Abstract
Recessive forms of catecholaminergic polymorphic ventricular tachycardia (CPVT) are induced by mutations in genes encoding triadin or calsequestrin, two proteins that belong to the Ca2+ release complex, responsible for intracellular Ca2+ release triggering cardiac contractions. To better understand the mechanisms of triadin-induced CPVT and to assay multiple therapeutic interventions, we used a triadin knockout mouse model presenting a CPVT-like phenotype associated with a decrease in calsequestrin protein level. We assessed different approaches to rescue protein expression and to correct intracellular Ca2+ release and cardiac function: pharmacological treatment with kifunensine or a viral gene transfer-based approach, using adeno-associated virus serotype 2/9 (AAV2/9) encoding the triadin or calsequestrin. We observed that the levels of triadin and calsequestrin are intimately linked, and that reduction of both proteins contributes to the CPVT phenotype. Different combinations of triadin and calsequestrin expression level were obtained using these therapeutic approaches. A full expression of each is not necessary to correct the phenotype; a fine-tuning of the relative re-expression of both triadin and calsequestrin is required to correct the CPVT phenotype and rescue the cardiac function. AAV-mediated gene delivery of calsequestrin or triadin and treatment with kifunensine are potential treatments for recessive forms of CPVT due to triadin mutations.
Keywords: cardiac arrhythmia, gene therapy, Ca2+ release, genetic disease, sarcoplasmic reticulum
Graphical Abstract

Catecholaminergic polymorphic ventricular tachycardia (CPVT) is induced by mutations in triadin or calsequestrin. Deletion/mutation of triadin is associated with reduction in calsequestrin and leads to CPVT. Cacheux and colleagues show that partial re-expression of triadin and calsequestrin through gene or pharmacological therapy reverses this cardiac arrhythmia.
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited sudden cardiac death (SCD) syndrome. This disorder is characterized by bidirectional/polymorphic ventricular tachycardia triggered in conditions of intense physical exercise or emotional stress, without any associated structural alterations of the heart or electrocardiogram (ECG) abnormalities at rest. SCD occurs in 30% of patients under the age of 30 years when CPVT is not promptly diagnosed and treated.1, 2 β-Blockers and flecainide are, to date, the main pharmacological treatments, although not fully effective, and can be combined with cardioverter-defibrillator implantation or left cardiac sympathetic denervation.3 Nevertheless, these treatments remain preventive rather than curative.
The sarcoplasmic Ca2+ release required for cardiac contraction is achieved by the Ca2+ release complex (CRC). CRC is composed of the ryanodine receptor type 2 (RyR2), calsequestrin 2 (CSQ2), and associated proteins such as triadin, FKBP12.6, calmodulin, and junctin. The pathophysiological mechanism described for CPVT is a stress-induced adrenergic stimulation leading to an enhanced sarcoplasmic reticulum (SR) Ca2+ reuptake responsible for a SR Ca2+ overload and an increased RyR2 permeability to Ca2+. The resulting RyR2-mediated Ca2+ leak may trigger spontaneous SR Ca2+ release responsible for ventricular tachycardia (VT), which in the worst cases leads to SCD.
The underlying genetic causes of dysregulation in intracellular Ca2+ handling mainly involve mutations in genes encoding CRC proteins. About 50% of CPVT cases have been attributed to mutations in the RYR2 gene4 and 5% to mutations in the CASQ2 gene.5 More recently, other genes have been associated with CPVT: the TRDN gene, encoding triadin, in which mutations lead to CPVT associated with skeletal muscle weakness,6, 7, 8 and the calmodulin genes.9 Finally, approximately one-third of CPVT cases have not been linked to any genetic cause so far.
Even with genetic screening of CPVT and the availability of preventive treatments, absence of recurring ventricular tachycardia events and SCD are not guaranteed. Therefore, a better understanding of the pathophysiological mechanisms and the development of new therapies are needed. Gene therapy appears to be a promising tool to consider as a therapeutic application in the treatment of this disease. Indeed, encouraging results were observed in different recessive CPVT mouse models. The CPVT phenotype in CSQ2R33Q/R33Q or calsequestrin null mouse models was reversed by injection of an adeno-associated virus (AAV) coding for the normal CSQ2.10, 11, 12 More recently, a promising approach based on the gene transfer of an engineered calmodulin has been described.13
Triadin is a protein associated with RyR2, which together with junctin anchors calsequestrin to the junctional SR.14 Triadin is expressed in striated muscle, and the alternative splicing of this single gene TRDN results in the production of different isoforms, the shorter one (Trisk 32 also called CT1) being expressed in cardiac and skeletal muscles.15 Knockout of triadin has been shown to cause cardiac arrhythmias and muscle weakness in mouse models.16, 17, 18 CPVT patients with TRDN mutations all experienced cardiac arrest at a very young age (<6 years), suggesting a severe CPVT phenotype caused by triadin mutations.6, 7, 8 To date, each TRDN mutation identified in CPVT patients, present either as homozygous or compound heterozygous, resulted in the total absence of the proteins.6, 7, 8
Here, we show that triadin and calsequestrin, both responsible for recessive forms of CPVT, are intimately linked in the pathophysiological mechanisms leading to arrhythmias because of their reduced expression or complete deletion. Based on the mechanisms identified, we tested the hypothesis that either a pharmacological treatment with kifunensine, or cardiac delivery of exogenous Trisk 32 (T32) or CSQ2 by viral gene transfer in our triadin knockout (KO) mouse model would, at least partially, rescue protein expression levels, leading to in vitro restoration of Ca2+ homeostasis and in vivo reversion of ECG abnormalities. Here we provided a proof of concept whereby either a pharmacological approach or a viral-induced gene therapy could be a viable approach for clinical application in TRDN and CASQ2 mutation-induced recessive CPVT.
