Abstract
Background:
The prognosis is poor for children and adolescents with recurrent osteosarcoma (OS). Glycoprotein non-metastatic B (gpNMB) is a glycoprotein highly expressed in OS cells. We conducted a Phase II study of Glembatumumab vedotin (GV), a fully human IgG2 monoclonal antibody (CR011) against gpNMB conjugated to the microtubule inhibitor, monomethyl auristatin E (MMAE).
Patients and Methods:
Patients ≥ 12 years and < 50 years with relapsed or refractory OS were eligible. GV 1.9 mg/kg/dose was administered on Day 1 of each 21 day cycle. Pharmacokinetics (PK) were mandatory in patients < 15 years. gpNMB expression was measured by immunohistochemistry. The primary endpoint was disease control at 4 months and RECIST response. A 2-stage design was used to determine efficacy.
Results:
Twenty-two patients were enrolled, and all were evaluable for response. Antibody drug conjugate levels were detectable in patients, although small numbers limit comparison to adult data. The toxicities observed were similar to the previous studies with GV. The most common Grade 3 adverse event was rash. One death from end organ failure occurred possibly related to GV. Of the twenty-two patients, 1 patient had a partial response and 2 had stable disease. There was no correlation between gpNMB expression and response to GV.
Conclusions:
GV was well tolerated in this population. Although there was some antitumor activity, the extent of disease control in Stage 1 did not meet the level required to proceed to Stage 2.
Trial registration numbers:
Keywords: Osteosarcoma, pediatrics, adolescent, young adult, clinical trial, molecular targeted therapy
Introduction
The 5-year overall survival of patients with newly diagnosed, localized osteosarcoma (OS) is 70% with multi-agent chemotherapy [1]. However, prognosis remains poor for patients with metastases or recurrent disease [2–3]. The 4-month event-free survival for patients with recurrent OS with measurable disease enrolled on previous Phase 2 studies from the Children’s Oncology Group (COG) is 12% (95% CI 619%) [4] indicating novel treatment strategies are needed.
Glycoprotein non-metastatic B (gpNMB) is a type I transmembrane glycoprotein normally expressed in a variety of cell types contributing to tissue repair, cellular adhesion, and regulation of cell growth [5]. Overexpression has been demonstrated in multiple cancers including melanoma, breast cancer and OS [5–8]. Glembatumumab Vedotin (GV) is an antibody drug conjugate (ADC) directed against gpNMB. Consisting of a fully human IgG2 monoclonal antibody (CR011) against gpNMB conjugated to the microtubule inhibitor, monomethyl auristatin E (MMAE) [5]. Three clinical trials of GV in patients with breast cancer and melanoma have been completed, and anti-tumor activity has been observed [9–11]. In pre-clinical testing, gpNMB was expressed in 92.5% of OS samples. GV induced cytotoxic effects in 74% of OS cell lines, and gpNMB protein levels correlated with GV in vitro cytotoxicity [6]. To evaluate the clinical activity of GV in OS, we conducted a single arm phase 2 trial, AOST1521, in patients with recurrent or refractory OS.
Material and methods
Eligibility
Eligible patients with recurrent or refractory OS included those ≥ 12 and < 50 years with measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [12]. Other eligibility criteria included normal renal, cardiac, and liver function along with adequate bone marrow function. Performance status corresponding to ECOG scores of 0, 1 or 2 and life expectancy ≥ 8 weeks was required. Patients were excluded for prior use of GV or other MMAE-containing products, peripheral neuropathy > Grade 2 using Common Terminology for Classifying Adverse Events (CTCAE) version 4.0, major surgery within 2 weeks of enrollment, were pregnant or breast feeding, or had brain metastasis. Archival tumor specimen from any previous biopsy/resection was required for evaluation of gpNMB expression measured by immunohistochemistry (IHC), however, documented expression was not required for enrollment. Based on accrual rates of patients with OS from three prior COG studies, ADVL0421, ADVL0524 and ADVL0525 [13–15], approximately 18 patients were expected to enroll annually. This trial was approved by the National Cancer Institute (NCI) Pediatric Central Institutional Review Board (CIRB), and by local regulatory boards at all sites not utilizing NCI Pediatric CIRB. Informed consent documentation was signed by all patients or their parent/legal guardian, and assent was obtained according to local institutional guidelines prior to enrollment.
