Supplemental Digital Content is available in the text.
Summary:
This article is a practical and technical guide for plastic surgeons interested in or practicing migraine surgery. It discusses the goals of migraine surgery including selection of appropriate candidates (screening form contained), pertinent anatomy, and surgical techniques with text summary, intraoperative photographs, and videos. In addition, pearls and pitfalls, the most common complications, and current procedural terminology (CPT) coding are detailed.
GOALS OF THE PROCEDURE
There is mounting evidence over the past 2 decades that migraine pain can be instigated by irritation of peripheral extracranial nerves, vessels, and possibly other soft tissues.1–9 This understanding has led to novel treatment approaches including cutting edge surgical techniques that challenge traditional thinking around this complex disease.10 The goals of surgical migraine treatment are identification of patients with anatomic triggers, accurate and comprehensive diagnosis of all irritation points, and successful decompression and/or selective neurectomy of nerves/vessels in affected areas. To achieve these objectives, correct patient selection, detailed anatomic knowledge, and technically well-executed surgery are paramount.11–26
For identification of candidates for migraine surgery, remember PAINS (Fig. 1)
Fig. 1.
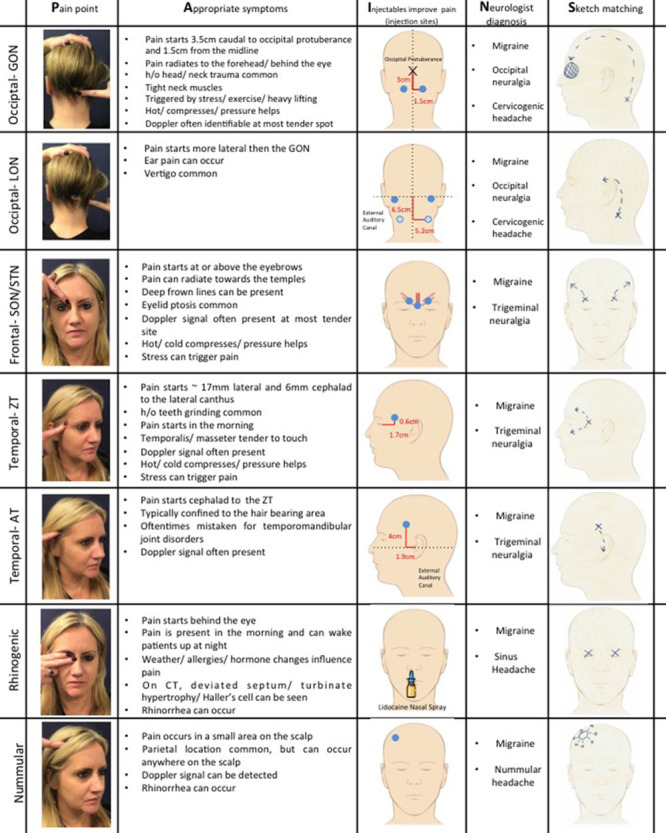
PAINS. P—Pain point. Patients are asked to point to where the pain starts with 1 finger. Characteristic pain points are illustrated. However, variability exists. For the GON, pain usually starts at the exit point of the GON 3 cm caudal to the occipital protuberance and 1.5 cm lateral to the midline. LON pain is very variable, but usually located lateral to GON pain. STN/SON pain is at or above the eyebrow. ZT pain is usually in the non–hair-bearing area of the scalp, whereas AT pain is in the hair-bearing scalp. Rhinogenic pain is perceived behind the eye. Nummular pain can be anywhere across the scalp, but is oftentimes seen in the parietal scalp. A—Appropriate symptoms. See this column for symptoms most commonly associated with triggers. I—Injectables improve pain. Blue points indicate injection sites. For the GON, start by injecting the point of maximum pain, which may be at or cephalad to the exit site of the GON from the semispinalis muscle (3 cm caudal to the occipital protuberance and 1.5 cm lateral to the midline). If the patient is still in pain, inject at the anatomic exit site of the nerve. For the LON, inject exactly to where the patient points with their finger. The usual injection point is higher (dark blue point) than the exit point of the LON from the sternocleidomastoid muscle (light blue point). SON/STN injection points are over the bilateral corrugator muscles and the procerus muscle. Again, patient pain points should guide the surgeon. ZT and AT injection sites are highly variable and correspond with the specific site of pain and oftentimes a positive Doppler signal. As an anatomic reference point, the ZT exits the temporalis fascia 17 mm lateral and 6 mm cephalad to the lateral canthus. This is oftentimes an area of pain. Similarly, the AT crosses the superficial temporal artery on average 19 mm lateral and 40 mm cephalad to the external auditory canal. Pain is often perceived in this area. At the rhinogenic site, lidocaine sprays can be used to determine pain relief. For nummular trigger sites, inject exactly at the point of pain/positive Doppler sign. N—Neurologist diagnosis. It is critical for patients to have a diagnosis of migraine by their neurologist before they are seen in the office. At the occipital site, occipital neuralgia or cervicogenic headache is also a common diagnosis. Patients are often misdiagnosed with trigeminal neuralgia and, therefore, should also be seen for evaluation. S—Sketch matching—as part of patient screening before the office visit, we find it very helpful to have patients draw where their pain starts and where it goes to. Oftentimes a diagnosis can be made just by looking at the sketch.
Pain point (identifiable with 1 finger),
Appropriate symptoms (constellation),
Injectables improve pain [nerve block/botulinum toxin, type A (BT-A)],
Neurologist confirmed diagnosis, and
Sketch matching.
