Abstract
Monosodium glutamate (MSG) is an umami substance widely used as flavor enhancer. Although it is generally recognized as being safe by food safety regulatory agencies, several studies have questioned its long-term safety. The purpose of this review was to survey the available literature on preclinical studies and clinical trials regarding the alleged adverse effects of MSG. Here, we aim to provide a comprehensive overview of the reported possible risks that may potentially arise following chronic exposure. Furthermore, we intend to critically evaluate the relevance of this data for dietary human intake.
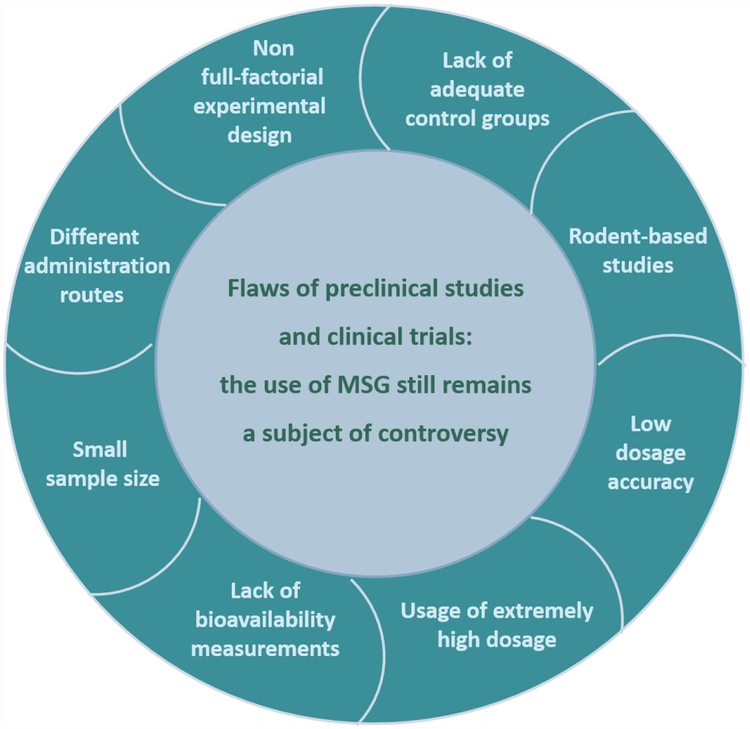
Preclinical studies have associated MSG administration with cardiotoxicity, hepatotoxicity, neurotoxicity, low-grade inflammation, metabolic disarray and premalignant alterations, along with behavioral changes. Moreover, links between MSG consumption and tumorigenesis, increased oxidative stress and apoptosis in thymocytes, as well as genotoxic effects in lymphocytes have been reported. However, in reviewing the available literature, we detected several methodological flaws, which led us to conclude that these studies have limited relevance for extrapolation to dietary human intakes of MSG risk exposure.
Clinical trials have focused mainly on the effects of MSG on food intake and energy expenditure. Besides its well-known impact on food palatability, MSG enhances salivary secretion and interferes with carbohydrate metabolism, while the impact on satiety and post-meal recovery of hunger varied in relation to meal composition. Reports on MSG hypersensitivity, also known as ‘Chinese restaurant syndrome’, or links of its use to increased pain sensitivity and atopic dermatitis were found to have little supporting evidence. Based on the available literature, we conclude that further clinical and epidemiological studies are needed, with an appropriate design, accounting for both added and naturally occurring dietary MSG. Critical analysis of existing literature, establishes that many of the reported negative health effects of MSG have little relevance for chronic human exposure and are poorly informative as they are based on excessive dosing that does not meet with levels normally consumed in food products.
Introduction
Monosodium glutamate (MSG) is a widely used flavor enhancer derived from L-glutamic acid, a naturally occurring amino acid in a variety of food products (Stanska & Krzeski, 2016; Wifall, Faes, Taylor-Burds, Mitzelfelt, & Delay, 2007). MSG possesses a specific taste – umami, which was first considered a predominant taste in Asia and much later in Western cultures (Kurihara, 2015; Stanska & Krzeski, 2016). This molecule was identified about one hundred years ago by Kikunae Ikeda as the fifth basic taste, in addition to sweet, sour, salty, and bitter. MSG is found in high-protein food products, such as meat or fish, and also in certain types of cheese (Roquefort and Parmesan) or vegetables (tomatos, mushrooms, broccoli) (Kochem & Breslin, 2017; Shigemura, Shirosaki, Sanematsu, Yoshida, & Ninomiya, 2009; Stanska & Krzeski, 2016; Wifall et al., 2007; Yamaguchi & Ninomiya, 2000). In addition to its basic specificity, the umami taste can enhance overall flavor intensity and improve food palatability. This effect is dependent on a variety of factors, the most important being the concentration of umami molecule and the food matrix (Masic & Yeomans, 2013).
In addition to MSG, other well-established umami substances are inosine 5’-monophosphate (IMP) and guanosine 5’-monophosphate (GMP). They can be found in a variety of natural sources and also as additives in certain food products, such as processed meat, canned vegetables, soups, sauces, dried bouillon cubes and salty flavored snacks (Conn, 1992; Scopp, 1991). Moreover, IMP is also used as a flavor enhancer to accentuate the umami taste of MSG (Shigemura et al., 2009; Wifall et al., 2007; Yamaguchi & Ninomiya, 2000).
Interestingly, L-glutamic acid itself and its disodium salt analog have mild umami properties, when compared to MSG. The umami taste is diminished by other chemical alterations, such as esterification or amide formation. Only acidic analogs of glutamic acid, such as L-aspartic acid, L-homocysteinic acid and succinic acid, were reported to have some umami effects (Kawai, Okiyama, & Ueda, 2002). The free carboxylic group hypothesis regarding structure-taste relationship, is confirmed by L-γ-glutamylethylamide, also known as theanine, a natural constituent of green tea, which exhibits umami properties (Sharma, Joshi, & Gulati, 2018; Turkozu & Sanlier, 2017; Williams et al., 2019; Zhang, Venkitasamy, Pan, Liu, & Zhao, 2016). Also, various cyclic derivatives of glutamic acid have umami properties, provided they contain a free carboxylic group, this being the case for pyroglutamic or ibotenic acid (Zhang et al., 2016).
MSG is supplemented in many processed foods (Conn, 1992; Scopp, 1991), with an estimated average daily human intake of around 0.3–1.0 g in European industrialized countries (Beyreuther et al., 2007). Although food safety regulatory agencies consider MSG consumption to be safe, some preclinical and clinical studies have challenged its safety, especially following chronic exposure. The controversy is likely fueled from the knowledge that endogenous glutamate plays a role in physiological as well as pathological processes. Glutamate has various physiological functions: it is a major substrate for energy production in enterocytes, an intermediary substance in protein metabolism, precursor of significant metabolites such as glutathione (GSH, oxidative stress modulator) or N-acetylglutamate (metabolic regulator), and also a central nervous system (CNS) excitatory neurotransmitter (Meldrum, 2000; Newsholme, 2001). An increase in CNS glutamate concentration is associated with brain damage, similar to status epilepticus, cerebral ischemia, or traumatic injuries, as well as with chronic neurodegeneration analogous to Huntington’s chorea (Meldrum, 2000).
Following oral administration, glutamte undergoes oxidation within enterocytes, in the small intestine (Blachier, Boutry, Bos, & Tome, 2009). Only a very small quantity is subsequently found in the portal blood and, most likely, this originates from glutamine catabolism as a result of intestinal glutaminase activity, rather than from the absorption of dietary glutamate (Battezzati, Brillon, & Matthews, 1995). In healthy human volunteers, nearly all of the enterally delivered glutamate is removed by the splanchnic bed on the first pass (Matthews, Marano, & Campbell, 1993). Following oxidation, glutamate is further converted to other amino acids or used as a precursor for the synthesis of different bioactive compounds (Reeds et al., 1997; Wakabayashi, Iwashima, Yamada, & Yamada, 1991; Wu, 2009).
The Joint FAO/WHO Expert Committee on Food Additives (JECFA), the US Food and Drug Administration (FDA) and the European Food Safety Association (EFSA) considered MSG to be a substance generally recognized as safe (GRAS). A food additive can be included on the GRAS list if widely used in food prior to 1958 (approval based on experience) or when its safety is confirmed by scientific toxicological reports based on its estimated dietary intake. However, some authors now argue that GRAS inclusion criteria, either for scientific-based or for experience-based procedures require updating, based on the developments made in toxicity testing (Barraj, Murphy, Tran, & Petersen, 2016; Burdock, Carabin, & Griffiths, 2006; Hartung, 2018). As a consequence, in 2006, EFSA established an acceptable daily intake (ADI) for several food additives taking into account their no-observed-adverse-effect level (NOAEL) (Committee on toxicity of chemicals in food, consumer products and the environment: Statement on food additives and developmental neurotoxicity, 2006). The considered additives were quinoline yellow (E104), sunset yellow (E110), carmoisine (E122), Ponceau 4R (E124), indigo carmine (E132), brilliant blue (E133), sodium benzoate (E211), sulfur dioxide (E220), monosodium glutamate (E621), acesulfame K (E950), aspartame (E951), and saccharin (E954). A similar review was undertaken in June 2017, when EFSA reassessed the safety of glutamic acid and glutamates and found a group ADI of 30 mg/kg/day, expressed as glutamic acid. All data are presented in Table 1 (Committee on toxicity of chemicals in food, consumer products and the environment: Statement on food additives and developmental neurotoxicity, 2006; EFSA Panel on Food Additives and Nutrient Sources added to Food, 2017).
Table 1.
Food additives NOAEL and ADI values
| Food additive | NOAEL | ADI |
|---|---|---|
| Quinoline yellow | 1000 mg/kg bw/day | 0–10 mg/kg bw/day |
| Sunset yellow | 500 mg/kg bw | 0–2.5 mg/kg bw/day |
| Carmoisine | 400 mg/kg bw/day | 0–4 mg/kg bw/day |
| Ponceau 4R | 375 mg/kg | 0–4 mg/kg bw/day |
| Indigo carmine | 500 mg/kg bw/day | 0–5 mg/kg bw/day |
| Brilliant blue | 2500 mg/kg bw/day | 0–12.5 mg/kg bw/day |
| Sodium benzoate | 500 mg/kg bw/day | 0–0.7 mg/kg |
| Sulfur dioxide | 70 mg/kg b.w | 0–0.7 mg/kg |
| MSG | 3200 mg/kg | 30 mg/kg |
| Acesulfame K | 900 mg/kg bw/day | 0–9 mg/kg bw/day |
| Aspartame | Not specified | 0–40 mg/kg bw/day |
| Saccharin | 500 mg/kg bw/day | 0–5 mg/kg bw |
Furthermore, EFSA’s scientific panel has also concluded this ADI exceeded levels for all population groups. This is worrisome since results from rodent studies associated its consumption with neurotoxicity, cardiotoxicity, hepatic and renal disturbances, as well as metabolic disorders. However, as mentioned, in our analysis of preclinical studies, we identified several issues regarding the employed protocols, and consequently, we question the relevance and merit of these data, as they fail to provide an estimate of safety parameters, such as margin of exposure. These reports led to multiple analyses (Geha et al., 2000b; Henry-Unaeze, 2017; Husarova & Ostatnikova, 2013; Obayashi & Nagamura, 2016; Stanska & Krzeski, 2016; Tarasoff & Kelly, 1993; Williams & Woessner, 2009) regarding the effects of MSG consumption on human health, as highlighted in Table 2.
Table 2.
Reviews regarding the impact of MSG on human health
| Objective | Conclusions | Observations | Reference |
|---|---|---|---|
| Evidence-based safety review | MSG is safe for all life-cycle stages. No alterations of nervous system function or of blood concentrations of pituitary hormones were found. |
The human body does not discriminate between naturally occurring glutamate in foods and added glutamate. Added MSG is much lower than daily dietary glutamate intake from natural sources. Thus, it is hard to attribute causal role in major central and endocrine pathologies only to MSG used as a flavor enhancer. | (Henry-Unaeze, 2017) |
| Systematic review assessing the incidence of headache after an oral administration of MSG. Studies were analyzed taking into consideration MSG administration in relation to meals, due to the significant differences in kinetics when taken with or without food. |
Five papers of MSG administered with food were analyzed and none showed a significant difference in the incidence of headache except for the female group in one study (dose: 3 g in 150 mL beef bouillon). Of seven studies without food, four studies showed a significant increase in the frequency of headaches. |
MSG was administered, being dissolved in beverages or soup at relatively high concentrations. Authors emphasize that most of the studies involved administration of MSG in high concentrations (>2%), and it could easily be identified, due to its unpleasant taste at concentrations over 1.3%, and, as such, these studies were not properly blinded. | (Obayashi & Nagamura, 2016) |
| Assessment of the effects of MSG consumption on human health | No effects of MSG administration on postprandial glycaemia and insulinemia were found. No correlation between MSG consumption and obesity was found. |
Results regarding the association of MSG intake with CRS and asthma were contradictory. Clinical trials with better design are needed for a conclusion. |
(Husarova & Ostatnikova, 2013) |
| Assessment of the possible role of MSG in the so-called ‘Chinese restaurant syndrome’ and in eliciting asthmatic bronchospasm, urticaria, angioedema, and rhinitis | Large doses of MSG (>3 g) ingested on an empty stomach without concomitant food ingestion may elicit some of the symptoms associated to CRS. It is unlikely that MSG consumption plays a role in the provocation of asthma. Dietary ingestion of MSG may be a rare cause for urticaria (accounting for under 3% of cases) and angioedema. |
There is no evidence that dietary MSG of a typical western diet induces symptoms of the CRS. There are not enough data to associate MSG consumption with rhinitis. The overall quality of the evidence supporting a relationship between MSG consumption and for urticaria and angioedema is low. |
(Williams & Woessner, 2009) |
| Assessment of correlation between MSG consumption and Chinese restaurant syndrome | Doses of 5 g of MSG given without food may determine symptoms (general weakness, muscle tightness, flushing, sweating, headache, numbness or tingling) in individuals who are self-proclaimed “MSG sensitive”. | The frequency of the responses was low and the responses were inconsistent and not reproducible. The results were not observed when MSG was given with food. |
(Geha et al., 2000b) |
| Literature review and original study of link between MSG consumption and Chinese restaurant syndrome | No evidence linking the CRS associated symptoms to MSG consumption was found. | MSG was administered in doses of 1.5, 3.0 and 3.15 g/person in capsules and specially formulated drinks, before breakfast. | (Tarasoff & Kelly, 1993) |
Taking all of the above into account, we surveyed the available data from preclinical studies and clinical trials regarding the adverse health effects of MSG administration, analyzing study design and methodology, and evaluating the relevance of these data for chronic dietary human exposure.
Methods
A systematic review of the literature for all relevant articles was performed using PUBMED. Articles were limited to those published in the English language, focusing on most recent work, between 2010 and 2018, (50% of the cited material), but not neglecting any older relevant studies. As such, 32.35% of articles were published between 2000 and 2010 and 13.52% in the 1990–2000 period, with 4.11% before 1990. For preclinical studies, the search strategy used the keywords and MeSH terms: “monosodium glutamate,” “flavor enhancer,” “umami substance,” “umami molecule,” AND “preclinical studies,” “mice,” “rats,” AND “toxicity” “effects”, “obesity”, “insulin resistance”, “metabolism”, “pain”, “heart toxicity”, “liver toxicity”, “neurotoxicity”, “oxidative balance”. A total of 76 papers were selected post eligibility analysis and cross-checking, following the rules of systematic review - PRISMA (Transparent reporting of systematic reviews and meta-analyzes - http://www.prisma-statement.org/). For clinical trials, the search strategy used the keywords and MeSH terms: “monosodium glutamate”, “flavor enhancer”, “umami substance”, “umami molecule” AND “clinical studies” AND “toxicity”, “health impact”, “effects”. A total of 40 papers were selected after eligibility analysis and cross-checking, as described above. For the effects of other umami molecules, the search strategy used the keywords and MeSH terms: “umami substance”, “umami molecule”, “flavor enhancer” AND “toxicity” “effects”, “health impact”, “effects”. A total of 16 studies were found eligible, as described above, and further analyzed.