Treatment and Response Assessment
NCI was the investigational new drug (IND) sponsor and supplied GV (Bethesda, MD). GV (1.9 mg/kg/dose) was administered as a 90-minute intravenous infusion on day one of each 21-day cycle until disease progression or intolerance. Cycles were repeated for a maximum of 14 months or 18 cycles.
Toxicity was evaluated using CTCAE version 4.0. Myeloid growth factor support for subsequent cycles was allowed for patients with Grade 4 neutropenia. Patients with continued Grade 4 neutropenia, Grade 4 thrombocytopenia or Grade >3 non-hematologic toxicity received dose reduction to 1.3 mg/kg/dose of GV in subsequent cycles. If toxicity did not resolve prior to the cycle following dose reduction, patients were removed from therapy.
Patients were evaluated for response following cycles 2, 4, and 6 and following every 3rd cycle thereafter using RECIST 1.1 [12].
To assess GV tolerability and toxicity in children, accrual of patients < 18 years was suspended after the sixth patient was enrolled until all completed cycle 1. If <1/3 of toxicity evaluable patients experienced dose limiting toxicity (DLT) during the first cycle of GV, subsequent enrolled patients would be treated at the same dose. All patients were monitored for DLT occurrence across all cycles considered evaluable for efficacy assessment. DLTs are defined in the protocol (supplementary materials).
Pharmacokinetics
Serum samples to assess pharmacokinetics (PK) were required for the first six evaluable patients < 15 years. Samples were obtained before treatment; at the end of infusion; at 1, 2, 4, 8, and 24 hours post-infusion on day 1; and 4, 7, and 21 days postinfusion. GV was quantitated in total antibody (TA) and antibody-drug conjugate (ADC) using enzyme-linked immunosorbent assays (ELISA). Free MMAE was quantified using liquid chromatography/mass spectrometry. All assays were described previously by Ott et al. [10].
Noncompartmental analysis was performed with WinNonlin 7.0 (Certara USA, Inc.) using nominal dosing for ADC and TA (1.9 mg/kg), total MMAE per dose was based on molar coupling ratio in drug product, and respective molecular weights of GV (150 kD) and auristatin E (718 Da). Infusion models were used for TA and ADC and an extravascular model was used for free MMAE. Exposure (Cmax, AUC0–3w) was determined for ADC and TA by linear trapezoidal approximation. Terminal elimination half-life was determined by least squares fitting of experimental concentrations in the terminal phase for TA and ADC analytes, as well as for free MMAE.
gpNMB Immunohistochemistry
Retrospective analysis of gpNMB expression in archived tumor specimens was conducted at a central laboratory (Mosaic Laboratories, Lake Forest, CA). gpNMB expression was assessed by IHC using a biotinylated CR011 monoclonal antibody. Stained slides were manually reviewed by a Mosaic pathologist using a standard bright field microscope. Maximum intensity of staining was graded on the following scale:1 + (weak), 2+ (moderate), 3+(strong).
Statistical Considerations
Primary endpoint included two outcomes: (1) RECIST response according to RECIST v 1.1 [12]; and (2) four-month disease control. Eligible patients who received at least one dose of GV were considered evaluable for response, unless the patient received non-protocol therapy after first demonstrating complete response (CR) or partial response (PR), but prior to the confirmatory evaluation. Any evaluable patient who demonstrated a RECIST-defined CR or PR before the end of the sixth cycle of therapy was considered a RECIST responder. Disease control evaluable patients who did not demonstrate RECIST-defined disease progression through six cycles of protocol therapy, or four months after study enrollment if GV therapy was stopped prior to the sixth cycle for any reason, were considered a disease control success. Otherwise, the patient was considered a disease control failure. Four-month disease control was selected based on Lagmay et al. [4].
Patients not evaluable for RECIST response or disease control could be replaced for the application of the statistical rule. Patients evaluable for response but not disease control were considered not to have experienced disease control. The definitions above did not provide for a patient to be evaluable for disease control but not RECIST-response.
The study had two stages. Nineteen outcome evaluable patients would be enrolled. If four or fewer disease control successes and one or fewer RECIST responses were observed, the study would be stopped, concluding GV was not associated with sufficient activity for further evaluation. Otherwise, an additional 10 outcome evaluable patients would be enrolled (AOST1521 protocol found in supplementary materials).