It is critical to confirm that patients carry the official diagnosis of migraine/headache/occipital neuralgia by a neurologist. Other causes of headache such as intracranial pathology, spinal disease, and brachial plexus injury should be ruled out. As part of the screening process, we recommend taking a detailed medical and migraine history, and asking patients to sketch their pain, whereby patients indicate where the pain starts and where it radiates. Classic pain patterns help guide the practitioner to the proper trigger site identification (Fig. 1). When patients present to the office, the most important question is where the pain starts, which is most effectively identified using the patient’s single index finger (Fig. 1).27 Diffuse pain that cannot be localized may indicate a different diagnosis. Each trigger has associated symptoms that confirm the diagnosis and should be specifically elicited during the interview and exam (Fig. 1).27–31 If patients have active pain at the time of the visit, a nerve block should be performed (Fig. 1). Initial injection often takes away the most severe pain and reveals additional triggers. Serial injections should be performed until the patient is pain-free. Alternatively, if the patient has no current pain, BT-A can be used. However, serial injections with BT-A can be time consuming and expensive. A positive response to injectables is optimal, whereas a negative response does not prohibit surgery given that injection may not be able to overcome longstanding chronic irritation. Doppler ultrasound is increasingly being used for nummular headaches and to confirm vessel involvement at other triggers for preoperative planning.32,33 Some surgeons routinely perform CT face/paranasal sinuses for preoperative planning at the rhinogenic and frontal trigger sites.34 The Migraine Surgery Council has developed a screening form that can be used for guidance in the screening process (SDC1; see survey, Supplemental Digital Content 1, which displays the migraine surgery intake questionnaire, http://links.lww.com/PRSGO/B117)
DESCRIPTION OF MOST-EFFECTIVE PROCEDURES
Trigger site release at all sites follows standard described principles.10,28–31 Well established techniques are described. However, other surgical techniques have been published.
Occipital Trigger Site
Greater Occipital Nerve/Third Occipital Nerve
Complete decompression of the greater occipital nerve (GON) involves addressing 6 compression points from proximal to distal31,35,36: (1) musculofascial tissue surrounding the obliquus capitis inferior muscle (point 1, 77 mm inferior to occipital protuberance (OP), and 20 mm from midline); (2) epimysium underlying the semispinalis, or the muscle itself (point 2, 59 mm inferior to OP); (3) exit point from the semispinalis muscle (point 3, 17 mm from midline and 30 mm inferior to OP, and 15 mm from midline); (4) the entrance to the trapezial tunnel (point 4, 24 mm inferior to OP, and 21 mm lateral to midline); (5) insertion into the nuchal line (point 5, 4 mm inferior to OP and 37 mm lateral to the midline); and (6) interaction with the occipital artery if present.
For exposure of the GON, make a vertical midline incision (~5 cm) as far distal as possible within the hairline (Fig. 2A).28,37 A transverse approach has also been described.37 After elevation of bilateral soft tissue flaps (Fig. 2B), the trapezius fascia is incised 1 cm lateral to the midline raphe and the GON is identified. If the third occipital nerve is encountered, it is either transected or decompressed. Third occipital nerve removal has not been shown to influence postoperative results and can therefore be deferred if dissection is difficult.38 The GON is then followed proximally through the obliquely oriented trapezius muscle to the vertically oriented semispinalis muscle (Fig. 2C). A rectangular cuff of semispinalis capitis muscle between the median raphe and nerve is removed as is a triangular segment lateral to the nerve (Fig. 2D, E). Fascial bands and the obliquus capitis at the base of the nerve are lysed/released. Next, a cuff of trapezius fascia/muscle at the entrance to the trapezial tunnel overlying the nerve is removed. By incising the overlying trapezius fascia, the nerve is then followed distally toward the subcutaneous plane (SDC2; see figure, Supplemental Digital Content 2, which displays GON decompression II, http://links.lww.com/PRSGO/B118).
Fig. 2.
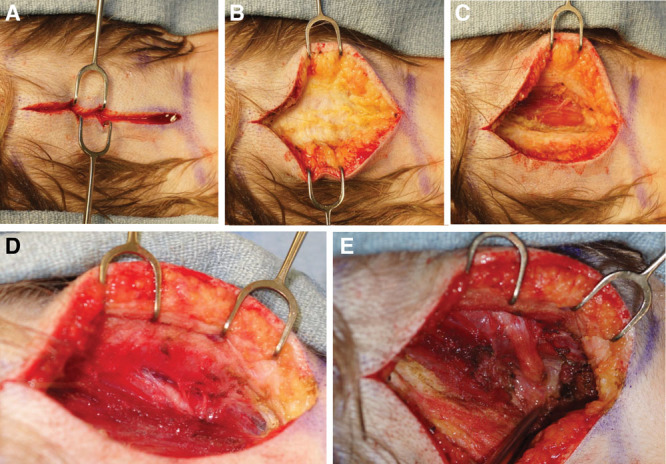
Greater occipital nerve decompression, I. A, Midline incision as far distal as possible within the hairline. B, Subcutaneous tissue dissection above trapezius fascia. C, Vertical semispinalis muscle fibers. D, GON exit from the semispinalis muscle. E, Removal of semispinalis muscle cuff.
If the occipital artery/dilated occipital veins transect or are intertwined with the nerve, vessel ligation/cauterization is performed (SDC2). An inferiorly based subcutaneous fat flap is raised (based caudally) or fat graft is placed under the nerve to prevent contact with the muscle and cushion the nerve (SDC3; see figure, Supplemental Digital Content 3, which displays GON decompression III, http://links.lww.com/PRSGO/B119). Use of a drain is recommended. To eliminate dead space, the subcutaneous tissue is sutured to the midline raphe (See Video 1, [online], which displays GON release.)
