Background:
Several steps to reduce the rate of postoperative surgical site infections (SSIs) have been implemented. The use of prophylactic antimicrobials targeting patient’s microbial flora has been associated with a decrease in postoperative infections. We evaluated the relationship between perioperative antimicrobials, baseline microbial flora, and occurrence of SSIs.
Methods:
We prospectively enrolled 241 patients scheduled to receive a postmastectomy implant-based reconstructive procedure between September 2015 and January 2018. Axillary swab cultures were obtained preoperatively, and all recovered bacteria were identified. Surgeons were blinded to these results. The use of prophylactic perioperative antimicrobials was defined as concordant if the baseline axillary flora were susceptible to the given antibiotic and discordant if not. As Staphylococcus species are the most common pathogen causative for breast implant-related infections, patients colonized with these organisms were analyzed in detail. All patients were followed up for at least 6 months postoperatively and evaluated for SSIs.
Results:
A total of 238 patients (99%) received both perioperative and postoperative oral antimicrobials. The most common preoperative staphylococci axillary flora recovered were methicillin-sensitive coagulase-negative Staphylococcus (67%), methicillin-resistant coagulase-negative Staphylococcus (35%), with only 1 case of methicillin-sensitive Staphylococcus aureus (0.4%). Thirty-three patients (14%) developed an SSI. Of those with a positive Staphylococcus culture, only 54% received a concordant antimicrobial regimen, but this was not associated with an increased risk for infection (P > 0.72).
Conclusions:
The use of perioperative antimicrobials whether concordant or discordant with the preoperative axillary microbial flora, specifically Staphylococci species, did not provide a significant impact on the risk of SSI.
The rate of breast cancer surgery has increased through the years, mainly due to the use of improved methods of cancer detection, such as radiological and genetic testing. Simultaneously, due to improved favorable economic factors, improvements in implant design, and the widespread successful use of acellular dermal matrix (ADM), breast reconstruction with tissue expanders (TEs) and implants is becoming increasingly popular among patients who have undergone mastectomy due to breast cancer prevention or treatment.1,2 The American Society of Plastic Surgeons reported that, in 2017, its members performed approximately 106,000 breast reconstructive procedures in the United States alone.3 Of these patients, approximately 74,000 underwent breast reconstruction with TEs and implants, whereas the remaining patients had autologous reconstructions.
Unfortunately, the rate of infection after breast implant reconstruction, which varies by hospital and region, remains unacceptably high, ranging from 2.5% to 24%.4–6 Infections that do not respond to antibiotic treatment alone usually require further surgery and explantation of the device, which is devastating for both patients and physicians.
Randomized controlled studies have demonstrated that prophylactic antibiotics are effective in preventing surgical wound infections.7 However, the most frequently utilized perioperative antimicrobials, mainly first-generation cephalosporins, do not cover commonly encountered skin pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) and coagulase-negative Staphylococcus resistant to methicillin (CNS-R), which are responsible for over two-thirds of all breast implant-related infections.8 The baseline skin and axillary microbial flora that are contiguous with the surgical incision site and the axillary surgical drains, which usually remain in place for 1–2 weeks, are the pathogens most likely to cause a surgical site infection (SSI).9 Therefore, selecting targeted, prophylactic perioperative antimicrobials on the basis of patients’ baseline microbial skin flora, instead of following a standard empirical “one size fits all” approach, seems a reasonable means of reducing SSIs. Several studies have demonstrated that the use of targeted perioperative antimicrobials, concordant with patients baseline microbial flora, has been associated with a decrease in postoperative SSI.10,11 We conducted this prospective observational study of patients undergoing postmastectomy implant-based reconstructive procedures to determine whether the use of antimicrobial prophylaxis concordant with patients’ baseline axillary microbial flora reduces the risk of postsurgical site infections.
METHODS
Patient Population
We prospectively enrolled 241 patients who were scheduled for a postmastectomy, 2-stage, implant-based breast reconstruction at our institution between September 2015 and January 2018. For all patients, baseline axillary swab (eSwab; Copan Diagnostics Inc., Murrieta, CA) cultures were obtained from the ipsilateral surgical site within 2 weeks before surgery, and every aerobic and anaerobic bacterial species recovered was identified. The surgeons who prescribed the patients’ perioperative antimicrobials were blinded to the results of the swab test. All patients were followed up for at least 6 months postoperatively and evaluated for potential surgical complications, including SSIs. Our study was approved by The University of Texas MD Anderson Cancer Center’s Institutional Review Board. Written informed consent was obtained from all participants.