Preclinical studies assessing MSG effects
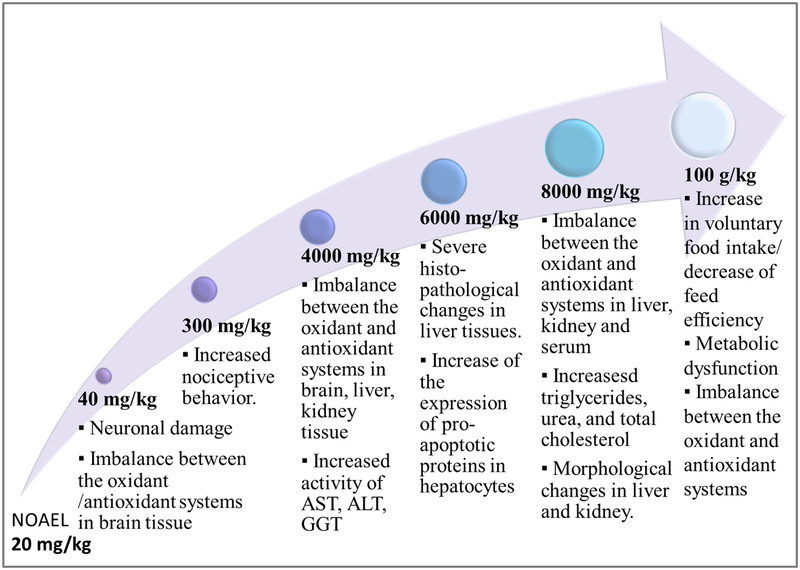
As for the preclinical data, we included in our search studies employing a single dose or chronical administration of MSG, in order analyze its effects on biochemical parameters (total plasma protein, HDL, LDL, glucose, triglycerides, oxidative stress biomarkers, insulin, leptin), as well as on the histology and morphology of several organs (heart, brain, ovaries, liver), and also behavioral tests-based studies evaluating the effects on CNS, with doses ranging from 0.04 g/kg to 8 g/kg administered orally, intraperitoneally (i.p.), intragastrically or subcutaneously (s.c.).
MSG consumption was associated with morphological alterations of the heart tissue, as well as changes in cardiac rhythm, and hepatotoxic effects, with fibrosis and neoplastic changes (Eweka, Igbigbi, & Ucheya, 2011; Insawang et al., 2012), diabetes, obesity (Collison et al., 2011), neurotoxicity (Izumi et al., 2009; Rivera-Cervantes et al., 2014; Swamy et al., 2014; Weil, Norman, DeVries, & Nelson, 2008), modified behavioral and physiological alterations, such as increased aggressivity, decreased locomotor activity, and loss of muscle strength (Campos-Sepulveda et al., 2009). Significant changes in the neuronal redox homeostasis (increased levels of lipid peroxidation, nitrite concentration, decreased levels of antioxidants), and in the neuronal histology of the hippocampus, along with an increase of brain and serum cholinesterase (ChE) levels, were also reported (Onaolapo, Onaolapo, Akanmu, & Gbola, 2016; Sadek, Abouzed, & Nasr, 2016).
Effects on the cardiovascular system
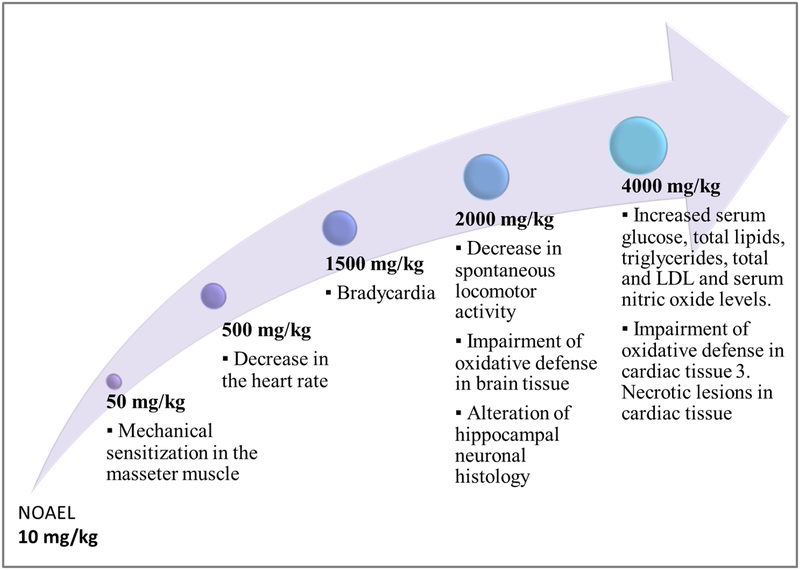
Four studies regarding MSG cardiovascular toxicity were identified and their results are summarized in Table 3. These indicated that MSG administration increased cardiac tissue oxidative stress and also determined biochemical changes, namely increasing some heart disease biomarkers, such as lactate dehydrogenase (LDH), aspartate transaminase (AST), and alanine transaminase (ALT) (Kumar & Bhandari, 2013). Doses between 0.5 g/kg and 1.5 g/kg induced changes of cardiac rhythm, as well as lethal tachyarrhythmia in myocardially infarcted rats (Liu et al., 2013).
Table 3.
Preclinical studies associating MSG exposure with cardiac toxicity
| Mode of administration | Species | MSG dosage/day | Duration of administration (days) | MSG effect (vs. control) | Reference |
|---|---|---|---|---|---|
| Subcutaneous | Laka-UK mice | 4 g/kg 8 g/kg |
6 | MSG induces dose-dependent oxidative stress in the cardiac tissue by:
|
(Singh & Pushpa, 2005) |
| Subcutaneous | Newborn Wistar rats | 4 g/kg | 7 (from day 2 to 14 after birth, on alternate days) |
|
(Kumar & Bhandari, 2013) |
| Intraperitoneal | Albino rat (the exact type is not specified) | 4 g/kg | 9 (three times/week, for three consecutive weeks) |
|
(Baky, 2009) |
| Intravenous | Wistar rat | 0.5 g/kg 1.5 g/kg |
Single administration | In normal rats:
|
(Liu et al., 2013) |
However, when analyzing whether these studies could indicate a threat to human health one must consider the high doses and routes of administration used. The subcutaneous, intraperitoneal, or intravenous administrations of doses which are a few-folds higher than the dietary intake of humans, (Beyreuther et al., 2007; Insawang et al., 2012; Shi et al., 2010) have little, if any, relevance for human exposure to MSG, as these routes overcome the normal metabolic pathway of ingested glutamate (Blood, 1969).
Evaluation of MSG’s hepatotoxicity
Several studies reported hepatotoxic effects following MSG administration, their key findings being summarized in Table 4. Dilatations of the central hepatic vein, with lysed erythrocytes and distorted hepatocytes, possibly due to impaired membrane permeability, were reported in adult Wistar rats, following controlled feeding with a mixture of food containing 0.04 g/kg and 0.08 g/kg MSG, on a daily basis for forty-two days. Furthermore, similar results were reported in an adult male Rattus norvegicus study, when MSG was given with food, but in a much higher daily dose of 6 g/kg. We carefully reviewed the hepatotoxic effects reported for the 0.04 g/kg and 0.08 g/kg doses, as they were extremely low, and found that the authors justified the employed dosages based on previous work. However, the quoted study comprised doses of 1.46 g/kg and, respectively, 2.92g/kg (Eweka & Om’iniabohs, 2007).
Table 4.
Summary of preclinical studies associating MSG exposure with hepatic toxicity
| Mode of administration | Species | MSG dosage/day | Duration of administration (days) |
MSG effect (vs. control) | Reference |
|---|---|---|---|---|---|
| Mixed with diet | Wistar rats | 0.04 mg/kg (n=8) 0.08 mg/kg (n=8) |
42 |
|
(Eweka et al., 2011) |
| Mixed with diet | Adult male Rattus norvigicus | 6 g/kg | 45 |
|
(El-Meghawry El-Kenawy et al., 2012) |
| Oral | Male Wistar Rats | 8 g/kg (n=6) | 20 |
|
(Paul et al., 2012) |
| Oral | Male Wistar Rats | 0.6 g/kg (n=6) | 10 |
|
(Onyema et al., 2006) |
| Subcutaneous | Newborn C57BL/6J male mice | 4 g/kg (n=6) | Single dose administration within 5 days of their birth | Characteristic liver histopathology of nonalcoholic steatohepatitis. Metabolic syndrome-like features:
|
(Takai et al., 2014) |
| Subcutaneous | ICR male mice | 2 g/kg (n=36) associated with unrestricted (n=18) or restricted dietary regimens (n=18) | From birth to day 5 of life |
|
(Fujimoto et al., 2014) |
| Intraperitoneal | Male albino Wistar-Norwegian brown hybrid rats | 4 g/kg | 10 |
|
(Farombi & Onyema, 2006) |
We consider studies in which MSG was given with food to be important, as their design is relevant to chronic human intake. However, before considering their results, we must emphasize that the actual quantity of MSG/day ingested by each individual animal was somewhat difficult to quantify.
Furthermore, one study (Fujimoto et al., 2014) had an inadequate control group. The authors administered 2 g/kg of MSG (n=36) associated with unrestricted (n=18), or restricted dietary regimens (n=18), but there was only one control group (distilled water s.c. 0.1 ml/10 g), which received only a restricted dietary regimen. Obesity, increased adiposity, hyperinsulinemia, increase in triglycerides, and LDL-cholesterol were reported for the MSG-treated groups, with unrestricted dietary regimen. Since restricted dietary regimens have a profound impact on glycemic and lipid profiles (Granholm et al., 2008), we consider this to be a noteworthy issue.
MSG administration was associated with increased hepatic lipid peroxidation, reduced GSH levels and decreased catalase (CAT) and superoxide dismutase (SOD) activity. Reports of diabetes and obesity, accompanied by steatosis, inflammation, and infiltration of lymphocytes, monocytes, and macrophages, with fibrosis and neoplastic alterations, nodular lesions, and deterioration of bile ducts, were also found. Moreover, it was reported that orally administered MSG determined an increase of liver oxidative stress at a dose which extrapolated for humans would be 0.6 mg/kg (El-Meghawry El-Kenawy, Osman, & Daghestani, 2012; Eweka et al., 2011; Nakanishi et al., 2008; Onyema, Farombi, Emerole, Ukoha, & Onyeze, 2006; Paul, Abhilash, Varghese, Alex, & Nair, 2012).
The seven studies included in this analysis indicated alterations in hepatic morphology and antioxidant defense, observed for different doses and routes of administration. Only one report (Onyema et al., 2006) of increased oxidative stress, following oral administration of doses which approach human dietary intake, seems substantial. The high dosing and routes of administration that fail to mimic the normal metabolic pathway of orally ingested glutamate make it difficult to extrapolate to plausible hepatotoxic effects associated with chronic dietary intake of MSG.
Impact of MSG on CNS function and morphology
The main findings of the preclinical studies, which reported behavioral and physiological alterations following MSG administration, are presented in Table 5.
Table 5.
Summary of preclinical studies investigating MSG neurotoxicity
| Mode of administration | Species | MSG dosage/day | Duration of administration (days) | MSG effect (vs. control) | Reference |
|---|---|---|---|---|---|
| Oral | Mice | 10 mg/kg (n=10) 20 mg/kg (n=10) 40 mg/kg (n=10) 80 mg/kg (n=10) |
28 | The doses of 40 and 80 mg/kg MSG determined:
|
(Onaolapo et al., 2016) |
| Subcutaneous | Newborn Wistar rats | 4 g/kg (n=8) | 4 (postnatal days 1, 3, 5, and 7) |
|
(Rivera-Cervantes et al., 2014) |
| Subcutaneous | Male Wistar rats | 5 mg/kg (n=8) | 30 |
|
(Sadek et al., 2016) |
| Subcutaneous | CFW strain mice | 2 g/kg on the 2nd and 4th postnatal days 4 g/kg, on the 6th, 8th and 10th postnatal days (n=8) |
5 | MSG postnatal treatment led to adult mice with:
|
(Campos-Sepulveda et al., 2009) |
| Intraperitoneal | Adult female Wistar rats | 2 g/kg | 7 |
|
(Shivasharan, Nagakannan, Thippeswamy, & Veerapur, 2014) |
| Intraperitoneal | Wistar albino rats of both sexes | 2 g/kg | 7 |
|
(Swamy et al., 2014) |
As for CNS function, increased aggressiveness, possibly due to overactivation of glutamate pathways, associated with decreased γ-aminobutyric acid (GABA) levels (Campos-Sepulveda et al., 2009; Swamy et al., 2014) and lower locomotor activity, possibly on account of free radical-induced dopaminergic neurodegeneration (Swamy et al., 2014), were reported. Administration of MSG was also correlated with alteration in antioxidant defense homeostasis, secondary to loss of integrity and functionality of neuronal membranes, with increased nonspecific permeability to several ions, and pathological changes of intracellular metabolic processes (Swamy et al., 2014).
Oral and subcutaneous administration of MSG was associated with changes in the hippocampus, such as a lower cyclic AMP-activated protein kinase (AMPK) activity and increased levels of apoptosis mediator Fas ligand (Dief, Kamha, Baraka, & Elshorbagy, 2014). Also, the upregulation of pro-apoptotic Bax (Bcl-2-associated X) protein (Sadek et al., 2016) was reported. Morphological changes, such as alterations of hippocampal neuronal histology or neuronal damage in the cerebrum and cerebellum, were reported following parenteral or oral MSG treatment in rodents (Onaolapo et al., 2016; Swamy et al., 2014).
Although a significant increase in plasma glutamate and glutamine were reported following oral administration in mice, no significant differences in total brain glutamate or glutamine levels were found (Onaolapo et al., 2016). Moreover, mammals have the ability to metabolize very large oral doses, and plasma glutamate concentrations fluctuate during the day as a result of changes in diet, metabolism, and protein turnover (Henry-Unaeze, 2017). Therefore, it seems unlikely that the use of lower and orally administered doses could induce histopathological alterations in the brain.
From the located six studies, five employed parenteral routes of administration and, in some, newborn rats/mice were used. In pups, the blood–brain barrier (BBB) is just forming and they are substantially more vulnerable to MSG effects, than dogs or primates (Walker & Lupien, 2000). Also, it was highlighted that infant mice are not equipped with enzymes necessary to metabolize MSG and that doses of 2 mg/g, administered to infant mice, were comparable to about 6 g in human infants (Blood, 1969). In adult rodents, brain regions with an intact BBB were not affected by changes in plasma glutamate (Price, Olney, Lowry, & Buchsbaum, 1981; Price et al., 1984). Only the nervous structures not protected by the BBB, such as the circumventricular organs, the dorsal root ganglia, and autonomic ganglia, could be vulnerable to acute fluctuations of large magnitude (Smith, 2000).
Taking all of the above into consideration, it remains to be proven that diet-added MSG could induce behavioral, biochemical, and morphological changes in structures such as cerebrum, hippocampus, and cerebellum of adult mammals.
Effects of MSG on fertility and fetal development
An important safety issue raised by preclinical studies is the effects of MSG chronic exposure on fertility and fetal development. Results of the preclinical studies found researching these matters are summarized in Tables 6 and 7.
Table 6.
Summary of preclinical studies investigating MSG administration effect on fertility
| Mode of administration | Species | MSG dosage/day | Duration of administration | MSG effect (vs. control) | Reference |
|---|---|---|---|---|---|
| Mixed with diet | Adult female Wistar rats | 0.04 mg/kg (n=8) 0.08 mg/kg (n=8) |
14 |
|
(Eweka & Om’iniabohs, 2011) |
| Mixed with diet | Adult female Wistar rats | 0.04 mg/kg (n=8) 0.08 mg/kg (n=8) |
14 |
|
(Eweka et al., 2010) |
| Intraperitoneal | Wistar male rats | 4 g/kg (n=24) | 14 |
|
(Nosseir et al., 2012) |
| Subcutaneous | Female Swiss Albino mice newborns | 2 g/kg (n=8) | 5 (2nd, 4th, 6th, 8th and 10th day of life) | After 75 days of recovery, the following results were reported for MSG groups:
|
(Das & Ghosh, 2011a) |
| Subcutaneous | Male Swiss Albino mice newborns | 2 g/kg (n=7) | 5: 2nd, 4th, 6th, 8th and 10th day of life. | After 75 days of recovery, the following results were reported for MSG groups:
|
(Das & Ghosh, 2011b) |
Table 7.
Summary of preclinical studies investigating the effect of MSG administration on fetal development
| Mode of administration | Species | MSG dosage/day | Duration of administration | MSG effect (vs. control) | Reference |
| Intragastric | Swiss Albino mice | Maternal mice were given 1.0, 2.0, 4.0 g/kg MSG | Maternal mice received MSG for 3 consecutive days: at 17–19 days of pregnancy. Offspring were assessed. |
MSG administration alters behavior, histopathology, genetic toxicity, and expression of apoptosis-related gene. | (Yu et al., 2006) |
| Oral | Kunming mice | Maternal mice were given 2.5 g/kg or 4.0 g/kg MSG | Maternal mice received MSG for 3 consecutive days: at 17–19 days of pregnancy. Offspring were assessed. |
|
(Yu et al., 1997) |
| Mixed with diet | Wistar rats male and female and their offspring | a) 2.5 g MSG/day representing 10% of dry weight of the average daily food ration b) 5 g MSG/day representing 20% of dry weight of the average daily food ration (in mother and offspring) |
In utero life + 90 days after | Maternal feeding with 5 g MSG per day results in:
|
(Hermanussen et al., 2006) |
In adult male rats, morphological changes in testes, as well as sperm abnormalities, were reported following intraperitoneal MSG administration. The changes were reversible, as improvements were observed after cessation of treatment (Nosseir, Ali, & Ebaid, 2012). Dietary exposure of adult female Wistar rats to 0.04 – 0.08 mg/kg MSG, determined pathological alterations of oocytes (Eweka & Om’iniabohs, 2011) and of the fallopian tubes (Eweka, Eweka, & Om’iniabohs, 2010), in a dose-dependent manner. Once again, we point out that there are some inconsistencies regarding these studies, one issue being the dosage employed. The authors have justified the dosages based on previous work done with this food additive. However, the previous study was comprised of doses of 1.46 g/kg and, respectively, 2.92g/kg (Eweka & Om’iniabohs, 2007). Furthermore, the studies did not include a report of the employed statistics and the exact number of pathological changes per group was not specified. Therefore, the relevance of these results to human health risk is questionable.