Video 1. This video displays greater occipital nerve release.
Lesser Occipital Nerve
Lesser occipital nerve (LON) location can be more inconsistent, although it is generally found lateral and caudal to the GON.31,39–42 Given anatomic variability, patients undergoing LON release are asked to point to the site of maximum pain preoperatively and the site is marked. A Doppler probe can be used to evaluate for vessel involvement. A vertical or transverse incision (~2 cm) is made directly overlying this area (SDC4; see figure, Supplemental Digital Content 4, which displays LON decompression, http://links.lww.com/PRSGO/B120). The nerve, with or without adjacent vessel, is identified deep to the trapezius fascia (SDC4). If a vessel is present, it is cauterized or ligated. Nerve avulsion, transection and neurotization into muscle or fascia, and decompression of the fascia along the nerve course without removal of the nerve have been described.
Frontal and Temporal (Zygomaticotemporal) Trigger Site
Isolated frontal surgery is typically performed through a transpalpebral incision.29,31,43 Frontal and zygomaticotemporal (ZT) trigger site surgery are oftentimes combined.44 In these cases, both sites can be accessed through an upper eyelid blepharoplasty incision, or through small incisions in the hair-bearing scalp using an endoscope.18,20,29,31 In patients with long foreheads or male pattern baldness, frontal release through the transpalpebral incision and endoscopic temple surgery is an option.29,31 Isolated temple surgery can be performed with an endoscope, or though an incision in the hair-bearing scalp.25,30,31 If patients desire a blepharoplasty, isolated ZT removal can be performed through a transpalpebral incision.18
At the frontal site, supraorbital (SON) and supratrochlear (STN) nerves may be irritated, compressed, or entrapped by a bony foramen or tight fascia crossing a notch, by fibers of the corrugator/depressor supercilii muscles, or by adjacent vessels (Fig. 3).20,34,45–49 At the temple, ZT pain usually originates from where the nerve pierces the deep temporal fascia 17 mm lateral and 6 mm cephalad to the lateral canthus.50,51
Fig. 3.
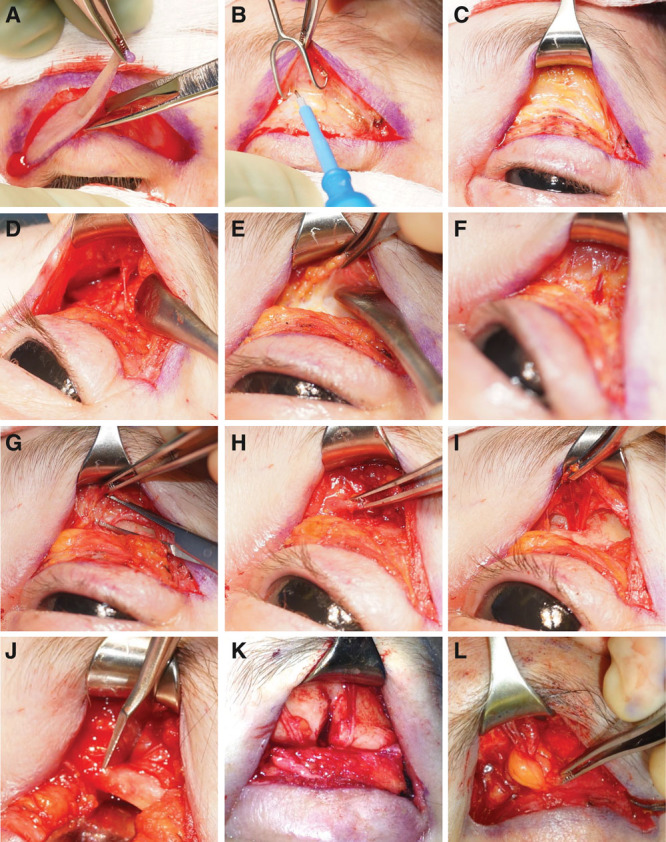
Frontal release. A, Blepharoplasty incision, B–F Dissection through the orbicularis muscle over the orbital rim until the depressor and corrugator supercilii become apparent. G–I, Removal of muscle. J–K, Foraminotomy. L, Adipose tissue flap.
Open Approach through the Eyelid
SON/STN are approached through an upper eyelid blepharoplasty incision (Fig. 3A).18,20,31,43 The incision is deepened through the orbicularis muscle to the orbital rim (Fig. 3B). Dissection over the orbital rim is carried out until the depressor and corrugator supercilii become apparent (Fig. 3C, F). Both muscles are removed carefully thereby releasing the SON and STN (Fig. 3G–I). Supraorbital and supratrochlear vessels are coagulated. If a supraorbital notch with fascial band is evident, the fascia is released. If a supraorbital foramen is present, it is opened with an osteotome or rongeur (Fig. 3J, K). A laterally based adipose flap from the medial orbital fat pad can be harvested and placed around the nerves (Fig. 3L). Alternatively, abdominal fat can be grafted around nerves and into the dead space created by muscle resection31 (See Video 2, [online], which displays frontal trigger site release (SON and STN).
If ZT decompression is performed simultaneously, the same blepharoplasty incision can be used18 (SDC5; see figure, Supplemental Digital Content 5, which displays ZT nerve release through blepharoplasty incision, http://links.lww.com/PRSGO/B121). Careful dissection is performed along the lateral orbital rim (inferior) over the deep temporal fascia (SDC5). The superficial temporal fascia is lifted off the deep temporal fascia using an elevator. Tissues are gently retracted to protect the frontal branch of the facial nerve. At this time, the sentinel vein can be seen (SDC5). Careful dissection around the vein reveals the ZT nerve right posterior to the vein (SDC5). Primary nerve avulsion and nerve decompression have been shown to alleviate migraine symptoms52 (See Video 3, [online], which displays ZT nerve avulsion.)