Data Collection and Definitions
We prospectively gathered information on patients’ demographics, baseline comorbidities, breast cancer characteristics, cancer treatments, timing of TE placement, operative approaches, axillary drain tube durations, and postoperative complications. We also recorded the use of all perioperative antimicrobials—including systemic perioperative, subpectoral pocket irrigation, and postoperative oral antimicrobials—and confirmed all of them via electronic chart reviews plus inpatient and outpatient pharmacy records. The use of perioperative antimicrobials was defined as concordant if the baseline axillary flora were susceptible to the antibiotic the patient received and discordant if they were not. We also identified all patients who developed an SSI, as defined by the Centers for Disease Control and Prevention.12
Microbiology
Once a patient’s axilla was swabbed, the specimen was submitted on the same day to the microbiology department for further processing. These specimens were inoculated onto solid media for aerobic and anaerobic culture. The aerobic culture media consisted of chocolate II agar (Gonococcus [GC] II Agar with hemoglobin and IsoVitalex; BD, Franklin Lakes, NJ; catalog 221267), trypticase soy agar with 5% sheep blood (BD; catalog 221261), MacConkey II agar (BD; catalog 221270), and Columbia Naladixic Acid (CNA) agar with 5% sheep blood (BD; catalog 221353). After inoculation, the aerobic culture was incubated at 35°C for 48 hours and observed for colony growth. Colonies growing on the culture were identified phenotypically to the genus and/or species level. The anaerobic culture consisted of prereduced media purchased from Anaerobe Systems (Morgan Hill, CA; catalog AS-303): Brucella agar, laked blood kanamycin-vancomycin agar, and phenylethyl alcohol agar. After inoculation, the anaerobic culture was incubated at 35°C in anaerobic conditions using the Anoxomat System (Advanced Instruments, Norwood, MA) with an atmosphere of 5% hydrogen, 10% carbon dioxide, and the balance of nitrogen. Cultures were held for 7 days and observed for colony growth. Colonies growing on the culture were identified phenotypically to the genus and/or species level. All isolated Staphylococcus species were tested for susceptibility to oxacillin, clindamycin, trimethoprim/sulfamethoxazole, and tetracycline using ETEST strips (bioMérieux, Inc., Durham, NC). All isolated Gram-negative bacilli were tested for susceptibility testing performed on the Vitek2 AST instrumentation (bioMérieux, Inc.) using the XN06 and GN69 cards (bioMérieux, Inc.).
Statistical Analysis