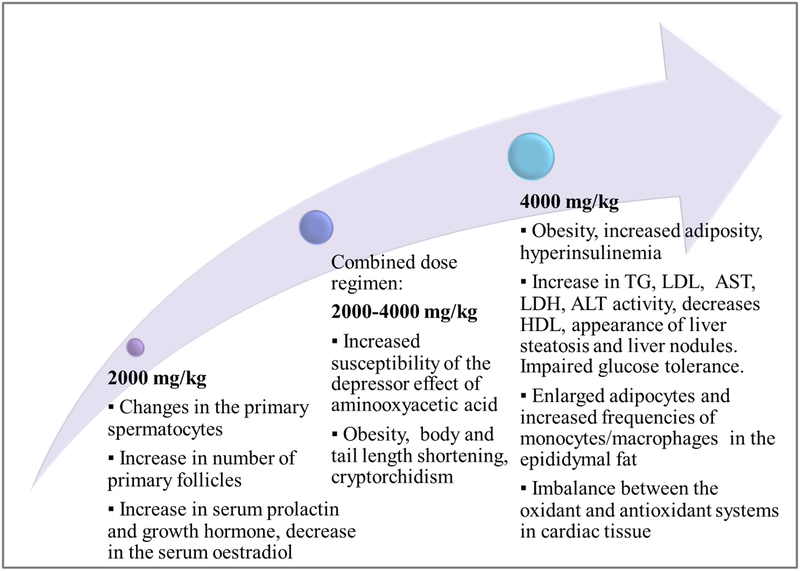
MSG intake was associated with an increased numbers of primary follicles and of primary spermatocytes in the pachytene stage, in two different studies, both of which employed newborn mice (Das & Ghosh, 2011a, 2011b). However, the control group of Das and Ghosh (2011-a) included only 5 mice. We believe that using a slightly higher number of animals could have offered higher reliability, from a statistical point of view. The same studies investigated the effects of MSG administration during the perinatal period, reporting pathological alterations (Das & Ghosh, 2011a, 2011b). Taking into consideration the high doses used, and the mode and duration of administration, as highlighted in Table 6, we consider them to have little impact on human toxicology following dietary intake.
Another important issue related to MSG is exposure during pregnancy. Negative health effects on offspring after MSG administration during gestation, such as decreased convulsion threshold, impaired Y-maze discrimination learning, increased body weight, and lower serum levels of growth hormone and insulin growth factor 1 (IGF-1) were reported (Yu et al., 2006; Yu, Zhao, Shi, Ma, & Yu, 1997). The offspring in utero-exposed to MSG, exhibited functional and morphological cerebral alterations, with increased expression of apoptosis-related genes and genetic toxicity (Yu et al., 2006).
With respect to MSG’s effects on fertility and fetal development, six of the eight studies included in the analysis used exceedingly high doses, with little relevance for human dietary intake, and/or parenteral routes of administration. We consider further investigations, using appropriate dosages and routes of administration, are necessary to assess the toxicity of chronic MSG exposure on the reproductive system and fetal development.
Promotion of tumor development in MSG-induced obesity
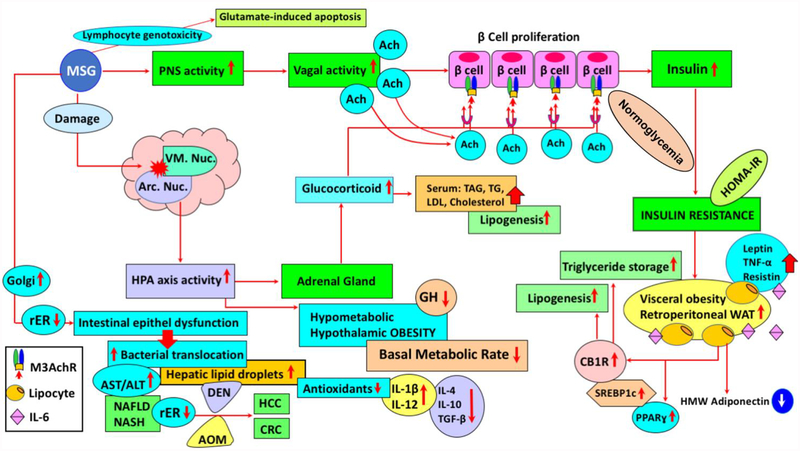
Various non-neuronal cells, including lymphocytes and thymocytes, have been shown to express glutamate receptor (Pavlovic, Cekic, Sokolovic, & Djindjic, 2006). Obesity models in mice can be established via treatment of newborn pups with MSG (2 mg/kg body weight for 4 days, followed by 4 mg/kg body weight for the next 6 days). A low-grade chronic inflammatory state, accompanied by pro-inflammatory cytokine increments and adiponectin gene expression reduction were observed (Figure 1) (Hernandez-Bautista et al., 2014; Tchkonia et al., 2010). Serum biochemical profile of MSG-treated mice showed hyperinsulinemia, hypercholesteremia, and hyperglycemia (Hata et al., 2012). Indeed, neonatal administration of MSG in mice provides a model of obesity with impaired glycemic control and increased serum levels of resistin and leptin (Roman-Ramos et al., 2011). An increase in mRNA expression of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNFα), resistin, and leptin was observed, also with the activation of both peroxisome proliferator-activated receptor α (PPARα) and γ (PPARγ), reflecting the MSG-induced pro-inflammatory profile (Figure 1) (Roman-Ramos et al., 2011). MSG-treated obese mice showed metabolic alterations, with differential gender susceptibility throughout a lifespan and during the aging process, as at 8 months male mice had a 13% higher body weight, while at 12 months females had significantly higher body weight than males (Hernandez-Bautista et al., 2014). In this context, obesity promoted-hepatic steatosis and inflammation were closely associated with liver carcinogenesis (Park et al., 2010; Siegel & Zhu, 2009). Additionally, MSG-induced obesity and diabetes, with steatosis and steatohepatitis, resemble human non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), with pre-neoplastic lesions (Figure 1) (Nakanishi et al., 2008). In fact, cellular hyperexcitability, via stimulation of mineral sodium voltage-gated channels and ligand excitatory glutamate, and non-neuronal excitatory receptors, could be a significant causative and pathogenic factor of cancer (Hoang, Levine, Pham, & Shaw, 2007). Increased levels of oxidative stress and aberrant hepatic lipogenesis, both of which are significantly linked to obesity and metabolic syndrome, were also dominantly observed during liver carcinogenesis and hepatocellular carcinoma progression (Ratziu et al., 2002; Siegel & Zhu, 2009). Elevated activities of drug-metabolizing enzymes, NAD(P)H:quinone oxidoreductase 1 and UDP-glucuronosyltransferases 1A, caused alterations in drug pharmacokinetics, in a MSG-mouse model of obesity (4 mg/g/day, s.c., MSG to newborn mice, in the first 6 days of life, followed by 10 mg/g day in days 7 and 8, and 20 mg/g in days 9 and 10). Moreover, decreased capacity of glutathione S-transferases, in obese animals, indicated a potentially reduced antioxidant defense and diminished chemoprotection (Dluzen & Lazarus, 2015; Matouskova et al., 2015). These enzymes can either inactivate carcinogens or, in some cases, generate reactive species with higher reactivity compared to the original compound. Data on the relation between the cytochrome P450, glutathione S-transferase, and UDP-glucuronosyltransferase activities and colorectal cancer, obtained from clinical and epidemiological studies in humans, have been recently reviewed (Beyerle, Frei, Stiborova, Habermann, & Ulrich, 2015).
Figure 1. Synergistic action of the HPA axis and PNS hyperactivity in MSG-induced obesity. Modulation of immune response and susceptibility to carcinogenic agents in MSG-induced obese subjects.
(MSG: monosodium glutamate, PNS: parasympathetic nerve, ACh: acetylcholine, M3AchR: M3 subtype of cholinergic muscarinic receptor, HPA: hypothalamus–pituitary–adrenal axis, CB1R: peripheral cannabinoid receptor 1, Arc Nuc: hypothalamic arcuate nucleus, rER: Rough Endoplasmic reticulum, VM Nuc: ventromedial nucleus, WAT: white adipose tissue, PPARɣ: peroxisome proliferator-activated receptor-gamma, TNFα: tumor necrosis factor-alpha, IL-6: interleukin-6, AST: aspartate aminotransferase, ALT: alanine aminotransferase, IL-1β: interleukin-1beta, NAFLD: nonalcoholic fatty liver disease, NASH: nonalcoholic steatohepatitis, DEN: diethylnitrosamine, AOM: azoxymethane, HCC: hepatocellular carcinoma, CRC: colorectal carcinogenesis, SREBP-1c: sterol regulatory element-binding protein-1c, TGF-β: transforming growth factor beta, HMW-adiponectin: high-molecular weight adiponectin, TAG: chylomicron triacylglycerol, TG: triglycerides, LDL: Low-density lipoproteins).
It was shown that diethylnitrosamine (DEN)-induced liver tumorigenesis, in MSG-treated mice, reflected steatosis-related liver carcinogenesis in human (Figure 1) (Miyazaki et al., 2016). In a model that aimed to investigate the involvement of obesity-related factors in colorectal cancer, newborn mice were administered MSG, 2 mg/g, s.c., for four days and azoxymethane (AOM), 15 mg/kg, i.p, a potent carcinogen that induces precancerous lesions in the colon. Hyperinsulinemia, hypercholesteremia and hyperglycemia were observed in MSG-treated mice, with or without AOM administration. The mRNA expression of IGF-1 receptor (IGF-1R) was increased in the MSG-AOM mice, when compared to the mice given AOM alone (Hata et al., 2012). The findings suggested that MSG-treated mice are highly susceptible to AOM-induced colorectal carcinogenesis.
As a result of the metabolic reprogramming of many types of cancer cells, glucose is mainly catabolized by aerobic glycolysis in tumors, while glutamine is converted to glutamate by glutaminase, which is further converted to α-ketoglutarate through the Krebs cycle. This reprogrammed pathway plays a major role in ATP synthesis, maintains redox balance, and regulates energy consumption in cancer cells (Scalise, Pochini, Galluccio, Console, & Indiveri, 2017).
Treatment of newborn rats with repeated doses of MSG resulted in altered morphology and functionality of alveolar macrophages. Intracellularly, numerous lipid vacuoles and lamellar structures were observed, in addition to an approximately 50% increase in total cellular lipid content. As a result, a decreased intracellular killing ability and inhibitory function on the growth and progression of tumor cells was reported (Liu, Wong, & Mak, 1989).
Prior to the implanting of Walker-256 tumor cells, newborn male Wistar rats were subcutaneously injected with 400 mg/kg MSG. The development of the tumor was higher in obese-MSG tumor rats compared to control tumor rats. Activation of the insulin-IR-ERK1/2 pathway and an anti-apoptotic effect might be the mechanisms involved in the higher development of tumors in obesity (de Queiroz, Akamine, de Carvalho, Sampaio, & Fortes, 2015; Fonseca et al., 2011).
Thus, MSG was shown to contribute to tumor progression in a preclinical setting. However, these experimental conditions do not mimic human dietary consumption of MSG and have little relevance for human tumorigenesis.
Effects of MSG on the immune system
The effect of MSG on the immune systemn was directly assessed in experiments employing cell cultures. MSG (250 – 8000 μg/ml) significantly, and dose-dependently, increased the frequencies of chromosome aberrations and sister-chromatid exchanges in human lymphocytes. However, replication and nuclear division indices were not affected. These results indicate that, at a cellular level, MSG may exert genotoxic effects on human peripheral blood lymphocytes (Figure 1) (Ataseven, Yuzbasioglu, Keskin, & Unal, 2016). Exposure to increasing MSG concentrations (1–100 mM) showed a dose-dependent effect on B cell viability. Glutamate induced apoptosis in both memory and naive B cell populations and this effect is most likely mediated through metabotropic glutamate receptor (mGluR) 7 receptors (Jovic et al., 2009). Likewise, naive and memory lymphocytes express different glutamate receptors. Differential expression profiles of glutamate receptors contribute to the induction of oxidative stress and apoptosis in immune cells (Jovic et al., 2009).
Neonatal administration of MSG (4 mg/g, s.c., days 2, 4, 6, 8, and 10 of life) to rats, led to elevated serum concentrations of interleukin (IL)-1β and IL-12, with a lowering of IL-4, IL-10, and tumor growth factor (TGF)-β levels (Falalyeyeva et al., 2017). During the acute phase response of inflammatory stress, in newborn MSG-treated (4 mg/g, i.p., day 2 of life) rats, the corticotropic-adrenal response, leptin, insulin, and triglyceride levels were higher, pro-inflammatory cytokine response was impaired, and anti-inflammatory cytokine response remained normal. These results indicate that metabolic and neuroendocrine-immune functions were altered in MSG-exposed rats (Castrogiovanni, Gaillard, Giovambattista, & Spinedi, 2008). Further, altered lipid absorption due to MSG (2% solution, intraduodenally) may be related to inflammatory responses and damage to the lining of the small intestine (Kohan, Yang, Xu, Lee, & Tso, 2016). The detrimental effects of postnatal MSG administration (2 mg/g, s.c., days 1, 2, 4, 6, 8, and 10 of life) may lead to impairment of defense mechanisms against pathogenic microorganisms (Nakadate, Motojima, Hirakawa, & Tanaka-Nakadate, 2016).
A decline in the percentage of blood lymphocytes, but without influencing the basal phagocytic activity of the neutrophils, was reported (Hriscu, Saulea, Vidrascu, & Baciu, 1997). In contrast, a neonatal MSG study (4 mg/g, s.c., day 4 of life) showed an increase in the number of macrophages, and their phagocytic activity as well (Pelaez, Blazquez, Pastor, Sanchez, & Amat, 1999).
MSG treatment (4 mg/g, i.p., 6 consecutive days) influenced thymocyte proliferation by enhancing apoptosis rate (Pavlovic et al., 2006), and it was also related to the increase of oxidative stress (Farombi & Onyema, 2006; Pavlovic et al., 2007). In addition to higher apoptotic rates, malondialdehyde (MDA) levels and xanthine oxidase (XO) activity increased, while CAT activity decreased (Pavlovic et al., 2007). Also, more MSG-induced thymocyte apoptosis can be ameliorated with high doses (250 μM) of ascorbic acid (Campbell, Cole, Bunditrutavorn, & Vella, 1999; Maellaro, Bello, & Comporti, 1996; Pavlovic, Cekic, Bojanic, Stojiljkovic, & Rankovic, 2005).
Analysis of the delayed-type hypersensitivity (DTH) depression in MSG-treated (2 mg/g, s.c., days 2–7 of life) mice, by the use of the macrophage migration inhibition assay, showed dysfunction of DTH effector T cells (Kato et al., 1986). A correlation between natural killer (NK) cell cytotoxicity depression and decreased numbers of large granular lymphocytes was also noted, in the MSG-treated mice (Belluardo, Mudo, & Bindoni, 1990).
Although we do not contest the results of the studies presented above, we cannot conclude that MSG exerts detrimental effects on the immune system in humans, as their design is inappropriate for extrapolation to human dietary exposure.
Other effects associated with MSG
Preferential consumption of water containing various concentrations of MSG, versus plain water, was reported in mice. The higher the concentration, the greater the preference (71% consumption difference in favor of 3% MSG solution). However, the total liquid consumption was unaffected by MSG addition, the control group and the tested groups consumed approximately 150 ml liquid/group (Buzescu, Cristea, Avram, & Chiriţă, 2013). Similar results were also seen in human volunteers, as described in the following section referring to clinical trials.
The addition of 100 g/kg MSG to standard diet increased food consumption. Aside from food overconsumption, the MSG group presented a metabolic dysfunction with increased levels of glucose, triacylglycerols, insulin, leptin, and homeostasis model assessment index (Diniz et al., 2005). However, this study used a mega-dose of the food additive (more than 25 times higher than the regular dietary intake), which could not be reached in real life.
An existing relation between neural taste processing and working memory networks was hypothesized (Meyer-Gerspach et al., 2016). Administration of 1 g MSG as a 30 mM solution, via a nasogastric tube, significantly altered brain activation patterns in the primary gustatory cortex, as well as in subcortical structures, previously reported to be involved in emotional learning and memory. Nevertheless, working memory performance was unaffected (Meyer-Gerspach et al., 2016). This aspect is important in establishing whether flavor enhancers have some bearing on affective responses and possibly behavior, thereby causing food overconsumption (Meyer-Gerspach et al., 2016).
Studies based on animal models have been inconsistent regarding a relationship between MSG consumption and body weight: while some reported a negative correlation (Kondoh & Torii, 2008), others suggested a direct link, associating MSG intake with higher energy intake and weight gain (Hirata, Andrade, Vaskevicius, & Dolnikoff, 1997). The observed differences might be a consequence of the different macronutrient composition of the preloads used in the different studies.
