Abstract
Background:
Estrogen signaling is essential for the sexual dimorphism of the skeleton, is required for normal bone remodeling balance in adults, and may influence the skeletal response to alcohol. High levels of alcohol consumption lower bone mass in ovary-intact but not ovariectomized (ovx) rats. However, the extremely rapid rate of bone loss immediately following ovx may obscure the effects of alcohol. We therefore determined (1) whether heavy alcohol consumption (35% caloric intake) influences bone in sexually mature ovx rats with established cancellous osteopenia, and (2) whether ICI 182,780 (ICI), a potent estrogen receptor-signaling antagonist, alters the skeletal response to alcohol.
Methods:
Three weeks following ovx, rats were randomized into five groups: (1) baseline, (2) control+vehicle, (3) control+ICI, (4) ethanol+vehicle, or (5) ethanol+ICI and treated accordingly for four weeks. Dual energy X-ray absorptiometry, micro-computed tomography, blood measurements of markers of bone turnover, and gene expression in femur and uterus were used to evaluate response to alcohol and ICI.
Results:
Rats consuming alcohol had lower bone mass and increased fat mass. Bone microarchitecture of the tibia and gene expression in femur were altered; specifically, there was reduced accrual of cortical bone, net loss of cancellous bone, and differential expression of 19/84 genes related to bone turnover. Furthermore, osteocalcin, a marker of bone turnover, was lower in alcohol-fed rats. ICI had no effect on weight gain, body composition, or cortical bone. ICI reduced cancellous bone loss and serum CTX-1, a biochemical marker of bone resorption; alcohol antagonized the latter two responses. Neither alcohol nor ICI affected uterine weight or gene expression.
Conclusion:
Alcohol exaggerated bone loss in ovx rats in the presence or absence of estrogen receptor blockade with ICI. The negligible effect of alcohol on uterus and limited effects of ICI on bone in alcohol-fed ovx rats suggests that estrogen receptor signaling plays a limited role in the action of alcohol on bone in a rat model for chronic alcohol abuse.
Keywords: Chronic alcohol abuse, osteoporosis, estrogen signaling, bone architecture, rat
Introduction
Alcohol consumption has context and dose-dependent effects on bone metabolism. In women, chronic alcohol abuse is associated with osteopenia and increased fracture risk (Gaddini et al., 2016). However, it has proven difficult to separate the specific effects of alcohol from the contributing effects of co-morbidity factors, such as smoking, poor nutrition and disease, often present in chronic alcohol abusers. These factors are controlled for in animal models (Gaddini et al., 2016). Alcohol levels similar to those experienced by alcohol abusers (Savola et al., 2004) slow bone accrual in growing rats and induce bone loss in skeletally mature rats (Hogan et al., 2001, Turner et al., 2001b, Sampson et al., 1996).
The mechanisms of action of alcohol on the skeleton are likely multifactorial. Bone metabolism is under tight endocrine control and there is evidence that alcohol is a nonspecific endocrine disrupter (Gaddini et al., 2016, Ronis et al., 2007). One of the hormones implicated as a target of alcohol action is estrogen (Turner and Sibonga, 2001, Chen et al., 2009). Estrogen is an important endocrine regulator of bone growth, where it plays an essential role in the sexual dimorphism of the skeleton. Estrogen is also critical for maintenance of bone remodeling balance in adults (Turner et al., 1994). The importance of this hormone for maintaining remodeling balance is illustrated by the rapid loss of cancellous bone following menopause in women and following ovariectomy (ovx) in skeletally mature rats (Turner et al., 1994).
Alcohol consumption could influence estrogen status by multiple non-mutually exclusive mechanisms, including (1) altered synthesis or metabolism of estrogen, leading to changes in circulating levels of the hormone or (2) changes in estrogen receptor signaling in estrogen target cells, altering the sensitivity of bone to the hormone (Kimble, 1997, Turner and Sibonga, 2001). There is evidence for both possibilities. Alcohol increased aromatase activity in rat liver and increased estrogen levels in some studies evaluating dietary alcohol in women (Chung, 1990, Purohit, 2000). In addition, in vitro studies suggest that high concentrations of alcohol increase estrogen receptor levels in cultured osteosarcoma cells (Chen et al., 2009).
In vivo, alcohol resulted in a dose-dependent reduction in bone turnover and at high levels resulted in bone loss in skeletally mature ovary-intact rats (Turner et al., 2001b). Pharmacological treatment with estrogen prevents osteopenia in growing rats during intragastric infusion of alcohol (Chen et al., 2006) and the detrimental skeletal response to alcohol is greater in cycling than pregnant rats (Shankar et al., 2006). These findings suggest that estrogen antagonizes the detrimental skeletal effects of alcohol. Interestingly, high levels of alcohol consumption did not alter the magnitude of bone loss following ovx (Kidder and Turner, 1998), whereas, intraperitoneal administration of alcohol resulted in additional bone loss (Callaci et al., 2006). The rate of bone loss after ovx is much more rapid than the rate of loss resulting from consuming high levels of alcohol (Hogan et al., 2001, Wronski et al., 1988). Therefore, it is possible that the effects of dietary alcohol on bone were obscured by the rapid bone loss occurring immediately following ovx. We evaluated this possibility in the present study by delaying alcohol treatment until ovx-induced osteopenia was well established and the rate of bone loss substantially diminished. We also evaluated the contribution of estrogen receptor signaling to a skeletal response by blockading signaling with the antiestrogen ICI 182,780 (ICI) (Turner et al., 2017). Specifically, we determined (1) whether heavy alcohol consumption influences whole body bone mass, density and turnover, and tibia microarchitecture in ovx rats subsequent to the initial rapid bone loss phase, and (2) whether ICI influences the skeletal response to alcohol.
Materials and Methods
Fifty 8-week-old ovx and ten 8-week-old ovary-intact Sprague Dawley rats were obtained from Harlan (Indianapolis, IN) and housed in plastic shoebox cages (1 rat/cage) in a temperature- and humidity-controlled room with a 12/12 hour light/dark cycle. Additional ovary-intact and ovx rats (n=5/group) were obtained from Harlan (Indianapolis, IN) and sacrificed 2 weeks following surgery to determine the effects of ovx on gene expression in uterus and femur. Animal care followed the guidelines found in the Guide for Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee at Oregon State University approved the animal protocol.
Experimental Design for Alcohol Study
After a 2-week period of acclimation (3 weeks following ovariectomy), the rats were randomized by body weight into one of five groups (n = 10/group): (1) baseline, (2) control+ vehicle, (3) control + ICI, (4) ethanol (EtOH) + vehicle, (5) EtOH + ICI and baseline animals sacrificed. Age-matched asynchronous baseline sham controls (n =10) were included as a reference group in order to confirm that ovx resulted in cancellous bone loss prior to initiation of ethanol feeding. Rats in the ethanol-fed groups were adapted to a liquid ethanol diet over a 1-week period, as recommended by the manufacturer (Lieber-DeCarli liquid diet, #F1258SP, Bio-Serve, Frenchtown, NJ). At the end of the adaptation period, 35% of caloric intake in these rats was derived from ethanol. Control rats consumed an isocaloric liquid diet with calories from ethanol being replaced with maltose-dextran (BioServe, #F1259SP). The liquid diet contained 1.36% Ca, 1.06% P and 0.13% Mg (wt/v). Control rats were pair fed to ethanol-fed rats to control for ethanol-induced reduction in food consumption. Subcutaneous injection of ICI (Sigma-Aldrich, St. Louis, MO) in sesame oil (0.1 ml/d; 1.5 mg/kg/d) or vehicle was started one day after completion of adaptation of rats to their liquid diets. Injections were then administered once a day for the duration of the experiment. The rats were weighed weekly and maintained on their respective treatment for 3 weeks.