Categorical variables were compared using the Chi-square or Fisher’s exact test, as appropriate. Continuous variables were compared using the Wilcoxon rank-sum test. Kaplan-Meier method was used to estimate the cumulative incidence curves of infection, and log-rank test was used for curve comparison. All tests were 2-sided tests with a P value <0.05 considered statistically significant. The data analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Clinical Characteristics
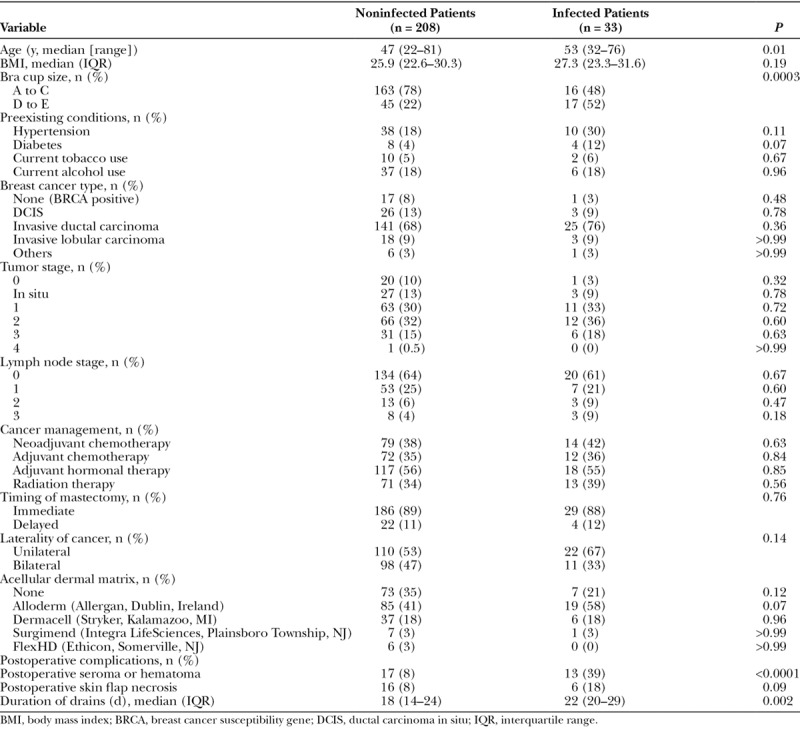
A total of 241 patients had a postmastectomy, implant-based breast reconstruction. Of these, 33 patients (14%) developed an SSI (Table 1). The mean age of the patients who developed a postoperative infection (53 years; range, 32–76 years) was higher than that of uninfected patients (47 years; range, 22–81 years; P = 0.01). A larger bra cup size (D to E) was also a risk factor for infection compared with smaller breast cup sizes (A to C) (P = 0.0003). Similarly, patients with a larger body mass index (median, 27.3; interquartile range [IQR], 23.3–31.6) were more likely to develop an SSI than were those with a smaller body mass index (median, 25.9; IQR, 22.6–30.3), although the difference was not significant (P = 0.19). Additionally, the development of a postoperative seroma or hematoma, which is usually associated with having a postsurgical drain for an extended duration (P = 0.002), was also associated with a higher risk of infection (P < 0.0001). The presence of skin flap necrosis (P = 0.09) and use of ADM (P = 0.12) were more common in patients with a postoperative infection but did not reach statistical significance. Furthermore, hypertension; diabetes; the use of tobacco or alcohol; the type of tumor or extension; the use of chemotherapy, radiotherapy, or hormonal therapy; and the timing of surgery were not risk factors for infection (P > 0.07 for each).
Table 1.
Patient Characteristics
Baseline Axillary Microbial Flora
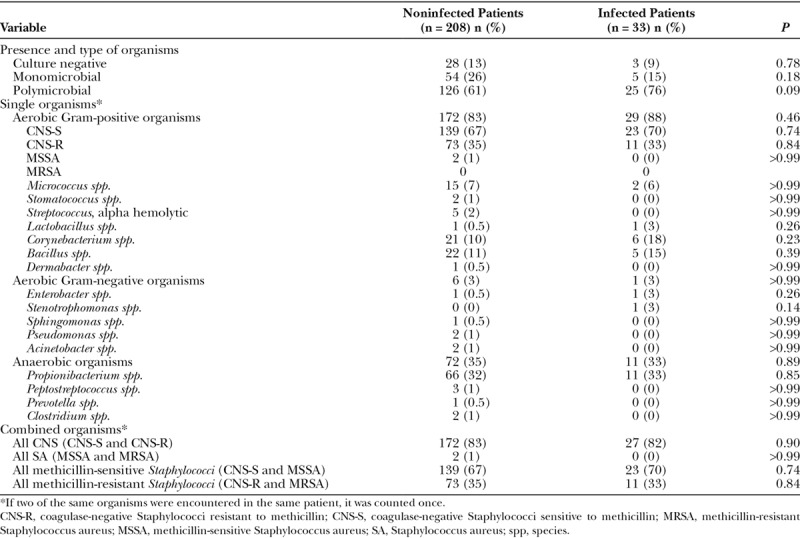
Axillary fossa swabs showed that 151 patients (63%) had polymicrobial flora, 59 (24%) had monomicrobial flora, and 31 (13%) had no positive culture. None of these results were associated with a greater risk of infection (P = 0.09, P = 0.18, and P = 0.78, respectively) (Table 2). Additionally, neither aerobic (Gram positive and Gram negative) nor anaerobic organisms were more common in patients who developed an infection than in those who did not (P ≥ 0.14 for all). This was also true if the organisms were grouped as CNS, regardless of resistance to methicillin (P = 0.90); Staphylococcus aureus (P > 0.99); or any Staphylococcus species (CNS and S. aureus), with (P = 0.84) or without (P = 0.74) resistance to methicillin.
Table 2.
