mechanisms. Consequently, determining the appropriate dose for phage therapy is challenging. Too few phages and these random contact events may fail to occur. Too many phages and they may show interference and reduced lytic capacity. Provided contact is made with the host, phages will replicate, with populations of phage rising and falling asynchronously with that of their host (Hallewell et al., 2014).
Phages are relatively resilient and have been administered directly into the blood stream, although they may be attacked by antibodies or removed from circulation by the reticuloendothelial system. Oral dosing of phages to control gastrointestinal pathogens is probably the most common use of phage therapy in livestock and has been used in poultry against Salmonella spp., both pre- and post-processing (Atterbury et al., 2007; Petsong and Vongkamjan, 2015). Phages chosen for phage therapy must also be easy to propagate in batch culture, and exhibit resilience during storage, even when absorbed to solid particles (Joerger, 2003). Bacteriophages do show promise as a method of pathogen control, particularly for those pathogens that already exhibit antibiotic resistance in livestock. However, finding phages appropriate for use in therapy for livestock is still in its infancy and additional studies are needed to verify efficacy of potential therapeutic cocktails.
Vaccines
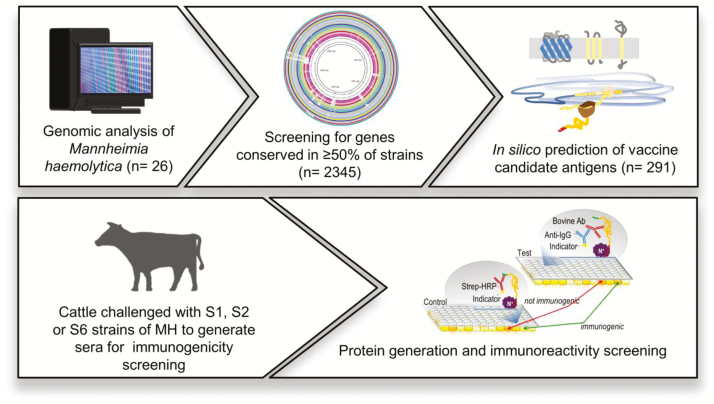
Vaccines are widely used to prevent bacterial and viral infections in livestock, poultry, and humans and are, at present, the most promising alternative to antibiotics. Vaccines have the added benefit that unlike antibiotics they can also be used to prevent viral infections. Conventional vaccines rely on the use of heat-killed, live modified or purified components typically derived from cultured bacteria or viruses. Reverse vaccinology involves the use of comparative genomics to identify those proteins in pathogens that are most closely linked to causing disease (Figure 5). We have used this technique to identify candidate proteins that could be used to design a more efficacious vaccine against M. haemolytica (Klima et al., 2018). This technology could revolutionize vaccine development and has already been used to develop a vaccine against tuberculosis in humans.
Figure 5.
Use of reverse vaccinology to identify appropriate protein candidates for inclusion in a vaccine against Mannheimia haemolytica. Comparative genomics is undertaken to identify candidate antigens based on genomic differences between virulent and commensal strains. Cattle are challenged with different serotypes of M. haemolytica. Proteins are expressed in a cell-free expression system and assessed for their immunoreactivity in a screening assay (Klima et al., 2018).
However, vaccines also have their limitations in that the host needs to be in a suitable state to mount an immune response against the infectious agent. Consequently, vaccines need to be administered well in advance of clinical signs of disease. Identification of a suitable adjuvant that promotes immunity can also be challenging and multiple vaccinations are often required to elicit a suitable immune response. Development of vaccines against microbes that reside in the intestinal tract is also notoriously difficult and only a few vaccines have shown efficacy against pathogens in this environment. Vaccination of a sufficient number of individuals within a population is also necessary to achieve population immunity; otherwise, infectious agents can continue to circulate within unvaccinated members of the population. From a One Health perspective, perhaps the greatest threat to the ability of vaccines to reduce the reliance on antibiotics arises from the anti-vaccine movement. The use of vaccines in humans has had wide spread value, reducing mortalities by two to three million per year. An aversion to the use of vaccines would not only lead to more human mortalities, but also to an inadvertent increase in the use of antibiotics.
Probiotics
A joint panel by the Food and Agriculture Organization and the World Health Organization defined probiotics as “live microorganisms administered in adequate amounts that confer a beneficial health effect on the host” (FAO/WHO, 2002). Though typically associated with functioning in the gastrointestinal tract (GIT) and delivery in food or feed, by definition, probiotics can also target microorganisms associated with the skin, respiratory and genitourinary tracts. The first concept of using live bacteria to maintain and improve human health was by Elie Metchnikoff, a comparative zoologist, who in the early 19th century advocated consumption of lactobacilli in milk to prolong life (Gordon, 2008). Research and use of probiotics for livestock and poultry dates back decades, well before the ban of subtherapeutic antibiotics for growth promotion (Vanbelle et al., 1990). However, with legislation limiting growth promoting claims of in-feed antibiotics, and increased consumer and scientific concerns over the use of antibiotics in livestock and poultry, probiotics have been increasingly seen as an alternative to antibiotics. However, inconsistent effects of probiotics in livestock and a lack of systematic design and testing have hampered their widespread adoption. Addressing these issues is critical if probiotics are to play a role in reducing antimicrobial use in agriculture.