Video 2. This video displays frontal trigger site release (supraorbital and supratrochlear nerve).
Video 3. This video displays zygomaticotemporal nerve avulsion.
Endoscopic Approach
In common, 5 ports are used.30,31 In patients with long foreheads, 2 paramedian incisions sites, rather than a single midline incision, may be necessary increasing the number of port sites to 6. In the 5 access incision approach, the midline incision is made 0.5 cm behind the hairline. If the patient is undergoing combined frontal and ZT release, one incision is made 7 cm, and other 10 cm from the midline in the temple area bilaterally. Assurance is made that the paramedian and temporal incisions straddle both sides of the temporal fusion line. All incisions are around 1.5 cm long. After injection of local anesthesia/epinephrine, the temporal incision is taken down through the superficial temporal fascia to the deep temporal fascia, and the paramedian incision is made down to periosteum. After sufficient dissection of the adjacent tissue with a periosteal elevator, an endoscopic access device (or silicone port) is inserted into the incisions. The plane superficial to the deep temporal fascia is dissected toward the supraorbital rim. Through the paramedian incision, the dissection is carried medially under the midline incision. Through the midline incision and the paramedian incisions on the other side, the dissected areas are connected subperiosteally.
Through the lateral temporal incision on one side, under direct vision through the endoscope, the dissection is continued above the deep temporal fascia toward the lateral orbital rim. The sentinel vein is preserved, and the ZT is avulsed or decompressed. For ZT decompression, the deep temporal fascia surrounding the nerve is incised and dilated. Vessels that accompany the nerve are cauterized. Fat from the deep temporal fat fad is harvested through a rent above the zygomatic arch lateral to the orbital wall and saved for later replacement of the corrugator muscle.
The arcus marginalis is released and the corrugator muscle and the lateral procerus muscle fibers are removed thoroughly. Supraorbital and supratrochlear arteries and veins are ablated. If a supraorbital foramen is present, an percutaneous osteotomy is performed. Otherwise, the fascial floor of the notch is carefully released, usually though a limited upper eyelid counterincision under direct visualization. The muscle site is filled with fat harvested from above the zygoma.
Isolated Temporal Trigger Site—ZT Nerve Surgery
Open Approach
A temporal hairline incision can be made 5–7 mm posterior to the temporal headline and taken down to the deep temporal fascia.18,25 The plane between the deep and superficial temporal fascia is elevated to expose the sentinel vein and ZT nerve. Again, the nerve can be decompressed or avulsed through this incision. If the patient desires a blepharoplasty, isolated ZT release can be performed through the upper eyelid as described above (SDC6; see figure, Supplemental Digital Content 6, which displays ZT nerve release through direct incision, http://links.lww.com/PRSGO/B123).
Endoscopic Approach
Endoscopic release of the ZT nerve uses 2 incisions.30,31 One is 7 cm from the midline, and the other is 10 cm from the midline (or 3 cm lateral to the paramedian access incision). With a periosteal elevator, tissue adjacent to the incision is freed up to insert an endoscopic access device. Through the upper temple incision, under direct vision through the endoscope, the dissection is continued above the deep temporal fascia toward the lateral orbital rim until the sentinel vein is visible. While preserving the vein, the ZT nerve is identified behind the vessel and either avulsed or decompressed. Concomitant vessels are cauterized. The nerve can either be avulsed or decompressed by incising and dilating the surrounding deep temporal fascia.
Temporal Trigger Site—Auriculotemporal Nerve
Compression points of the auriculotemporal nerve are variable.31,53–55 Patients are asked to point to the area of maximum pain, and handheld Doppler is used to evaluate presence of a vessel. If a single point can be isolated, resection/ligation of the neurovascular bundle at this site can be performed. If patients have multiple tender points along the nerve course with positive Doppler, recurrent pain at this site after primary decompression, or more vague pain in the hair-bearing area, neurectomy of the main auriculotemporal nerve and main superficial temporal artery may be required, usually near the helical root.31
Release of the auriculotemporal nerve is performed through an open incision (SDC7 and Video 4; see figure, Supplemental Digital Content 7, which displays auriculotemporal nerve release, http://links.lww.com/PRSGO/B124 and See Video 4, [online], which displays Auriculotemporal Nerve Excision). Neurectomy and arterectomy/ligation of the superficial temporal artery can be performed under local anesthesia. The patient is asked to point to the area of pain, which is usually located closer to the ear than the ZT trigger site. Doppler is used to confirm vessel involvement. A small incision is made at the point of maximal tenderness and the neurovascular bundle can be easily identified. Nerve and artery are transected and buried in the temporalis muscle.
Video 4. This video displays auriculotemporal nerve excision.
Rhinogenic Trigger Site
At the rhinogenic site, turbinate hypertrophy, and other intranasal pathology such as deviated septum, Haller’s cell, concha bullosa, and septal spurs result in contact/compression points that can result in headaches.31,56–58 Surgical management depends on findings on CT face/paranasal sinuses. The most common procedures performed are septoplasty and turbinate reduction. Typically, a septoplasty approach through a Killian or hemitransfixion incision is used. Turbinates can be outfractured or reduced, being careful to avoid empty nose syndrome from overaggressive resection.