Baseline Axillary Microbial Flora
Prophylactic Antimicrobial Regimens
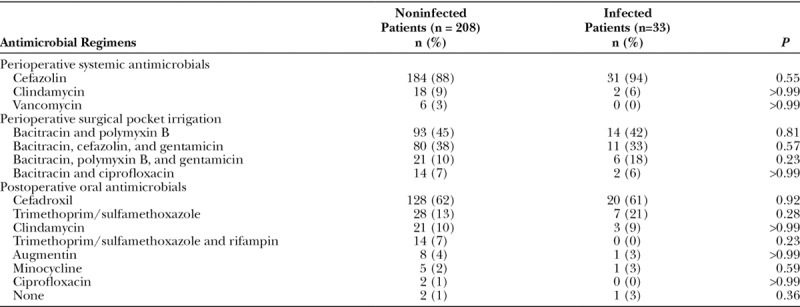
All patients received standardized perioperative systemic antimicrobial therapy (Table 3). The most common drugs used were cefazolin (215 patients; 89%), clindamycin (20 patients; 8%), and vancomycin (6 patients; 3%). Thereafter, at the discretion of each surgeon, all patients received subpectoral pocket irrigation with a broad-spectrum antimicrobial solution. The most commonly used regimen was bacitracin plus polymyxin B (44%), followed by bacitracin, cefazolin plus gentamicin (38%) and bacitracin, polymyxin B plus gentamicin (11%). After surgery, oral prophylactic antibiotics were used in 99% of cases, either for a week or until the drainage catheters were removed. The most commonly prescribed postsurgical antimicrobial drugs were cefadroxil (61%), trimethoprim/sulfamethoxazole (15%), and clindamycin (10%). None of the antimicrobials utilized were statistically associated with a higher rate for postsurgical site infections (P > 0.23).
Table 3.
Perioperative and Postoperative Prophylactic Antimicrobial Regimens
Concordance Between Prophylactic Antimicrobials and Baseline Axillary Staphylococci Flora
A total of 31 patients (13%) did not have any bacterial growth upon baseline axillary cultures, and 11 patients (5%) did not have any Staphylococci growth, for which these patients were not utilized in this part of the analysis (Table 4). Only 108 patients (54%) received a concordant systemic perioperative antimicrobial, whereas 107 patients (54%) received a concordant postoperative oral antimicrobial. In other words, in both the perioperative and the postoperative periods, approximately half of the patients received an antimicrobial to which the baseline axillary Staphylococci flora were resistant. Moreover, the probability that the combination of both peri- and postoperative antimicrobials was discordant was 40%. However, whether the patient received a concordant or discordant antimicrobial combination did not predict for infection (P ≥ 0.72; Fig. 1).
Table 4.
Concordance Between Prophylactic Antimicrobials and Baseline Axillary Staphylococci Flora
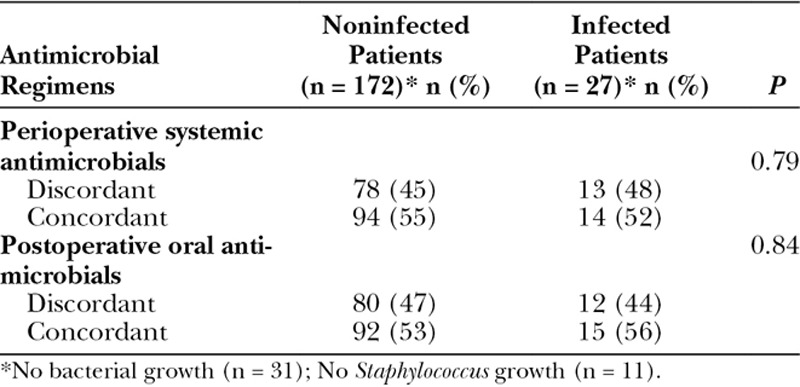
Fig. 1.