Dual Energy X-ray Absorptiometry
Prior to necropsy, rats were anesthetized with isoflurane delivered in oxygen and lean mass (g), fat mass (g), percent fat (%), total body bone mineral content (BMC, g), total body bone area (cm2), and total body bone mineral density (BMD, g/cm2) were measured using dual-energy X-ray absorptiometry (DXA, Hologic QDR-4500A Elite, Waltham, MA) equipped with small animal software. Bone area, BMC and BMD were also measured ex vivo (Piximus 2, Lunar Corporation, Madison, WI) in individual tibiae.
Tissue Collection
Death in anesthetized rats was induced by exsanguination from the heart, followed by cardiac excision. Abdominal white adipose tissue (WAT) and uteri were excised, weighed and the latter stored in RNAlater prior to extraction and gene expression analysis. Tibiae were excised and placed in 70% ethanol for DXA and micro-computed tomography (μCT) evaluation. Femora were excised, snap frozen in liquid N2, and stored at −80°C until analyzed.
μCT Analysis
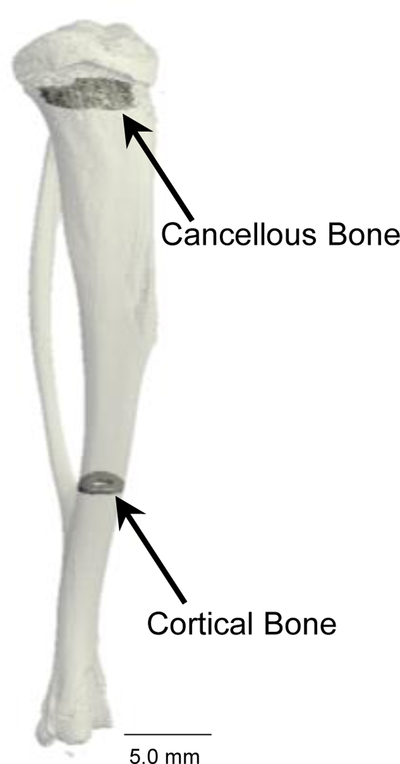
μCT was used to measure cancellous and cortical bone architecture in response to treatment. The proximal tibia and distal tibia (vicinity of tibio-fibular junction) were scanned at a voxel size of 16 × 16 × 16 μm using a Scanco Medical μCT 40 scanner (Scanco Medical AG, Basserdorf, Switzerland). The threshold for analysis was determined empirically and set at 245 (0–1,000 range). Tibia length was measured in ScoutView as the distance between the intercondyloid eminence and the medial malleolus. Fifty slices (800 μm) of cancellous bone were analyzed in the tibial metaphysis and 10 slices (160 μm) 1 mm proximal to the tibio-fibular junction were analyzed in the tibial diaphysis (Figure 1). Cancellous bone measurements included cancellous bone volume fraction (bone volume/tissue volume, %), trabecular number (mm−1), trabecular spacing (μm), and trabecular thickness (μm). Cortical bone measurements included cross-sectional volume (cortical volume + marrow volume, mm3), cortical bone volume (mm3), marrow volume (mm3), and cortical thickness (μm). Polar moment of inertia (Ipolar, mm4) was calculated as a surrogate measure of bone strength in torsion.
Figure 1.
μCT image of tibia showing the regions of interest evaluated in the proximal tibia metaphysis (cancellous bone) and distal tibia diaphysis (cortical bone).
Serum ethanol and markers of bone turnover
Serum ethanol was measured at necropsy as described (Gaddini et al., 2015). Serum CTX-1 and osteocalcin were measured using standard ELISA. CTX-1 was quantified using Rat CTX-1 ELISA kit (Novatein Biosciences, Woburn, MA). Osteocalcin was quantified using Rat Gla-Osteoalcin High Sensitive EIA kit (Takara Bio, Mountain View, CA).
Gene expression
Expression levels of a panel of 4 estrogen-regulated genes (Esr1, Esr2, Igf1 and Pgr) in uterus of rats were evaluated in the alcohol study as described (Gingery et al., 2017). For reference, the expression levels of the same genes were measured in age-matched ovary intact and ovx rats.
Total RNA was isolated from whole femur as described (Tennant et al., 2017). cDNA was prepared using SuperScript 277 III First-Strand Synthesis SuperMix for qRT-PCR (ThermoFisher). The expression of 84 gene were determined using a pathway focused RT² Profiler™ PCR Array (Rat Osteoporosis Array – PARN-170Z) according to the manufacturer’s protocol (Qiagen, Carlsbad, CA). Gene expression was normalized to Gapdh and relative quantification was determined using the ΔΔCt method and RT2 Profiler PCR Array Data Analysis software version 3.5 (Qiagen).
Statistical Analysis
The main experiment was performed according to a 2 × 2 factorial design with categorical variables for treatment group (ethanol and control) and estrogen receptor blockade (ICI and vehicle). Two-factor analysis of variance with an interaction between treatment and estrogen receptor blockade was used to compare mean values. T-tests were used to compare the baseline group to the control + vehicle group or ovary intact group to ovx group (uterine gene expression). Goodness of fit was evaluated based on Levene’s test for homogeneity of variance, plots of residuals versus fitted values, normal quantile plots, and Anderson-Darling tests of normality. The Benjamini and Hochberg method (Benjamini and Hochberg, 1995) for maintaining the false discovery rate at 5% was used to adjust for multiple comparisons. Differences were considered significant at p ≤ 0.05. All data are presented as mean ± SE. Data analysis was performed using R version 3.4.3.
Results
Body Composition
The effects of ethanol and ICI on body composition are shown in Table 1. Starting weights did not differ among treatment groups (data not shown). Compared to 11-week-old baseline rats, 15-week-old vehicle-treated control rats had higher body weight due to increased lean mass and fat mass (total, percent fat, and abdominal white adipose tissue weight). Uterine weight was lower in the vehicle-treated rats compared to baseline rats. Total body bone area, BMC, and BMD were all higher in vehicle-treated rats compared to baseline rats. Despite pair-feeding, ethanol resulted in lower body weight due to lower lean mass and lower abdominal adipose tissue weight. Moreover, total fat mass and percent fat were greater in ethanol rats. Significant differences in uterine weight were not detected with ethanol treatment. Ethanol resulted in lower total body bone area and BMC. However, significant differences in BMD were not detected with ethanol treatment. ICI had no effect on body weight or body composition and no ethanol x ICI interactions were noted for any of the endpoints evaluated.
Table 1.