Nummular Trigger Site
Nummular triggers at locations that do not correspond to the classic triggers mentioned above are often associated with vessels.31,32 Patients are asked to point to the area of the scalp where they are experiencing pain and the site is marked. In most cases, a Doppler signal can be detected at the site and this area is marked. Under local anesthesia, an incision is designed over the marked area (~1 cm). The vessel is identified and either cauterized or ligated. Small nerves associated with the vessels are removed.
NERVE TRANSECTION
Currently, there is no consensus among migraine surgeons regarding choice between nerve decompression, neurolysis, and neurectomy. Some surgeons choose to ablate smaller trigger nerves such as the ZT, auriculotemporal, third occipital, and LONs during primary trigger deactivation.18,25,52,53 Others will decompress these nerves. In a prospective randomized controlled study, Guyuron et al. showed that there is no difference between neurectomy and decompression of the ZT nerve in terms of migraine headache frequency, duration, and pain.52 However, more data is needed to come to a final conclusion.
In general, bigger nerves such as the GON and STN/SON undergo decompression as first line treatment. However, in patients with long-term symptoms, direct injury to the nerve, or neuroma, nerve decompression may not be sufficient to alleviate symptoms.28,59–61 When patients fail primary decompression, neurectomy can be considered.19 Permanent consequences of nerve transection have to be discussed with the patient in detail. Ducic19 described transection of the GON after failed decompression, with 70% of patients experiencing improved symptoms after surgery with >50% reduction in Migraine Headache Index (migraine frequency × duration ×pain). His technique further describes burial of the proximal stump in the nuchal musculature to prevent stump migration/neuroma formation.19,59 Other common nerve reconstruction techniques have also been employed after transection.
AVOIDING AND MANAGING MOST-DANGEROUS COMPLICATIONS
For all sites, wound complications are extremely rare and can be avoided with sterile technique and tension-free closure.17,31 If nerves are avulsed, burial in adjacent tissues should be considered to prevent neuroma formation. At the occipital site, placement of a drain is recommended to avoid fluid collections. Frontal trigger release can be complicated by contour deformities after removal of the glabellar muscle group. This can be avoided by placement of fat into the area of concern. When performing an osteotomy for a supraorbital foramen, be careful to protect the intraorbital contents. Decompression of the ZT nerve should never result in injury to the facial nerve. Ensure that tissue dissection is carried out superficial to the deep temporal fascia. To confirm the correct layer of dissection, an incision can be made in the deep temporal fascia to reveal temporalis muscle, if needed. Complications at the rhinogenic site include rare excessive bleeding, which can be minimized with intraoperative use of desmopressin or tranexamic acid.62 Another potential complication is infection, which can be avoided with prophylactic antibiotics. Septal perforation can occur if bilateral septal tears on the same level are not repaired intraoperatively.
PEARLS AND PITFALLS
The most common reason for failure after migraine surgery is incomplete primary surgery.63 Preoperative diagnosis of all triggers is essential for successful surgery. Sometimes, secondary triggers do not appear until the primary trigger is eliminated and patients may require staged procedures. Incomplete nerve release will also result in failure. It is important to be familiar with the anatomy and decompress nerves at all points. Given variable intraoperative anatomy, exploration of all nerve branches is recommended. If nerve diameter is small, dissection may be too superficial and the nerve should be followed to the main branch.31 If symptoms persist although the trigger was properly diagnosed and decompression was complete, consider nerve transection.19
WHAT PATIENTS SHOULD KNOW BEFORE HAVING THIS PROCEDURE
Procedures are performed under general anesthesia on an outpatient basis. Isolated LON, auriculotemporal nerve (AT), and nummular headache surgery can potentially be performed under local anesthesia in the office. Recovery depends on how many trigger sites are operated on. In general, the more triggers, the longer the recovery. For procedures under local anesthesia, patients can return to their usual activities the day after surgery. For outpatient procedures, patients can expect to return to light activities 1 week after surgery and full activities 3 weeks after surgery. Immediately after surgery, some patients experience migraine headache that resolves soon after surgery. Transient numbness is expected after nerve release at all sites and can last for up to 1 year. Transient shooting pain is expected during this period. If nerves are avulsed or transected, permanent numbness will result. Bleeding, infection, and wound healing problems are extremely rare. At the frontal site, periorbital swelling and bruising is expected and will resolve within weeks. All scars are hidden in hair-bearing areas and alopecia would be extremely uncommon.
CPT CODING
The CPT codes listed in this section are not legally binding, just reflective of the consensus opinion of the individuals listed in the acknowledgement section that helped construct the Migraine Surgery Council CPT guidelines.
Greater Occipital Nerve
-
Decompression, and
64716—Neuroplasty and/or transposition, cranial nerve.
-
Neurectomy
64744—Transection or avulsion of neuroma or major peripheral nerve, except ischial.
±64787—Implantation of nerve end into bone or muscle.
Lesser Occipital Nerve
-
Decompression, and
64716—Neuroplasty and/or transposition, cranial nerve.
-
Neurectomy
64784—Transection of neuroma or major peripheral nerve, except ischial.
±64787—Implantation of nerve end into bone or muscle.
Third Occipital Nerve
-
Decompression, and
64716—Neuroplasty and/or transposition, cranial nerve.
-
Neurectomy
64772—Transection or avulsion of other spinal nerve, extradural.
±64787—Implantation of nerve end into bone or muscle.
Auriculotemporal Nerve
-
Decompression, and
64722—Decompression of unspecified nerve.
-
Neurectomy
64784—Transection of neuroma or major peripheral nerve, except ischial.
±64787—Implantation of nerve end into bone or muscle.
±37609—Ligation or biopsy, temporal artery.