MSG administration seemed to have an impact on glucose metabolism. When given to healthy (Bertrand, Puech, Loubatieres-Mariani, & Bockaert, 1995) and, respectively, type 2 diabetic rats (Bertrand, Ravier, Puech, Loubatieres Mariani, & Bockaert, 1997), during an oral glucose tolerance test, insulin secretion was increased and glucose tolerance was improved. MSG seemed to interfere with hepatic gene expression. In a feline model (MSG diet: 30% protein and 1,125% MSG, with an average MSG intake of 201.4 ± 18.65 mg/kg), MSG exposure in utero in the first months of life led to obesity, steatosis, insulin secretion impairment, and alterations in the expression of genes involved in lipid metabolism (Collison et al., 2011; Tomankova et al., 2015).
Systemic administration of MSG (10 and 50 mg/kg) led to a 2- to 3-fold elevation in interstitial glutamate levels, in the rat masseter muscle, and a decrease in afferent mechanical threshold. These results indicate that even small elevations of interstitial glutamate concentration can induce afferent mechanical sensitization and alter musculoskeletal pain sensitivity (Cairns et al., 2007).
Our research group investigated the effects of oral administration of various doses of MSG on the nociceptive threshold. Our results indicated that a 300 mg/kg MSG dose, but not a 150 mg/kg dose, administered orally for 21 days, reduced significantly the thermal nociceptive threshold, the effect also being correlated with an increase in brain nitrates concentration (Zanfirescu, Cristea, Nitulescu, Velescu, & Gradinaru, 2017). However, these effects were observed for high doses, unattainable in normal human diets. We believe that an interesting future direction of research could represent the assessment of chronic low-dose MSG exposure on nociceptive threshold.
A synopsis of the in vivo studies mentioned above are summarized in Table 8. The five studies included in this section indicate that the use of MSG might be associated with different types of pain, but at very high doses, and also that it could influence metabolism and weight.
Table 8.
Summary of preclinical studies investigating other effects of MSG administration
| Mode of administration | Species | MSG dosage/day | Duration of administration | MSG effect (vs. control) | Reference |
|---|---|---|---|---|---|
| Mixed with diet | Male Wistar rats | a) 100 g/kg in standard diet b) 100 g/kg in high-fiber diet |
45 days |
|
(Diniz et al., 2005) |
| Mixed with diet | Cats | Average intake 201.4 ± 18.65 mg/kg | In utero exposure and 9 months after birth |
|
(Collison et al., 2011) |
| Subcutaneous | Newborn male Wistar rats | 4 g/kg (n=10) | First 10 days of life (injection 5 times, every other day) |
|
(Hirata et al., 1997) |
| Intravenous | Sprague-Dawley rats of either sex (n=16) | 10 mg/kg 50 mg/kg |
Single dose administration | MSG, 50 mg/kg, determined:
|
(Cairns et al., 2007) |
| Oral | Male NMRI mice | 150 mg/kg 300 mg/kg |
ays | MSG, 300 mg/kg, determined:
|
(Zanfirescu et al., 2017) |
Clinical trials on MSG exposure
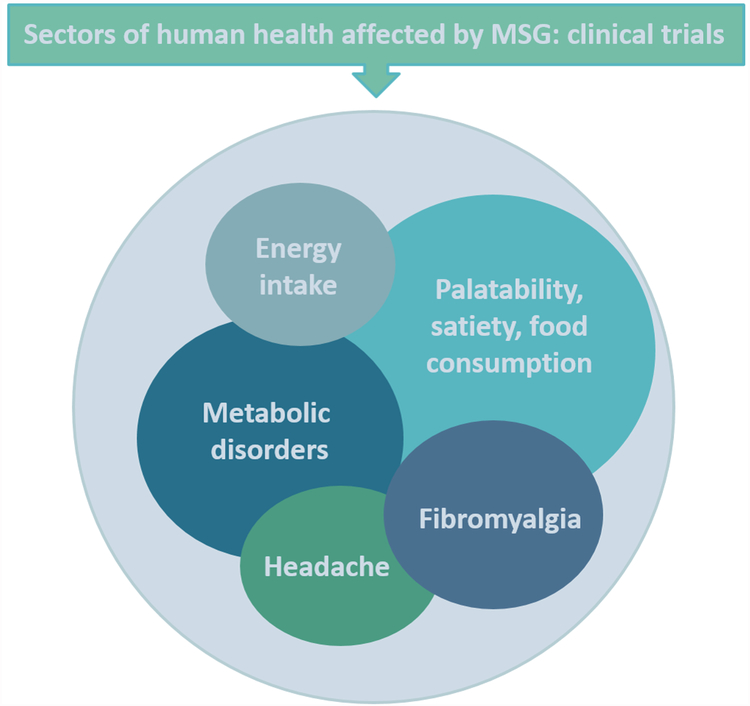
Effects on food consumption and energy intake
MSG increases food palatability, when added in low concentrations, with an expected effect of reducing satiety. However, most studies reported the opposite effect: a satiety enhancement, which could be partially sensory-specific (Masic & Yeomans, 2014b). Sensory-specific satiety consists in the decline of palatability for a specific taste after exposure to a food product (Wilkinson & Brunstrom, 2016).
Data on MSG effects on energy intake are contradictory. Some clinical studies showed no significant differences in hunger ratings or subsequent energy intake. Other studies, undertaken in nursing homes and institutions for the elderly, reported a similar dietary intake of foods with and without MSG. However, it should be taken into consideration that the chemosensory perception diminishes with age, and that the heterogeneity of the study population could have masked small differences between the treatment and control group (Essed, Kleikers, van Staveren, Kok, & de Graaf, 2009; Essed et al., 2009; Essed, van Staveren, Kok, & de Graaf, 2007; Rogers & Blundell, 1990).
The addition of 0.6% MSG to foods of medium palatability increased their spontaneous intake, without affecting overall energy intake, due to a reduction of non-MSG-enriched foods consumption, in both healthy and diabetic elderly groups (Carter, Monsivais, Perrigue, & Drewnowski, 2011). Similar results were found when supplementing chicken broth with MSG, with an increase in subjective ratings for satiety, but no alteration of energy intake at the next meal, in young adult normal-weight women, aged 20 to 40 years old (Luscombe-Marsh, Smeets, & Westerterp-Plantenga, 2008).
However, these results contradict those in other studies, which reported an increased energy intake for the MSG-supplemented soup group, versus a control broth (Imada, Hao, Torii, & Kimura, 2014; Luscombe-Marsh, Smeets, & Westerterp-Plantenga, 2009). The time between MSG presentation and the test meal were similar in both studies study (45 min). These contradictory results suggest that the influence of MSG on appetite is directly linked to the macronutrient content of meals to which it is added: a low level of protein (3 g) in the first study versus a high amount of protein in the second one (30 g). Similar results were observed regarding MSG effects on satiety, which appear to be dependent upon nutrient content, as an increase in satiety was reported only for MSG-enriched protein meals, and not for carbohydrate meals (Kochem & Breslin, 2017). Interestingly, individuals who preferred higher concentrations of MSG solution had a lower nutritional/protein status than those who preferred lower concentrations, suggesting the taste detection threshold for this food enhancer, when choosing a food product, may be correlated with a preference for protein and with habitual protein intake (Masic & Yeomans, 2017). Furthermore, the protein content of the chosen food product might be impacting satiety – for example, there is evidence suggesting leucine could suppress food intake by increasing satiety (Casperson, Sheffield-Moore, Hewlings, & Paddon-Jones, 2012; Laeger et al., 2014).
Furthermore, associating MSG, dissolved in chicken broth, with high-fat foods or sweet snacks reduced energy intake from these products in middle-aged healthy women, and reduced added sugar intake (Boutry et al., 2010).
Administration of a 2g MSG-supplemented meal for 6 days determined a significant increased antral distension for the 2-h post-ingestion, compared to sodium chloride, and led to significantly increased levels of circulating leucine, isoleucine, valine, lysine, cysteine, alanine, tyrosine and tryptophan (Boutry et al., 2010).
The clinical studies assessing MSG consumption impact on food consumption and energy intake are summarized in Table 9. The eleven clinical trials we identified investigating the effect of MSG on food consumption and energy intake, indicate that adding MSG increases food palatability and enhances satiety, partially due to a sensory-specific mechanism. While consumption of MSG-enriched meals may increase food intake, a reduction of non-MSG-enriched food products was concomitantly observed. However, the macronutrient composition is a key regulator of MSG’s effect on satiety and energy intake, as MSG-enriched protein meals, but not carbohydrate meals, increase satiety. The nutritional status of the individual is also important, as individuals with lower nutritional/protein status preferred higher concentrations of MSG (Luscombe-Marsh et al., 2008, 2009; Masic & Yeomans, 2017). Magerowski G et al examined the neurocognitive mechanisms underlying this effect. The most striking observation was that adding MSG to soups increased the engagement of a brain region associated with successful self-control during dietary decisions in a group of healthy women, suggesting that glutamate might play a significant role in cognitive executive processes regulating eating behaviors and food choices (Magerowski et al., 2018).
Table 9.
Summary of clinical studies on MSG impact on food consumption and energy intake
| Number of participants (characteristics) | Vehicle for MSG | Study protocol | Results | Reference |
|---|---|---|---|---|
| 26 volunteers (age 18–34 years old; mean BMI 22.7 kg/m2) | Spiced carrot soup | 3 hours after standardized breakfast, volunteers received 450 g of (a) low energy or (b) high-energy, high-carbohydrate and protein soup preload with added MSG/IMP [0.6% MSG (w/w) and 0.25% (w/w) IMP] or without MSG/IMP. Changes in appetite during soup intake and at a subsequent (after 45 minutes) ad libitum lunch were recorded. | Increase flavor and immediate appetite but reduced subsequent ad libitum test meal intake regardless of the protein content of the soup. | (Masic & Yeomans, 2014b) |
| 35 volunteers (age: 18–28 years old; mean BMI: 22 kg/m2) | Spiced carrot soup | 3 hours after standardized breakfast, volunteers received 450 g of (a) low energy, (b) high-energy high-carbohydrate or (c) high-energy high-protein soup preload with added MSG [1 % (w/w) MSG] or without MSG. Changes in appetite during soup intake and at a subsequent (after 45 minutes) ad libitum lunch were recorded. | MSG addition enhances significantly compensation for energy added as protein There were no differences between MSG or specific macronutrient conditions in rated satiety over the course of testing after preload intake. |
(Masic & Yeomans, 2014a) |
| 30 infants (age: 1.0–3.7 months; Weight-for-length percentile at study entry: 63.9 ± 4.9) | Milk formula | Infants received iso-caloric formulas: (a) CMF (cow milk formula) - low in free amino acids and small peptides (b) ePHF (extensive protein hydrolysate formula) - abundant in free aminoacids and small peptides or (c) cow milk formula with added free glutamate 84 mg/100 mL. A second meal of CMF was given when hunger was signaled again | Infants consumed less of formulas higher in free glutamate than of an iso-caloric formula lower in free glutamate, yet showed equivalent levels of satiation and greater levels of satiety. | (Ventura, Beauchamp, & Mennella, 2012) |
| 33 elderly individuals (age: 74.6–87 years old; BMI: 21.3±2 kg/m2) and 29 young subjects (age: 19.1–23.5; BMI: 27.4±4.9 kg/m2)b) | Mashed potatoes, spinach and ground beef | Volunteers rated pleasantness (10-point scale) of the 3 foods each with 0 g, 0.5 g, 0.8 g, 1.3 g and 2.0 g of MSG/100 g | 0.5% MSG (p<0.05) was preferred in mash potatoes. No optimal concentration was found for the other courses. | (Essed, Oerlemans, et al., 2009) |
| 53 elderly (age: 74.6–87 years old; BMI: 26.5±4.2 kg/m2) | Volunteers received 2 cooked meals with MSG (0.5% in mashed potatoes, 2% in spinach and ground meat) and without MSG in random order (single blind, cross-over design) within four weeks. | MSG 0.5% and 2% did not increase food intake in elderly people. | ||
| 83 elderly individuals institutionalized in a nursing home (age: 79.2–94.1 years old) | Cooked meal | The participants were randomly assigned to the control group (placebo 1 g maltodextrine; n=25), the MSG group (300 mg MSG + 700 mg maltodextrine; n=24), the flavor group (700 mg flavor + 300 mg maltodextrine; n=26) or to the flavor plus MSG group ((700 mg flavor + 300 mg MSG; n=25) for 16 weeks. Anthropometry data (body weight, body composition), dietary intake of the cooked meal, pleasantness and appetite data were assessed |
|
(Essed et al., 2007) |
| 120 elderly adults (age: 72±6 years old) | Tomato soup | Participants received (a) 1,200 mg/L MSG (0.12% MSG) + 3 g/L celery powder versus (b) non-enhanced soup. The effect on intake and pleasantness were assessed. |
|
(Essed, Kleikers, et al., 2009) |
| 35 women (age: 20–40 years old; BMI: 18.5–24.9 kg/m2) | Chicken broth | Participants received:
|
|
(Carter et al., 2011) |
| 60 volunteers (age: 19–63; BMI: 20–30 kg/m2) | Soup stock | On two separate experimental sessions, each subject determined (using a single blinded design) the lowest detected concentration of (a) MSG or (b) MSG +IMP. MSG was added in concentrations of 0.1%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7%, 0.8% (w/w) and IMP’5 0.25% (w/w). On each day, subjects assessed the sensory properties of the soup and their ‘liking’ and ‘eating frequency’ of high carbohydrate, fat and protein food items. |
|
(Luscombe-Marsh et al., 2008) |
| 22 volunteers (age: 19–63; BMI: 20–30 kg/m2) | Vegetable soup and rolls filled with minced meat | On five experimental sessions (single-blinded, randomized cross-over design), subjects consumed:
|
|
(Luscombe-Marsh et al., 2009) |
| 86 women (age: 30–45; BMI: 20–26 kg/m2) | Chicken flavor broth | Volunteers received
|
|
(Imada et al., 2014) |
| 13 volunteers (age: 30–50 years; BMI: 23–28 kg/m2) | Capsule | Dietary adaptation for 2×7days (cross-over, single-blinded design) Volunteers received with food (a) MSG 2 g/day or (b) NaCl 0.87 g On test day they received a test meal (38 g milk protein, 27 g fat, 99 g carbohydrate final volume of 600 mL) + (1) MSG 2g or (2) NaCl 0.87g. |
|
(Boutry et al., 2010) |
The epidemiological studies assessing the correlation between MSG consumption and food intake, satiety, and body weight are presented in Table 10. The INTERMAP cross-sectional study positively associated MSG consumption with increased body mass, in Chinese population (He et al., 2008). A prospective open-cohort, nationwide health and nutrition survey, China Health and Nutrition Survey (CHNS), seem to confirm these results (He et al., 2011). MSG intake was associated with increased BMI and prevalence of metabolic syndrome, in a dose-dependent manner, independent of the total energy intake and level of physical activity (Insawang et al., 2012). However, analysis of other subgroups included in the CHNS, as well as studies in other regions of Asia, found no association between MSG intake and weight gain (Shi et al., 2010; Thu Hien, Thi Lam, Cong Khan, Wakita, & Yamamoto, 2012).
Table 10.
Summary of epidemiological studies regarding the correlation between MSG consumption and obesity
| Study type | Number of volunteers | Protocol | Results | Reference |
|---|---|---|---|---|
| China Health and Nutrition Survey a prospective open-cohort study (1991–2006) | 10,095 healthy Chinese adults (age 18–65 years old) | Diet, including MSG and other condiments, was assessed with a weighed food inventory in combination with three 24-h recalls. The cumulative mean (±SD) MSG intake was 2.2 ± 1.6 g/day | MSG consumption was positively associated with BMI, in a dose-dependent manner, after adjustment for age, physical activity, total energy intake, and other major lifestyle factors). The adjusted hazard ratio of overweight was 1.33 (95% CI: 1.01, 1.75; p for trend < 0.01). | (He et al., 2011) |
| Cross‐sectional study INTERMAP (1997) | 752 healthy Chinese adults (age 40–59 years old) | Diet was assessed with four in‐depth multipass 24‐h recalls. Average intake was 0.33 ± 0.40 g/day. | MSG consumption was positively associated with BMI, in a dose-dependent manner, after adjustment for age, physical activity, total energy intake, and other major lifestyle factors. The multivariable‐adjusted odds ratios of overweight (BMI ≥23.0 and ≥25.0) were 2.10 (p = 0.03) and 2.75 (p= 0.04). | (He et al., 2008) |
| Epidemiological survey (2009 – 2010) | 349 healthy Thai adults (age 33–55 years old) | MSG was given as the sole source for the use in meal preparation for 10 days. Average MSG intake was 4.0 ± 2.2 g/day. The prevalence of overweight and obesity (BMI ≥ 25 kg/m2), insulin resistance (HOMA-IR >3), and the metabolic syndrome (ATP III criteria) were assessed. | MSG intake increases the prevalence of the metabolic syndrome or of BMI in a dose-dependent manner, independent of the total energy intake and the level of physical activity. | (Insawang et al., 2012) |
| Jiangsu Nutrition Study. (1st data set: 2002, 2nd data set: 2007) | 1,277 healthy Chinese adults (average age 49.3 years old) | Dietary intake patterns during the previous year were investigated by a series of detailed questions about the usual frequency and quantity of intake of thirty-three food groups and beverages. Average intake was 3.80 ± 4.30 g/day | MSG intake was not associated with significant weight gain, after adjusting for age, sex, multiple lifestyle factors and energy intake. | (Shi et al., 2010) |
| Cross-sectional survey (2008) | 1,528 Vietnamese adults (age ≥20 years old) | Dietary intake was assessed by the 24 h recall method for 3 d. MSG intake was evaluated by the weighing method on three consecutive days. Average intake was 2.2 ± 1.8 g/day. | MSG intake was not associated with significant weight gain, after adjusting for age, sex, multiple lifestyle factors and energy intake. | (Thu Hien et al., 2012) |
One of the reasons underlying the different results observed could be that the studies used different methods, especially regarding MSG and food records. The method of measurement seems highly imprecise. In the INTERMAP study, participants were asked to demonstrate the amount of MSG shaken out during preparation of a meal, this being weighed by trained interviewers. If participants went to a restaurant, interviewers asked chefs to estimate the amount of MSG added to the served dish. In the CHNS, although the number of subjects was large, the MSG intake was estimated based on the proportion of each individual’s food consumption and it is not clear how many times it was assessed. Another issue of these studies involved extremely large differences in the mean MSG intakes (0.33±4 g/day in the INTERMAP versus 2.2±1.6 g/day in the CHNS). The mean intake of the INTERMAP was similar to the lowest group in the CHNS study.