Effects of ethanol and ICI 182,780 on body composition in overiectomized rate.
| Control | EtOH | ANOVA P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | Baseline | Veh | ICI | Veh | ICI | EtOH | ICI | Interaction |
| Terminal body weight (g) | 234 ± 2 | 300 ± 2* | 297 ± 3 | 278 ± 3 | 276 ± 5 | 0.000 | 0.654 | 0.924 |
| Abdominal WAT weight (g) | 1.3 ± 0.2 | 5.0 ± 0.3* | 4.9 ± 0.3 | 3.7 ± 0.3 | 4.1 ± 0.5 | 0.009 | 0.681 | 0.639 |
| Uterine weight (mg) | 122 ± 8 | 93 ± 7* | 85 ± 2 | 83 ± 3 | 80 ± 4 | 0.204 | 0.339 | 0.679 |
| Densitometry (total body) | ||||||||
| Lean mass (g) | 230 ± 2 | 279 ± 3* | 278 ± 4 | 248 ± 2 | 251 ± 3 | 0.000 | 0.924 | 0.661 |
| Fat mass (g) | 19 ± 1 | 30 ± 1* | 30 ± 1 | 39 ± 1 | 38 ± 2 | 0.000 | 0.906 | 0.724 |
| Percent fat | 7.4 ± 0.3 | 9.3 ± 0.4* | 9.5 ± 0.3 | 13.2 ± 0.4 | 12.8 ± 0.7 | 0.000 | 0.876 | 0.654 |
| Bone area (cm2) | 44.97 ± 0.38 | 56.01 ± 0.67* | 55.20 ± 0.48 | 50.98 ± 0.45 | 51.37 ± 0.56 | 0.000 | 0.826 | 0.482 |
| BMC (g) | 6.88 ± 0.08 | 8.84 ± 0.11* | 8.81 ± 0.11 | 8.18 ± 0.10 | 8.16 ± 0.13 | 0.000 | 0.903 | 0.990 |
| BMD (g/cm2) | 0.153 ± 0.001 | 0.158 ± 0.001* | 0.160 ± 0.001 | 0.161 ± 0.001 | 0.159 ± 0.002 | 0.574 | 0.985 | 0.348 |
Data are mean ± SE, n = 9 – 10/group
Vehicle-treated Control different from Baseline, P<0.05
Food and ethanol consumption
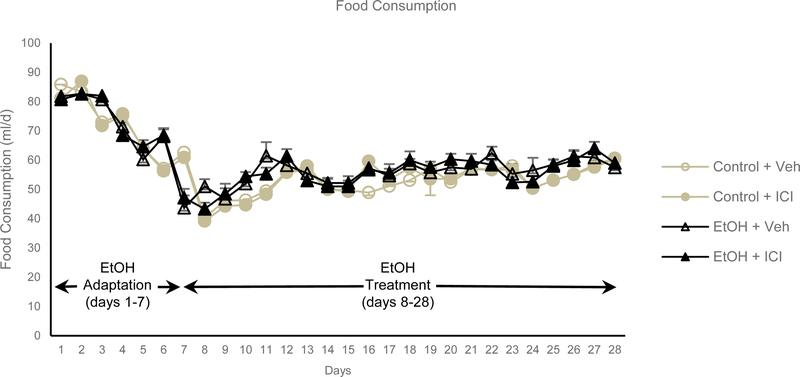
Food consumption did not differ among treatment groups (Figure 2). During the 3-week experiment, ethanol treated rats consumed 55 ml/d diet (2.8 g/d ethanol). The Lieber-DeCarli liquid diet results in blood ethanol concentrations of 0.9 ± 0.2 g/l when measured shortly after lights. At necropsy, blood ethanol levels had fallen to 0.3 ± 0.1 g/l with no difference between ethanol + veh and ethanol + ICI treatment groups.
Figure 2.
Food consumption in rats during adaptation-to-ethanol phase (days 1–7) and treatment phase (days 8–28). During the treatment phase, 35% of caloric intake was derived from ethanol. Control rats were pair fed to ethanol-fed rats. Data are mean ± SE. N = 10/group.
Tibia Mass, Density and Microarchitecture
The effects of ethanol and ICI on tibia length, area, bone mineral content, and bone mineral density are shown in Table 2. Compared with 11-week-old baseline rats, 15-week-old vehicle-treated rats had longer tibiae and higher tibial bone area, BMC and BMD. Ethanol had no effect on tibia length but resulted in lower bone area, BMC and BMD. ICI had no independent effect on tibia length, bone area, BMC or BMD and there were no ethanol x ICI interactions for any of the endpoints evaluated.
Table 2.
Effects of ethanol and ICI 182,780 on tibia length area, bone mineral content and bone mineral density in overiectomized rats.
| Control | EtOH | ANOVA P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | Baseline | Veh | ICI | Veh | ICI | EtOH | ICI | Interaction |
| Length (mm) | 37.31 ± 0.22 | 39.67 ± 0.48* | 39.39 ± 0.34 | 39.03 ± 0.19 | 39.38 ± 0.23 | 0.526 | 0.985 | 0.553 |
| Densitometry (tibia) | ||||||||
| Bone Area (cm2) | 2.01 ± 0.03 | 2.29 ± 0.03* | 2.31 ± 0.02 | 2.24 ± 0.01 | 2.22 ± 0.02 | 0.006 | 0.990 | 0.595 |
| BMC (g) | 0.227 ± 0.006 | 0.289 ± 0.004* | 0.297 ± 0.005 | 0.269 ± 0.002 | 0.268 ± 0.004 | 0.000 | 0.608 | 0.428 |
| BMD (g/cm2) | 0.113 ± 0.002 | 0.126 ± 0.001* | 0.129 ± 0.001 | 0.120 ± 0.001 | 0.121 ± 0.001 | 0.000 | 0.526 | 0.574 |
Data are mean ± SE, n = 8 – 10/group
Vehicle-treated Control different from Baseline, P<0.05
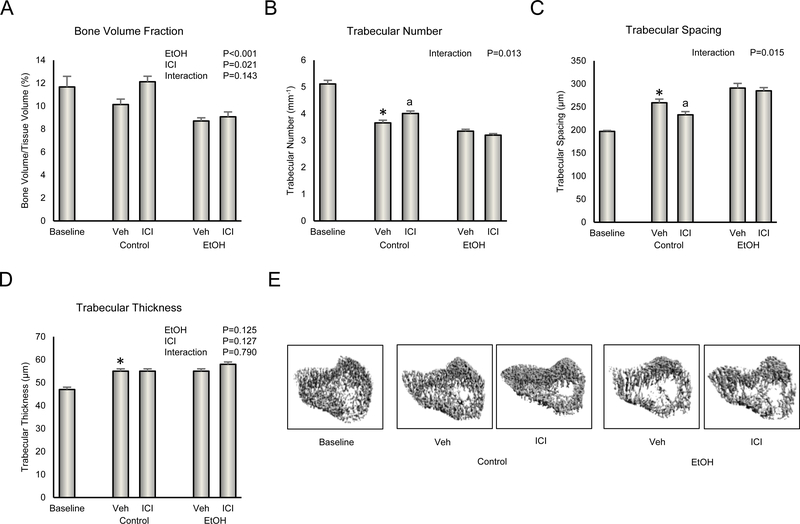
The effects of ethanol and ICI on cancellous bone architecture in proximal tibial metaphysis are shown in Figure 3. Significant differences in cancellous bone volume fraction (Figure 3A) were not detected between baseline and vehicle-treated rats. However, cancellous bone volume fraction in ovx rats at baseline (11.7 ± 0.9%) was much lower than that in asynchronous age-matched ovary-intact rats (22.2 ± 1.3%), indicating that severe cancellous osteopenia developed prior to initiation of treatment with ethanol. The vehicle-treated rats had lower trabecular number (Figure 3B), higher trabecular spacing (Figure 3C), and higher trabecular thickness (Figure 3D) than baseline rats. Administration of ethanol resulted in lower cancellous bone volume fraction while administration of ICI resulted in the opposite effect. A significant ethanol x ICI interaction was noted for trabecular number and trabecular spacing; treatment with ICI resulted in higher trabecular number and lower trabecular spacing in control but not ethanol-treated rats. Neither ethanol nor ICI had an independent effect on trabecular thickness nor was there an ethanol x ICI interaction for this endpoint. The differences in bone architecture in response to treatment can be readily appreciated in Figure 3E.