Lactobacillus, Bifidobacterium, and Enterococcus are the most common genera that have been evaluated for their ability to replace the use of antibiotics for growth promotion. In order to serve as an alternative, probiotics should function in a similar capacity and achieve comparable results to antibiotics in terms of disease prevention and control in livestock. For antibiotic growth promoters (AGP), the mechanisms whereby growth is improved is unclear and may include (i) modulating the gastrointestinal microbiome to enhance digestion and metabolism, or (ii) reducing intestinal mucosal inflammation through immunomodulation (Brown et al., 2017). While the mechanisms may differ, typically probiotics have been shown to behave similarly to metaphylactic antibiotics and AGP in that they inhibit pathogens, alter the metabolism of the microbiome and modulate immunity.
Probiotics antagonize pathogens in several ways and in many cases through a combination of mechanisms. Certain probiotics produce bacteriocins, antimicrobial peptides which can have a broad or narrow spectrum of activity. They inhibit growth by altering the cell envelope of Gram-positive bacteria or interfering with DNA, RNA, and protein metabolism in Gram-negative bacteria (Cotter et al., 2013). Corr et al. (2007) compared the protective effects of a bacteriocin-producing Lactobacillus salivarius UCC118 strain or a genetically equivalent mutant that could not produce bacteriocins against pathogenic Listeria monocytogenes in mice. The UCC118 strain prevented L. monocytogenes infection, whereas the mutant strain did not. Probiotics can also produce other antimicrobials including organics acids which lower cellular pH and hydrogen peroxide which can cause oxidative damage, both of which inhibit pathogens (Nair et al., 2017). Competition for nutrients and reducing adhesion are other ways in which probiotics interfere with pathogens. For example, the probiotic Escherichia coli strain Nissle 1917 assimilates iron through a mechanism similar to Salmonella enterica serovar Typhimurium. When administered together to mice, E. coli Nissle 1917, out competed S. enterica for iron, reducing its numbers (Deriu et al., 2013). Adherence to the mucosa is a necessary step for both pathogens and probiotics to modulate the host immune system (Collado et al., 2006; Wall, 2008). Probiotics can reduce pathogen adherence through competition for binding sites on epithelial cells, with strains that exhibit high affinity being more antagonistic (Guglielmetti et al., 2010).
Probiotics do not only affect pathogens and it is likely that commensal bacteria are also altered as a result of probiotic interactions with the microbiome. For example, using probiotics to modify the rumen microbiome to improve digestion has been a key focus in ruminant nutrition. A meta-analysis of the data from yeast studies showed that this additive increased milk and fat-corrected milk yields in dairy cattle (Poppy et al., 2012). There is also data to show that yeast can improve feed efficiency and alter rumen metabolism (Alugongo et al., 2017). Although the mechanisms for these responses are unclear, one hypothesis proposes that yeast stimulates the growth and activity of fibre-digesting bacteria.
Alterations in the microbiome are not always directly associated with the probiotic administered. For example, inoculation of weaned pigs with Enterococcus faecalis LAB31 improved growth performance, reduced the incidence of diarrhea, and increased Lactobacillus in the feces (Hu et al., 2015). While many previous probiotic studies specifically analyzed Lactobacillus, Bifidobacterium, and coliforms using culturing or low-throughput DNA identification techniques, the advent of next generation sequencing has provided greater insight into how probiotics alter the host microbiome. Comparison of Enterococcus faecalis to a mixture of in-feed antimicrobials (bacitracin, chlortetracycline, and colistin) in pigs showed that the probiotic shifted the gut microbiome in a manner similar to the antimicrobial mixture (Li et al., 2017). If this shift is linked to growth promotion, studying the impact of probiotics on the microbiome could be ideal for selecting viable antibiotic alternatives. In contrast, chickens inoculated with Lactobacillus plantarum possessed a fecal microbiome that differed from those fed a diet containing a mixture of chlortetracycline and salinomycin (Gao et al., 2017). The L. plantarum appeared to accelerate the maturation of the fecal microbiota, improving feed efficiency and eliciting a greater immune response than that observed in chickens receiving antibiotics. These studies show that while analyzing the effects of probiotics on the host microbiota can inform our understanding of these additives, the results can be highly variable depending on the animal and probiotic species, strain, and class of antibiotics studied.
Probiotics can affect animal health directly though interacting with the GIT mucosa, where the lumen is in contact with commensal and potentially pathogenic bacteria. The mucosa serves as a barrier to pathogens and is in a constant state of controlled inflammation (Brown et al., 2017). Disruption of the mucosal epithelial lining can lead to pathogenesis. Probiotics can enhance epithelial barrier function by inducing mucin-binding proteins that inhibit pathogen colonization and by modulating genes that control epithelial barrier function so as to reduce pathogen translocation (Nair et al., 2017). For example, administration of Pediococcus acidilactici and Saccharomyces cerevisiae boulardii to pigs reduced E. coli translocation to mesenteric lymph nodes as compared with those that did not receive probiotics (Lessard et al., 2009). Certain probiotic strains can also reduce intestinal inflammation by inducing anti-inflammatory cytokines (Vanderpool et al., 2008). This promotes intestinal barrier integrity, and as with AGP, may reduce the energetic costs associated with inflammation. Anti-inflammatory responses to probiotics may not always need to arise as a result of their direct colonization of the GIT. Yin et al. (2017) showed that production of a bacteriocin by L. plantarum elicited an anti-inflammatory response in mice, possibly through a shift in the GIT microbiome. Similarly, administration of Enterococcus durans caused an increase in Faecalibacterium prausnitzii in mice, a bacterium proposed to contribute to anti-inflammatory responses (Carasi et al., 2017).