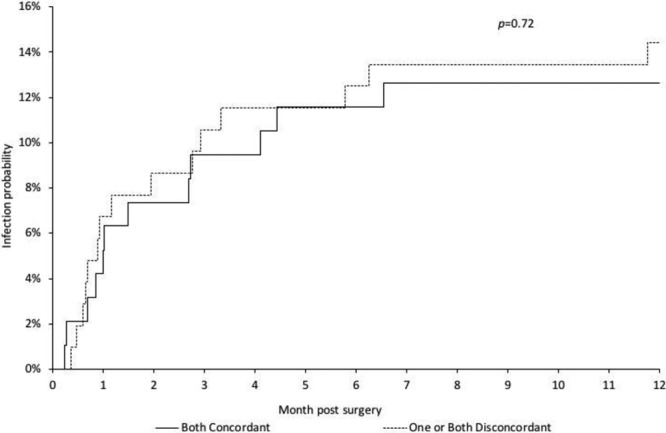
Cumulative incidence curves for infection in relation to baseline axillary Staphylococci flora and concordance of perioperative antimicrobials.
Concordance Between Prophylactic Antimicrobials and Baseline Gram-Negative Axillary Flora
A total of 7 patients (3%) had Gram-negative rods on their baseline preoperative axillary cultures (Table 2). Although all patients received discordant antimicrobials (6 patients received perioperative cefazolin followed by postoperative cefadroxil, and 1 patient received perioperative and postoperative clindamycin), only 1 patient developed an infection. In this individual, no cultures were done at the time of infection owing to the lack of any abnormal drainage; however, the implant was successfully salvaged with use of our standardized red breast protocol,13 which utilizes empiric ciprofloxacin, doxycycline, and rifampin.
Surgical Site Infections
A total of 33 patients (14%) developed an SSI, of whom 15 (45%) eventually needed TE explantation because of lack of response to antimicrobial treatment or advanced stage of infection. The median time from initial surgery to infection was 35 days (IQR, 21–95 days). Twelve (36%) of the patients with an SSI had an axillary drain in place at the onset of infection.
Of the 33 patients who developed a postoperative infection, 8 patients (24%) did not have any cultures performed owing to the lack of drainage or surgery (100% of implants were salvaged); 7 patients (21%) had cultures done, but with negative bacterial growth (71% of implants were salvaged); and 18 patients (55%) had cultures that showed positive microbial growth (28% of implants were salvaged). Of the latter group of 18 patients, only 5 (28%) had postoperative cultures which grew the same pathogen as that seen in the patient’s baseline axillary cultures (CNS-R in all cases). Two of these patients had received concordant perioperative and postoperative antimicrobials, 2 had received 1 concordant antimicrobial, and 1 patient had received both discordant antimicrobials. The remaining 13 patients (72%) had a different pathogen than that seen in the baseline axillary culture. Five of these patients had Gram-positive organisms (CNS-R, MRSA, Corynebacterium, and Bacillus species, 1 patient each); 6 patients had Gram-negative organisms (Serratia marcescens and Pseudomonas species, 3 patients each); 1 patient had a Mycobacterium abscess; and 1 patient had a mixed infection with CNS-R plus Pseudomonas.
DISCUSSION
Our study reveals that the use of concordant or discordant systemic perioperative and/or postoperative oral antimicrobial prophylaxis targeting the most common organisms colonizing the axillary flora, specifically Staphylococci species, did not reflect in a statistically significant decrease or increase in SSIs. However, because approximately half of the patients received discordant antimicrobials and did not develop an SSI, it is possible that (a) the baseline axillary flora are not risk factors for infection, (b) modern sterile surgical techniques plus intraoperative broad-spectrum antimicrobial pocket irrigations are adequate for preventing SSIs, or (c) the multiple risk factors for infection, as discussed below, may have confounded our findings for which a larger sample size would be needed to observe a statistically significant difference.