The Jiangsu Nutrition Study (JNS), showing a negative association between MSG consumption and body weight, also used an imprecise method for assessing MSG consumption: Members of each household were interviewed about their monthly consumption of MSG and other seasonings (Shi et al., 2010). These subjects were surveyed in 2002 and then followed for 5 years. In 2007, interviewers did not query the amount of MSG consumed in a month as in 2002, but estimated it based on a food frequency questionnaire.
The Thai epidemiological study is the only one in which participants were provided with a fixed amount of MSG (250 g) as the sole source of MSG for food preparation for 10 days. The amount used was determined on day 10, based on the remaining quantity. The MSG consumption was calculated by dividing the number of subjects over 10 years of age, in order to estimate grams/person/day.
Therefore, large clinical trials are needed to assess the effects of the long-term exposure to this food additive on satiety and food consumption, in relation to body weight and BMI, in which the daily MSG intake is closely monitored.
MSG sensitivity
The “Chinese restaurant syndrome” (CRS), later described as the “MSG symptom complex” (MSC), as MSG was incriminated as the main culprit (Freeman, 2006), consists of a series of symptoms such as weakness, flushing, dizziness, headache, difficulty in breathing, numbness, muscle tightness, and even syncope (Freeman, 2006; Niaz, Zaplatic, & Spoor, 2018; Walker, 1999; Williams & Woessner, 2009; Yang, Drouin, Herbert, Mao, & Karsh, 1997). MSG sensitivity is estimated to be less than 1% in the general population (Yang et al., 1997). In individuals that had a history of CRS or were MSG-sensitive, the administration of this flavor enhancer has been shown to cause mild to severe late onset (1–2 hours post ingestion) of asthmatic symptoms (Freeman, 2006). However, MSG sensitivity is usually self-assessed and placebo response is thought to play an important part in these reports (Freeman, 2006; Yang et al., 1997). Also, the cumulative effects that two or more additives can exert, must be taken into account, even if they are found at lower concentrations than their NOAEL (Kostoff, Goumenou, & Tsatsakis, 2018).
The first studies linking MSG to CRS had flawed designs and a low number of subjects. When the study population was increased in subsequent double-blind studies, no differences were detected between study and placebo groups (Freeman, 2006; Geha et al., 2000a; Tarasoff & Kelly, 1993; Williams & Woessner, 2009).
The administration of high doses (5 g) of MSG on an empty stomach to individuals with self-assessed MSG sensitivity induced CRS-associated symptoms (Freeman, 2006; Yang et al., 1997), and intake of a high dose of MSG in the absence of solid food was linked to a higher incidence of several CRS symptoms (Bawaskar, Bawaskar, & Bawaskar, 2017).
Some case reports have linked MSG consumption to urticaria and angioedema (Williams & Woessner, 2009). In patients with chronic urticaria, a high MSG dose caused no changes, even in those who reported a previous exacerbation believed to be MSG-related (Simon, 2000). Other contradictory studies had design shortcomings, such as discontinuing chronic medication and failure to disguise the taste associated with MSG (Williams & Woessner, 2009), which must be taken into account since high-dose MSG solutions have an unpleasant taste and may cause gastrointestinal discomfort, affecting a “blind” study design (Obayashi & Nagamura, 2016).
Similarly, studies linking MSG consumption to asthma exacerbation had a small sample size and questionable study design, which involved the withholding of asthma medication (Freeman, 2006; Niaz et al., 2018; Williams & Woessner, 2009). A study enrolling a large number of Chinese adults found no association between MSG consumption and asthma (Shi et al., 2012). In addition, other smaller clinical studies found no link between MSG (1 g or 5 g each) and asthmatic symptoms (Woods, Weiner, Thien, Abramson, & Walters, 1998). Other studies have noted an association between MSG and rhinitis, rhinorrhea, sneezing, and nasal itching, with improvement of symptoms following an additive-free diet and relapse when MSG was administered (Williams & Woessner, 2009). However, due to lack of reliable clinical studies, no conclusions may be drawn as to the link between MSG and rhinitis symptoms.
Impact on atopic dermatitis
Intake of MSG was alleged to aggravate atopic dermatitis, a chronic remittent skin disease common in infancy and early childhood. The undesirable effects of MSG and other food additives on atopic dermatitis were previously highlighted (Van Bever, Docx, & Stevens, 1989, Worm, Ehlers, Sterry, & Zuberbier, 2000). Adult patients with atopic dermatitis, who consumed a diet low in pseudoallergens, including MSG, showed a significant improvement of their symptoms (Worm, Ehlers, Sterry, & Zuberbier, 2000). Also, when breast-feeding mothers of children with atopic dermatitis avoided certain nut-related products and fermented foods such as soy sauce, 73% of children had significantly reduced symptoms (Uenishi, Sugiura, Tanaka, & Uehara, 2011).
Restoration of nutritional deficiencies, and personalized dietary plan excluding MSG from the diet, resulted in the improvement of skin lesions in over 85% of the cases in a study which enrolled 30 children with atopic dermatitis (Tsoukalas, 2018).
Since atopic dermatitis is a complex disease, with inflammatory and immune-mediated pathogenesis, with a variety of environmental factors, contact allergens and food additives that can act as trigger molecules (Kim, 2015; Overgaard et al., 2017). It seems plausible that food additives may worsen symptomatology, while an individualized, closely monitored additive-free diet might greatly benefit these patients. However, a direct link between MSG consumption and atopic dermatitis has yet to be established.
Influence of MSG on pain-associated conditions
Several claims have been made as to the potentiating effect of MSG regarding headaches and migraines, although strong clinical evidence in support of such claims is lacking (Freeman, 2006). In studies with MSG administration with food, no significant differences were observed at doses of 1.5 to 3 g (Obayashi & Nagamura, 2016).
Oral administration of MSG in doses of 75 or 150 mg/kg to healthy subjects brought about a significant increase in the frequency of headache reports and subjectively reported pericranial muscle tenderness. The results of this double-blinded, placebo-controlled crossover study, also showed an elevation of systolic blood pressure for the higher dose. No muscle pain or robust changes in mechanical sensitivity were reported (Baad-Hansen, Cairns, Ernberg, & Svensson, 2009). Other studies using MSG quantities ranging between 2.5–5 g also reported an increase in the incidence of headache (four out of five clinical studies) (Geha et al., 2000b; Shimada et al., 2016; Tarasoff & Kelly, 1993; Yang et al., 1997). These studies are summarized in Table 11. Nevertheless, given the small sample size, it is difficult to extrapolate these results to the general population (Baad-Hansen et al., 2009; Shimada et al., 2016) and to determine a dose that exceeded the taste threshold, thus affecting the “blind” study design (Geha et al., 2000b). Taken together, there seems to be some evidence in support of the hypothesis that MSG might trigger headaches, but again, so may other food-derived molecules (Finocchi & Sivori, 2012; Taheri, 2017).
Table 11.
Clinical studies regarding the impact of MSG consumption on headache
| Study design | MSG vehicle | Number of participants | Protocol | Results | Author |
|---|---|---|---|---|---|
| Double-blinded, placebo-controlled, crossover study | 400 mL sugar-free soda | 14 healthy participants | Volunteers received (a) MSG (75 or 150 mg/kg) or (b) NaCl (24 mg/kg, placebo) in three separate sessions | Significant increase in the incidence of headache (p<0.05) for MSG group 75 mg/kg, but not for MSG 150 mg/kg. Systolic BP was elevated in the high MSG session. |
(Baad-Hansen et al., 2009) |
| Double-blinded, placebo-controlled, crossover study | 400 mL sugar-free lemon soda | 14 healthy participants | Volunteers received (a) MSG 5g or (b) NaCl (24 mg/kg), in two distinct 5-days sessions. | Significant increase in the incidence of headache (p<0.05) for MSG group vs placebo. | (Shimada et al., 2016) |
| Multicenter, multiphase, double-blind, placebo-controlled crossover study | 200 mL citrus soda | 130 self-reported MSG-reactive participants |
Volunteers received (a) MSG 5 g one day or (b) no MSG (placebo) the following day | Significant increase in the frequency of headache (p<0.05) for MSG group vs placebo. | (Geha et al., 2000b) |
| Double-blinded, placebo-controlled, crossover study | 200 mL citrus-tasting soda | 36 participants | Volunteers received (a) MSG 1.25 g, (b) MSG 2.5 g, (c) MSG 5 g or (d) placebo (no MSG), in four distinct 1-day sessions. | Significant increase in the incidence of headache for MSG 2.5 g and 5 g vs placebo (p<0.05), not for MSG 1.25 g. | (Yang et al., 1997) |
| Randomized double-blind, placebo controlled crossover study | 71 participants | (Tarasoff & Kelly, 1993) | |||
| Soft drink | The following 2 days, 300 mL soda containing (1\a) 3.15 g MSG or (b) placebo. | No correlation between MSG consumption and headache. |
The results of a randomized, double-blinded, placebo-controlled study, showed that a single MSG administration (oral administration, 150 mg/kg) in myofascial temporomandibular disorder (TMD) patients, led to increased interstitial glutamate concentration in the masseter muscle, significantly increasing the intensity of spontaneous pain (Kitamura, Sato, Uneyama, Torii, & Niijima, 2010).
MSG administration may also impact patients suffering from fibromyalgia. One study reported that the administration of 5 g of MSG, over 3 consecutive days, led to a worsening of fibromyalgia severity, as determined by the revised fibromyalgia impact questionnaire, and a non-significant trend toward worsening fibromyalgia pain, as based on a visual analog scale (Vellisca & Latorre, 2013). However, the administration of MSG and aspartame-free meals, did not improve symptoms of fibromyalgia in a trial enrolling 72 female patients suffering from this disease (Holton, Taren, Thomson, Bennett, & Jones, 2012). The results of the above-mentioned studies suggest a pausible link between MSG use and pain-associated conditions.
Implications for other umami molecules
The best-known umami molecule is MSG. Nonetheless, 5’-mononucleotides, such as IMP and GMP, can also be included in this category and, to some extent, other L-amino acids (Kitamura et al., 2010; Kochem & Breslin, 2017; Stanska & Krzeski, 2016; Wifall et al., 2007).
It is well-established that IMP and GMP have umami effects on their own, yielding responses similar to MSG (Wifall et al., 2007). However, the pathway triggered by IMP ingestion, and central nervous integration of signals, is still only partially known (Tsurugizawa, Uematsu, Uneyama, & Torii, 2011). G-protein-coupled taste receptors TIR1/TIR3 and glutamate receptor 1, found mainly in the oral cavity and the gastrointestinal tract, but also in the pancreas, liver, adipose tissue, skeletal muscles, and hypothalamus, were shown to be activated, besides L-glutamate, by 5’-ribonucleotides (Blonde & Spector, 2017; Kitamura et al., 2010; Kochem & Breslin, 2017; Tsurugizawa et al., 2011).
The 5’-monophosphate nucleotides were found to augment glutamate effects in taste receptor cells and cortex (Festring & Hofmann, 2011; Nakamura et al., 2011; Narukawa et al., 2011; Tsurugizawa et al., 2011; Wifall et al., 2007), as the presence of IMP and GMP increases TIR1/TIR3 activation by glutamate (Blonde & Spector, 2017; Kinnamon & Vandenbeuch, 2009; Kochem & Breslin, 2017). GMP was found to be 2.3 times more effective than IMP in enhancing MSG stimulation, due to its ability to undergo Maillard-type reactions, yielding molecules with umami properties about 6 times higher than IMP (Festring & Hofmann, 2011). In addition to the MSG umami sensitivity enhancement, 5’-ribonucleotides increased sensations of sweetness and saltiness, while decreasing sourness and bitterness (Shim et al., 2015).
When testing IMP and GMP effects in rats, the two nucleotides were shown to possess similar detection thresholds and taste/hedonic qualities, with no capacity of discriminating between them (Wifall et al., 2007). However, although yielding analogous responses to MSG, the experimental animals were able to discriminate between 5’-mononucleotides and glutamate, as the nucleotides exhibited a lower detection threshold than MSG (Wifall et al., 2007).
Studies on taste receptors in solitary tract cells of rats showed that some receptors are activated by both MSG and GMP while others responded to either MSG or 5’-ribonucleotides (Kinnamon & Vandenbeuch, 2009; Wifall et al., 2007). Receptors for IMP and MSG are present in the oral cavity. Gastric administration of IMP and MSG has been shown to stimulate distinct brain areas, indicating the existence of some differences in their signaling pathways (Tsurugizawa et al., 2011). Effects on the activity of the celiac vagus nerve and on the adrenal splanchnic nerve were analogous to those of glutamate, and blocking the vagal gastric innervation resulted in blockage of IMP and glutamate signaling. Thus, 5’-ribonucleotides are implicated in controlling intestinal motility and secretory activity in the gastric phase of digestion (Kitamura et al., 2010). Moreover, it was found that gastrically administrated IMP can interfere, in a dose-dependent manner, with the activity of the vagal gastric afferent nerve, resulting in triggering vago-vagal and vago-sympathetic reflexes, while glucose, sucrose, and sodium chloride administration failed to do so (Kitamura et al., 2010).
Another molecule which potentiates MSG effect on palatability is sodium chloride. Dietary use of sodium chloride (NaCl) was associated with noticeable odor and flavor enhancement effects (Ventanas, 2010). However, it is well known that high dietary sodium (Na+) intake is linked to an increase of blood pressure, and more than 85% of Na+ is being consumed as table salt (McCallum, Lip, & Padmanabhan, 2015). MSG can be used to substitute NaCl as a flavor enhancer in low-sodium products, as it does not substantially increase the total Na+ content (Roininen, Lahteenmaki, & Tuorila, 1996). The same authors reported that the pleasantness of reduced-salt foods was increased upon the addition of MSG, relationship which could be used to regulate food consumption and energy intake, improving nutrition habits.
Both sodium and glutamate contribute to the flavor-enhancement effect in an independent manner (Okiyama & Beauchamp, 1998). It was reported that when NaCl levels were reduced from their optimal concentration of 0.92%, the palatability score decreased dramatically. However, a concentration of 0.38% MSG and 0.41% NaCl caused the recovery of the initial 0.92% NaCl-yielded palatability rating. Moreover, a 32.5% reduction in sodium levels was possible by adding 0.7% MSG to the spicy soups (Jinap et al., 2016; Yamaguchi & Takahashi, 1984). As such, a reduction in total sodium intake is possible by partly substituting dietary NaCl with MSG, while maintaining of food palatability.
MSG administration had no influence on the postprandial glucose, insulin, glucagon-like peptide (GLP)-1, and ghrelin when compared to NaCl-supplemented meals. Subjective assessments of hunger and fullness were neither affected by MSG supplementation. It seems that MSG supplementation promoted greater postprandial elevations of several indispensable amino acids in plasma suggesting a sparing effect on the uptake of some amino acids (Di Sebastiano et al., 2013).
Addition of MSG, alone or in combination with IMP, to a high-protein meal did not lead to changes in appetite and in GLP-1, glucose or insulin levels (Imada et al., 2014).
In addition to MSG, other molecules also possess noteworthy umami properties and can be used for enhancing MSG effects, or for reducing the quantity of MSG/NaCl added in food products for the improvement of palatability, thereby diminishing any possible undesired effects.
Discussion
MSG was originally defined by the FDA as a GRAS substance and its safety has been repeatedly reaffirmed by various sources within the scientific community, including the JECFA and EFSA. However, in June 2017, EFSA reassessed the safety of dietary glutamates and found a group ADI of 30 mg/kg/day (expressed as glutamic acid), highlighting that this is frequently exceeded in all age groups.