The intestinal microbiota is critical to immune development as germ-free mice have a compromised immune system (Round and Mazmanian, 2009). Like host bacteria, probiotics are also capable of modulating innate (non-specific) and adaptive (acquired) immune responses through interactions with gut-associated lymphoid cells. For example, Lactobacillus strains were shown to influence regulatory T cells in mice (Petersen et al., 2012). T-cells have a role in cell-mediated (acquired) immunity and regulator T-cells help suppress inflammation and maintain immune-tolerance. Administration of Lactobacillus fermentum and Saccharomyces cerevisiae to chickens, stimulated T-cell immunity compared with controls and a group fed chlortetracycline (Bai et al., 2013), while daily gain and feed efficiency between these groups were similar. Further evidence of immunomodulation by probiotics comes from studies that have measured their value as adjuvants. A recent review analyzing the effects of probiotics on vaccine responses in humans indicated that they could enhance both the efficacy and the duration of the response to vaccination (Zimmermann and Curtis, 2018). Similarly, administration of a probiotic in conjunction with a vaccine against coccidia provided greater protection against this parasite than the vaccine alone (Ritzi et al., 2016).
Meta-analysis and clinical studies have shown that probiotics may counteract or prevent intestinal dysbioses (Patel and Dupont, 2015; Parker et al., 2018). Meta-analysis studies have also shown that probiotics can reduce the occurrence and duration of upper respiratory tract infections (URTI) in adults and children (King et al., 2014; Hao et al., 2015), lowering antibiotic use (Hao et al., 2015). It should be noted that most URTI are most commonly caused by viruses, but misdiagnosis can still cause an increase in antibiotic use (Lenoir-Wihnkoop et al., 2016).
Despite the variability in response of livestock and poultry to probiotics, the fact that some studies have documented equivalent or even better responses than antibiotics (Zhang and Kim, 2014; Gao et al., 2017) highlights their potential as alternatives within some production systems. Most probiotic strains were isolated from the gut or fermented foods and were selected for their ability to produce high yields and be resilient during production, as opposed to confer health benefits (O’Toole et al., 2017). Frequently, strains used for livestock were first used in humans and may not be suitably adapted for livestock or poultry. There are ongoing efforts to identify strains of bacteria within the livestock host’s commensal microbiota that may be more suitable for use in various species of livestock. Strains of this nature differ from conventional probiotic genera such as Lactobacillus and Bifidobacterium and would be most likely used for therapeutic purposes. These have been coined “new-generation probiotics” (Patel and DuPont, 2015), or biotherapeutics. For example, the relative abundance of Akkermansia muciniphila has been inversely correlated with body weight and can reduce caloric uptake and weight gain in mice (Fabbiano et al., 2017). While this is a desirable response in humans, a strain that elicits the opposite response in livestock and poultry could improve production efficiency. This type of research employs an informed approach to probiotic design. We have recently been identifying commensal bacteria within the respiratory tract that exhibit activity against, M. haemolytica. Most feedlots in North America use metaphylactic administration of antibiotics to mitigate BRD in arriving cattle. To identify potential new probiotics, we started by comparing the microbiota in the upper respiratory tract of cattle that remain healthy to those that developed BRD upon feedlot arrival (Holman et al., 2015). From this, we determined that healthy cattle had a greater abundance of Bacillus and Lactobacillus than those that developed BRD. These bacteria were isolated and characterized for their ability to inhibit the adherence of M. haemolytica to bovine respiratory (Figure 6) and immuno-modulate cell lines. We also assessed the antibiotic susceptibility of the top six isolates as some of the currently registered probiotics encode antibiotic resistance genes (Wong et al., 2015). The next step in development will be to test the efficacy of intranasal application of the probiotic to reduce M. haemolytica colonization. Ultimately, probiotics developed in this fashion will need to be validated in clinical trials or field testing and will also require greater regulatory scrutiny (e.g., virulence, antimicrobial resistance, mobile genetic elements) to receive approval for a disease prevention claim. Increased knowledge on how microbial ecosystems affect host health, digestion, and growth, will lead to opportunities for developing probiotics that specifically replace antibiotic use in livestock.