Results
T32 and CSQ2 Expressions Are Intimately Linked in the Heart
The molecular consequences of triadin deletion on the cardiac CRC proteins were first studied using quantitative western blot (Figures 1A and 1B) and qRT-PCR (Figure S1). As expected for a TRDN KO model, T32 protein and mRNA levels were virtually null. A moderate reduction of 33% ± 4% in RyR2 protein amount was detected in triadin KO mice, whereas no modification of dihydropyridine receptor (DHPR-Cav1.2) or FKBP12.6 protein expressions was observed (Figure 1B). Transcript levels of RyR2, FKPB12.6, and junctin were not significantly modified (Figure S1). In contrast, triadin deletion induced an 80% reduction of CSQ2 expression (CSQ2 protein level compared with wild-type [WT]: 20.5% ± 3%) (Figure 1B), although the transcript level of CSQ2 remained unchanged (Figure S1). This result points out a possible instability of CSQ2 protein in the absence of triadin due to inefficient mRNA translation or increased protein degradation. Similar CSQ2 and RyR2 reductions were previously reported in another triadin KO mouse model,16 in which triadin deletion was associated with slightly different compensatory modifications of the CRC proteins.
Figure 1.
Proteins and mRNA Levels in TRDN KO Cardiac Muscle before and after Triadin Re-expression
(A and B) Representative immunoblots (A) and quantification of the relative amount of proteins of the CRC (B), compared with WT, in cardiac muscle homogenates of triadin KO mice (five WT and five KO mice, and for each mouse, the value presented is the mean of two to three western blots). Student’s t tests corrected for multiple comparisons using the Holm-Sidak method: RyR2, p = 0.003; DHPR, p = 0.525; FKBP12, p = 0.590; CSQ2, p < 0.001; T32, p < 0.001. (C and D) Representative immunoblots (C) and quantification of CSQ2 and T32 expression in triadin KO mice (D) after AAV2/9-T32 injection (3–4 mice in each group), Student’s t tests corrected for multiple comparisons using the Holm-Sidak method: CSQ2, p = 0.002; T32, p = 0.001. (E) Relative T32 mRNA expression (qPCR) in triadin KO mice after AAV2/9-T32 injection. Data obtained from qPCR were analyzed with the ΔΔCt method. Quantification is displayed relative to WT mice (three mice in each group). GAPDH was used as a reference gene. ANOVA followed by Holm-Sidak selected comparisons tests: WT versus KO, p < 0.001; KO versus KO+T32, p < 0.001.
We next injected an AAV2/9 virus encoding the rat cardiac T32 into the triadin KO mice to re-express the triadin protein. As previously reported, triadin re-expressed in the heart was correctly targeted to the dyads and co-localized with RyR2.6 The triadin re-expression level was quantified in heart homogenates and showed a level of T32 transcript of 35.5% ± 5.1% (Figure 1E), as well as a 18% ± 2% re-expression of the protein compared with absence of expression in KO (Figures 1C and 1D). This T32 expression level was sufficient to induce a 2-fold increase in CSQ2 protein expression, from 27% ± 6% to 56% ± 2% (Figures 1C and 1D), demonstrating a tight link between these two proteins in the heart.
The Expression of a CPVT Mutant Triadin T32-T59R Impairs Cardiac Ca2+ Homeostasis
We previously identified the first triadin mutation (mutation p.T59R) in a CPVT family and demonstrated that this mutation most probably resulted in the absence of triadin.6 We further analyzed the functional consequences of this mutation on Ca2+ homeostasis in basal conditions. Ca2+ transients, sparks, and the SR Ca2+ content were measured in isolated cardiomyocytes from non-transduced triadin-KO mice or from triadin-KO mice transduced with the WT T32 (KO+T32) or with the mutant T32-T59R (KO+T32T59R) (Figure 2). Both viral gene transfers have the same efficiency and resulted in similar transcript level for T32 and T32-T59R.6 We first confirmed that in this triadin KO mouse model, the cardiomyocytes clearly displayed a remodeling in Ca2+ handling (Figure 2A), as already observed in the other triadin KO model,16 with a decrease in Ca2+ transient amplitude (Figure 2B) and a slowdown in SR Ca2+ release (Figure 2C). The decay time of Ca2+ transient was not affected by triadin modifications (Figure 2D), the SR Ca2+ content was reduced (Figure 2F), and a non-significant increase in sparks frequency (Figure 2E) was observed. All of these alterations were reversed by the re-expression of T32, but not by the mutant T32-T59R. Therefore, re-expression of only 19% of normal triadin levels is sufficient to normalize the Ca2+ cycling as well as the SR Ca2+ content (Figure 2, KO+T32).
Figure 2.
Ca2+ Homeostasis in Triadin KO Cardiomyocytes after In Vivo WT and Mutant T32 Re-expression
(A) Representative Ca2+ transient measured in cardiomyocytes isolated from WT mice (n = 50 cardiomyocytes analyzed from five different mice), triadin KO mice (KO, n = 44 cardiomyocytes analyzed from six mice), triadin KO injected with normal triadin (KO+T32, n = 71 cardiomyocytes from six mice), and triadin KO injected with mutated triadin (KO+T32T59R, n = 73 cardiomyocytes from seven mice), with a magnification in the upper inset. (B) Mean Ca2+ transient amplitude, ANOVA followed by Holm-Sidak multiple comparisons tests: WT versus KO, p < 0.05; KO versus KO+T32, p < 0.05; KO versus KO+T32T59R, not significant (ns). (C) Mean Ca2+ transient rise time, ANOVA followed by Holm-Sidak multiple comparisons tests: WT versus KO, p < 0.001; KO versus KO+T32, p < 0.01; KO versus KO+T32T59R, p < 0.05. (D) Mean Ca2+ transient decay time, non-significantly different using an ANOVA. (E) Average Ca2+ sparks frequency from WT mice (n = 50 cardiomyocytes), triadin KO mice (KO, n = 44 cardiomyocytes), triadin KO injected with normal triadin (KO+T32, n = 71 cardiomyocytes), and triadin KO injected with mutated triadin (KO+T32T59R, n = 59 cardiomyocytes), ANOVA followed by Holm-Sidak multiple comparisons tests: WT versus KO, ns; KO versus KO+T32, p < 0.05; KO versus KO+T32T59R, ns. (F) SR Ca2+ load represented by mean values of peak amplitude of caffeine-induced SR-Ca2+ release measured on n = 7 WT mice, n = 7 KO mice, n = 7 KO+T32 mice, and n = 7 KO+T32T59R mice. ANOVA followed by Holm-Sidak multiple comparisons tests, compared with KO: WT versus KO, p < 0.05; KO versus KO+T32, p < 0.01; KO versus KO+T32T59R, ns.