Figure 3.
Effect of ethanol and ICI 182 780 (ICI) on cancellous bone architecture in proximal tibia metaphysis: (A) cancellous bone volume fraction, (B) trabecular number, (C) trabecular spacing, and (D) trabecular thickness. Representative μCT images of cancellous bone in the proximal tibia metaphysis are shown in E. Data are mean ± SE. N = 8–10/group. *Vehicle-treated Control different from Baseline, P<0.05. aDifferent from Veh within treatment, P<0.05.
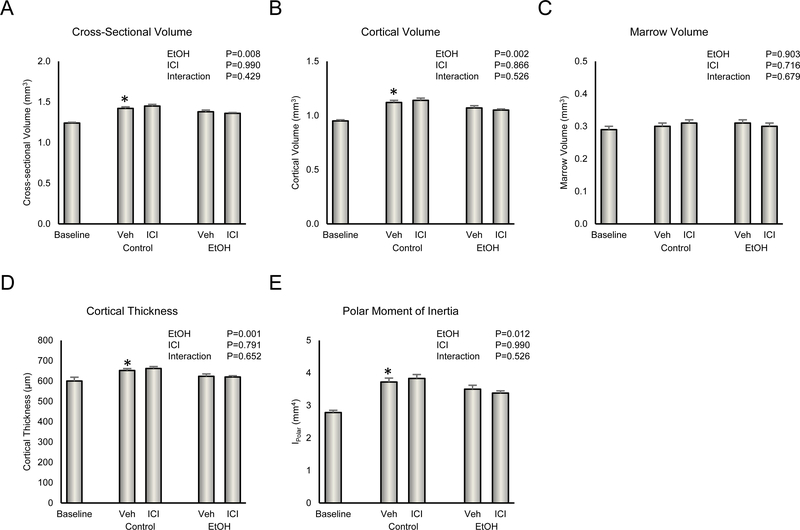
The effects of ethanol and ICI on distal tibia cortical bone architecture are shown in Figure 4. Compared with baseline, vehicle-treated rats had higher cross sectional volume (Figure 4A), cortical volume (Figure 4B), cortical thickness (Figure 4D), and polar moment of inertia (Figure 4E). Significant differences in marrow volume (Figure 4C) were not detected between the baseline and vehicle-treated rats. Administration of ethanol resulted in lower cross sectional volume, cortical volume, cortical thickness, and polar moment of inertia. ICI had no independent effect on midshaft architecture and there were no ethanol x ICI interactions for any of the endpoints evaluated.
Figure 4.
Effect of ethanol and ICI 182 780 (ICI) on cortical bone architecture in distal tibia diaphysis: (A) cross-sectional volume, (B) cortical volume, (C) marrow volume, (D) cortical thickness, and (E) polar moment of inertia. Data are mean ± SE. N = 9–10/group. *Vehicle-treated Control different from Baseline, P<0.05.
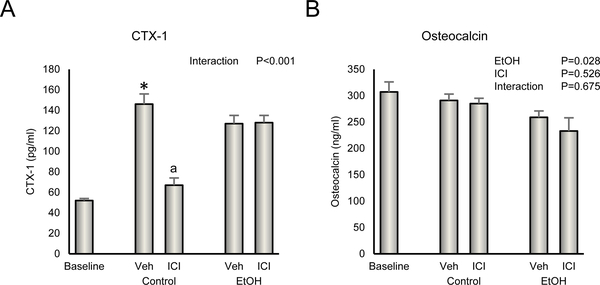
Biochemical Markers of Bone Turnover
The effects of ethanol and ICI on serum biochemical markers of bone turnover are shown in Figure 5. Vehicle-treated rats had higher levels of CTX (Figure 5A) than baseline rats. Significant differences in osteocalcin (Figure 5B) were not detected between baseline and vehicle-treated rats. A significant ethanol x ICI interaction was noted for CTX; treatment with ICI resulted in lower CTX levels in control but not ethanol-treated animals. Administration of ethanol resulted in lower levels of osteocalcin. ICI had no independent effect on serum osteocalcin and no ethanol x ICI interaction was noted.
Figure 5.
Effect of ethanol and ICI 182 780 (ICI) on (A) plasma CTX-1, a marker of global bone resorption and (B) plasma osteocalcin, a marker of global bone formation. Data are mean ± SE. N = 10/group. *Vehicle-treated Control different from Baseline, P<0.05. aDifferent from Veh within treatment, P<0.05.