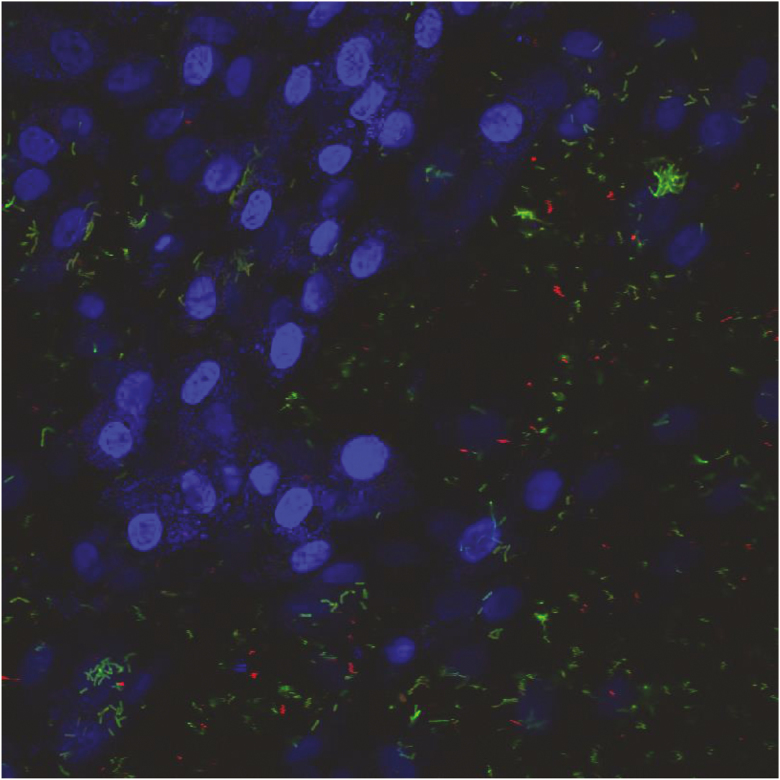
Figure 6.
Adherence of the bovine respiratory pathogen Mannheimia haemolytica (stained red with Alexa Fluor 488) and the probiotic Lactobacillus lactis (stained green with Alexa Fluor 594) to bovine turbinate cells (stained blue with DAPI). M. haemolytica and L. lactis were added to the turbinate cells in equal amounts. After 3 h, unbound bacteria were washed.
Conclusion
It is clear that there is no single alternative that can replace the current use of antibiotics throughout the One Health continuum. Of the above approaches, disease prevention through proper nutrition, vaccination, adequate housing, and limiting stressors that compromise immunity are likely the most effective means of reducing antibiotic use. Bacteria are masters of adaptation and can become resistant to plant bioactives, phages, probiotics, and even develop mechanisms to combat the immune system. Although novel antibiotics could offset resistance in the short term, it is inevitable that bacterial resistance to these new molecules will also arise. Genetic selection of the host for disease resistance using emerging genomic tools is one strategy to reduce the present reliance on antibiotics to maintain livestock health. However, for progress to be made, the entire arsenal of alternatives, in conjunction with precision use of antibiotics, is needed to combat disease in both humans and livestock. What is needed is a more surgical approach to the use of antibiotics throughout the One Health continuum. This would include designation of the most important antibiotics for exclusive use in humans, but still allow the use of other antibiotics in livestock. A severe restriction of the use of all antibiotics could have serious negative consequences for the health and welfare of livestock. A greater focus on health promotion and disease prevention in combination with some of the above alternatives throughout the One Health continuum is likely the most effective strategy to lower antibiotic use and the occurrence of resistance.
Acknowledgments
Our research has been partially funded by Agriculture and Agri-Food Canada through the Agricultural Policy Framework and the Growing Forward programs. We thank the many technologists, students, and postdoctoral fellows who have made invaluable contributions to our research. The editorial assistance of Krysty Munns is also gratefully appreciated.
About the Authors

Dr. Tim McAllister, Principal Research Scientist, AAFC Lethbridge. Tim McAllister received an M. Sc. in Animal Biochemistry from the University of Alberta in 1987 and a Ph.D. in Microbiology and Nutrition from the University of Guelph in 1991. He has worked as a research scientist with Agriculture and Agri-Food Canada since 1996 where he now holds the position of principal research scientist in microbiology and beef cattle production. His team addresses a broad-range of topics as they relate to antibiotic resistance including the surveillance of antibiotic resistance in beef production systems, comparative genomics of antibiotic resistance in indicator species and characterization of the resistome in beef cattle and the environment. His team has been the recipient of numerous societal awards for their contribution to beef cattle production in North America.

Dr. Yuxi Wang, Senior Research Scientist, AAFC Lethbridge. Dr. Yuxi Wang received an M. Sc. in Animal Nutrition from Shandong Agricultural University, China in 1989 and a Ph.D. in Ruminant Nutrition and Microbiology from Massey University, NZ in 1995. He has worked at the Lethbridge Research and Development Centre of Agriculture and Agri-Food Canada since 1996 and now holds a senior research scientist position in ruminant nutrition and rumen microbiology. His research addresses a broad-range of topics relating to beef production which includes exploring the use of naturally occurring plant compounds such as condensed tannins as alternatives to in-feed antibiotics. His team has numerous scientific publications and reviews in this research area.

Dr. Moussa Diarra, Research Scientist, AAFC Guelph. Moussa S. Diarra completed his Master degree in Animal Sciences in 1992 and his PhD in Microbiology-Immunology in 1995 from the Laval University (QC). Since 2002, he is a Research Scientist with Agriculture and Agri-Food Canada. His research is focussed on mitigating antibiotic resistance in food production systems, understating bacterial pathogenesis and the interactions between pathogenic bacteria and their hosts. He also is looking for the discovery and the devolvement of novel alternatives to antibiotics in livestock and poultry production. Dr. Diarra combines animal production, basic and advanced molecular microbiology technics to reach his research objectives. He received several research grants and co-authored several publications including patents, peer reviewed articles and conferences. He is a member of several scholarly societies.