A Pharmacological Approach to Correct Cardiac Arrhythmias
We previously reported that the CPVT mutation T32-T59R induces protein instability and proteasomal degradation of the mutant,6 as demonstrated by increased protein levels in transfected cells incubated with the proteasome inhibitor MG132. In order to further assess the possible in vivo therapeutic benefit of blocking protein degradation, we used kifunensine instead of MG132 to reduce the in vivo side effects. Kifunensine is an inhibitor of mannosidase-I, a key enzyme in the eukaryotic N-glycosylation pathway that targets misfolded proteins for proteasomal degradation. Inhibitors of mannosidase-I have been shown to improve the folding and activity of a number of unstable mutant proteins19 and may represent a therapeutic approach for certain mutations.20 After an in vitro validation of the kifunensine efficiency to correct the mutant triadin instability, the kifunensine treatment was applied in vivo to the AAV2/9-T32-T59R-injected mice. Twenty days after AAV injection, the mice were treated with kifunensine for 12 days and then ECGs were monitored in conscious animals (Figure 3; Figures S2 and S3). The protein expression was quantified in cardiac muscle homogenates by western blot (Figure 4; Figure S4). No epinephrine-induced CPVT was detected in any of the 12 WT mice (Figure 3A). In the absence of kifunensine treatment, 9 out of 11 triadin KO mice (82%; Figures 3B and 3C) exhibited epinephrine-induced CPVT. T32 re-expression significantly abolished the arrhythmias (p = 0.012), whereas T32-T59R did not (Figures 3C and S2B). After kifunensine treatment, triadin re-expression in the KO+T32-T59R mice was unchanged (Figure 4A); however, we observed that only two out of six T32-T59R-injected mice triggered arrhythmias after epinephrine stimulation (non-adjusted p = 0.046, this value did not reach the level of significance set to 0.05, after correction for multiple comparison), whereas kifunensine alone was ineffective in KO mice (Figure 3C; Figures S2C and S2D).
Figure 3.
In Vivo ECG Recordings after Kifunensine Treatment
(A) Representative ECG recording in WT mice. In the WT mice, none of the 12 mice developed CPVT after epinephrine injection. (B) Representative ECG recording of CPVT in triadin KO mouse after epinephrine injection (2 mg/kg i.p.). (C) Percentage and number of mice developing CPVT in KO (n = 9/11), KO+T32 (n = 3/12), KO+T3259R (n = 4/6), KO+T32-T59R+kifunensine (n = 2/6), and KO+kifunensine (n = 7/10). Chi-square tests: KO versus KO+T32, p = 0.0064; KO versus KO+T32T59R, ns; KO versus KO+T32T59R+kifu, p = 0.046, non-significant after correction for multiple comparisons; KO versus KO+kifu, not significant (ns).
Figure 4.

Effect of Kifunensine Treatment on T32 and CSQ2 Expression Levels
(A and B) Representative immunoblot of the triadin (A) expressed at baseline level in cardiac muscle homogenates of WT mice, KO mice, or KO mice injected with AAV2/9-T32 or AAV2/9-T32T59R ± treatment with kifunensine, and of CSQ2 (B) in the same conditions. (C) Relative amount of CSQ2 compared with myosin evaluated with western blot as represented in (B) on n = 5 KO mice, n = 4 KO+T32 mice, n = 5 KO+T32T59R mice, n = 3 KO+T32T59R+Kifu mice, and n = 3 KO+Kifu mice. ANOVA followed by Holm-Sidak selected comparisons tests: KO versus KO+T32, not significant (ns); KO versus KO+T32T59R, ns; KO versus KO+T32T59R+Kifu, p < 0.001; and KO versus KO+Kifu, p < 0.001.
Because kifunensine gave promising results in the reduction of CPVT occurrence, and because of the relationship between triadin and CSQ2, we further studied the impact of this treatment on CSQ2 expression (Figures 4B and 4C; additional western blots are presented in Figure S4). Although AAV2/9-T32-T59R injection did not enhance triadin expression to a detectable amount (Figure 4A), it increased the CSQ2 protein expression level from 28% ± 3% to 45% ± 6% compared with WT level. In T32-T59R-expressing mice, kifunensine treatment induced a further increase of CSQ2 protein expression to 144% ± 8.5%. Similarly, kifunensine treatment in triadin KO mice (Figure 4, KO+Kifu) was also able to increase CSQ2 expression to 117% ± 32%. This could point out the instability of CSQ2 in the absence of triadin, probably due to incorrect folding of the protein. Triadin may act as a chaperone, interacting with CSQ2 and promoting its correct folding in the SR and anchoring to the triad. Based on this assumption, we hypothesized that the mutant triadin T32-T59R would be expressed at sufficient levels to perform part of its chaperone function before being targeted for degradation. To test this hypothesis, HEK293 cells were transfected with both CSQ2 and triadin (WT-T32 or mutant T32-T59R), and the interaction between CSQ2 and triadin was assessed by immunoprecipitation. As observed in Figure S5, T32 or T32-T59R co-immunoprecipitated with CSQ2, thus confirming that the mutation T59R did not abolish the ability of triadin to interact with CSQ2 and perform its putative chaperone function on CSQ2.