The numerous negative effects reported by preclinical studies (Figure 2) are a matter of debate, as several shortcomings of these studies (Figure 3) are apparent, namely: lack of adequate control groups, small sample size, methodological flaws, lack of dosage accuracy, or usage of extremely high doses far exceeding those unattained in normal diets. The route of administration is another important issue that must be addressed, with effects varying accordingly, as presented in Figures 4, 5 and 6. Subcutaneous or intraperitoneal administration of high doses in preclinical studies has little, if any, relevance to dietary human exposure, as they bypass the normal metabolic pathway of orally ingested MSG. Another issue that would benefit from further studies is the impact of dietary MSG on fetal development. Preclinical studies indicated behavioral changes in the rats born from mothers exposed to MSG, but due to the design of these studies, is hard to establish their relevance for human prenatal exposure.
Figure 2.
Possible impact on human health - extrapolation from preclinical studies
Figure 3.
Flaws of preclinical studies and clinical trials
Figure 4. Acute exposure in newborn rodents – parenteral administration.
TG – triglycerides, LDL – low-density lipoproteins, HDL – high-density lipoproteins, AST – aspartate aminotransferase, ALT – alanine aminotransferase
Figure 5. Subchronic exposure in adult rodents – oral administration.
NOAEL – no-observed-effects level, AST – aspartate aminotransferase, ALT – alanine aminotransferase, GGT – gamma glutamyl transferase
Figure 6. Acute exposure in adult rodents – parenteral administration.
NOAEL – no-observed-effects level
Most clinical trials have focused on food consumption and energy intake effects (Figure 7). In addition to its impact on food palatability, MSG exhibited the ability to enhance salivary secretion and to interfere with carbohydrate metabolism, while its impact on satiety and post-meal recovery of hunger varied in relation to meal composition.
Figure 7.
Clinical trials reported effects of monosodium glutamate
Reports on MSG hypersensitivity or links of its use to atopic dermatitis were found to have little supporting evidence. Furthermore, MSG was considered a possible trigger agent for some types of pain, including headache and fibromyalgia-associated pain, although strong clinical evidence supporting these claims is lacking. We consider that the influence of chronic exposure to low doses of this flavoring agent on nociception is still not fully understood and that further investigations could help clarify this matter. Moreover, this is also intriguing from the pharmacological point of view: could MSG consumption interfere with the pharmacodynamic effect of analgesics?
Conclusions
Based on a critical analysis of existing literature, we posit that many of the reported negative health effects of MSG have little relevance for chronic human exposure to low doses. In order for preclinical studies to be significant for human dietary intake, they must mimic the real context of exposure to flavor enhancers (adequate species, dosage, route of administration). Moreover, clinical and epidemiological studies need to have an optimal design, accounting for both added and natural originating MSG, thereby reporting accurate and significant data, that truly impart relevant information on its health effects. Furthermore, we consider that the assessment of MSG impact on nociception and on fetal neurodevelopment, following chronic exposure to dietary doses, represent valid research directions that should be considered.
Acknowledgements
MA was supported by grants from the National Institute of Environmental Health Sciences, NIEHS R01ES07331, NIEHS R01ES10563 and NIEHS R01ES020852.
Footnotes
The authors have no affiliation with entities/organizations with any interest in the subject matter addressed in this paper and have no conflict of interest to declare.
References
- Ataseven N, Yuzbasioglu D, Keskin AC, & Unal F (2016). Genotoxicity of monosodium glutamate. Food and Chemical Toxicology, 91, 8–18. doi: 10.1016/j.fct.2016.02.021 [DOI] [PubMed] [Google Scholar]
- Baad-Hansen L, Cairns B, Ernberg M, & Svensson P (2009). Effect of systemic monosodium glutamate (MSG) on headache and pericranial muscle sensitivity. Cephalalgia, 30(1), 68–76. doi: 10.1111/j.1468-2982.2009.01881.x [DOI] [PubMed] [Google Scholar]
- Baky NA, Mohamed AM, & Faddah LM (2009). Protective effect of N-acetyl cysteine and/or pro vitamin A against monosodium glutamate-induced cardiopathy in rats. Journal of Pharmacology and Toxicology, 4(5), 178–193. [Google Scholar]
- Barraj L, Murphy M, Tran N, & Petersen B (2016). Chemistry, manufacturing and exposure assessments to support generally recognized as safe (GRAS) determinations. Regulatory Toxicology and Pharmacology, 79 Suppl 2, S99–S104. doi: 10.1016/j.yrtph.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Battezzati A, Brillon DJ, & Matthews DE (1995). Oxidation of glutamic acid by the splanchnic bed in humans. American Journal of Physiology, 269(2 Pt 1), E269–276. doi: 10.1152/ajpendo.1995.269.2.E269 [DOI] [PubMed] [Google Scholar]
- Bawaskar HS, Bawaskar PH, & Bawaskar PH (2017). Chinese restaurant syndrome. Indian Journal of Critical Care Medicine, 21(1), 49–50. doi: 10.4103/0972-5229.198327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, & Bindoni M (1990). Effects of early destruction of the mouse arcuate nucleus by monosodium glutamate on age-dependent natural killer activity. Brain Research, 534(1–2), 225–233. [DOI] [PubMed] [Google Scholar]
- Bertrand G, Puech R, Loubatieres-Mariani MM, & Bockaert J (1995). Glutamate stimulates insulin secretion and improves glucose tolerance in rats. American Journal of Physiology, 269(3 Pt 1), E551–556. doi: 10.1152/ajpendo.1995.269.3.E551 [DOI] [PubMed] [Google Scholar]
- Bertrand G, Ravier M, Puech R, Loubatieres Mariani MM, & Bockaert J (1997). Effects of glutamate on glucose tolerance and insulin secretion in a rat model of type II diabetes. Diabetologia, 40, 514–514. [Google Scholar]
- Beyerle J, Frei E, Stiborova M, Habermann N, & Ulrich CM (2015). Biotransformation of xenobiotics in the human colon and rectum and its association with colorectal cancer. Drug Metabolism Reviews, 47(2), 199–221. doi: 10.3109/03602532.2014.996649 [DOI] [PubMed] [Google Scholar]
- Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, … Walker R (2007). Consensus meeting: monosodium glutamate - an update. European Journal of Clinical Nutrition, 61(3), 304–313. doi:1602526 [pii] 10.1038/sj.ejcn.1602526 [DOI] [PubMed] [Google Scholar]
- Blachier F, Boutry C, Bos C, & Tome D (2009). Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. The American Journal of Clinical Nutrition, 90(3), 814S–821S. doi: 10.3945/ajcn.2009.27462S [DOI] [PubMed] [Google Scholar]
- Blonde GD, & Spector AC (2017). An examination of the role of L-glutamate and inosine 5’-monophosphate in hedonic taste-guided behavior by mice lacking the T1R1 + T1R3 receptor. Chemical Senses, 42(5), 393–404. doi: 10.1093/chemse/bjx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood FR, Oser BL, White PL, & Olney JW. (1969). Monosodium glutamate. Science, 165(3897), 1028–1029. [DOI] [PubMed] [Google Scholar]
- Boutry C, Matsumoto H, Airinei G, Benamouzig R, Tome D, Blachier F, & Bos C (2010). Monosodium glutamate raises antral distension and plasma amino acid after a standard meal in humans. American Journal of Physiology-Gastrointestinal and Liver Physiology, 300(1), G137–145. doi:ajpgi.00299.2010 [pii] 10.1152/ajpgi.00299.2010 [DOI] [PubMed] [Google Scholar]
- Burdock GA, Carabin IG, & Griffiths JC (2006). The importance of GRAS to the functional food and nutraceutical industries. Toxicology, 221(1), 17–27. doi: 10.1016/j.tox.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Buzescu A, Cristea AN, Avram L, & Chiriţă C (2013). The addictive behaviour induced by food monosodium glutamate. Romanian Journal Of Medical Practice, 8(4), 229–233. [Google Scholar]
- Cairns BE, Dong X, Mann MK, Svensson P, Sessle BJ, Arendt-Nielsen L, & McErlane KM (2007). Systemic administration of monosodium glutamate elevates intramuscular glutamate levels and sensitizes rat masseter muscle afferent fibers. Pain, 132(1–2), 33–41. doi:S0304–3959(07)00047–4 [pii] 10.1016/j.pain.2007.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JD, Cole M, Bunditrutavorn B, & Vella AT (1999). Ascorbic acid is a potent inhibitor of various forms of T cell apoptosis. Cellular Immunology, 194(1), 1–5. doi: 10.1006/cimm.1999.1485 [DOI] [PubMed] [Google Scholar]
- Campos-Sepulveda AE, Martinez Enriquez ME, Rodriguez Arellanes R, Pelaez LE, Rodriguez Amezquita AL, & Cadena Razo A (2009). Neonatal monosodium glutamate administration increases aminooxyacetic acid (AOA) susceptibility effects in adult mice. Proceedings of the Western Pharmacology Society, 52, 72–74. [PubMed] [Google Scholar]
- Carter BE, Monsivais P, Perrigue MM, & Drewnowski A (2011). Supplementing chicken broth with monosodium glutamate reduces hunger and desire to snack but does not affect energy intake in women. British Journal of Nutrition, 106(9), 1441–1448. doi:S0007114511001759 [pii] 10.1017/S0007114511001759 [DOI] [PubMed] [Google Scholar]
- Casperson SL, Sheffield-Moore M, Hewlings SJ, & Paddon-Jones D (2012). Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clinical Nutrition, 31(4), 512–519. doi: 10.1016/j.clnu.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrogiovanni D, Gaillard RC, Giovambattista A, & Spinedi E (2008). Neuroendocrine, metabolic, and immune functions during the acute phase response of inflammatory stress in monosodium L-glutamate-damaged, hyperadipose male rat. Neuroendocrinology, 88(3), 227–234. doi: 10.1159/000124131 [DOI] [PubMed] [Google Scholar]
- Collison KS, Zaidi MZ, Saleh SM, Makhoul NJ, Inglis A, Burrows J, … Al-Mohanna FA (2011). Nutrigenomics of hepatic steatosis in a feline model: effect of monosodium glutamate, fructose, and trans-fat feeding. Genes & Nutrition, 7(2), 265–280. doi: 10.1007/s12263-011-0261-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on toxicity of chemicals in food, consumer products and the environment: Statement on food additives and developmental neurotoxicity. (2006). Retrieved from https://cot.food.gov.uk/sites/default/files/cot/cotstatementadditives.pdf
- Conn H (1992). “Umami”: the fifth basic taste. Food Science, 92(2), 21–23. doi: 10.1108/EUM0000000000953 [DOI] [Google Scholar]
- Das RS, & Ghosh SK (2011a). Long-term effects in ovaries of the adult mice following exposure to monosodium glutamate during neonatal life - a histological study. Nepal Medical College Journal, 13(2), 77–83. [PubMed] [Google Scholar]
- Das RS, & Ghosh SK (2011b). Long-term effects of monosodium glutamate on spermatogenesis following neonatal exposure in albino mice - a histological study. Nepal Medical College Journal, 12(3), 149–153. [PubMed] [Google Scholar]
- de Queiroz EA, Akamine EH, de Carvalho MH, Sampaio SC, & Fortes ZB (2015). Metformin reduces the Walker-256 tumor development in obese-MSG rats via AMPK and FOXO3a. Life Sciences, 121, 78–87. doi: 10.1016/j.lfs.2014.11.028 [DOI] [PubMed] [Google Scholar]
- Di Sebastiano KM, Bell KE, Barnes T, Weeraratne A, Premji T, & Mourtzakis M (2013). Glutamate supplementation is associated with improved glucose metabolism following carbohydrate ingestion in healthy males. British Journal of Nutrition, 110(12), 2165–2172. doi:S0007114513001633 [pii] 10.1017/S0007114513001633 [DOI] [PubMed] [Google Scholar]
- Dief AE, Kamha ES, Baraka AM, & Elshorbagy AK (2014). Monosodium glutamate neurotoxicity increases beta amyloid in the rat hippocampus: a potential role for cyclic AMP protein kinase. Neurotoxicology, 42, 76–82. doi:S0161-813X(14)00062-X [pii] 10.1016/j.neuro.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Diniz YS, Faine LA, Galhardi CM, Rodrigues HG, Ebaid GX, Burneiko RC, … Novelli EL (2005). Monosodium glutamate in standard and high-fiber diets: metabolic syndrome and oxidative stress in rats. Nutrition, 21(6), 749–755. doi:S0899–9007(05)00088–2 [pii] 10.1016/j.nut.2004.10.013 [DOI] [PubMed] [Google Scholar]
- Dluzen DF, & Lazarus P (2015). MicroRNA regulation of the major drug-metabolizing enzymes and related transcription factors. Drug Metabolism Reviews, 47(3), 320–334. doi: 10.3109/03602532.2015.1076438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food. (2017). Re-evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives. EFSA Journal, 15(7). doi: 10.2903/j.efsa.2017.4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Meghawry El-Kenawy A, Osman HE, & Daghestani MH (2012). The effect of vitamin C administration on monosodium glutamate induced liver injury. An experimental study. Experimental and Toxicologic Pathology, 65(5), 513–521. doi:S0940–2993(12)00028–0 [pii] 10.1016/j.etp.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Essed NH, Kleikers S, van Staveren WA, Kok FJ, & de Graaf C (2009). No effect on intake and liking of soup enhanced with mono-sodium glutamate and celery powder among elderly people with olfactory and/or gustatory loss. International Journal of Food Sciences and Nutrition, 60 Suppl 5, 143–154. doi:911592918 [pii] 10.1080/09637480802710216 [DOI] [PubMed] [Google Scholar]
- Essed NH, Oerlemans P, Hoek M, Van Staveren WA, Kok FJ, & De Graaf C (2009). Optimal preferred MSG concentration in potatoes, spinach and beef and their effect on intake in institutionalized elderly people. The Journal of Nutrition Health and Aging, 13(9), 769–775. [DOI] [PubMed] [Google Scholar]
- Essed NH, van Staveren WA, Kok FJ, & de Graaf C (2007). No effect of 16 weeks flavor enhancement on dietary intake and nutritional status of nursing home elderly. Appetite, 48(1), 29–36. doi:S0195–6663(06)00505–8 [pii] 10.1016/j.appet.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Eweka A, Igbigbi P, & Ucheya R (2011). Histochemical studies of the effects of monosodium glutamate on the liver of adult wistar rats. Annals of Medical and Health Science Research, 1(1), 21–29. [PMC free article] [PubMed] [Google Scholar]
- Eweka A, & Om’iniabohs F (2011). Histological studies of the effects of monosodium glutamate on the ovaries of adult wistar rats. Annals of Medical and Health Science Research, 1(1), 37–43. [PMC free article] [PubMed] [Google Scholar]
- Eweka AO, Eweka A, & Om’iniabohs FA (2010). Histological studies of the effects of monosodium glutamate of the fallopian tubes of adult female Wistar rats. North American Journal of Medicine and Science, 2(3), 146–149. doi: 10.4297/najms.2010.3146 NAJMS-2–146 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eweka AO, & Om’iniabohs FA (2007). Histological studies of the effects of monosodium glutamate on the small intestine of adult Wistar rat. Electronic Journal of Biomedicine, 2, 14–18. [Google Scholar]
- Falalyeyeva TM, Leschenko IV, Beregova TV, Lazarenko LM, Savchuk OM, Sichel LM, … Spivak MY(2017). Probiotic strains of lactobacilli and bifidobacteria alter pro- and anti-inflammatory cytokines production in rats with monosodium glutamate-induced obesity. Fiziologichnyi Zhurnal, 63(1), 17–25. [DOI] [PubMed] [Google Scholar]
- Farombi EO, & Onyema OO (2006). Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E and quercetin. Human & Experimental Toxicology, 25(5), 251–259. doi: 10.1191/0960327106ht621oa [DOI] [PubMed] [Google Scholar]
- Festring D, & Hofmann T (2011). Systematic studies on the chemical structure and umami enhancing activity of Maillard-modified guanosine 5’-monophosphates. Journal of Agricultural and Food Chemistry, 59(2), 665–676. doi: 10.1021/jf103849e [DOI] [PubMed] [Google Scholar]
- Finocchi C, & Sivori G (2012). Food as trigger and aggravating factor of migraine. Neurological Sciences, 33 Suppl 1, S77–80. doi: 10.1007/s10072-012-1046-5 [DOI] [PubMed] [Google Scholar]