Enrollment involved two groups. Stratum 1 included patients who had not received eribulin prior to enrollment and stratum 2 included patients who had received eribulin prior to enrollment, thus minimizing potential cross resistance that could develop from prior microtubule inhibitor exposure e.g. eribulin. The efficacy of GV would be evaluated according to the design stated above for patients enrolled in stratum 1. Stratum 2 enrollment would be stopped when the evaluation of stratum 1 was complete or when sufficient data were obtained to fully evaluate GV in stratum 2, whichever occurred first. If the adult dose of GV was considered tolerable in patients < 18 years, those patients were included in the stratum indicated by the patient’s history of eribulin treatment prior to enrollment.
By study design, the relationship between gpNMB expression measured by IHC and the probability of 4-month disease control was assessed only in patients without prior exposure to eribulin. The hypothesis of no association between strength of IHC expression and probability of disease control was assessed by the exact conditional test of proportions. The hypothesis of a linear trend was assessed using logistic regression with IHC reading taken as a continuous variable [16].
Results
Patient Characteristics
Twenty-two adolescents and young adults (AYA) were enrolled (3 with prior eribulin therapy) at 17 centers throughout the United States and Canada between February 2016 to August 2016. As all patients had completed therapy, data current to June 2017 were used in this analysis. Enrollment occurred more rapidly than anticipated; Stage I completed within six months. Seven patients < 18 years were enrolled instead of the planned six due to a data entry error in a patient’s date of birth. The patient was allowed to remain on study. Median age of enrollment was 20 years. All patients were evaluable for response, and the median number of RECIST lesions was 2 (range 1 – 5). Lung and bone were the most common sites of disease (Table 1 and Supplementary Table 1).
Table 1.
Patient Characteristics Of 22 Patients Enrolled On AOST1521
| Characteristic | ||
|---|---|---|
| Age in Years at Study Enrollment | Mean | 20 Years |
| Median | 20 Years | |
| Range | 12 Years – 31 Years | |
| Number ≥ 18 Years | 15 | |
| Patient Sex | Number Male | 15 |
| Number Female | 7 | |
| Race | Number White | 20 |
| Number African-American | 2 | |
| Number American Indian, Aleutian or Eskimo | 0 | |
| Ethnicity | Number Hispanic | 3 |
| Number Not Hispanic | 19 | |
| Number of Lesions Measured for RECIST Evaluation | Mean | 2.5 |
| Median | 2 | |
| Range | 1 – 5 | |
| Tumor Burden of largest lesion in millimeters | Mean | 35.1 |
| Median | 33 | |
| Range | 10 – 146 | |
| Sites of Measurable Disease | ||
| Lung | 10 | |
| Bone | 3 | |
| Lung + Bone | 2 | |
| Lung + Other | 6 | |
| Lung + Bone + Other | 1 |
Treatment Tolerability
GV was well tolerated. The most common Grade 3 adverse events included rash and hypokalemia (Table 2). One of the seven patients < 18 years had a DLT, Grade 3 anaphylaxis, during the first cycle. GV administered at 1.9 mg/kg was considered tolerable in younger patients. DLTs occurred in six of the sixty-one cycles in 6 patients, indicating no excessive rate of DLT (Supplementary Table 2). One patient death occurred during cycle 1 at day 16 following therapy.
Table 2.
Adverse Event (AE) Count During the 61 Cycles of Chemotherapy Administered to 22 patients on AOST1521.
| Patient Cycles (N=61) | |
|---|---|