CSQ2, a Possible Option for Gene Therapy in Triadin Mutation-Induced CPVT
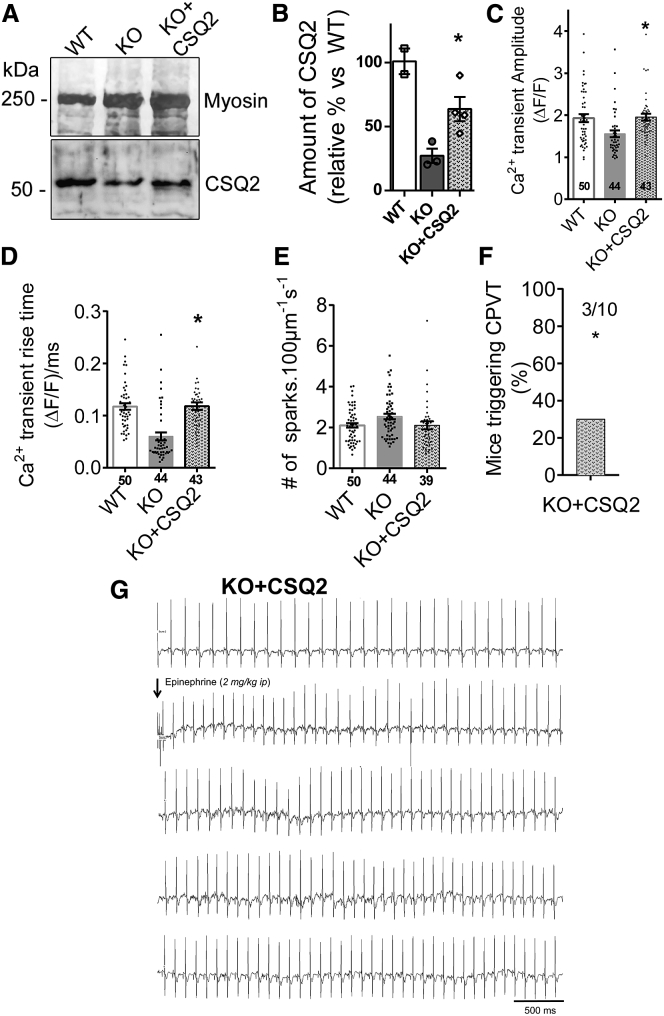
Because CSQ2 seems to mediate the functional effect observed after triadin injection and kifunensine treatment, we directly re-expressed CSQ2 in the triadin KO mice. After injection of an AAV2/9 encoding calsequestrin, cardiac arrhythmia was monitored by in vivo ECG recording (Figure 5). The amount of CSQ2 protein expressed after this AAV-CSQ2 injection was evaluated in cardiac muscle homogenates and found to be an average of 63% ± 9% of the WT level (Figures 5A and 5B), which is similar to the CSQ2 amount found after T32 re-expression in triadin KO mice (Figure 1D). It represents a 2-fold increase compared with CSQ2 expression level in KO mice. CSQ2 re-expression was able to increase the amplitude of calcium transient (Figure 5C) and to prevent the slowing of Ca2+ transient rise time (Figure 5D) compared with KO mice. This shows that CSQ2 was able to partially compensate for the lack of triadin and was sufficient to rescue the Ca2+ homeostasis in cardiomyocytes. As a confirmation, ECG recording demonstrated that CSQ2 re-expression reduced CPVT (only 3 mice out of 10 triggered CPVT compared with 9 out of 11 for KO mice, p = 0.0165) after epinephrine injection (Figures 5F and 5G).
Figure 5.
Effect of CSQ2 Re-expression in TRDN KO Mice
(A and B) Representative immunoblot (A) and quantification of the amount of CSQ2 (B) expressed at baseline level in WT (n = 2) and KO (n = 3) mice and re-expressed in cardiac muscle homogenates of triadin KO mouse 6 weeks after injection of AAV2/9-CSQ2 (n = 4 mice). Student t test: KO versus KO+CSQ2, p = 0.030. (C–E) Calcium homeostasis in isolated cardiomyocytes of KO+CSQ2 mice. The addition of a new group of four mice KO+CSQ2 to data previously displayed in Figure 2 did not modify the significance of any ANOVA. Data for WT and KO groups from Figure 2 are recalled here. (C) Mean calcium transient amplitude. The Holm-Sidak post hoc comparison following the same ANOVA as performed in Figure 2C indicated that the KO+CSQ2 group was significantly different from the KO group, p < 0.05. (D) Mean Ca2+ transient rise time. The Holm-Sidak post hoc comparison following the same ANOVA as performed in Figure 2C indicated that the KO+CSQ2 group was significantly different from the KO group, p < 0.001. (E) Average Ca2+ sparks frequency (KO+CSQ2: n = 39 cardiomyocytes from four mice). (F) Percentage and number of mice triggering CPVT in KO+CSQ2 (n = 3/10), chi-square test compared with the KO data presented in Figure 3C, p = 0.0165. (G) Representative ECG recording in triadin KO + CSQ2 mouse after epinephrine injection (2 mg/kg i.p.).