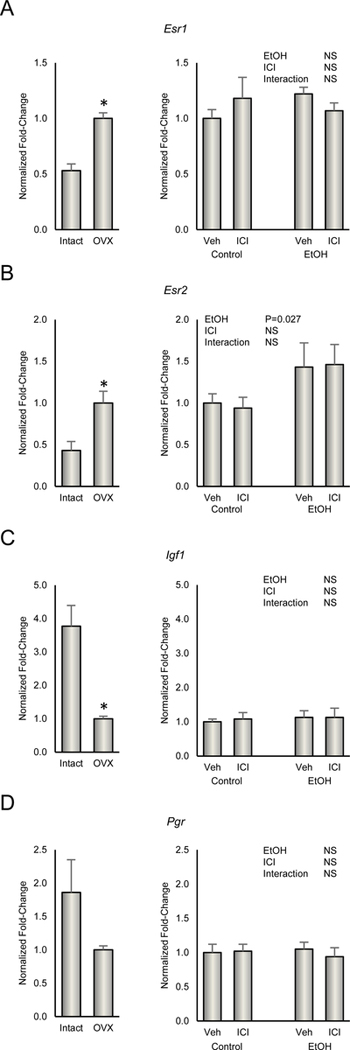
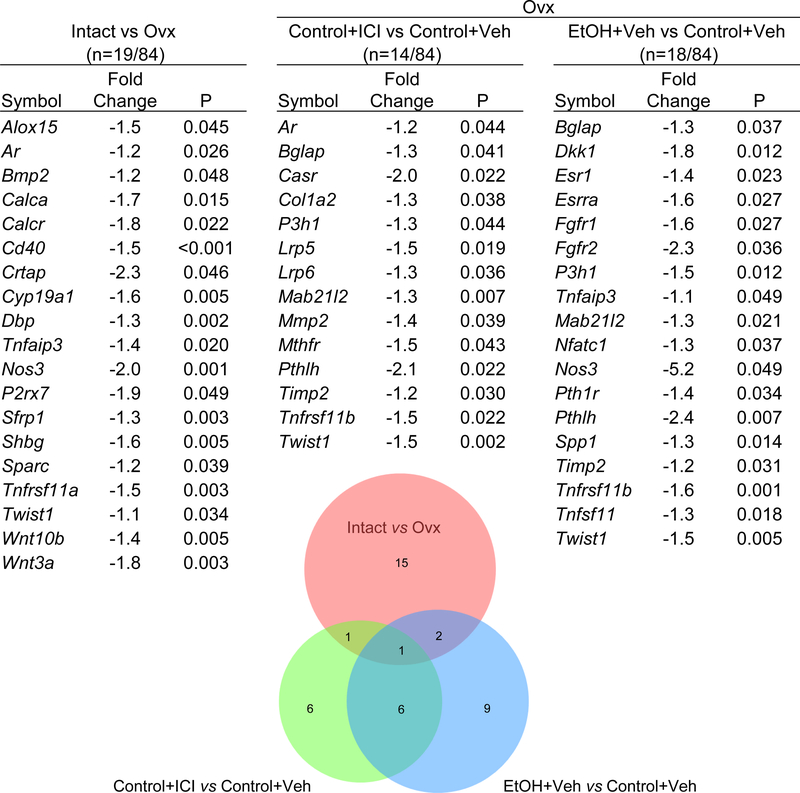
Gene Expression in Uterus and Femur
The effects of ovx, ethanol and ICI on gene expression in uterus and femur are shown in Figure 6 and 7, respectively. Esr1 and Esr2 levels were lower in ovary intact animals whereas Igf1 was higher. mRNA levels for Pgr did not change. A total of 19/84 genes were differentially expressed in femur when ovx rats were compared to ovary intact animals. 18/84 genes were differentially expressed in femur when ethanol treated ovx rats were compared with ovx rats and 14 genes differentially expressed in femur when ICI treated rats were compared with ovx rats. As shown in the Venn diagram, there was moderate overlap in differentially expressed genes comparing ICI and ethanol (7 genes) but low overlap comparing ICI and ovarian status (2 genes) or ethanol and ovarian status (3 genes). A complete listing of differentially expressed genes (compared to ovx) in femur is shown in Figure 7.
Figure 6.
Effect of ethanol and ICI 182 780 (ICI) on gene expression of (A) Esr1, (B) Esra2, (C) Igf1, and (D) Pgr in uterus. Gene expression in ovary-intact and ovx rats is presented for reference. Data are mean ± SE. N = 7/group (ethanol study) and N = 5/group (reference study). *Different from ovx, P<0.05.
Figure 7.
Effect of ethanol and ICI 182 780 (ICI) on gene expression for 84 genes related to bone growth and turnover in femur normalized to Gapdh. ovx mice were used to compare the effects of gonadal hormone insufficiency (red circle; 19 genes differentially expressed), ethanol treatment (blue circle; 18 genes differentially expressed) and ICI treatment (green circle; 14 genes differentially expressed). Differentially expressed genes for each treatment condition are tabulated. The numbers depicted where circles overlap represent shared genes.
Discussion
Heavy alcohol consumption beginning 4 weeks following ovx had no effect on uterine weight, slowed accrual of total body mass, lean mass and abdominal WAT mass but resulted in increased total body fat mass and percent fat mass. Alcohol lowered serum osteocalcin, a biochemical marker of bone turnover, decreased accrual of cortical bone mass in tibia of ovx rats by reducing addition of bone onto the periosteal bone surface and accentuated further ovx-induced cancellous bone loss. ICI treatment had no effect on uterine weight, body weight, body composition, tibia bone mass, density, or cortical bone architecture. ICI prevented further cancellous bone loss, a response antagonized by alcohol.
The time course effects of ovx on bone growth and turnover in Sprague Dawley rats are well-established (Wronski et al., 1988). Ovx accelerates longitudinal and radial tibial growth in young rats, resulting in longer bones with increased cross sectional area (Turner et al., 1989). Cancellous bone turnover increases following ovx and osteopenia occurs at sites such as the proximal tibial metaphysis where the increase in bone resorption exceeds the coupled increase in bone formation (Westerlind et al., 1997). Time-course studies show that ovx in young rats (e.g. 2-month-old) prevents further accrual of cancellous bone and, as in the present study, can result in bone loss (Turner et al., 2013). The most dramatic changes in cancellous microarchitecture occur during the initial few weeks following ovx (Wronski et al., 1988). Alcohol results in dose-dependent cancellous bone loss in ovary-intact skeletally mature rats, but the magnitude is lower and rate of bone loss is slower than bone loss following ovx (Turner et al., 2001b). The rapid development of cancellous osteopenia in response to acute onset of gonadal hormone insufficiency may explain our prior failure to detect an independent effect of dietary alcohol following ovx (Kidder and Turner, 1998).
In the present study, cross-sectional volume, cortical volume, cortical thickness and polar moment of inertia (surrogate measure of strength in torsion) increased in the control rats compared to baseline, indicating that the rats, in spite of ovx, were accruing cortical bone mass and increasing cortical bone strength. Alcohol reduced the accrual of bone; the observed changes in cortical bone microarchitecture, particularly the lower cross-sectional volume and no change in medullary volume, imply reduced periosteal bone formation as the likely explanation for the lower cortical bone volume in alcohol-fed rats. The present findings are consistent with prior studies assessing the skeletal effects of alcohol in growing intact female rats and intact male rats (Maddalozzo et al., 2009, Turner et al., 2001a, Brown et al., 2002, Sampson et al., 1997). We performed the present studies in sexually mature but growing rats, in part, because bone accrual during late adolescence is especially important for achieving optimal peak bone mass (McCormack et al., 2017). An inhibitory effect of alcohol on periosteal expansion during late adolescence and early adulthood, should it occur, would likely lower peak bone mass and consequently increase fracture risk later in life (Langdahl et al., 2016).
Our findings in the present study suggest that chronic heavy alcohol consumption can accentuate cancellous osteopenia induced by gonadal insufficiency. This study design differs from our earlier work (Kidder and Turner, 1998) in that in the present study we did not begin alcohol treatment until development of ovx-induced cancellous osteopenia. Under these experimental conditions, modest additional cancellous bone loss in rats fed alcohol was apparent. Fracture risk in women increases dramatically following menopause and is associated with the magnitude of bone loss (Trajanoska et al., 2018). Therefore, even modest changes in magnitude of bone loss may influence fracture risk in this population. This possibility could be further explored using skeletally mature (>8-month-old) rats (Martin et al., 2003).