Dr. Trevor Alexander, Research Scientist, AAFC Lethbridge. Trevor Alexander is a Research Scientist at Agriculture and Agri-Food Canada, Lethbridge. He received a B.Sc. and Ph.D. in Nutrition and Metabolism from the University of Alberta. His research program focuses on feedlot cattle, with an emphasis on bovine respiratory disease and environmental microbiology. Ongoing projects include the development of antibiotic alternatives for mitigating respiratory disease and the epidemiology of veterinary and human bacterial pathogens throughout the beef continuum.

Dr. Kim Stanford, Research Scientist, Alberta Agriculture and Forestry. Kim Stanford received an MSc in Animal Genetics from the University of Alberta in 1987 and a PhD in Animal Science from the University of Alberta in 1998. She has been working as a research Scientist for Alberta Agriculture and Forestry since 1990 first specializing in sheep production and more recently working with food and feed-borne pathogens. Her laboratory has been investigating bacteriophage for the control of pathogenic E. coli since 2007.
Literature Cited
- Alugongo G.M., Xiao J., Wu Z., Li S., Wang Y., and Cao Z.. 2017. Review: utilization of yeast of Saccharomyces cerevisiae origin in artificially raised calves. J. Anim. Sci. Biotechnol. 8:34. doi:10.1186/s40104-017-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atturbury R.J., Van Bergen M.A.P., Ortiz F., Lovell M.A., Harris J.A., De Boer A., Wagenaar J.A., Allen V.M., and Barrow P.A.. 2007. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 73:4543–4549. doi: 10.1128/AEM.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baby S., and George V.. 2009. Essential oils and new antimicrobial strategies. In: Ahmad, I and E. Aqil, editors. New strategies combating bacterial infection.Weinheim (Germany):Wiley-VCH Verlag MmbH & Co. KGaA; p. 165–203. [Google Scholar]
- Bai S.P., Wu A.M., Ding X.M., Lei Y., Bai J., Zhang K.Y., and Chio J.S.. 2013. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 92:663–670. doi:10.3382/ps.2012-02813. [DOI] [PubMed] [Google Scholar]
- Brown K., Uwiera R.R.E., Kalmokoff M.L., Brooks S.P. J., and Inglis G.D.. 2017. Antimicrobial growth promoter use in livestock: a requirement to understand their modes of action to develop effective alternatives. Int. J. Antimicrob. Agents. 49:12–24. doi:10.1016/j.ijantimicag.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Carasi P., Racedo S.M., Jacquot C., Elie A.M., Serradell M.L., and Urdaci M.C.. 2017. Enterococcus durans EP1 a promising anti-inflammatory probiotic able to stimulate sIgA and to increase Faecalibacterium prausnitzii abundance. Front. Immunol. 8:88. doi: 10.3389/fimmu.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona F., Andrés-Lacueva C., Tulipani S., Tinahones F.J., and Queipo-Ortuño M.I.. 2013. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 24:1415–1422. doi:10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Carson C.F., Mee B.J., and Riley T.V.. 2002. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 46:1914–1920. doi:10.1128/AAC.46.6.1914-1920.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cássia da Silveira e Sá R., Andrade L.N., dos Reis Barreto de Oliveira R., and Pergentino de Sousa D.. 2014. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules. 19:1459–1480; doi:10.3390/molecules19021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan S., Sharma K., and Guleria S.. 2017. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 4:58. doi:10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddensa A., Loosa M., Vanrompay D., Remonc J. P., and Coxa E.. 2017. Cranberry extract inhibits in vitro adhesion of F4 and F18+ Escherichia coli to pig intestinal epithelium and reduces in vivo excretion of pigs orally challenged with F18+ verotoxigenic E. coli. Vet. Microbiol. 202:64–71. doi:10.1016/j.vetmic.2017.01.019 [DOI] [PubMed] [Google Scholar]
- Collado M.C., Gueimonde M., Sanz Y., and Salminen S.. 2006. Adhesion properties and competitive pathogen exclusion ability of bifidobacteria with acquired acid resistance. J. Food Prot. 69:1675–1679. doi:10.4315/0362-028X-69.7.1675 [DOI] [PubMed] [Google Scholar]
- Corr S.C., Li Y., Riedel C.U., O’Toole P.W., Hill C., and Gahan C.G.. 2007. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl Acad. Sci. USA. 104:7617–7621. doi:10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P.D., Ross R.P., and Hill C.. 2013. Bacteriocins—a viable alternative to antibiotics?Nat. Rev. Microbiol. 11:95–105. doi:10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- Das Q., Islam M.R., Marcone M.F., Warriner G. K., and Diarra M.S.. 2017. Potential of berry extracts to control foodborne pathogens. Food Control. 73:650–662. doi: 10.1016/j.foodcont.2016.09.019. [Google Scholar]
- Deriu E., Liu J.Z., Pezeshki M., Edwards R.A., Ochoa R.J., Contreras H., Libby S.J., Fang F.C., and Raffatellu M.. 2013. Probiotic bacteria reduce Salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 14:26–37. doi:10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra M.S., Block G., Rempel H., Oomah B.D., Harrison J., McCallum J., Boulanger S., Brouillette É., Gattuso M., and Malouin F.. 2013. In vitro and in vivo antibacterial activities of cranberry press cake extracts alone or in combination with β-lactams against Staphylococcus aureus. BMC Complement. Altern. Med. 13:90. doi:10.1186/1472-6882-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA) 2017. Safety of cranberry extract powder as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA J. 15:4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbiano S., Suárez-Zamorano N., and Trajkovski M.. 2017. Host-microbiota mutualism in metabolic diseases. Front. Endocrinol. 8:267. doi:10.3389/fendo.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, Food and Agriculture Organization of the United Nation and World Health Organization 2002. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food [online] http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed October, 2017).