Patients undergoing implant-based reconstruction have several intrinsic risk factors that place them at a higher risk for infection than patients undergoing nonreconstructive breast surgeries. Our study, like others, identified that older age, greater body mass index, larger breast cup size, longer duration of axillary surgical drains, and development of a postsurgical seroma and/or hematoma were all associated with a higher risk for infection.14–16 Furthermore, we identified a trend toward developing SSIs among patients who developed a postsurgical skin flap necrosis or in whom ADMs were used.17,18 However, we did not observe a statistically significant correlation between SSIs and other risk factors identified in the literature, such as diabetes, the use of chemotherapy or radiotherapy, and concurrent axillary lymphadenectomy.6,14,18,19
Sterile surgical and postsurgical aseptic techniques, as well as the use of perioperative chlorhexidine and appropriate timing of perioperative antimicrobials, have all been validated as important pillars for the prevention of SSIs.7,20,21 Unless the patients in our study had a specific β-lactam allergy or due to physicians preference, all received cefazolin which would not provide adequate prophylaxis for those patients colonized with methicillin-resistant Staphylococcus species. Several clinical studies and economic models have compared the use of β-lactam and glycopeptide prophylactic antimicrobials. Although the effectiveness of both of these antimicrobial groups has been shown to be adequate, taking into account environmental selective pressure and the potential for glycopeptide-resistant organisms, these antimicrobials (including vancomycin) are the preferred prophylactic antimicrobials in institutions that have a high prevalence of MRSA infections.22–27 However, there is insufficient evidence to determine whether there is a threshold prevalence of MRSA at which vancomycin would be considered clinically useful and cost-effective.25,26
The use of preoperative MRSA screening and targeted decolonization has also shown promising results in orthopedic and cardiac surgeries.28 However, despite the fact that decolonization is utilized in clinical practice, to our knowledge, there has not been any study evaluating MRSA decolonization in patients undergoing implant-based breast reconstruction. Several other studies have evaluated the use of culture-based targeted antimicrobial prophylaxis for patients colonized with resistant pathogens, instead of the provision of a standardized empiric antimicrobial. For example, it has been shown that the presence of fluoroquinolone-resistant Escherichia coli in rectal swab cultures of patients undergoing transrectal prostate biopsy is a risk factor for subsequent septicemia, but targeted antimicrobial can reduce the risk.10,29 A cost-effectiveness analysis revealed that targeted prophylaxis yielded a cost savings of $4,499 for every infection-related complication of post-transrectal ultrasound-guided prostate biopsy that was averted and that the number needed to treat to prevent 1 infectious complication was 38.11 Targeted antimicrobials have also had favorable results in patients undergoing hepatobiliary reconstruction.30 However, some studies have not been associated with a significant statistical difference between baseline microbial flora, use of targeted antimicrobials, and risk for infection.31
The main limitation of our study is that this was an observational and not an interventional study, in which we were not able to evaluate whether the provision of a targeted antimicrobial prophylaxis based on the patient baseline axillary flora might have decreased the likelihood for SSIs. Additionally, we did not perform cultures from the anterior nares to evaluate whether or not the patients were colonized with MRSA. Furthermore, as several prophylactic antimicrobials utilized during subpectoral surgical pocket irrigation are not commonly tested in routine determinations of antimicrobial susceptibilities, these antimicrobials were not evaluated for concordance.
In summary, after an extended patient follow-up period, we found that the use of concordant or discordant antimicrobials did not impact the risk of SSIs. Therefore, to determine whether targeted antimicrobial prophylaxis based on baseline axillary flora protects against SSIs, a large-scale prospective randomized controlled study is warranted.
ACKNOWLEDGMENTS
We thank The University of Texas MD Anderson Cancer Center’s Department of Scientific Publications for their editorial assistance.
Footnotes
Published online 24 July 2019.
Presented in part at the 29th European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), April 16, 2019, Amsterdam, The Netherlands.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This research was supported by the University Cancer Foundation via the Institutional Research Grant program at The University of Texas MD Anderson Cancer Center, as well as by the National Institutes of Health/National Cancer Institute under award number P30CA016672.
REFERENCES
- 1.Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69:195–208. [DOI] [PubMed] [Google Scholar]
- 2.Halvorson EG, Disa JJ, Mehrara BJ, et al. Outcome following removal of infected tissue expanders in breast reconstruction: a 10-year experience. Ann Plast Surg. 2007;59:131–136. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Plastic Surgeons 2017 Statistics Report. [Available from: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf.