| Hematologic (AE Grade 4 or greater) | n (%) |
| Neutrophil count decreased | 2 (3.3) |
| Platelet count decreased | 1 (1.6) |
| White blood cell decreased | 1 (1.6) |
| Non-Hematologic (AE Grade 3 or greater) | |
| Abdominal pain | 2 (3.3) |
| Anaphylaxis | 1 (1.6) |
| Anorexia | 1 (1.6) |
| Back pain | 2 (3.3) |
| Constipation | 1 (1.6) |
| Febrile neutropenia | 1 (1.6) |
| Headache | 1 (1.6) |
| Hypertension | 1 (1.6) |
| Hypocalcemia | 1 (1.6) |
| Hypokalemia | 4 (6.6) |
| Hypophosphatemia | 2 (3.3) |
| Hypotension | 1 (1.6) |
| Infections and infestations - Other, specify: UTI | 1 (1.6) |
| Mucositis oral | 2 (3.3) |
| Myalgia | 1 (1.6) |
| Nausea | 1 (1.6) |
| Neoplasms benign, malignant and unspecified* | 1 (1.6) |
| Pain | 2 (3.3) |
| Pain in extremity | 1 (1.6) |
| Peripheral sensory neuropathy | 1 (1.6) |
| Pneumothorax | 1 (1.6) |
| Rash acneiform | 1 (1.6) |
| Rash maculo-papular | 6 (9.8) |
| Respiratory failure | 1 (1.6) |
| Somnolence | 1 (1.6) |
the term neoplasm is describing progressive osteosarcoma
Pharmacokinetics
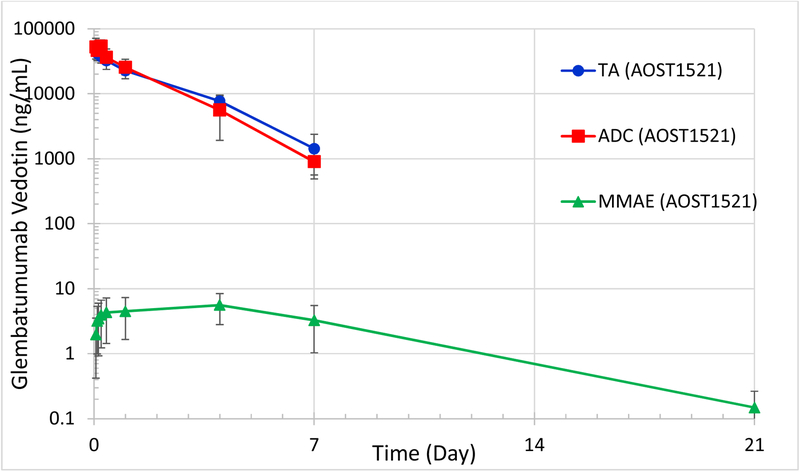
GV PK were studied in four patients 12–14 years (Table 3). TA and ADC were detectable through 4–7 days, while free MMAE was detectable 7–21 days following infusion. The half-life of TA ranged from 28.6–41.1 hours, and ADC half-life ranged from 17.3–36.8 hours. Maximum free unconjugated MMAE levels occurred 4 days after dosing, ranging from 2.4–8.0 ng/mL (Figure 1).
Table 3.
Summary Of Glembatumumab Vedotin Pharmacokinetics In Patients 12 – 14 Years Of Age
| n | Cmax (μg/mL) | Tmax (hr) | T1/2 (hr) | AUC0–3w (hr-μg/mL) | Vz (mL/kg) | Cl (mL/hr/kg) | |
|---|---|---|---|---|---|---|---|
| TA | 4 | 48.8 ± 10.0 | 1.8 ± 0.5 | 35.2 ± 6.4 | 2319 ± 334 | 42.5 ± 11.7 | 0.8 ± 0.1 |
| ADC | 4 | 59.8 ± 16.3 | 3.3 ± 1.7 | 29.1 ± 8.4 | 2260 ± 750 | 37.9 ± 16.3 | 0.9 ± 0.3 |
| MMAE | 4 | 0.006 ± 0.003 | 96 ± 0.0 | 62.6 ± 14.6 | 1.28 ± 0.88 | 42,802 ± 28,786 | 533 ± 468 |
TA – total antibody; ADC – antibody drug conjugate; MMAE - monomethyl auristatin E; n – number; Cmax – maximum concentration; T1/2 – half life; AUC – area under the curve; Vz – volume of distribution; Cl – clearance
Figure 1.
Glembatumumab Vedotin Exposure Among Pediatric Osteosarcoma Patients (n=4) enrolled in AOST1521.
TA = total antibody, ADC = Antibody Drug Conjugate, MMAE = monomethyl auristatin E Serum Concentrations are represented as arithmetic mean (n=4) with y-axis in log-base-10 scale. Error bars represent +/− Standard Deviation.
Disease Control, Response Rate
Nineteen patients were considered in the analysis of disease control and response rate. One patient demonstrated both 4-month disease control and a RECIST PR. Two patients achieved the primary outcome measure of 4-month disease control without RECIST response. The activity level was inadequate to proceed to stage 2 based on our trial design.
gpNMB Expression
Tumor samples were submitted for 21 of 22 patients for gpNMB expression. IHC analysis was completed on all specimens with H-scores ranging from 0–3+. Fifteen (68%) specimens expressed a score of 3+ indicating strong gpNMB expression. The patient with a RECIST PR and one of the two patients with 4-month disease control response both had score of 3+. The other patient with 4-month disease control had an score of 1+. There was no correlation between gpNMB expression and disease control at 4 months (P=0.68). The odds ratio for disease control success for non-eribulin patients with at most 2+ staining compared with 3+ staining was 0.91 (95% CI, .66 to 13) (Supplementary Table 3).