Whether alcohol can increase aromatization of androgens to estrogens is unclear (Purohit, 2000). Acute administration of moderate alcohol to early postmenopausal women reduced biochemical markers of bone turnover (Marrone et al., 2012), a finding consistent with the known actions of estrogen on bone turnover (Turner et al., 1994). However, serum estradiol levels did not change following administration of alcohol and there was no association between estradiol levels and serum biochemical markers of bone turnover. Some studies (reviewed by (Gaddini et al., 2016) suggest that the alcohol-induced increase in estrogen levels are transient and only detectable when assayed a short interval following alcohol consumption. Uterine weight increases in ovx rats in response to increased circulating estrogen levels, providing a functional assay for changes in estrogen status in the animal model (Lotinun et al., 2003, Sibonga et al., 2003). The absence of an increase in uterine weight in the present study provides strong evidence that heavy consumption of alcohol did not lead to increased estrogen signaling in the growing rats. The absence of changes in mRNA levels for representative estrogen regulated genes provides further support for this conclusion (Gingery et al., 2017). Our findings in postmenopausal women and ovary-intact rats (Turner et al., 1998) indicate that alcohol has rapid effects on bone metabolism not requiring increased estradiol levels but they do rule out the possibility that alcohol influences metabolism by bone cells, in part, by modulating estrogen signaling at the estrogen receptor level. However, the weak overlap between ethanol and genes differentially expressed in femur following ovx does not provide strong support for this possibility.
We did not design our analysis of gene expression in femur to determine mechanisms of action of ovarian hormones, ethanol or ICI on bone metabolism. The resulting data instead provides a useful “read out” of tissue level changes in response to treatment. As expected, ICI had little effect on expression of genes differentially expressed following ovx. Ethanol treatment resulted in lower mRNA levels for Esr1 and Essra, two receptors involved in estrogen signaling. However, ethanol also lowered expression levels for other receptors (Fgfr1, Fgfr2 and Pth1r), as well as genes related to cell signaling (Dkk1, Tnfsf11, Tnfrs11b, Pthlh, Nos3) and transcription (Nfatc1, Tnfaip3, Twist1), findings consistent with prior evidence that alcohol acts on the skeleton through multiple pathways. In addition to estrogen levels and/or signaling discussed above, alcohol consumption is associated with changes in the levels and/or skeletal response to a wide variety of bone regulatory factors. These include mechanical loading, androgens, vitamin D, parathyroid hormone, growth hormone, and leptin (Hefferan et al., 2003, Maddalozzo et al., 2009, Turner et al., 1988, Turner et al., 2010, Ronis et al., 2007, Shankar et al., 2008). Each play an important role in bone metabolism and disturbances in associated signaling would likely result in alterations in bone response.
In the present study, we investigated the skeletal actions of alcohol in ovx rats in which estrogen receptor signaling was blocked by treatment with ICI (Wakeling and Bowler, 1992). ICI has two modes of action to block estrogen receptor mediated signaling: (1) competition for estrogen binding sites on the receptor and (2) acceleration of receptor degradation (Robertson, 2001). Treatment with ICI results in uterine atrophy and osteopenia in ovary-intact rats (Gallagher et al., 1993, Sibonga et al., 1998) but as shown in the present study, decreases magnitude of cancellous osteopenia in ovx rats (Gallagher et al., 1993, Sibonga et al., 1998). Notably, administration of ICI to 10-week-old ovary-intact rats, using the same protocol as in the present study, decreased uterine weight to values that did not differ from ovx animals. Due to its exclusion from brain and growth plate at doses used in the present study (Gallagher et al., 1993, Sibonga et al., 1998), ICI treatment had no effect on body weight gain or longitudinal bone growth in sham or ovx rats. ICI treatment of ovary-intact animals actually increases the circulating level of estrogen in ovary intact animals (Kennedy et al., 2005), demonstrating the efficacy of the treatment protocol in blocking activation of estrogen receptors by endogenous estrogens. Thus, it is possible that the partial reduction in cancellous bone loss in ICI-treated ovx rats occurs via ICI mimicking estrogen, with both acting through a non-estrogen receptor mediated pathway. Specifically, we have shown that estradiol activates signal transducer and activator of transcription-1 in estrogen receptor negative human fetal osteoblasts and estrogen receptor negative tumor cells by a mechanism that is dependent on Src kinase activity (Kennedy et al., 2005). Notably, ICI also regulates Src kinase activity, a step critical for estrogen receptor degradation (Yeh et al., 2013). In that there was moderate overlap in differentially expressed genes in femur by ethanol and ICI, it is plausible that they share a common estrogen receptor independent pathway for their opposing actions on bone in ovx rats.
Taken as a whole, our prior findings in women and present findings in rats suggest that the majority of skeletal effects of alcohol on bone metabolism are not dependent upon estrogen receptor-mediated signaling. However, ICI increased trabecular number and decreased trabecular spacing as well as serum CTX in control but not ethanol-treated ovx animals. ICI completely blocked the effects of estrogenic compounds on osteoblasts in vitro, suggesting that a non-estrogen receptor dependent alcohol sensitive pathway mediates these actions (Maran et al., 2006, Robinson et al., 2000). Because ICI is used to treat advanced breast cancer (Lee et al., 2017) where it appears to have a bone sparing effect (Journe et al., 2008), our results raise a concern that high levels of alcohol consumption may interfere with therapeutic actions of the drug (Journe et al., 2008).
There is additional evidence that estrogen signaling has a limited role in mediating the skeletal effects of alcohol. Alcohol has similar effects on bone metabolism in male and female rats (Turner, 2000), and although there is compelling evidence that estrogen plays an important role in skeletal maturation in human males (Khosla et al., 2001), this seems not be the case in rats. In contrast to female rats, administration of ICI had virtually no effect on the skeleton in male rats and did not antagonize the bone anabolic effects of testosterone, indicating that bone growth and turnover proceeds normally in spite of estrogen receptor blockade (Sibonga et al., 1998, Turner et al., 2000, Vandenput et al., 2002).
In summary, alcohol exaggerated cancellous bone loss in ovx rats with established osteopenia suggesting that chronic heavy alcohol consumption may aggravate bone loss associated with gonadal insufficiency. Additionally, alcohol reduced accrual of cortical bone during the latter stages of bone growth. Co-treatment of post ovx rats with alcohol and the potent estrogen receptor antagonist ICI revealed that most of the skeletal effects of alcohol occur independent of estrogen receptor signaling. However, alcohol antagonized the bone sparing action of ICI on cancellous bone in ovx rats, likely through a non-estrogen receptor dependent pathway. Additional studies will be required to determine whether estrogen signaling influences the skeletal response to moderate alcohol consumption and whether the effects are influenced by gonadal status.
Acknowledgments
Support: This work was supported by a grant from the Department of Defense (DR043181) and the National Institutes of Health (AA02689).
Footnotes
Conflict of interest: The authors have no conflict of interest.