- Fonseca EA, de Oliveira MA, Lobato Nde S, Akamine EH, Colquhoun A, de Carvalho MH, … Fortes ZB (2011). Metformin reduces the stimulatory effect of obesity on in vivo Walker-256 tumor development and increases the area of tumor necrosis. Life Sciences, 88(19–20), 846–852. doi: 10.1016/j.lfs.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Freeman M (2006). Reconsidering the effects of monosodium glutamate: a literature review. Journal of the American Academy of Nurse Practitioners, 18(10), 482–486. doi: 10.1111/j.1745-7599.2006.00160.x [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Tsuneyama K, Nakanishi Y, Salunga TL, Nomoto K, Sasaki Y, … Selmi C (2014). A dietary restriction influences the progression but not the initiation of MSG-Induced nonalcoholic steatohepatitis. Journal of Medicinal Food, 17(3), 374–383. doi: 10.1089/jmf.2012.0029 [DOI] [PubMed] [Google Scholar]
- Geha RS, Beiser A, Ren C, Patterson R, Greenberger PA, Grammer LC, … Saxon A (2000a). Multicenter, double-blind, placebo-controlled, multiple-challenge evaluation of reported reactions to monosodium glutamate. Journal of Allergy and Clinical Immunology, 106(5), 973–980. doi: 10.1067/mai.2000.110794 [DOI] [PubMed] [Google Scholar]
- Geha RS, Beiser A, Ren C, Patterson R, Greenberger PA, Grammer LC, … Saxon A (2000b). Review of alleged reaction to monosodium glutamate and outcome of a multicenter double-blind placebo-controlled study. The Journal of Nutrition, 130(4S Suppl), 1058S–1062S. doi: 10.1093/jn/130.4.1058S [DOI] [PubMed] [Google Scholar]
- Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, & Sambamurti K (2008). Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. Journal Of Alzheimers Disease, 14(2), 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T (2018). Rebooting the generally recognized as safe (GRAS) approach for food additive safety in the US. Alternatives to animal experimentation, 35(1), 3–25. doi: 10.14573/altex.1712181 [DOI] [PubMed] [Google Scholar]
- Hata K, Kubota M, Shimizu M, Moriwaki H, Kuno T, Tanaka T, … Hirose Y (2012). Monosodium glutamate-induced diabetic mice are susceptible to azoxymethane-induced colon tumorigenesis. Carcinogenesis, 33(3), 702–707. doi: 10.1093/carcin/bgr323 [DOI] [PubMed] [Google Scholar]
- He K, Du S, Xun P, Sharma S, Wang H, Zhai F, & Popkin B (2011). Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). The American Journal of Clinical Nutrition, 93(6), 1328–1336. doi:ajcn.110.008870 [pii] 10.3945/ajcn.110.008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Zhao L, Daviglus ML, Dyer AR, Van Horn L, Garside D, … Stamler J (2008). Association of monosodium glutamate intake with overweight in Chinese adults: the INTERMAP Study. Obesity (Silver Spring), 16(8), 1875–1880. doi:oby2008274 [pii] 10.1038/oby.2008.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry-Unaeze HN (2017). Update on food safety of monosodium L-glutamate (MSG). Pathophysiology, 24(4), 243–249. doi:S0928–4680(17)30072-X [pii] 10.1016/j.pathophys.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Hermanussen M, Garcia AP, Sunder M, Voigt M, Salazar V, & Tresguerres JA (2006). Obesity, voracity, and short stature: the impact of glutamate on the regulation of appetite. European Journal of Clinical Nutrition, 60(1), 25–31. doi:1602263 [pii] 10.1038/sj.ejcn.1602263 [DOI] [PubMed] [Google Scholar]
- Hernandez-Bautista RJ, Alarcon-Aguilar FJ, Del CE-VM, Almanza-Perez JC, Merino-Aguilar H, Fainstein MK, & Lopez-Diazguerrero NE (2014). Biochemical alterations during the obese-aging process in female and male monosodium glutamate (MSG)-treated mice. International Journal of Molecular Sciences, 15(7), 11473–11494. doi: 10.3390/ijms150711473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata AE, Andrade IS, Vaskevicius P, & Dolnikoff MS (1997). Monosodium glutamate (MSG)-obese rats develop glucose intolerance and insulin resistance to peripheral glucose uptake. Brazilian Journal of Medical and Biological Research, 30(5), 671–674. [DOI] [PubMed] [Google Scholar]
- Hoang BX, Levine SA, Pham P, & Shaw DG (2007). Hypothesis of the cause and development of neoplasms. European Journal of Cancer Prevention, 16(1), 55–61. doi: 10.1097/01.cej.0000220636.15976.4c [DOI] [PubMed] [Google Scholar]
- Holton KF, Taren DL, Thomson CA, Bennett RM, & Jones KD (2012). The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clinical and Experimental Rheumatology, 30(6 Suppl 74), 10–17. doi:5359 [pii] [PubMed] [Google Scholar]
- Hriscu M, Saulea G, Vidrascu N, & Baciu I (1997). Effects of monosodium glutamate on blood neutrophils phagocytic activity and phagocytic response in mice. Romanian Journal of Physiology, 34(1–4), 95–101. [PubMed] [Google Scholar]
- Husarova V, & Ostatnikova D (2013). Monosodium glutamate toxic effects and their implications for human intake: a review. The Journal of Medical Research, 1–12. doi: 10.5171/2013.608765 [DOI] [Google Scholar]
- Imada T, Hao SS, Torii K, & Kimura E (2014). Supplementing chicken broth with monosodium glutamate reduces energy intake from high fat and sweet snacks in middle-aged healthy women. Appetite, 79, 158–165. doi:S0195–6663(14)00176–7 [pii] 10.1016/j.appet.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Insawang T, Selmi C, Cha’on U, Pethlert S, Yongvanit P, Areejitranusorn P, … Hammock BD (2012). Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutrition & Metabolism, 9(1), 50. doi:1743-7075-9-50 [pii] 10.1186/1743-7075-9-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Yamamoto N, Matsuo T, Wakita S, Takeuchi H, Kume T, … Akaike A (2009). Vulnerability to glutamate toxicity of dopaminergic neurons is dependent on endogenous dopamine and MAPK activation. Journal of Neurochemistry, 110(2), 745–755. doi:JNC6178 [pii] 10.1111/j.1471-4159.2009.06178.x [DOI] [PubMed] [Google Scholar]
- Jinap S, Hajeb P, Karim R, Norliana S, Yibadatihan S, & Abdul-Kadir R (2016). Reduction of sodium content in spicy soups using monosodium glutamate. Food & Nutrition Research, 60, 30463. doi: 10.3402/fnr.v60.30463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovic Z, Veselinovic M, Vasic K, Stankovic-Djordjevic D, Cekic S, Milovanovic M, & Sarac M (2009). Monosodium glutamate induces apoptosis in naive and memory human B cells. Bratislava Medical Journal, 110(10), 636–640. [PubMed] [Google Scholar]
- Kato K, Hamada N, Mizukoshi N, Yamamoto K, Kimura T, Ishihara C, … Matsuura N (1986). Depression of delayed-type hypersensitivity in mice with hypothalamic lesion induced by monosodium glutamate: involvement of neuroendocrine system in immunomodulation. Immunology, 58(3), 389–395. [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Okiyama A, & Ueda Y (2002). Taste enhancements between various amino acids and IMP. Chemical Senses, 27(8), 739–745. [DOI] [PubMed] [Google Scholar]
- Kim K (2015). Influences of Environmental Chemicals on Atopic Dermatitis. Toxicological Research, 31(2), 89–96. doi: 10.5487/TR.2015.31.2.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon SC, & Vandenbeuch A (2009). Receptors and transduction of umami taste stimuli. Annals of the New York Academy of Sciences, 1170, 55–59. doi: 10.1111/j.1749-6632.2009.04106.x [DOI] [PubMed] [Google Scholar]
- Kitamura A, Sato W, Uneyama H, Torii K, & Niijima A (2010). Effects of intragastric infusion of inosine monophosphate and L-glutamate on vagal gastric afferent activity and subsequent autonomic reflexes. The Journal of Physiological Sciences, 61(1), 65–71. doi: 10.1007/s12576-010-0121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochem M, & Breslin PA (2017). Clofibrate inhibits the umami-savory taste of glutamate. PLoS One, 12(3), e0172534. doi: 10.1371/journal.pone.0172534 PONE-D-16–32950 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan AB, Yang Q, Xu M, Lee D, & Tso P (2016). Monosodium glutamate inhibits the lymphatic transport of lipids in the rat. American Journal of Physiology-Gastrointestinal and Liver Physiology, 311(4), G648–G654. doi: 10.1152/ajpgi.00342.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh T, & Torii K (2008). MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague-Dawley rats. Physiology & Behavior, 95(1–2), 135–144. doi:S0031–9384(08)00158–3 [pii] 10.1016/j.physbeh.2008.05.010 [DOI] [PubMed] [Google Scholar]
- Kostoff RN, Goumenou M, & Tsatsakis A (2018). The role of toxic stimuli combinations in determining safe exposure limits. Toxicology Reports, 5, 1169–1172. doi: 10.1016/j.toxrep.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, & Bhandari U (2013). Protective effect of Trigonella foenum-graecum Linn. on monosodium glutamate-induced dyslipidemia and oxidative stress in rats. Indian Journal of Pharmacology, 45(2), 136–140. doi: 10.4103/0253-7613.108288 IJPharm-45–136 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara K (2015). Umami the Fifth Basic Taste: History of Studies on Receptor Mechanisms and Role as a Food Flavor. BioMed Research International, 2015, 189402. doi: 10.1155/2015/189402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Reed SD, Henagan TM, Fernandez DH, Taghavi M, Addington A, … Morrison CD (2014). Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 307(3), R310–320. doi: 10.1152/ajpregu.00116.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WK, Wong CC, & Mak NK (1989). Effects of neonatal monosodium-L-glutamate treatment on rat alveolar macrophages. Chemico-Biological Interactions, 69(2–3), 193–201. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou L, Xu HF, Yan L, Ding F, Hao W, … Gao X (2013). A preliminary experimental study on the cardiac toxicity of glutamate and the role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor in rats. Chinese Medical Journal, 126(7), 1323–1332. [PubMed] [Google Scholar]
- Luscombe-Marsh ND, Smeets AJ, & Westerterp-Plantenga MS (2008). Taste sensitivity for monosodium glutamate and an increased liking of dietary protein. British Journal of Nutrition, 99(4), 904–908. doi:S000711450788295X [pii] 10.1017/S000711450788295X [DOI] [PubMed] [Google Scholar]
- Luscombe-Marsh ND, Smeets AJ, & Westerterp-Plantenga MS (2009). The addition of monosodium glutamate and inosine monophosphate-5 to high-protein meals: effects on satiety, and energy and macronutrient intakes. British Journal of Nutrition, 102(6), 929–937. doi:S0007114509297212 [pii] 10.1017/S0007114509297212 [DOI] [PubMed] [Google Scholar]
- Maellaro E, Bello BD, & Comporti M (1996). Protection by ascorbate against apoptosis of thymocytes: implications of ascorbate-induced nonlethal oxidative stress and poly(ADP-ribosyl)ation. Experimental Cell Research, 226(1), 105–113. doi: 10.1006/excr.1996.0208 [DOI] [PubMed] [Google Scholar]
- Magerowski G, Giacona G, Patriarca L, Papadopoulos K, Garza-Naveda P, Radziejowska J, & Alonso-Alonso M (2018). Neurocognitive effects of umami: association with eating behavior and food choice. Neuropsychopharmacology, 43(10), 2009–2016. doi: 10.1038/s41386-018-0044-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masic U, & Yeomans MR (2013). Does monosodium glutamate interact with macronutrient composition to influence subsequent appetite? Physiology & Behavior, 116–117, 23–29. doi:S0031–9384(13)00067-X [pii] 10.1016/j.physbeh.2013.03.017 [DOI] [PubMed] [Google Scholar]
- Masic U, & Yeomans MR (2014a). Monosodium glutamate delivered in a protein-rich soup improves subsequent energy compensation. Journal of Nutritional Science, 3, e15. doi: 10.1017/jns.2014.1500015 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masic U, & Yeomans MR (2014b). Umami flavor enhances appetite but also increases satiety. The American Journal of Clinical Nutrition, 100(2), 532–538. doi:ajcn.113.080929 [pii] 10.3945/ajcn.113.080929 [DOI] [PubMed] [Google Scholar]
- Masic U, & Yeomans MR (2017). Does acute or habitual protein deprivation influence liking for monosodium glutamate? Physiology & Behavior, 171, 79–86. doi: 10.1016/j.physbeh.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Matouskova P, Bartikova H, Bousova I, Levorova L, Szotakova B, & Skalova L (2015). Drug-metabolizing and antioxidant enzymes in monosodium L-glutamate obese mice. Drug Metabolism & Disposition, 43(2), 258–265. doi: 10.1124/dmd.114.061176 [DOI] [PubMed] [Google Scholar]
- Matthews DE, Marano MA, & Campbell RG (1993). Splanchnic bed utilization of glutamine and glutamic acid in humans. American Journal of Physiology, 264(6 Pt 1), E848–854. doi: 10.1152/ajpendo.1993.264.6.E848 [DOI] [PubMed] [Google Scholar]
- McCallum L, Lip S, & Padmanabhan S (2015). The hidden hand of chloride in hypertension. Pflügers Archiv - European Journal of Physiology, 467(3), 595–603. doi: 10.1007/s00424-015-1690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS (2000). Glutamate as a neurotransmitter in the brain: review of physiology and pathology. The Journal of Nutrition, 130(4S Suppl), 1007S–1015S. doi: 10.1093/jn/130.4.1007S [DOI] [PubMed] [Google Scholar]
- Meyer-Gerspach AC, Suenderhauf C, Bereiter L, Zanchi D, Beglinger C, Borgwardt S, & Wolnerhanssen BK (2016). Gut Taste Stimulants Alter Brain Activity in Areas Related to Working Memory: a Pilot Study. Neurosignals, 24(1), 59–70. doi:000442612 [pii] 10.1159/000442612 [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Shirakami Y, Kubota M, Ideta T, Kochi T, Sakai H, … Shimizu M (2016). Sodium alginate prevents progression of non-alcoholic steatohepatitis and liver carcinogenesis in obese and diabetic mice. Oncotarget, 7(9), 10448–10458. doi: 10.18632/oncotarget.7249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakadate K, Motojima K, Hirakawa TT, & Tanaka-Nakadate S (2016). Progressive Depletion of Rough Endoplasmic Reticulum in Epithelial Cells of the Small Intestine in Monosodium Glutamate Mice Model of Obesity. BioMed Research International, 2016:5251738. doi: 10.1155/2016/5251738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Goto TK, Tokumori K, Yoshiura T, Kobayashi K, Nakamura Y, … Yoshiura K (2011). Localization of brain activation by umami taste in humans. Brain Research, 1406, 18–29. doi: 10.1016/j.brainres.2011.06.029 [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Tsuneyama K, Fujimoto M, Salunga TL, Nomoto K, An JL, … Gershwin ME (2008). Monosodium glutamate (MSG): a villain and promoter of liver inflammation and dysplasia. Journal of Autoimmunity, 30(1–2), 42–50. doi: 10.1016/j.jaut.2007.11.016 [DOI] [PubMed] [Google Scholar]
- Narukawa M, Morita K, Uemura M, Kitada R, Oh SH, & Hayashi Y (2011). Nerve and behavioral responses of mice to various umami substances. Bioscience, Biotechnology, and Biochemistry, 75(11), 2125–2131. doi: 10.1271/bbb.110401 [DOI] [PubMed] [Google Scholar]
- Newsholme P (2001). Glutamine metabolism: nutritional and clinical significance. The Journal of Nutrition, 131 (9 Suppl), 2515–2522. [PubMed] [Google Scholar]
- Niaz K, Zaplatic E, & Spoor J (2018). Extensive use of monosodium glutamate: A threat to public health? Experimental and Clinical Sciences, 17, 273–278. doi: 10.17179/excli2018-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosseir NS, Ali MHM, & Ebaid HM (2012). A histological and morphometric study of monosodium glutamate toxic effect on testicular structure and potentiality of recovery in adult albino rats. Research Journal of Biological Sciences, 2(2), 66–78. [Google Scholar]
- Obayashi Y, & Nagamura Y (2016). Does monosodium glutamate really cause headache?:a systematic review of human studies. The Journal of Headache and Pain, 17, 54. doi: 10.1186/s10194-016-0639-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiyama A, & Beauchamp GK (1998). Taste dimensions of monosodium glutamate (MSG) in a food system: role of glutamate in young American subjects. Physiology & Behavior, 65(1), 177–181. [DOI] [PubMed] [Google Scholar]
- Onaolapo OJ, Onaolapo AY, Akanmu MA, & Gbola O (2016). Evidence of alterations in brain structure and antioxidant status following ‘low-dose’ monosodium glutamate ingestion. Pathophysiology, 23(3), 147–156. doi:S0928–4680(16)30022–0 [pii] 10.1016/j.pathophys.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Onyema OO, Farombi EO, Emerole GO, Ukoha AI, & Onyeze GO (2006). Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian Journal of Biochemistry & Biophysics, 43(1), 20–24. [PubMed] [Google Scholar]