- Gao P., Ma C., Sun Z., Wang L., Huang S., Su X., Xu J., and Zhang H.. 2017. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. 5:91. doi:10.1186/s40168-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, S. 2008. Elie metchnikoff: father of natural immunity. Eur. J. Immunol. 38:3257–3264. doi:10.1002/eji.200838855. [DOI] [PubMed] [Google Scholar]
- Guglielmetti S., Taverniti V., Minuzzo M., Arioli S., Stuknyte M., Karp M., and Mora D.. 2010. Oral bacteria as potential probiotics for the pharyngeal mucosa. Appl. Environ. Microbiol. 76:3948–3958. doi:10.1128/AEM.00109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallewell H., Niu Y. D., Munns K., McAllister T.A., Johnson R.P., Ackermann H.W., Thomas J.E., and Stanford K.. 2014. Differing populations of endemic bacteriophages in cattle shedding high and low numbers of Escherichia coli O157:H7 bacteria in feces. Appl. Environ. Microbiol. 80: 3819–3823. doi:10.1128/AEM.00708-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q., Dong B., and Wu T.. 2015. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 3(2):CD006895. doi:10.1002/14651858.CD006895.pub3 [DOI] [PubMed] [Google Scholar]
- Harrison J.E., Oomah B.D., Diarra M. S., and Ibarra-Alvarado C.. 2013. Bioactivities of pilot scale extracted cranberry juice and pomace. J. Food Process. Preservat. 37:356–365. 10.1111/j.1745-4549.2011.00655.x [DOI] [Google Scholar]
- Hendrix R.W. 2002. Bacteriophages: evolution of the majority. Theor. Popul. Biol. 61:471–480. 10.1006/tpbi.2002.1590 [DOI] [PubMed] [Google Scholar]
- Holman D.B., McAllister T.A., Topp E., Wright A.D., and Alexander T.W.. 2015. The nasopharyngeal microbiota of feedlot cattle that develop bovine respiratory disease. Vet. Microbiol. 180:90–95. doi:10.1016/j.vetmic.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Hsu Y.H., Cook S.R., Alexander T.W., Klima C.L., Niu Y.D., Selinger L.B., and McAllister T.A.. 2013. Investigation of Mannheimia haemolytica bacteriophages relative to host diversity. J. Appl. Microbiol. 114:1592–1603. doi:10.1111/jam.12185. [DOI] [PubMed] [Google Scholar]
- Hu Y., Dun Y., Li S., Zhang D., Peng N., Zhao S., and Liang Y.. 2015. Dietary Enterococcus faecalis LAB31 improves growth performance, reduces diarrhea, and increases fecal lactobacillus number of weaned piglets. PLoS One 10:e0116635. doi:10.1371/journal.pone.0116635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.Q., Liu X.L., Zhao G.Q., Hu T., and Wang Y.. 2017a. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. (Review). Anim. Nutr. doi:10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.Q., Holman D.B., Alexander T., Hu T., Jin L., Xu Z., McAllister T.A., Acharya S., Zhao G.Q., and Wang Y.. 2017b. Fecal microbiota of lambs fed purple prairie clover (Dalea purpurea Vent.) and alfalfa (Medicago Sativa). Arch. Microbiol. doi:10.1007/s00203-017-1427-5. [DOI] [PubMed] [Google Scholar]
- Islam R., Oomah D.B., and Diarra M.D.. 2017. Potential immunomodulatory effect of non-dialyzable materials of cranberry extracts in poultry production. Poult. Sci. 96:341–350. dx.doi.org/10.3382/ps/pew302. [DOI] [PubMed] [Google Scholar]
- Joerger R.D. 2003. Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 82:640–647. 10.1093/ps/82.4.640 [DOI] [PubMed] [Google Scholar]
- Juskiewicz J., Jankowski J., Zielinski H., Zdunczyk Z., Mikulski D., Antoszkiewicz Z., Kosmala M., and Zdunczyk P.. 2017. The fatty acid profile and oxidative stability of meat from turkeys fed diets enriched with n-3 polyunsaturated fatty acids and dried fruit pomaces as a source of polyphenols. PLoS One. 12:e0170074. doi:10.1371/journal.pone.0170074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S., Glanville J., Sanders M.E., Fitzgerald A., and Varley D.. 2014. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br. J. Nutr. 112:41–54. doi:10.1017/S0007114514000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klima C.L., Zaheer R., Cook S.R., Rasmussen J., Alexander T.W., Potter A., Hendrick S., and McAllister T.A.. 2018. In silico identification and high throughput screening of antigenic proteins as candidates for a Mannheimia haemolytica vaccine. Vet. Immunol. Immunopathol. 195:19–24. doi:10.1016/j.vetimm.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Kollanoor-Johny A., Mattson T., Baskaran S.A., Amalaradjou M.A., Babapoor S., March B., Valipe S., Darre M., Hoagland T., Schreiber D., et al. 2012. Reduction of Salmonella enterica serovar enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 78:2981–2987. doi:10.1128/AEM.07643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir-Wijnkoop I., Gerlier L., Roy D., and Reid G.. 2016. The clinical and economic impact of probiotics consumption on respiratory tract infections: projections for Canada. PLoS One. 11:e0166232. doi:10.1371/journal.pone.0166232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard M., Dupuis M., Gagnon N., Nadeau E., Matte J.J., Goulet J., and Fairbrother J.M.. 2009. Administration of Pediococcus acidilactici or Saccharomyces cerevisiae boulardii modulates development of porcine mucosal immunity and reduces intestinal bacterial translocation after Escherichia coli challenge. J. Anim. Sci. 87:922–934. doi:10.2527/jas.2008-0919. [DOI] [PubMed] [Google Scholar]