Discussion
Alterations of CSQ2 and Triadin Are Associated in the Different Recessive CPVT Models
Because we have identified the first mutations in the TRDN gene in CPVT patients,6 this gene has been further involved in other very severe CPVT7, 8 or long-QT syndrome patients.21 Most of the triadin mutations identified to date result in the absence of the protein.6, 8 Numerous recessive CPVT mouse models have been studied within the last decade. Decrease in, or complete absence of, CSQ2 resulted in an increase of RyR2 and calmodulin expression, as well as a reduction of triadin and junctin in the hearts of CSQ2-related CPVT mouse models.10, 11, 22, 23, 24 Here we show in our triadin-deficient mouse model that the loss of triadin is associated with an 80% reduction in CSQ2 protein and a 30% reduction in RyR2, without modification of the other proteins tested in the CRC. This probably explains why this triadin KO model shares so many similarities with CSQ2 KO models. Because of the lack of commercially available specific anti-junctin antibody, junctin was assessed in our model only at the mRNA level, and no modification was observed. Our model is therefore slightly different from the other triadin KO model, in which a 60% reduction in the amount of CSQ2 and a 50% reduction in the amount of RyR2 was associated with triadin deletion.16 In line with the other CPVT mouse models developed previously with CSQ2 mutations,22, 24 our triadin KO mouse model presents a reduction in the SR Ca2+ load, pointing out a common pathophysiological mechanism.
We previously showed that the T32-T59R mutation induces the instability of the protein and its degradation by proteasome.6 However, we observed here that the amount of CSQ2 is increased from 20% in triadin-deficient mice to 45% after viral gene transfer of the mutant T32-T59R, although the amount of triadin was too low to be detected by western blot. Therefore, this triadin mutant is involved in the folding and stability of CSQ2 before being targeted to degradation. As it has been shown in vitro, the trafficking and translation of triadin and CSQ2 are codependent.25 Here, our results suggest that the deficiency of CSQ2 alone would be responsible for the cardiac arrhythmia, as proposed recently,12 and triadin would be involved in CSQ2 folding and/or trafficking and anchoring.
A Common Mechanism for Triadin- and CSQ2-Related CPVT
At the cellular level, it is commonly reported that CPVTs occur via a mechanism of store overload-induced calcium release (SOCIR; for review, see Priori and Chen26). In our model, the absence of triadin and the reduction in CSQ2 result in a reduction in the SR content (both CSQ2-buffered calcium and free luminal calcium) and SR calcium release process (i.e., kinetics of release and transient amplitude). According to the model of SOCIR, lack of SR calcium buffering due to the absence of CSQ2 contributes, during catecholaminergic stress, to an excess of free luminal calcium leading to SOCIR. Nevertheless, decrease in SR Ca2+ release kinetics can be interpreted as an alteration of RyR2 gating properties. According to the work of Györke et al.,27 CSQ2 represents a major luminal Ca2+ sensor responsible for the regulation of RyR2 by luminal Ca2+. The proposed mechanism is that CSQ2 monomers inhibit RyR2 at low SR Ca2+ concentrations by binding to the complex formed by RyR2, triadin, and junctin. At high SR Ca2+ concentrations, CSQ2 dissociates from the RyR2 macromolecular complex and polymerizes, leading to RyR2 activation and desynchronization in SR Ca2+ release.28 The absence of CSQ2 interaction with RyR2 may also account for a reduced duration of Ca2+ signaling refractoriness (for review, see Györke29) and lower SOCIR threshold as is commonly reported for RyR2-mediated CPVT.26
Both T32 and CSQ2 Proteins Are Required for Optimal Function
At the functional level, all of the CSQ2-related CPVT mouse models showed an alteration in Ca2+ homeostasis, as well as stress-induced Ca2+ release leading to CPVT.10, 11, 22, 23, 24 We find similar cardiac alterations in our triadin KO mice, which are corrected by T32 re-expression. A balance between the relative expression level of triadin and CSQ2 seems to be a key point for functional restoration, and the precise triadin-to-CSQ2 protein ratio has already been demonstrated as instrumental in an optimal heart function.30 An interplay between the expressions of these two proteins seems to exist in the heart as observed in many studies,31, 32 because the modification of one of these proteins results in the concomitant modification of the other. Most notably, for an optimal heart function, the full expression of each protein may not be necessary. Only 18% of T32 expression almost fully restored Ca2+ homeostasis. This functional recovery is not only due to T32 re-expression but also to the simultaneous increase of CSQ2 from 27% to 56%. However, CSQ2 alone, re-expressed at 63% of the WT level after AAV-CSQ2 gene transfer in TRDN KO mice, improves Ca2+ homeostasis and arrhythmia, but to a lower extent than in the presence of a small amount of T32, confirming that triadin not only improves the expression level of CSQ2 but also allows the correct CSQ2 anchoring to the triad and thus improves the whole function of the CRC. Because triadin mutations leading to triadin deletion are associated with very severe CPVT with early onset (2–3 years), it can be hypothesized that patients with the T59R mutation present with a less severe form of the disease (onset around 10 years)6 because of the higher amount of CSQ2. On the other side, excess in CSQ2 has been shown to be deleterious for heart function,33, 34 and it has been proposed that triadin helps to hold CSQ2 in a condensed configuration in the junctional SR and therefore maintained normal SR Ca2+ content and release. Along this line, kifunensine treatment, which induced a higher increase in CSQ2, reaching WT level or above, seems either inefficient or only slightly efficient in restoring normal heart function, possibly depending on the amount of triadin. It could be hypothesized that kifunensine treatment might slightly improve the cardiac phenotype only in the presence of unstable mutant triadin because it induced a small increase in triadin expression resulting in improved targeting and folding of CSQ2. In the case of a full triadin KO, with the overexpressed CSQ2 after kifunensine treatment being not associated to any triadin molecule, it would have no beneficial effect.
These data show that a fine threshold of CSQ2 expression at approximately 50% of WT protein expression level reverses the CPVT-like phenotype. Furthermore, this threshold is lowered in the presence of triadin, which seems to profoundly compensate and restore Ca2+ homeostasis and the cardiac function.