ZT Nerve
-
Decompression, and
64722—Decompression of unspecified nerve.
-
Neurectomy
64771—Transection or avulsion of other cranial nerve, extradural.
±64787—Implantation of nerve end into bone or muscle.
Supratrochlear Nerve
-
Decompression, and
64716—Neuroplasty and/or transposition, cranial nerve.
-
Neurectomy
64771—Transection or avulsion of other cranial nerve, extradural.
±64787—Implantation of nerve end into bone or muscle.
Supraorbital Nerve
-
Decompression, and
64716—Neuroplasty and/or transposition, cranial nerve.
±21137—Reduction forehead, contouring only (if significant bony work is required).
±20926—Tissue graft, other (if a single tissue graft is used).
±15770—Dermis/fat/fascia graft (if a composite graft is used, eg, dermis, fat, and fascia).
±14060–14061 Adjacent tissue transfer for rearrangement eyelids, 10 cm2 or less, 10–30 cm2 (if local tissue rearrangement is used, eg, pedicled fat flap from the upper, lateral compartment).
-
Neurectomy
64732—Transection or avulsion of supraorbital nerve.
±64787—Implantation of nerve end into bone or muscle.
±14060–14061 Adjacent tissue transfer for rearrangement eyelids, 10 cm2 or less, 10–30 cm2 (if local tissue rearrangement is used, eg, pedicled fat flap from the upper, lateral compartment).
Endonasal
-
30140—Submucous resection inferior turbinate, partial or complete, any method.
31231—Nasal endoscopy, diagnostic, unilateral or bilateral.
30520—Septoplasty or submucous resection with or without cartilage graft scoring, contouring or replacement with graft.
30465—Repair of nasal vestibular stenosis (eg, spreader graft, lateral nasal wall reconstruction).
21235—Graft: ear cartilage, autogenous to nose or ear.
ACKNOWLEDGEMENTS
We would like to acknowledge the founding members of the Migraine Surgery Council for their work on guidelines for the Migraine Surgery Council Screening Forms and CPT coding guidelines (Austen William Gerald, Jr, Afifi Ahmed, Averitt Paul, Branch David, Ducic Ivan, Gfrerer Lisa, Guyuron Bahman, Hagan Robert, Hochman Marcelo, Hulsen, John, Janis Jeffrey, Khansa Ibrahim, Kung Ted, John B. Moore, Totonchi Ali, and Peled Ziv).
Supplementary Material
Footnotes
Published online 31 July 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Guyuron B, Yohannes E, Miller R, et al. Electron microscopic and proteomic comparison of terminal branches of the trigeminal nerve in patients with and without migraine headaches. Plast Reconstr Surg. 2014;134:796e–805e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstein R, Blake P, Schain A, et al. Extracranial origin of headache. Curr Opin Neurol. 2017;30:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gfrerer L, Hansdorfer M, Ortiz R, et al. Occipital Neuralgia/Migraine: Intra-operative Evidence for Extracranial Pathology. 2018Boston, MA: Northeastern Society of Plastic Surgeons. [Google Scholar]
- 4.Ducic I, Felder JM, III, Janis JE. Occipital artery vasculitis not identified as a mechanism of occipital neuralgia-related chronic migraine headaches. Plast Reconstr Surg. 2011;128:908–912. [DOI] [PubMed] [Google Scholar]
- 5.Shevel E, Spierings EH. Role of the extracranial arteries in migraine headache: a review. Cranio. 2004;22:132–136. [DOI] [PubMed] [Google Scholar]
- 6.Del Fiacco M, Quartu M, Boi M, et al. TRPV1, CGRP and SP in scalp arteries of patients suffering from chronic migraine. J Neurol Neurosurg Psychiatry. 2015;86:393–397. [DOI] [PubMed] [Google Scholar]
- 7.Schueler M, Neuhuber WL, De Col R, et al. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache. 2014;54:996–1009. [DOI] [PubMed] [Google Scholar]
- 8.Perry CJ, Blake P, Buettner C, et al. Upregulation of inflammatory gene transcripts in periosteum of chronic migraineurs: implications for extracranial origin of headache. Ann Neurol. 2016;79:1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosaras B, Jakubowski M, Kainz V, et al. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009;515:331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyuron B, Kriegler JS, Davis J, et al. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg. 2005;115:1–9. [PubMed] [Google Scholar]
- 11.Guyuron B, Tucker T, Davis J. Surgical treatment of migraine headaches. Plast Reconstr Surg. 2002;109:2183–2189. [DOI] [PubMed] [Google Scholar]
- 12.Guyuron B, Reed D, Kriegler JS, et al. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg. 2009;124:461–468. [DOI] [PubMed] [Google Scholar]
- 13.Guyuron B, Kriegler JS, Davis J, et al. Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg. 2011;127:603–608. [DOI] [PubMed] [Google Scholar]
- 14.Dirnberger F, Becker K. Surgical treatment of migraine headaches by corrugator muscle resection. Plast Reconstr Surg. 2004;114:652–657; discussion 658. [DOI] [PubMed] [Google Scholar]
- 15.Poggi JT, Grizzell BE, Helmer SD. Confirmation of surgical decompression to relieve migraine headaches. Plast Reconstr Surg. 2008;122:115–122; discussion 123. [DOI] [PubMed] [Google Scholar]