Discussion
Our study was conducted in refractory or relapsed heavily pre-treated adolescents and young adults with osteosarcoma reflecting current FDA draft guidance that adolescents 12 – 17 years be eligible for enrollment in adult oncology clinical trials at all stages of drug development when the histology and biologic behavior of the cancer under investigation is the same in, or the molecular target of the drug is relevant to, cancers in both adult and adolescent patients [17]. While some antitumor activity was observed in stage 1, it did not meet criteria to initiate stage 2. However, one patient had a RECIST response. In contrast, five previous COG phase 2 trials enrolling patients with recurrent OS with a primary endpoint of RECIST response, had no observable RECIST responses [4] and the recently completed COG clinical trial AOST1322 utilizing eribulin in patients with relapsed OS exhibited zero responses to therapy [18]. Additionally, one patient in the current study had prolonged stable disease receiving all 18 cycles of GV. This indicates GV has activity in some patients with OS and further study of GV could be considered if a biomarker predicting benefit were identified.
In preclinical experiments using patient derived xenograft (PDX) models of osteosarcoma, IHC testing showed expression of gpNMB (1+ to 3+) in six out of seven samples. These six PDX models received GV and three maintained a CR and had IHC 2+ to 3+ for gpNMB expression [8]. Despite fifteen of our patient samples expressing high levels (IHC 3+) of gpNMB only two met the disease control endpoint. Further, one patient with prolonged stable disease had IHC 1+, suggesting IHC expression of gpNMB did not predict response.
There are multiple possible explanations for discrepancy between pre-clinical evidence and our trial results. Microtubule inhibitors may not be as active in OS. There may be differential competition for GV between non-tumor GPNMB in PDX models versus humans as a result of different affinities for human versus mouse GPNMB. This may be inferred from the longer half-life observed in PDX models versus those observed in clinical trials [10, 19]. PDX models may not represent the high likelihood of tumor heterogeneity in patients that have already received multiple forms of systemic therapy and gpNMB expression in archival tissue may be not a reliable predictor of OS tumor response. Recent research has identified alternatives to difficult to obtain biospecimens for biomarker studies such as serum microRNAs, long non-coding RNAs, circulating tumor cells and circulating tumor DNA [20–22]. In future OS clinical trials, it would be ideal to evaluate blood-based assays for their ability to serve as biomarkers to facilitate patient selection.
Toxicities with GV were as expected and similar to previous GV studies [9–11]. Our patients < 18 years tolerated GV well and if Stage 2 had proceeded, enrollment would have been opened again to younger patients. The patient who died had advanced metastatic disease prior to enrollment including lung involvement and a disease-related pericardial effusion. The patient received colchicine for pericardial effusion treatment prior to starting protocol therapy and developed, neutropenic fever, mucositis, and acute kidney injury following GV. Cause of death was end organ failure and GV was considered to be a contributing factor. As both colchicine and GV are microtubule inhibitors there may have been an interaction between the two drugs.
PK data was minimal and comparison of concentration versus time profiles against historical data from the EMERGE trial in breast cancer [9] indicate similar exposure of components (Supplementary Figure 1), however the small population size makes direct comparisons difficult.
Along with signs of early activity, another positive outcome of this study was a more rapid than predicted patient accrual. Additionally, the median age of enrollment was 20 years, demonstrating our success in enrolling AYA patients ≥ 18 years to a trial led by the COG. This is encouraging as traditionally AYAs have poor representation on clinical trials [23–24].
Conclusions
Although our trial did not meet sufficient disease control in Stage I to proceed to Stage 2, some patients had meaningful responses, indicating there may be a role for GV in OS. In order to determine which patients with OS will benefit, further exploration of predictive biomarkers would need to be done.
Supplementary Material
Highlights.
Glembatumumab vedotin was well tolerated with no unexpected toxicities in adolescents and young adults with recurrent osteosarcoma
AOST1521 successfully and rapidly enrolled adolescents and young adults (median age 20 years) with recurrent osteosarcoma
Although AOST1521 did not meet sufficient disease control in Stage I to proceed to Stage 2, some patients had meaningful responses, indicating there may be a role for Glembatumumab Vedotin in patients with Osteosarcoma
Acknowledgements
We are grateful to all the adolescents and young adults and their families that we have the privilege of caring for who participated in our study. Also, we are very thankful to the study teams at the participating centers as well as NIH/CTEP, Celldex and Mosaic Laboratories.