The involvement of CSQ2 in CPVT was highlighted years ago, and since then, more clinically applicable, advanced studies have been undertaken. Indeed, the efficiency of viral gene transfer has been shown in vitro in human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes from a CSQ2-deficient CPVT patient.35 Recently, AAV9-based gene transfer with a modified calmodulin has also been shown to improve the CPVT-phenotype in a CSQ2R33Q mouse model.13 This supports the concept that gene therapy is a promising clinical approach to cure recessive CPVTs. Our work enables hypothesizing that the viral gene transfer of CSQ2 in recessive CPVT patients presenting with a mutation in either CASQ2 or TRDN genes could be considered as an efficient therapeutic approach. In conclusion, either pharmacological treatment with kifunensine in the presence of mutations leading to an unstable protein or gene therapy, as reported here, are possible alternatives to current therapeutic strategies in CPVT. Kifunensine deserved additional investigations, specifically assessing CPVT-related CSQ2 mutations. Although kifunensine appears as a promising molecule in those initial experiments, its use in humans could be restrained by low efficiency and/or side effects. Indeed, the improvement was not as efficient as gene transfer, or it was masked by deleterious side effects. Among the deleterious effects, the uncontrolled kifunensine-induced overexpression of CSQ2 in the absence of triadin might play a major role and could be responsible for the low efficiency of kifunensine treatment, because large overexpression of CSQ2 has previously been shown to be deleterious.33, 34 Nevertheless, in the presence of a mutant form of triadin, the overexpression of CSQ2 induced by kifunensine appears protective, highlighting the interplay between triadin and CSQ2 in the pathogenesis of CPVT. In the situation described here, gene transfer seems to be a better option. Gene transfer using AAV appears safe and is already used in many clinical trials,36 and the major concern is the reduced efficiency of gene transfer by immune response, which has to be taken into account and handled correctly.37, 38
Materials and Methods
Study Approval
All procedures using animals were approved by the institutional ethics committee (CEEA-GIN 04, N°134) and followed the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Plasmids and AAV
Rat cardiac triadin T32 and human CSQ2 (GenBank: NM_001232.3) were cloned into pcDNA3.1 (Invitrogen) for cell transfection and pZac2.1 for AAV development as previously described.6 Three recombinant AAVs of serotype 2/9, encoding the WT T32 (AAV-T32), the mutant T32-T59R (AAV-T32-T59R), or the human CSQ2 (AAV-CSQ2) were produced by the Penn Vector Core (Philadelphia, PA, USA).
Antibodies
Anti-RyR antibody,39 anti-Triadin N-terminal end antibody,40 and anti-T32 C-terminal end antibody41 have been described previously. Anti-Cav1.2 was obtained from Alomone (ACC-003), anti-CSQ2 from Abcam (ab3516), and anti-FKBP12 from Thermo Scientific (PA1-026A).
qRT-PCR
Total RNA was isolated from WT and Triadin KO hearts or isolated cardiomyocytes using TRIzol reagent (Life Technologies, Saint Aubin, France) and PureLink RNA Mini Kit (Life Technologies, Saint Aubin, France). First-strand cDNA was obtained using oligo(dT) primed reverse transcription from 500 ng of RNA. mRNAs real-time quantification was performed using iQ SYBR Green Supermix (Bio-Rad, Marnes la Coquette, France) with an IQ iCycler detection system (Bio-Rad, Marnes la Coquette, France). Genes and primer sequences are listed in Table S1. The following experimental protocol was used: denaturation at 95°C for 3 min followed by 40 cycles of 95°C for 10 s and 55°C for 45 s. Melting curve analysis showed specific melting temperatures. Data were analyzed with the ΔΔCt method. Relative gene expression was quantified as follows: fold change = 2 − Δ(ΔCt), where ΔCt = Cttarget − Ctreference and Δ(ΔCt) = ΔCtsample − ΔCtcontrol. Ct is the fractional cycle number at which the fluorescence passes the fixed threshold. The target gene represents TRDN, CASQ2, RYR2, JCN, and FKBP1B genes, and the reference gene is the GAPDH.
HEK293 Cell Transfection and Immunoprecipitation
HEK293T cells (ATCC) were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (Invitrogen). Cells were transfected with pcDNA3.1-CSQ2 alone or cotransfected with either pcDNA3.1-T32 or pcDNA3.1-T32-T59R using Exgen 500 (Euromedex). Cells were collected 28 h later. Solubilization and immunoprecipitation were performed as previously described39 using anti-T32 antibody and Dynabeads Protein-G (Life Technologies). The immunoprecipitated proteins were analyzed by western blot.
Mouse In Vivo Transduction and Kifunensine Treatment
Triadin KO mice were previously described.17 Two-week-old triadin KO male mice (5–7 g) were injected intraperitoneally with 1.5 × 1011 viral genome copies diluted in 100 μL. The mice were used after 6 weeks of transgene expression, and transgene expression has been checked by western blot analysis. Kifunensine (Enzo Life Sciences) was diluted at 0.8 mg/mL in water, and the mice were treated daily by gastric feeding at 4 mg/kg/day for the last 12 days before experiments.
Western Blot
Western blot analysis has been performed on isolated cardiomyocytes or on cardiac muscle homogenates as stated in the figure legends. Whole cardiac muscle homogenates were prepared by homogenization as previously described.17 One hundred micrograms of cardiomyocyte homogenates or 40 μg of cardiac muscle homogenates was analyzed by quantitative western blot analysis as described previously17 using myosin heavy chain as a reference protein and horseradish peroxidase (HRP)-labeled secondary antibodies (Jackson ImmunoResearch Laboratories). The quantification was performed on a ChemiDoc XRS apparatus using Quantity One software (Bio-Rad). For each lane, the protein amount measured was normalized by that of myosin. For each blot, WT homogenates were used as the reference, i.e., their mean protein amount was set to 100% while every individual homogenate of the blot was given a value relative to 100%. The value presented for each mouse is the mean of two to three independent western blots. For each protein of interest, the final value is the mean ± SEM of the n animals tested.