- 16.Janis JE, Dhanik A, Howard JH. Validation of the peripheral trigger point theory of migraine headaches: single-surgeon experience using botulinum toxin and surgical decompression. Plast Reconstr Surg. 2011;128:123–131. [DOI] [PubMed] [Google Scholar]
- 17.Janis JE, Barker JC, Javadi C, et al. A review of current evidence in the surgical treatment of migraine headaches. Plast Reconstr Surg. 2014;134(4 Suppl 2):131S–41S. [DOI] [PubMed] [Google Scholar]
- 18.Gfrerer L, Maman DY, Tessler O, et al. Nonendoscopic deactivation of nerve triggers in migraine headache patients: surgical technique and outcomes. Plast Reconstr Surg. 2014;134:771–778. [DOI] [PubMed] [Google Scholar]
- 19.Ducic I, Felder JM, III, Khan N, et al. Greater occipital nerve excision for occipital neuralgia refractory to nerve decompression. Ann Plast Surg. 2014;72:184–187. [DOI] [PubMed] [Google Scholar]
- 20.Hagan RR, Fallucco MA, Janis JE. Supraorbital rim syndrome: definition, surgical treatment, and outcomes for frontal headache. Plast Reconstr Surg Glob Open. 2016;4:e795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gfrerer L, Guyuron B. Surgical treatment of migraine headaches. Acta Neurol Belg. 2017;117:27–32. [DOI] [PubMed] [Google Scholar]
- 22.Gfrerer L, Lans J, Faulkner HR, et al. Ability to cope with pain puts migraine surgery patients in perspective. Plast Reconstr Surg. 2018;141:169–174. [DOI] [PubMed] [Google Scholar]
- 23.Larson K, Lee M, Davis J, et al. Factors contributing to migraine headache surgery failure and success. Plast Reconstr Surg. 2011;128:1069–1075. [DOI] [PubMed] [Google Scholar]
- 24.Ducic I, Felder JM, III, Fantus SA. A systematic review of peripheral nerve interventional treatments for chronic headaches. Ann Plast Surg. 2014;72:439–445. [DOI] [PubMed] [Google Scholar]
- 25.Peled ZM. A novel surgical approach to chronic temporal headaches. Plast Reconstr Surg. 2016;137:1597–1600. [DOI] [PubMed] [Google Scholar]
- 26.Gfrerer L, Hulsen JH, McLeod MD, et al. Migraine surgery: an all or nothing phenomenon? Prospective evaluation of surgical outcomes. Ann Surg. 2019;269:994–999. [DOI] [PubMed] [Google Scholar]
- 27.Guyuron B, Nahabet E, Khansa I, et al. The current means for detection of migraine headache trigger sites. Plast Reconstr Surg. 2015;136:860–867. [DOI] [PubMed] [Google Scholar]
- 28.Ascha M, Kurlander DE, Sattar A, et al. In-depth review of symptoms, triggers, and treatment of occipital migraine headaches (Site IV). Plast Reconstr Surg. 2017;139:1333e–1342e. [DOI] [PubMed] [Google Scholar]
- 29.Kurlander DE, Ascha M, Sattar A, et al. In-depth review of symptoms, triggers, and surgical deactivation of frontal migraine headaches (Site I). Plast Reconstr Surg. 2016;138:681–688. [DOI] [PubMed] [Google Scholar]
- 30.Kurlander DE, Punjabi A, Liu MT, et al. In-depth review of symptoms, triggers, and treatment of temporal migraine headaches (Site II). Plast Reconstr Surg. 2014;133:897–903. [DOI] [PubMed] [Google Scholar]
- 31.Guyuron B. Migraine Surgery. 2018New York: Thieme Publishers Inc.. [Google Scholar]
- 32.Guyuron B, Gatherwright J, Reed D, et al. Treatment of dopplerable nummular headache with minimally invasive arterectomy under local anesthesia. J Plast Reconstr Aesthet Surg. 2018;71:1010–1014. [DOI] [PubMed] [Google Scholar]
- 33.Guyuron B, Riazi H, Long T, et al. Use of a Doppler signal to confirm migraine headache trigger sites. Plast Reconstr Surg. 2015;135:1109–1112. [DOI] [PubMed] [Google Scholar]
- 34.Pourtaheri N, Guyuron B. Computerized tomographic evaluation of supraorbital notches and foramen in patients with frontal migraine headaches and correlation with clinical symptoms. J Plast Reconstr Aesthet Surg. 2018;71:840–846. [DOI] [PubMed] [Google Scholar]
- 35.Janis JE, Hatef DA, Ducic I, et al. The anatomy of the greater occipital nerve: part II. Compression point topography. Plast Reconstr Surg. 2010;126:1563–1572. [DOI] [PubMed] [Google Scholar]
- 36.Mosser SW, Guyuron B, Janis JE, et al. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg. 2004;113:693–697; discussion 698. [DOI] [PubMed] [Google Scholar]
- 37.Ducic I, Hartmann EC, Larson EE. Indications and outcomes for surgical treatment of patients with chronic migraine headaches caused by occipital neuralgia. Plast Reconstr Surg. 2009;123:1453–1461. [DOI] [PubMed] [Google Scholar]
- 38.Lee M, Lineberry K, Reed D, et al. The role of the third occipital nerve in surgical treatment of occipital migraine headaches. J Plast Reconstr Aesthet Surg. 2013;66:1335–1339. [DOI] [PubMed] [Google Scholar]
- 39.Ducic I, Moriarty M, Al-Attar A. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg. 2009;123:859–863; discussion 864. [DOI] [PubMed] [Google Scholar]
- 40.Lee M, Brown M, Chepla K, et al. An anatomical study of the lesser occipital nerve and its potential compression points: implications for surgical treatment of migraine headaches. Plast Reconstr Surg. 2013;132:1551–1556. [DOI] [PubMed] [Google Scholar]