References Cited
- BENJAMINI Y & HOCHBERG Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Scoiety Series B, 57, 289–300. [Google Scholar]
- BROWN EC, PERRIEN DS, FLETCHER TW, IRBY DJ, ARONSON J, GAO GG, HOGUE WJ, SKINNER RA, SUVA LJ, RONIS MJ, HAKKAK R, BADGER TM & LUMPKIN CK JR. 2002. Skeletal toxicity associated with chronic ethanol exposure in a rat model using total enteral nutrition. J Pharmacol Exp Ther, 301, 1132–8. [DOI] [PubMed] [Google Scholar]
- CALLACI JJ, JUKNELIS D, PATWARDHAN A & WEZEMAN FH 2006. Binge alcohol treatment increases vertebral bone loss following ovariectomy: compensation by intermittent parathyroid hormone. Alcohol Clin Exp Res, 30, 665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN JR, HALEY RL, HIDESTRAND M, SHANKAR K, LIU X, LUMPKIN CK, SIMPSON PM, BADGER TM & RONIS MJ 2006. Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. J Pharmacol Exp Ther, 319, 1182–90. [DOI] [PubMed] [Google Scholar]
- CHEN JR, LAZARENKO OP, HALEY RL, BLACKBURN ML, BADGER TM & RONIS MJ 2009. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. J Bone Miner Res, 24, 221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG KW 1990. Effects of chronic ethanol intake on aromatization of androgens and concentration of estrogen and androgen receptors in rat liver. Toxicology, 62, 285–95. [DOI] [PubMed] [Google Scholar]
- GADDINI GW, GRANT KA, WOODALL A, STULL C, MADDALOZZO GF, ZHANG B, TURNER RT & IWANIEC UT 2015. Twelve months of voluntary heavy alcohol consumption in male rhesus macaques suppresses intracortical bone remodeling. Bone, 71, 227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GADDINI GW, TURNER RT, GRANT KA & IWANIEC UT 2016. Alcohol: a simple nutrient with complex actions on bone in the adult skeleton. Alcohol Clin Exp Res, 40, 657–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLAGHER A, CHAMBERS TJ & TOBIAS JH 1993. The estrogen antagonist ICI 182,780 reduces cancellous bone volume in female rats. Endocrinology, 133, 2787–91. [DOI] [PubMed] [Google Scholar]
- GINGERY A, IWANIEC UT, SUBRAMANIAM M, TURNER RT, PITEL KS, MCGOVERN RM, REID JM, MARLER RJ, INGLE JN, GOETZ MP & HAWSE JR 2017. Skeletal and uterotrophic effects of endoxifen in female rats. Endocrinology, 158, 3354–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEFFERAN TE, KENNEDY AM, EVANS GL & TURNER RT 2003. Disuse exaggerates the detrimental effects of alcohol on cortical bone. Alcohol Clin Exp Res, 27, 111–7. [DOI] [PubMed] [Google Scholar]
- HOGAN HA, ARGUETA F, MOE L, NGUYEN LP & SAMPSON HW 2001. Adult-onset alcohol consumption induces osteopenia in female rats. Alcohol Clin Exp Res, 25, 746–54. [PubMed] [Google Scholar]
- JOURNE F, BODY JJ, LECLERCQ G & LAURENT G. 2008. Hormone therapy for breast cancer, with an emphasis on the pure antiestrogen fulvestrant: mode of action, antitumor efficacy and effects on bone health. Expert Opin Drug Saf, 7, 241–58. [DOI] [PubMed] [Google Scholar]
- KENNEDY AM, SHOGREN KL, ZHANG M, TURNER RT, SPELSBERG TC & MARAN A. 2005. 17beta-estradiol-dependent activation of signal transducer and activator of transcription-1 in human fetal osteoblasts is dependent on Src kinase activity. Endocrinology, 146, 201–7. [DOI] [PubMed] [Google Scholar]
- KHOSLA S, MELTON LJ 3RD & RIGGS BL 2001. Estrogens and bone health in men. Calcif Tissue Int, 69, 189–92. [DOI] [PubMed] [Google Scholar]
- KIDDER LS & TURNER RT 1998. Dietary ethanol does not accelerate bone loss in ovariectomized rats. Alcohol Clin Exp Res, 22, 2159–64. [PubMed] [Google Scholar]
- KIMBLE RB 1997. Alcohol, cytokines, and estrogen in the control of bone remodeling. Alcohol Clin Exp Res, 21, 385–91. [DOI] [PubMed] [Google Scholar]
- LANGDAHL B, FERRARI S & DEMPSTER DW 2016. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis, 8, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE CI, GOODWIN A & WILCKEN N. 2017. Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst Rev, 1, CD011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOTINUN S, WESTERLIND KC, KENNEDY AM & TURNER RT 2003. Comparative effects of long-term continuous release of 16 alpha-hydroxyestrone and 17 beta-estradiol on bone, uterus, and serum cholesterol in ovariectomized adult rats. Bone, 33, 124–31. [DOI] [PubMed] [Google Scholar]
- MADDALOZZO GF, TURNER RT, EDWARDS CH, HOWE KS, WIDRICK JJ, ROSEN CJ & IWANIEC UT 2009. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int, 20, 1529–38. [DOI] [PubMed] [Google Scholar]
- MARAN A, SHOGREN K, ZHANG M, YASZEMSKI MJ, HEFFERAN TE, SPELSBERG TC, KLOOSTERBOER HJ & TURNER RT 2006. Effects of stable transfection of human fetal osteoblast cells with estrogen receptor-alpha on regulation of gene expression by tibolone. Bone, 39, 523–9. [DOI] [PubMed] [Google Scholar]
- MARRONE JA, MADDALOZZO GF, BRANSCUM AJ, HARDIN K, CIALDELLA-KAM L, PHILBRICK KA, BREGGIA AC, ROSEN CJ, TURNER RT & IWANIEC UT 2012. Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause, 19, 974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN EA, RITMAN EL & TURNER RT 2003. Time course of epiphyseal growth plate fusion in rat tibiae. Bone, 32, 261–7. [DOI] [PubMed] [Google Scholar]
- MCCORMACK SE, COUSMINER DL, CHESI A, MITCHELL JA, ROY SM, KALKWARF HJ, LAPPE JM, GILSANZ V, OBERFIELD SE, SHEPHERD JA, WINER KK, KELLY A, GRANT SFA & ZEMEL BS 2017. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr, 171, e171769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUROHIT V. 2000. Can alcohol promote aromatization of androgens to estrogens? A review. Alcohol, 22, 123–7. [DOI] [PubMed] [Google Scholar]
- ROBERTSON JF 2001. Faslodex (ICI 182, 780), a novel estrogen receptor downregulator--future possibilities in breast cancer. J Steroid Biochem Mol Biol, 79, 209–12. [DOI] [PubMed] [Google Scholar]
- ROBINSON JA, WATERS KM, TURNER RT & SPELSBERG TC 2000. Direct action of naturally occurring estrogen metabolites on human osteoblastic cells. J Bone Miner Res, 15, 499–506. [DOI] [PubMed] [Google Scholar]