- 4.Armstrong RW, Berkowitz RL, Bolding F. Infection following breast reconstruction. Ann Plast Surg. 1989;23:284–288. [DOI] [PubMed] [Google Scholar]
- 5.Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg. 2006;118:825–831. [DOI] [PubMed] [Google Scholar]
- 6.Nahabedian MY, Tsangaris T, Momen B, et al. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112:467–476. [DOI] [PubMed] [Google Scholar]
- 7.Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–286. [DOI] [PubMed] [Google Scholar]
- 8.Viola GM, Baumann DP, Mohan K, et al. Improving antimicrobial regimens for the treatment of breast tissue expander-related infections. Plast Reconstr Surg Glob Open. 2016;4:e704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degnim AC, Scow JS, Hoskin TL, et al. Randomized controlled trial to reduce bacterial colonization of surgical drains after breast and axillary operations. Ann Surg. 2013;258:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cussans A, Somani BK, Basarab A, et al. The role of targeted prophylactic antimicrobial therapy before transrectal ultrasonography-guided prostate biopsy in reducing infection rates: a systematic review. BJU Int. 2016;117:725–731. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AK, Zembower TR, Nadler RB, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187:1275–1279. [DOI] [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 13.Viola GM, Selber JC, Crosby M, et al. Salvaging the infected breast tissue expander: a standardized multidisciplinary approach. Plast Reconstr Surg Glob Open. 2016;4:e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis SH, Ruberg RL, Stevenson KB, et al. Independent risk factors for infection in tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124:1790–1796. [DOI] [PubMed] [Google Scholar]
- 15.Moyer KE, Potochny JD. Technique for seroma drainage in implant-based breast reconstruction. J Plast Reconstr Aesthet Surg. 2012;65:1614–1617. [DOI] [PubMed] [Google Scholar]
- 16.Ozturk CN, Ozturk C, Soucise A, et al. Expander/implant removal after breast reconstruction: analysis of risk factors and timeline. Aesthetic Plast Surg. 2018;42:64–72. [DOI] [PubMed] [Google Scholar]
- 17.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. [DOI] [PubMed] [Google Scholar]
- 18.Weichman KE, Clavin NW, Miller HC, et al. Does the use of biopatch devices at drain sites reduce perioperative infectious complications in patients undergoing immediate tissue expander breast reconstruction? Plast Reconstr Surg. 2015;135:9e–17e. [DOI] [PubMed] [Google Scholar]
- 19.Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877–881. [DOI] [PubMed] [Google Scholar]
- 20.Darouiche RO, Wall MJ, Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26. [DOI] [PubMed] [Google Scholar]
- 21.Bratzler DW, Dellinger EP, Olsen KM, et al. ; American Society of Health-System Pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. [DOI] [PubMed] [Google Scholar]
- 22.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278; quiz 279. [DOI] [PubMed] [Google Scholar]
- 23.Zanetti G, Goldie SJ, Platt R. Clinical consequences and cost of limiting use of vancomycin for perioperative prophylaxis: example of coronary artery bypass surgery. Emerg Infect Dis. 2001;7:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott RA, Weatherly HL, Hawkins NS, et al. An economic model for the prevention of MRSA infections after surgery: non-glycopeptide or glycopeptide antibiotic prophylaxis? Eur J Health Econ. 2010;11:57–66. [DOI] [PubMed] [Google Scholar]
- 25.Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al. A systematic review and economic model of switching from non-glycopeptide to glycopeptide antibiotic prophylaxis for surgery. Health Technol Assess. 2008;12:iii–iv, xixii,, 1147. [DOI] [PubMed] [Google Scholar]
- 26.Crawford T, Rodvold KA, Solomkin JS. Vancomycin for surgical prophylaxis? Clin Infect Dis. 2012;54:1474–1479. [DOI] [PubMed] [Google Scholar]
- 27.Alexander JW, Solomkin JS, Edwards MJ. Updated recommendations for control of surgical site infections. Ann Surg. 2011;253:1082–1093. [DOI] [PubMed] [Google Scholar]
- 28.Schweizer M, Perencevich E, McDanel J, et al. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. BMJ. 2013;346:f2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryu JW, Jung SI, Ahn JH, et al. Povidone-iodine rectal cleansing and targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound-guided prostate biopsy are associated with reduced incidence of postoperative infectious complications. Int Urol Nephrol. 2016;48:1763–1770. [DOI] [PubMed] [Google Scholar]
- 30.Okamura K, Tanaka K, Miura T, et al. Randomized controlled trial of perioperative antimicrobial therapy based on the results of preoperative bile cultures in patients undergoing biliary reconstruction. J Hepatobiliary Pancreat Sci. 2017;24:382–393. [DOI] [PubMed] [Google Scholar]
- 31.Liss MA, Kim W, Moskowitz D, et al. Comparative effectiveness of targeted vs empirical antibiotic prophylaxis to prevent sepsis from transrectal prostate biopsy: a retrospective analysis. J Urol. 2015;194:397–402. [DOI] [PubMed] [Google Scholar]