- Leusink G., Rempel H., Skura B., Berkyto M., White W., Yang Y., Rhee J.Y., Xuan S.Y., Chiu S., Silversides F., et al. 2010. Growth performance, meat quality, and gut microflora of broiler chickens fed with cranberry extract. Poult. Sci. 89:1514–1523. doi:10.3382/ps.2009-00364. [DOI] [PubMed] [Google Scholar]
- Li P., Niu Q., Wei Q., Zhang Y., Ma X., Kim S.W., Lin M., and Huang R.. 2017. Microbial shifts in the porcine distal gut in response to diets supplemented with Enterococcus faecalis as alternatives to antibiotics. Sci. Rep. 7:41395. doi:10.1038/srep41395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Diarra M.S., Zhang Y., Wang Q., Yu H., Nie S.P., Xie M.Y., and Gong J.. 2016. Effect of encapsulated carvacrol on the incidence of necrotic enteritis in broiler chickens. Avian Pathol. 45:357–364. doi:10.1080/03079457.2016.1138281. [DOI] [PubMed] [Google Scholar]
- McMurdo M.E., Argo I., Phillips G., Daly F., and Davey P.. 2009. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J. Antimicrob. Chemother. 63:389–395. doi:10.1093/jac/dkn489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.H., and Su X.. 2011. Artemisinin: discovery from the Chinese herbal garden. Cell. 146:855–858. doi:10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Harvey I. 2006. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agri. 86: 2010–2037. 10.1002/jsfa.2577 [DOI] [Google Scholar]
- Nair M. S., Amalaradjou M.A., and Venkitanarayanan K.. 2017. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv. Appl. Microbiol. 98:1–29. doi:10.1016/bs.aambs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Niu Y.D., Stanford K., Kropinski A.M., Ackermann H.W., Johnson R.P., She Y.M., Ahmed R., Villegas A., and McAllister T.A.. 2012. Genomic, proteomic and physiological characterization of a T5-like bacteriophage for control of shiga toxin-producing Escherichia coli O157:H7. Plos One. 7:e34585. doi:10.1371/journal.pone.0034585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotarska S.W., Nowotarski K., Grant I.R., Elliott C.T., Friedman M., and Situ C.. 2017. Mechanisms of antimicrobial action of cinnamon and oregano oils, cinnamaldehyde, carvacrol, 2,5-dihydroxybenzaldehyde, and 2-hydroxy-5-methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods. 6:72. doi:10.3390/foods6090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole P.W., Marchesi J.R., and Hill C.. 2017. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2:17057. doi:10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- Özcan E., Sun J., Rowley D.C., and Sela D.A.. 2017. A human gut commensal ferments cranberry carbohydrates to produce formate. Appl. Environ. Microbiol. 83: https://doi.org/10.1128/AEM.01097-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker E.A., Roy T., D’Adamo C.R., and Wieland L.S.. 2018. Probiotics and gastrointestinal conditions: an overview of evidence from the cochrane collaboration. Nutrition. 45:125–134.e11. doi:10.1016/j.nut.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., and DuPont H.L.. 2015. New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 60(Suppl 2):S108–S121. doi:10.1093/cid/civ177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patraa A. K. and Yu Z.. 2012. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. App. Environ. Microbiol. 78:4271–4280. doi:10.1128/AEM.00309-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K., Huang Q.Q., Xu Z., McAllister T.A., Acharya S., Wang S., Drake C., Mueller-Harvey I., and Wang Y.. 2017. Characterization of condensed tannins from freeze-dried, silage or hay purple prairie clover (Dalea purpurea Vent.): structure composition, protein precipitation and anti-Escherichia coli properties. J. Anim. Sci. 95(Suppl 4):140–141. [Google Scholar]
- Perricone M., Arace E., Corbo M.R., Sinigaglia M., and Bevilacqua A.. 2015. Bioactivity of essential oils: a review on their interaction with food components. Front. Microbiol. 6:76. doi:10.3389/fmicb.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E.R., Claesson M.H., Schmidt E.G., Jensen S.S., Ravn P., Olsen J., Ouwehand A.C., and Kristensen N.N.. 2012. Consumption of probiotics increases the effect of regulatory T cells in transfer colitis. Inflamm. Bowel Dis. 18:131–142. doi:10.1002/ibd.21709. [DOI] [PubMed] [Google Scholar]