- RONIS MJ, WANDS JR, BADGER TM, DE LA MONTE SM, LANG CH & CALISSENDORFF J. 2007. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res, 31, 1269–85. [DOI] [PubMed] [Google Scholar]
- SAMPSON HW, CHAFFIN C, LANGE J & DEFEE B 2ND 1997. Alcohol consumption by young actively growing rats: a histomorphometric study of cancellous bone. Alcohol Clin Exp Res, 21, 352–9. [PubMed] [Google Scholar]
- SAMPSON HW, PERKS N, CHAMPNEY TH & DEFEE B 2ND 1996. Alcohol consumption inhibits bone growth and development in young actively growing rats. Alcohol Clin Exp Res, 20, 1375–84. [DOI] [PubMed] [Google Scholar]
- SAVOLA O, NIEMELA O & HILLBOM M. 2004. Blood alcohol is the best indicator of hazardous alcohol drinking in young adults and working-age patients with trauma. Alcohol Alcohol, 39, 340–5. [DOI] [PubMed] [Google Scholar]
- SHANKAR K, HIDESTRAND M, HALEY R, SKINNER RA, HOGUE W, JO CH, SIMPSON P, LUMPKIN CK JR., ARONSON J, BADGER TM & RONIS MJ 2006. Different molecular mechanisms underlie ethanol-induced bone loss in cycling and pregnant rats. Endocrinology, 147, 166–78. [DOI] [PubMed] [Google Scholar]
- SHANKAR K, LIU X, SINGHAL R, CHEN JR, NAGARAJAN S, BADGER TM & RONIS MJ 2008. Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1,25 dihydroxyvitamin D3–24-hydroxylase (CYP24A1). Endocrinology, 149, 1748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIBONGA JD, DOBNIG H, HARDEN RM & TURNER RT 1998. Effect of the high-affinity estrogen receptor ligand ICI 182,780 on the rat tibia. Endocrinology, 139, 3736–42. [DOI] [PubMed] [Google Scholar]
- SIBONGA JD, LOTINUN S, EVANS GL, PRIBLUDA VS, GREEN SJ & TURNER RT 2003. Dose-response effects of 2-methoxyestradiol on estrogen target tissues in the ovariectomized rat. Endocrinology, 144, 785–92. [DOI] [PubMed] [Google Scholar]
- TENNANT KG, LEONARD SW, WONG CP, IWANIEC UT, TURNER RT & TRABER MG 2017. High-dietary alpha-tocopherol or mixed tocotrienols have no effect on bone mass, density, or turnover in male rats during skeletal maturation. J Med Food, 20, 700–708. [DOI] [PubMed] [Google Scholar]
- TRAJANOSKA K, SCHOUFOUR JD, DE JONGE EAL, KIEBOOM BCT, MULDER M, STRICKER BH, VOORTMAN T, UITTERLINDEN AG, OEI EHG, ARFAN IKRAM M, CAROLA ZILLIKENS M, RIVADENEIRA F & OEI L. 2018. Fracture incidence and secular trends between 1989 and 2013 in a population based cohort: The Rotterdam Study. Bone, 114, 116–124. [DOI] [PubMed] [Google Scholar]
- TURNER RT 2000. Skeletal response to alcohol. Alcohol Clin Exp Res, 24, 1693–701. [PubMed] [Google Scholar]
- TURNER RT, ALOIA RC, SEGEL LD, HANNON KS & BELL NH 1988. Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcohol Clin Exp Res, 12, 159–62. [DOI] [PubMed] [Google Scholar]
- TURNER RT, EVANS GL & DOBNIG H. 2000. The high-affinity estrogen receptor antagonist ICI 182,780 has no effect on bone growth in young male rats. Calcif Tissue Int, 66, 461–4. [DOI] [PubMed] [Google Scholar]
- TURNER RT, EVANS GL, ZHANG M. & SIBONGA JD 2001a. Effects of parathyroid hormone on bone formation in a rat model for chronic alcohol abuse. Alcohol Clin Exp Res, 25, 667–71. [PubMed] [Google Scholar]
- TURNER RT, HANNON KS, DEMERS LM, BUCHANAN J. & BELL NH 1989. Differential effects of gonadal function on bone histomorphometry in male and female rats. J Bone Miner Res, 4, 557–63. [DOI] [PubMed] [Google Scholar]
- TURNER RT, IWANIEC UT, ANDRADE JE, BRANSCUM AJ, NEESE SL, OLSON DA, WAGNER L, WANG VC, SCHANTZ SL & HELFERICH WG 2013. Genistein administered as a once-daily oral supplement had no beneficial effect on the tibia in rat models for postmenopausal bone loss. Menopause, 20, 677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER RT, KIDDER LS, KENNEDY A, EVANS GL & SIBONGA JD 2001b. Moderate alcohol consumption suppresses bone turnover in adult female rats. J Bone Miner Res, 16, 589–94. [DOI] [PubMed] [Google Scholar]
- TURNER RT, PHILBRICK KA, KUAH AF, BRANSCUM AJ & IWANIEC UT 2017. Role of estrogen receptor signaling in skeletal response to leptin in female ob/ob mice. J Endocrinol, 233, 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER RT, RIGGS BL & SPELSBERG TC 1994. Skeletal effects of estrogen. Endocr Rev, 15, 275–300. [DOI] [PubMed] [Google Scholar]
- TURNER RT, ROSEN CJ & IWANIEC UT 2010. Effects of alcohol on skeletal response to growth hormone in hypophysectomized rats. Bone, 46, 806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER RT & SIBONGA JD 2001. Effects of alcohol use and estrogen on bone. Alcohol Res Health, 25, 276–81. [PMC free article] [PubMed] [Google Scholar]
- TURNER RT, WRONSKI TJ, ZHANG M, KIDDER LS, BLOOMFIELD SA & SIBONGA JD 1998. Effects of ethanol on gene expression in rat bone: transient dose-dependent changes in mRNA levels for matrix proteins, skeletal growth factors, and cytokines are followed by reductions in bone formation. Alcohol Clin Exp Res, 22, 1591–9. [DOI] [PubMed] [Google Scholar]
- VANDENPUT L, SWINNEN JV, VAN HERCK E, VERSTUYF A, BOONEN S, BOUILLON R & VANDERSCHUEREN D. 2002. The estrogen receptor ligand ICI 182,780 does not impair the bone-sparing effects of testosterone in the young orchidectomized rat model. Calcif Tissue Int, 70, 170–5. [DOI] [PubMed] [Google Scholar]
- WAKELING AE & BOWLER J. 1992. ICI 182,780, a new antioestrogen with clinical potential. J Steroid Biochem Mol Biol, 43, 173–7. [DOI] [PubMed] [Google Scholar]
- WESTERLIND KC, WRONSKI TJ, RITMAN EL, LUO ZP, AN KN, BELL NH & TURNER RT 1997. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci U S A, 94, 4199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRONSKI TJ, CINTRON M & DANN LM 1988. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int, 43, 179–83. [DOI] [PubMed] [Google Scholar]
- YEH WL, SHIODA K, COSER KR, RIVIZZIGNO D, MCSWEENEY KR & SHIODA T. 2013. Fulvestrant-induced cell death and proteasomal degradation of estrogen receptor alpha protein in MCF-7 cells require the CSK c-Src tyrosine kinase. PLoS One, 8, e60889. [DOI] [PMC free article] [PubMed] [Google Scholar]