- Overgaard LEK, Main KM, Frederiksen H, Stender S, Szecsi PB, Williams HC, & Thyssen JP (2017). Children with atopic dermatitis and frequent emollient use have increased urinary levels of low-molecular-weight phthalate metabolites and parabens. Allergy, 72(11), 1768–1777. doi: 10.1111/all.13157 [DOI] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, … Karin M (2010). Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell, 140(2), 197–208. doi: 10.1016/j.cell.2009.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MV, Abhilash M, Varghese MV, Alex M, & Nair RH (2012). Protective effects of alpha-tocopherol against oxidative stress related to nephrotoxicity by monosodium glutamate in rats. Toxicology Mechanisms and Methods, 22(8), 625–630. doi: 10.3109/15376516.2012.714008 [DOI] [PubMed] [Google Scholar]
- Pavlovic V, Cekic S, Bojanic V, Stojiljkovic N, & Rankovic G (2005). Ascorbic acid modulates spontaneous thymocyte apoptosis. Acta Medica Medianae, 44, 21–23. [Google Scholar]
- Pavlovic V, Cekic S, Sokolovic D, & Djindjic B (2006). Modulatory effect of monosodium glutamate on rat thymocyte proliferation and apoptosis. Bratislava Medical Journal, 107(5), 185–191. [PubMed] [Google Scholar]
- Pavlovic V, Pavlovic D, Kocic G, Sokolovic D, Jevtovic-Stoimenov T, Cekic S, & Velickovic D (2007). Effect of monosodium glutamate on oxidative stress and apoptosis in rat thymus. Molecular and Cellular Biochemistry, 303(1–2), 161–166. doi: 10.1007/s11010-007-9469-7 [DOI] [PubMed] [Google Scholar]
- Pelaez B, Blazquez JL, Pastor FE, Sanchez A, & Amat P (1999). Lectinhistochemistry and ultrastructure of microglial response to monosodium glutamate-mediated neurotoxicity in the arcuate nucleus. Histology and Histopathology, 14(1), 165–174. doi: 10.14670/HH-14.165 [DOI] [PubMed] [Google Scholar]
- Price MT, Olney JW, Lowry OH, & Buchsbaum S (1981). Uptake of exogenous glutamate and aspartate by circumventricular organs but not other regions of brain. Journal of Neurochemistry, 36(5), 1774–1780. [DOI] [PubMed] [Google Scholar]
- Price MT, Pusateri ME, Crow SE, Buchsbaum S, Olney JW, & Lowry OH (1984). Uptake of exogenous aspartate into circumventricular organs but not other regions of adult mouse brain. Journal of Neurochemistry, 42(3), 740–744. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, … Poynard (2002). Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology, 35(6), 1485–1493. doi: 10.1053/jhep.2002.33324 [DOI] [PubMed] [Google Scholar]
- Reeds PJ, Burrin DG, Stoll B, Jahoor F, Wykes L, Henry J, & Frazer ME (1997). Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. American Journal of Physiology, 273(2 Pt 1), E408–415. doi: 10.1152/ajpendo.1997.273.2.E408 [DOI] [PubMed] [Google Scholar]
- Rivera-Cervantes MC, Castaneda-Arellano R, Castro-Torres RD, Gudino-Cabrera G, Feria y Velasco AI, Camins A, & Beas-Zarate C (2014). P38 MAPK inhibition protects against glutamate neurotoxicity and modifies NMDA and AMPA receptor subunit expression. Journal of Molecular Neuroscience, 55(3), 596–608. doi: 10.1007/s12031-014-0398-0 [DOI] [PubMed] [Google Scholar]
- Rogers PJ, & Blundell JE (1990). Umami and appetite: effects of monosodium glutamate on hunger and food intake in human subjects. Physiology & Behavior, 48(6), 801–804. doi:0031–9384(90)90230–2 [pii] [DOI] [PubMed] [Google Scholar]
- Roininen K, Lahteenmaki L, & Tuorila H (1996). Effect of umami taste on pleasantness of low-salt soups during repeated testing. Physiology & Behavior, 60(3), 953–958. [DOI] [PubMed] [Google Scholar]
- Roman-Ramos R, Almanza-Perez JC, Garcia-Macedo R, Blancas-Flores G, Fortis-Barrera A, Jasso EI, … Alarcon-Aguilar FJ (2011). Monosodium glutamate neonatal intoxication associated with obesity in adult stage is characterized by chronic inflammation and increased mRNA expression of peroxisome proliferator-activated receptors in mice. Basic & Clinical Pharmacology & Toxicology, 108(6), 406–413. doi: 10.1111/j.1742-7843.2011.00671.x [DOI] [PubMed] [Google Scholar]
- Sadek K, Abouzed T, & Nasr S (2016). Lycopene modulates cholinergic dysfunction, Bcl-2/Bax balance, and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in a rat model. Canadian Journal of Physiology and Pharmacology, 94(4), 394–401. doi: 10.1139/cjpp-2015-0388 [DOI] [PubMed] [Google Scholar]
- Scalise M, Pochini L, Galluccio M, Console L, & Indiveri C (2017). Glutamine transport and mitochondrial metabolism in cancer cell growth. Frontiers in Oncology, 7, 306. doi: 10.3389/fonc.2017.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopp AL (1991). MSG and hydrolyzed vegetable protein induced headache: review and case studies. Headache, 31(2), 107–110. [DOI] [PubMed] [Google Scholar]
- Sharma E, Joshi R, & Gulati A (2018). l-Theanine: An astounding sui generis integrant in tea. Food Chemistry, 242, 601–610. doi: 10.1016/j.foodchem.2017.09.046 [DOI] [PubMed] [Google Scholar]
- Shi Z, Luscombe-Marsh ND, Wittert GA, Yuan B, Dai Y, Pan X, & Taylor AW (2010). Monosodium glutamate is not associated with obesity or a greater prevalence of weight gain over 5 years: findings from the Jiangsu Nutrition Study of Chinese adults. British Journal of Nutrition, 104(3), 457–463. doi:S0007114510000760 [pii] 10.1017/S0007114510000760 [DOI] [PubMed] [Google Scholar]
- Shi Z, Yuan B, Wittert GA, Pan X, Dai Y, Adams R, & Taylor AW (2012). Monosodium glutamate intake, dietary patterns and asthma in Chinese adults. PLoS One, 7(12), e51567. doi: 10.1371/journal.pone.0051567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemura N, Shirosaki S, Sanematsu K, Yoshida R, & Ninomiya Y (2009). Genetic and molecular basis of individual differences in human umami taste perception. PLoS One, 4(8), e6717. doi: 10.1371/journal.pone.0006717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Son HJ, Kim Y, Kim KH, Kim JT, Moon H, … Rhyu MR (2015). Modulation of sweet taste by umami compounds via sweet taste receptor subunit hT1R2. PLoS One, 10(4), e0124030. doi: 10.1371/journal.pone.0124030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Castrillon EE, Baad-Hansen L, Ghafouri B, Gerdle B, Wahlen K, … Svensson P (2016). Increased pain and muscle glutamate concentration after single ingestion of monosodium glutamate by myofascial temporomandibular disorders patients. European Journal of Pain, 20(9), 1502–1512. doi: 10.1002/ejp.874 [DOI] [PubMed] [Google Scholar]
- Shivasharan BD, Nagakannan P, Thippeswamy BS, & Veerapur VP (2014). Protective effect of Calendula officinalis L. flowers against monosodium glutamate induced oxidative stress and excitotoxic brain damage in rats. Indian Journal of Biochemistry & Biophysics, 28(3), 292–298. doi: 10.1007/s12291-012-0256-1 256 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AB, & Zhu AX (2009). Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer, 115(24), 5651–5661. doi: 10.1002/cncr.24687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RA (2000). Additive-induced urticaria: experience with monosodium glutamate (MSG). The Journal of Nutrition, 130(4S Suppl), 1063S–1066S. doi: 10.1093/jn/130.4.1063S [DOI] [PubMed] [Google Scholar]
- Singh K, & Pushpa A (2005). Alteration in some antioxidant enzymes in cardiac tissue upon monosodium glutamate [MSG] administration to adult male mice. Indian Journal of Biochemistry & Biophysics, 20(1), 43–46. doi: 10.1007/BF02893040 BF02893040 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith QR (2000). Transport of glutamate and other amino acids at the blood-brain barrier. The Journal of Nutrition, 130(4S Suppl), 1016S–1022S. doi: 10.1093/jn/130.4.1016S [DOI] [PubMed] [Google Scholar]
- Stanska K, & Krzeski A (2016). The umami taste: from discovery to clinical use. Otolaryngologia Polska, 70(4), 10–15. doi:1199991 [pii] 10.5604/00306657.1199991 [DOI] [PubMed] [Google Scholar]
- Swamy AH, Patel NL, Gadad PC, Koti BC, Patel UM, Thippeswamy AH, & Manjula DV (2014). Neuroprotective activity of Pongamia pinnata in monosodium glutamate-induced neurotoxicity in rats. Indian Journal of Pharmaceutical Sciences, 75(6), 657–663. [PMC free article] [PubMed] [Google Scholar]
- Taheri S (2017). Effect of exclusion of frequently consumed dietary triggers in a cohort of children with chronic primary headache. Nutrition and Health, 23(1), 47–50. doi: 10.1177/0260106016688699 [DOI] [PubMed] [Google Scholar]
- Takai A, Kikuchi K, Kajiyama Y, Sugiura A, Negishi M, Tsunashima H, … Miyakawa H (2014). Serological and histological examination of a nonalcoholic steatohepatitis mouse model created via the administration of monosodium glutamate. International Scholarly Research Notices, 2014, 725351. doi: 10.1155/2014/725351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasoff L, & Kelly MF (1993). Monosodium L-glutamate: a double-blind study and review. Food and Chemical Toxicology, 31(12), 1019–1035. doi:0278–6915(93)90012-N [pii] [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, … Kirkland JL (2010). Fat tissue, aging, and cellular senescence. Aging Cell, 9(5), 667–684. doi: 10.1111/j.1474-9726.2010.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu Hien VT, Thi Lam N, Cong Khan N, Wakita A, & Yamamoto S (2012). Monosodium glutamate is not associated with overweight in Vietnamese adults. Public Health Nutrition, 16(5), 922–927. doi:S1368980012003552 [pii] 10.1017/S1368980012003552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomankova V, Liskova B, Skalova L, Bartikova H, Bousova I, Jourova L, … Anzenbacherova E (2015). Altered cytochrome P450 activities and expression levels in the liver and intestines of the monosodium glutamate-induced mouse model of human obesity. Life Sciences, 133, 15–20. doi:S0024–3205(15)00255–6 [pii] 10.1016/j.lfs.2015.04.014 [DOI] [PubMed] [Google Scholar]
- Tsoukalas D (2018). Advances in clinical application of metabolomics: Treating children with atopic dermatitis Primaderma Proceedings, 23–27 May 2018. [Google Scholar]
- Tsurugizawa T, Uematsu A, Uneyama H, & Torii K (2011). Different BOLD responses to intragastric load of L-glutamate and inosine monophosphate in conscious rats. Chemical Senses, 36(2), 169–176. doi: 10.1093/chemse/bjq107 [DOI] [PubMed] [Google Scholar]
- Turkozu D, & Sanlier N (2017). L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Critical Reviews in Food Science and Nutrition, 57(8), 1681–1687. doi: 10.1080/10408398.2015.1016141 [DOI] [PubMed] [Google Scholar]
- Uenishi T, Sugiura H, Tanaka T, & Uehara M (2011). Aggravation of atopic dermatitis in breast-fed infants by tree nut-related foods and fermented foods in breast milk. The Journal of Dermatology, 38(2), 140–145. doi: 10.1111/j.1346-8138.2010.00968.x [DOI] [PubMed] [Google Scholar]
- Van Bever HP, Docx M, & Stevens WJ (1989). Food and food additives in severe atopic dermatitis. Allergy, 44(8), 588–594. [DOI] [PubMed] [Google Scholar]
- Vellisca MY, & Latorre JI (2013). Monosodium glutamate and aspartame in perceived pain in fibromyalgia. Rheumatology International, 34(7), 1011–1013. doi: 10.1007/s00296-013-2801-5 [DOI] [PubMed] [Google Scholar]
- Ventanas S, Mustonen S, Puolanne E, & Tuorila H (2010). Odour and flavour perception in flavoured model systems: Influence of sodium chloride, umami compounds and serving temperature. Food Quality and Preference, 21(5), 453–462. doi: 10.1016/j.foodqual.2009.11.003 [DOI] [Google Scholar]
- Ventura AK, Beauchamp GK, & Mennella JA (2012). Infant regulation of intake: the effect of free glutamate content in infant formulas. The American Journal of Clinical Nutrition, 95(4), 875–881. doi:ajcn.111.024919 [pii] 10.3945/ajcn.111.024919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Iwashima A, Yamada E, & Yamada R (1991). Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate. II. N-acetylglutamate synthase. Archives of Biochemistry and Biophysics, 291(1), 9–14. [DOI] [PubMed] [Google Scholar]
- Walker R (1999). The significance of excursions above the ADI. Case study: monosodium glutamate. Regulatory Toxicology and Pharmacology, 30(2 Pt 2), S119–121. doi: 10.1006/rtph.1999.1337 [DOI] [PubMed] [Google Scholar]
- Walker R, & Lupien JR (2000). The safety evaluation of monosodium glutamate. The Journal of Nutrition, 130(4S Suppl), 1049S–1052S. doi: 10.1093/jn/130.4.1049S [DOI] [PubMed] [Google Scholar]
- Weil ZM, Norman GJ, DeVries AC, & Nelson RJ (2008). The injured nervous system: a Darwinian perspective. Progress in Neurobiology, 86(1), 48–59. doi:S0301–0082(08)00058–0 [pii] 10.1016/j.pneurobio.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wifall TC, Faes TM, Taylor-Burds CC, Mitzelfelt JD, & Delay ER (2007). An analysis of 5’-inosine and 5’-guanosine monophosphate taste in rats. Chemical Senses, 32(2), 161–172. doi:bjl043 [pii] 10.1093/chemse/bjl043 [DOI] [PubMed] [Google Scholar]
- Wilkinson LL, & Brunstrom JM (2016). Sensory specific satiety: More than ‘just’ habituation? Appetite, 103, 221–228. doi: 10.1016/j.appet.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AN, & Woessner KM (2009). Monosodium glutamate ‘allergy’: menace or myth? Clinical & Experimental Allergy, 39(5), 640–646. doi: 10.1111/j.1365-2222.2009.03221.x [DOI] [PubMed] [Google Scholar]
- Williams J, Sergi D, McKune AJ, Georgousopoulou EN, Mellor DD, & Naumovski N (2019). The beneficial health effects of green tea amino acid l-theanine in animal models: Promises and prospects for human trials. Phytotherapy Research. doi: 10.1002/ptr.6277 [DOI] [PubMed] [Google Scholar]
- Woods RK, Weiner JM, Thien F, Abramson M, & Walters EH (1998). The effects of monosodium glutamate in adults with asthma who perceive themselves to be monosodium glutamate-intolerant. Journal of Allergy and Clinical Immunology, 101(6 Pt 1), 762–771. [DOI] [PubMed] [Google Scholar]
- Worm M, Ehlers I, Sterry W, & Zuberbier T (2000). Clinical relevance of food additives in adult patients with atopic dermatitis. Clinical & Experimental Allergy, 30(3), 407–414. doi:cea722 [pii] [DOI] [PubMed] [Google Scholar]
- Wu G (2009). Amino acids: metabolism, functions, and nutrition. Amino Acids, 37(1), 1–17. doi: 10.1007/s00726-009-0269-0 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, & Ninomiya K (2000). Umami and food palatability. The Journal of Nutrition, 130(4S Suppl), 921S–926S. doi: 10.1093/jn/130.4.921S [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, & Takahashi C (1984). Interactions of monosodium glutamate and sodium chloride on saltiness and palatability of a clear soup. Journal of Food Science, 65(1), 82–85. doi: 10.1111/j.1365-2621.1984.tb13675.x [DOI] [Google Scholar]
- Yang WH, Drouin MA, Herbert M, Mao Y, & Karsh J (1997). The monosodium glutamate symptom complex: assessment in a double-blind, placebo-controlled, randomized study. Journal of Allergy and Clinical Immunology, 99(6 Pt 1), 757–762. doi:S0091674997001437 [pii] [DOI] [PubMed] [Google Scholar]
- Yu L, Zhang Y, Ma R, Bao L, Fang J, & Yu T (2006). Potent protection of ferulic acid against excitotoxic effects of maternal intragastric administration of monosodium glutamate at a late stage of pregnancy on developing mouse fetal brain. European Neuropsychopharmacology, 16(3), 170–177. doi:S0924-977X(05)00133-1 [pii] 10.1016/j.euroneuro.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Yu T, Zhao Y, Shi W, Ma R, & Yu L (1997). Effects of maternal oral administration of monosodium glutamate at a late stage of pregnancy on developing mouse fetal brain. Brain Research, 747(2), 195–206. doi:S0006–8993(96)01181-X [pii] [DOI] [PubMed] [Google Scholar]
- Zanfirescu A, Cristea AN, Nitulescu GM, Velescu BS, & Gradinaru D (2017). Chronic monosodium glutamate administration-induced hyperalgesia in mice. Nutrients, 10(1). doi:nu10010001 [pii] 10.3390/nu10010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitasamy C, Pan Z, Liu W, & Zhao L (2016). Novel umami ingredients: Umami peptides and their taste. Journal of Food Science, 82(1), 16–23. doi: 10.1111/1750-3841.13576 [DOI] [PubMed] [Google Scholar]