- 41.Peled ZM, Pietramaggiori G, Scherer S. Anatomic and compression topography of the lesser occipital nerve. Plast Reconstr Surg Glob Open. 2016;4:e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dash KS, Janis JE, Guyuron B. The lesser and third occipital nerves and migraine headaches. Plast Reconstr Surg. 2005;115:1752–1758; discussion 1759. [DOI] [PubMed] [Google Scholar]
- 43.Guyuron B, Son JH. Transpalpebral corrugator resection: 25-year experience, refinements and additional indications. Aesthetic Plast Surg. 2017;41:339–345. [DOI] [PubMed] [Google Scholar]
- 44.Seyed Forootan NS, Lee M, Guyuron B. Migraine headache trigger site prevalence analysis of 2590 sites in 1010 patients. J Plast Reconstr Aesthet Surg. 2017;70:152–158. [DOI] [PubMed] [Google Scholar]
- 45.Janis JE, Hatef DA, Hagan R, et al. Anatomy of the supratrochlear nerve: implications for the surgical treatment of migraine headaches. Plast Reconstr Surg. 2013;131:743–750. [DOI] [PubMed] [Google Scholar]
- 46.Janis JE, Ghavami A, Lemmon JA, et al. Anatomy of the corrugator supercilii muscle: part I. Corrugator topography. Plast Reconstr Surg. 2007;120:1647–1653. [DOI] [PubMed] [Google Scholar]
- 47.Janis JE, Ghavami A, Lemmon JA, et al. The anatomy of the corrugator supercilii muscle: part II. Supraorbital nerve branching patterns. Plast Reconstr Surg. 2008;121:233–240. [DOI] [PubMed] [Google Scholar]
- 48.Ortiz R, Gfrerer L, Hansdorfer MA, et al. Intraoperative anatomy associated with migraines at the frontal site. 2018; Presented at: American Society of Peripheral Nerve Meeting; Palm Desert, CA. [Google Scholar]
- 49.Fallucco M, Janis JE, Hagan RR. The anatomical morphology of the supraorbital notch: clinical relevance to the surgical treatment of migraine headaches. Plast Reconstr Surg. 2012;130:1227–1233. [DOI] [PubMed] [Google Scholar]
- 50.Totonchi A, Pashmini N, Guyuron B. The zygomaticotemporal branch of the trigeminal nerve: an anatomical study. Plast Reconstr Surg. 2005;115:273–277. [PubMed] [Google Scholar]
- 51.Janis JE, Hatef DA, Thakar H, et al. The zygomaticotemporal branch of the trigeminal nerve: part II. Anatomical variations. Plast Reconstr Surg. 2010;126:435–442. [DOI] [PubMed] [Google Scholar]
- 52.Guyuron B, Harvey D, Reed D. A prospective randomized outcomes comparison of two temple migraine trigger site deactivation techniques. Plast Reconstr Surg. 2015;136:159–165. [DOI] [PubMed] [Google Scholar]
- 53.Sanniec K, Borsting E, Amirlak B. Decompression-avulsion of the auriculotemporal nerve for treatment of migraines and chronic headaches. Plast Reconstr Surg Glob Open. 2016;4:e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chim H, Okada HC, Brown MS, et al. The auriculotemporal nerve in etiology of migraine headaches: compression points and anatomical variations. Plast Reconstr Surg. 2012;130:336–341. [DOI] [PubMed] [Google Scholar]
- 55.Janis JE, Hatef DA, Ducic I, et al. Anatomy of the auriculotemporal nerve: variations in its relationship to the superficial temporal artery and implications for the treatment of migraine headaches. Plast Reconstr Surg. 2010;125:1422–1428. [DOI] [PubMed] [Google Scholar]
- 56.Lee M, Erickson C, Guyuron B. Intranasal pathology in the migraine surgery population: incidence, patterns, and predictors of surgical success. Plast Reconstr Surg. 2017;139:184–189. [DOI] [PubMed] [Google Scholar]
- 57.Afifi AM, Kempton SJ, Gordon CR, et al. Evaluating current functional airway surgery during rhinoplasty: a survey of the American Society of Plastic Surgeons. Aesthetic Plast Surg. 2015;39:181–190. [DOI] [PubMed] [Google Scholar]
- 58.Farmer RaA, AM A systematic review of functional rhinoplasty and improvement in facial pain and headache. 2017; Presented at: Midwestern Association of Plastic Surgeons Annual Meeting; Chicago, IL. [Google Scholar]
- 59.Ducic I, Felder JM, III, Endara M. Postoperative headache following acoustic neuroma resection: occipital nerve injuries are associated with a treatable occipital neuralgia. Headache. 2012;52:1136–1145. [DOI] [PubMed] [Google Scholar]
- 60.Ducic I, Larson EE. Posttraumatic headache: surgical management of supraorbital neuralgia. Plast Reconstr Surg. 2008;121:1943–1948. [DOI] [PubMed] [Google Scholar]
- 61.Ducic I, Sinkin JC, Crutchfield KE. Interdisciplinary treatment of post-concussion and post-traumatic headaches. Microsurgery. 2015;35:603–607. [DOI] [PubMed] [Google Scholar]
- 62.Faber C, Larson K, Amirlak B, et al. Use of desmopressin for unremitting epistaxis following septorhinoplasty and turbinectomy. Plast Reconstr Surg. 2011;128:728e–732e. [DOI] [PubMed] [Google Scholar]
- 63.Punjabi A, Brown M, Guyuron B. Emergence of secondary trigger sites after primary migraine surgery. Plast Reconstr Surg. 2016;137:712e–716e. [DOI] [PubMed] [Google Scholar]