Measurement of Intracellular Ca2+ Transients, Ca2+ Sparks, and SR Ca2+ Content
Heart excisions were performed after cervical dislocation of mice by authorized personnel. According to “The AVMA Guidelines for the Euthanasia of Animals: 2013 Edition,” this method allows a rapid loss of consciousness and does not chemically contaminate tissue. Cardiac ventricular myocytes were enzymatically dissociated as previously reported.42 Freshly isolated cardiomyocytes were then loaded with Fluo-4 AM (5 μM; Molecular Probes) and field stimulated at 1 Hz with 1-ms current pulse. To obtain a similar dye loading, the cell concentration was set to 40,000 cells/μL during the loading, and the few cells with a very low intensity (none responsive to stimulation) or very high fluorescence and calcium waves were discarded. Changes in fluorescence (ΔF/F0) were recorded using an LSM 510 Meta Zeiss confocal microscope at room temperature with identical settings (pinhole, PMT, offset) in line-scan mode (1.5 ms/line) during field stimulation or after caffeine application (10 mM) to assess the SR Ca2+ load. Ca2+ transients were analyzed using PeakInspector software (https://asalykin.github.io/PeakInspector/). Spontaneous Ca2+ sparks were recorded in quiescent cells following 5-min stimulations in order to reach steady-state SR-Ca2+ content and analyzed with the SparkMaster plug-in under ImageJ software (https://imagej.nih.gov/ij/plugins/).
Telemetric ECG Recording and Analyses
ECGs were recorded using subcutaneous teletransmitters (TAE-F20; Data Sciences International) in accordance to the Lambeth conventions from the housing of animals to determination of arrhythmic events.43 After a preanesthetic (physical) evaluation, the transmitter (TAE-F20; Data Sciences International, St. Paul, MN, USA) was inserted in mice subcutaneously along the back under general anesthesia (2% inhaled isoflurane/O2; Aerrane, Baxter, France), and two ECG electrodes were placed hypodermically in the region of the right shoulder (negative pole) and toward the lower left chest (positive pole) to approximate lead II of the Einthoven surface ECG. During the procedure, respiratory and cardiac rate and rhythm, adequacy of anesthetic depth, muscle relaxation, body temperature, and analgesia were monitored to avoid anesthesia-related complications. ECG monitoring was performed 2 weeks after recovery from surgery in the home cage with a signal transmitter-receiver (RPC-1) connected to a data acquisition system (IOX, EMKA, France). The data were collected continuously over 24 h at a sampling rate of 2,000 Hz. Continuous digital recordings were analyzed off-line with version 1.5.12 of the software ECG-auto (EMKA Technologies, Paris, France) after to be digitally filtered between 0.1 and 1,000 Hz. ECG were scanned by hand in blind conditions to detect catecholamine-induced polymorphic ventricular tachycardia after epinephrine injection (intraperitoneally [i.p.], 2 mg/kg body weight in 0.9% NaCl sterile solution; Baxter, France) over 1.5-h ECG.44
Statistical Analysis
The statistical analysis has been done with GraphPad Prism 6.0 software. The number of samples and the name of the parametric test applied are indicated in each figure legend. Because most results were obtained after multiple comparisons or from scarce samples, significant results are presented as *p < 0.05, whatever the real value for p, indicated in the figure legends. All data are shown as mean ± SEM.
Author Contributions
M.C., A.O., N.R.-B., and Julie Brocard performed the molecular analyses and the mice treatments. J.F. performed the calcium analysis. J.T. performed the in vivo analysis. Jacques Brocard performed the statistical analysis. A.L., J.F., and I.M. designed the study, supervised the project, and wrote the paper. All authors analyzed the data and reviewed the manuscript.
Acknowledgments
We thank Dr. B. Strauss for the proofreading of the manuscript, and Drs. I. Richard and T. Toursel for their help with the kifunensine treatment. This work was supported by grants from INSERM, Centre National de la Recherche Scientifique (CNRS), and Association Française contre les Myopathies (AFM-Téléthon).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.09.012.
Contributor Information
Alain Lacampagne, Email: alain.lacampagne@inserm.fr.
Isabelle Marty, Email: isabelle.marty@univ-grenoble-alpes.fr.
Supplemental Information
References
- 1.Jiménez-Jáimez J., Peinado R., Grima E.Z., Segura F., Moriña P., Sánchez Muñoz J.J., Mazuelos F., Cózar R., Gimeno J.R., Heras R.P. Diagnostic approach to unexplained cardiac arrest (from the FIVI-Gen Study) Am. J. Cardiol. 2015;116:894–899. doi: 10.1016/j.amjcard.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi M., Denjoy I., Extramiana F., Maltret A., Buisson N.R., Lupoglazoff J.M., Klug D., Hayashi M., Takatsuki S., Villain E. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 3.De Ferrari G.M., Dusi V., Spazzolini C., Bos J.M., Abrams D.J., Berul C.I., Crotti L., Davis A.M., Eldar M., Kharlap M. Clinical management of catecholaminergic polymorphic ventricular tachycardia: the role of left cardiac sympathetic denervation. Circulation. 2015;131:2185–2193. doi: 10.1161/CIRCULATIONAHA.115.015731. [DOI] [PubMed] [Google Scholar]