This work was supported by the National Clinical Trials Network Operations Center Grant, the National Clinical Trials Network Statistics and Data Center Grant and the St. Baldrick’s Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Lisa M. Kopp is an employee of the contract research organization Covance. Mark Krailo is a consultant for Merck. Katherine A. Janeway has received travel reimbursement from Loxo oncology. Richard Gorlick has received laboratory research funding from Eisai. Thomas Hawthorne and Elizabeth Crowley are employees of Celldex. Remaining authors report no conflicts of interest.
References:
- [1].Ward E, et al. , Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin, 2014. 64(2): p. 83–103. [DOI] [PubMed] [Google Scholar]
- [2].Meyers PA, et al. , Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol, 1993. 11(3): p. 449–53. [DOI] [PubMed] [Google Scholar]
- [3].Bielack SS, et al. , Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol, 2002. 20(3): p. 776–90. [DOI] [PubMed] [Google Scholar]
- [4].Lagmay JP, et al. , Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol, 2016. 34(25): p. 3031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rose AAN, et al. , Targeting GPNMB with glembatumumab vedotin: Current developments and future opportunities for the treatment of cancer. Pharmacol Ther, 2017. [DOI] [PubMed] [Google Scholar]
- [6].Roth M, et al. , Targeting Glycoprotein NMB With Antibody-Drug Conjugate, Glembatumumab Vedotin, for the Treatment of Osteosarcoma. Pediatr Blood Cancer, 2016. 63(1): p. 32–8. [DOI] [PubMed] [Google Scholar]
- [7].Maric G, et al. , Glycoprotein non-metastatic b (GPNMB): A metastatic mediator and emerging therapeutic target in cancer. Onco Targets Ther, 2013. 6: p. 839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kolb EA, et al. , Initial testing (stage 1) of glembatumumab vedotin (CDX-011) by the pediatric preclinical testing program. Pediatr Blood Cancer, 2014. 61(10):p. 1816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yardley DA, et al. , EMERGE: A Randomized Phase II Study of the Antibody-Drug Conjugate Glembatumumab Vedotin in Advanced Glycoprotein NMB-Expressing Breast Cancer. J Clin Oncol, 2015. 33(14): p. 1609–19. [DOI] [PubMed] [Google Scholar]
- [10].Ott PA, et al. , Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma. J Clin Oncol, 2014. 32(32): p. 3659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bendell J, et al. , Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J Clin Oncol, 2014. 32(32): p. 3619–25. [DOI] [PubMed] [Google Scholar]
- [12].Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- [13].Beaty O 3rd, et al. , A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer, 2010. 55(3): p. 440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jacobs S, et al. , Phase II trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: a report from the children’s oncology group. Clin Cancer Res, 2010. 16(2): p. 750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Warwick AB, et al. , Phase 2 trial of pemetrexed in children and adolescents with refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer, 2013. 60(2): p. 237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bishop Y, Fienberg S, and Holland P, Discrete Multivariate Analysis: Theory and Practice 1 ed Discrete Multivariate Analysis: Theory and Practice. 1975:The MIT Press; 551. [Google Scholar]
- [17].National, Cancer, and Institute. Protocol Templates and Guidelines 2018. 11/23/18 [cited January 2019; Available from https://ctep.cancer.gov/protocoldevelopment/templates_applications.htm.
- [18].Isakoff MS, et al. , A phase II study of eribulin in recurrent or refractory osteosarcoma: A report from the Children’s Oncology Group. Pediatr Blood Cancer, 2019. 66(2): p. e27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pollack VA, et al. , Treatment parameters modulating regression of human melanoma xenografts by an antibody-drug conjugate (CR011-vcMMAE) targeting GPNMB. Cancer Chemother Pharmacol, 2007. 60(3): p. 423–35. [DOI] [PubMed] [Google Scholar]
- [20].Bishop MW, Janeway KA, and Gorlick R, Future directions in the treatment of osteosarcoma. Curr Opin Pediatr, 2016. 28(1): p. 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Evola FR, et al. , Biomarkers of Osteosarcoma, Chondrosarcoma, and Ewing Sarcoma. Front Pharmacol, 2017. 8: p. 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Raimondi L, et al. , Circulating biomarkers in osteosarcoma: new translational tools for diagnosis and treatment. Oncotarget, 2017. 8(59): p. 100831–100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Davis LE, et al. , Clinical trial enrollment of adolescents and young adults with sarcoma. Cancer, 2017. 123(18): p. 3434–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keegan TH, et al. , Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer, 2016. 122(7): p. 1009–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.