- Petsong K., and Vongkamjan, K. 2015. Application of Salmonella bacteriophages in the food production chain. Pages 275–283 in Mendes-Vilas, A., editor. The battle against microbial pathogens. Formatex Research Center, Badajz, Spain. [Google Scholar]
- Poppy G.D., Rabiee A.R., Lean I.J., Sanchez W.K., Dorton K.L., and Morley P.S.. 2012. A meta-analysis of the effects of feeding yeast culture produced by anaerobic fermentation of Saccharomyces cerevisiae on milk production of lactating dairy cows. J. Dairy Sci. 95:6027–6041. doi:10.3168/jds.2012-5577. [DOI] [PubMed] [Google Scholar]
- Redondo L.M., Chacana P.A., Dominguez J.E., and Fernandez Miyakawa M.E.. 2014. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 5:118. doi:10.3389/fmicb.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi M.M., Abdelrahman W., van-Heerden K., Mohnl M., Barrett N.W., and Dalloul R.A.. 2016. Combination of probiotics and coccidiosis vaccine enhances protection against an eimeria challenge. Vet. Res. 47:111. doi:10.1186/s13567-016-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K.A., Ehret D., Godfrey D., Fukumoto L., and Diarra M.S.. 2017. Characterization of pilot scale processed Canadian organic cranberry (Vaccinium macrocarpon) and blueberry (Vaccinium angustifolium) juice pressing residues and phenolic enriched extractives. Inter. J. Fruit Sci. doi: 10.1080/15538362.2017.1285264. [Google Scholar]
- Round J.L., and Mazmanian S.K.. 2009. The gut microbiome shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9:313–323. doi:10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W., Gong J., Chanas C., Cui S., Yu H., Caballero C., and Friendship R.M.. 2006. In vitro assessment of antimicrobial activity of carvacrol, thymol and cinnamaldehyde towards Salmonella typhimurium DT104: effects of pig diets and emulsification in hydrocolloids. J. Appl. Microbiol. 101:1282–1291. doi:10.1111/j.1365-2672.2006.03045.x. [DOI] [PubMed] [Google Scholar]
- Smith A.H., and Mackie R.I.. 2004. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract. Appl. Environ. Microbiol. 70:1104–1115. doi:10.1128/AEM.70.2.1104-1115.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Chmiel R., Wernicki A., Stęgierska D., Dec M., Dudzic A., and Puchalski A.. 2015. Isolation and characterization of lytic properties of bacteriophages specific for m. Haemolytica strains. PLoS One. 10:e0140140. doi:10.1371/journal.pone.0140140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbelle M., Teller E., and Focant M.. 1990. Probiotics in animal nutrition: a review. Arch. Tierernahr. 40:543–567. [DOI] [PubMed] [Google Scholar]
- Vanderpool C., Yan F., and Polk D.B.. 2008. Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 14:1585–1596. doi:10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- Wall R., Hussey S.G., Ryan C.A., O’Neill M., Fitzgerald G., Stanton C., and Ross R.P.. 2008. Presence of two Lactobacillus and Bifidobacterium probiotic strains in the neonatal ileum. ISME J. 2:83–91. doi:10.1038/ISMEJ.2007.69. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jin L., Ominski K.H., He M., Xu Z., Krause D.O., Acharya S.N., Wittenberg K.M., Liu X.L., Stanford K., et al. 2013. Screening of condensed tannins from Canadian Prairie forages for anti-Escherichia coli O157:H7 with an emphasis on purple prairie clover (Dalea purpurea vent). J. Food Prot. 76:560–567. doi:10.4315/0362-028X.JFP-12-259. [DOI] [PubMed] [Google Scholar]
- Wang Y., McAllister T.A., and Acharya S.. 2015. Condensed tannins in sainfoin: composition, concentration, and effects on nutritive and feeding value of sainfoin forage. Crop Sci. 55: 13–22. [Google Scholar]
- Wong A., Ngu D.Y., Dan L.A., Ooi A., and Lim R.L.. 2015. Detection of antibiotic resistance in probiotics of dietary supplements. Nutr. J. 14:95. doi:10.1186/s12937-015-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanga Y., Wang Q., Diarra M.S., Yu H., Hua Y., and Gong J.. 2016. Functional assessment of encapsulated citral for controlling necrotic enteritis in broiler chickens. Poult Sci. 95:780–789. doi: 10.3382/ps/pev375. [DOI] [PubMed] [Google Scholar]
- Yin X., Heenev D., Srisengfa Y., Golomb B., Griffey S., and Marco M.. 2017. Bacteriocin biosynthesis contributes to the anti-inflammatory capacities of probiotic Lactobacillus plantarum. Benef. Microbes. 25: 1–12. doi: 10.3920/BM2017.0096. [DOI] [PubMed] [Google Scholar]
- Zhang Z.F., and Kim I.H.. 2014. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult. Sci. 93:364–370. doi:10.3382/ps.2013-03314. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., and Curtis N.. 2018. The influence of probiotics on vaccine responses—a systematic review. Vaccine. 36:207–213. http://dx.dor.org/10.1016/j.vaccine.2017.08.069. [DOI] [PubMed] [Google Scholar]