- 4.Priori S.G., Napolitano C., Tiso N., Memmi M., Vignati G., Bloise R., Sorrentino V., Danieli G.A. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 5.Lahat H., Pras E., Olender T., Avidan N., Ben-Asher E., Man O., Levy-Nissenbaum E., Khoury A., Lorber A., Goldman B. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am. J. Hum. Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roux-Buisson N., Cacheux M., Fourest-Lieuvin A., Fauconnier J., Brocard J., Denjoy I., Durand P., Guicheney P., Kyndt F., Leenhardt A. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum. Mol. Genet. 2012;21:2759–2767. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh M.A., Stuart A.G., Schlecht H.B., James A.F., Hancox J.C., Newbury-Ecob R.A. Compound heterozygous triadin mutation causing cardiac arrest in two siblings. Pacing Clin. Electrophysiol. 2016;39:497–501. doi: 10.1111/pace.12813. [DOI] [PubMed] [Google Scholar]
- 8.Rooryck C., Kyndt F., Bozon D., Roux-Buisson N., Sacher F., Probst V., Thambo J.B. New family with Catecholaminergic Polymorphic Ventricular Tachycardia linked to the triadin gene. J. Cardiovasc. Electrophysiol. 2015;26:1146–1150. doi: 10.1111/jce.12763. [DOI] [PubMed] [Google Scholar]
- 9.Nyegaard M., Overgaard M.T., Søndergaard M.T., Vranas M., Behr E.R., Hildebrandt L.L., Lund J., Hedley P.L., Camm A.J., Wettrell G. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am. J. Hum. Genet. 2012;91:703–712. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denegri M., Bongianino R., Lodola F., Boncompagni S., De Giusti V.C., Avelino-Cruz J.E., Liu N., Persampieri S., Curcio A., Esposito F. Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age. Circulation. 2014;129:2673–2681. doi: 10.1161/CIRCULATIONAHA.113.006901. [DOI] [PubMed] [Google Scholar]
- 11.Denegri M., Avelino-Cruz J.E., Boncompagni S., De Simone S.A., Auricchio A., Villani L., Volpe P., Protasi F., Napolitano C., Priori S.G. Viral gene transfer rescues arrhythmogenic phenotype and ultrastructural abnormalities in adult calsequestrin-null mice with inherited arrhythmias. Circ. Res. 2012;110:663–668. doi: 10.1161/CIRCRESAHA.111.263939. [DOI] [PubMed] [Google Scholar]
- 12.Kurtzwald-Josefson E., Yadin D., Harun-Khun S., Waldman M., Aravot D., Shainberg A., Eldar M., Hochhauser E., Arad M. Viral delivered gene therapy to treat catecholaminergic polymorphic ventricular tachycardia (CPVT2) in mouse models. Heart Rhythm. 2017;14:1053–1060. doi: 10.1016/j.hrthm.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Liu B., Walton S.D., Ho H.T., Belevych A.E., Tikunova S.B., Bonilla I., Shettigar V., Knollmann B.C., Priori S.G., Volpe P. Gene transfer of engineered calmodulin alleviates ventricular arrhythmias in a calsequestrin-associated mouse model of catecholaminergic polymorphic ventricular tachycardia. J. Am. Heart Assoc. 2018;7:e008155. doi: 10.1161/JAHA.117.008155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., Kelley J., Schmeisser G., Kobayashi Y.M., Jones L.R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- 15.Marty I. Triadin regulation of the ryanodine receptor complex. J. Physiol. 2015;593:3261–3266. doi: 10.1113/jphysiol.2014.281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chopra N., Yang T., Asghari P., Moore E.D., Huke S., Akin B., Cattolica R.A., Perez C.F., Hlaing T., Knollmann-Ritschel B.E. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc. Natl. Acad. Sci. USA. 2009;106:7636–7641. doi: 10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oddoux S., Brocard J., Schweitzer A., Szentesi P., Giannesini B., Brocard J., Fauré J., Pernet-Gallay K., Bendahan D., Lunardi J. Triadin deletion induces impaired skeletal muscle function. J. Biol. Chem. 2009;284:34918–34929. doi: 10.1074/jbc.M109.022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen X., Franzini-Armstrong C., Lopez J.R., Jones L.R., Kobayashi Y.M., Wang Y., Kerrick W.G., Caswell A.H., Potter J.D., Miller T. Triadins modulate intracellular Ca(2+) homeostasis but are not essential for excitation-contraction coupling in skeletal muscle. J. Biol. Chem. 2007;282:37864–37874. doi: 10.1074/jbc.M705702200. [DOI] [PubMed] [Google Scholar]
- 19.Wang F., Song W., Brancati G., Segatori L. Inhibition of endoplasmic reticulum-associated degradation rescues native folding in loss of function protein misfolding diseases. J. Biol. Chem. 2011;286:43454–43464. doi: 10.1074/jbc.M111.274332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartoli M., Gicquel E., Barrault L., Soheili T., Malissen M., Malissen B., Vincent-Lacaze N., Perez N., Udd B., Danos O., Richard I. Mannosidase I inhibition rescues the human alpha-sarcoglycan R77C recurrent mutation. Hum. Mol. Genet. 2008;17:1214–1221. doi: 10.1093/hmg/ddn029. [DOI] [PubMed] [Google Scholar]
- 21.Altmann H.M., Tester D.J., Will M.L., Middha S., Evans J.M., Eckloff B.W., Ackerman M.J. Homozygous/compound heterozygous triadin mutations associated with autosomal-recessive long-QT syndrome and pediatric sudden cardiac arrest: elucidation of the triadin knockout syndrome. Circulation. 2015;131:2051–2060. doi: 10.1161/CIRCULATIONAHA.115.015397. [DOI] [PubMed] [Google Scholar]
- 22.Kalyanasundaram A., Viatchenko-Karpinski S., Belevych A.E., Lacombe V.A., Hwang H.S., Knollmann B.C., Gyorke S., Periasamy M. Functional consequences of stably expressing a mutant calsequestrin (CASQ2D307H) in the CASQ2 null background. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H253–H261. doi: 10.1152/ajpheart.00578.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valle G., Boncompagni S., Sacchetto R., Protasi F., Volpe P. Post-natal heart adaptation in a knock-in mouse model of calsequestrin 2-linked recessive catecholaminergic polymorphic ventricular tachycardia. Exp. Cell Res. 2014;321:178–189. doi: 10.1016/j.yexcr.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Song L., Alcalai R., Arad M., Wolf C.M., Toka O., Conner D.A., Berul C.I., Eldar M., Seidman C.E., Seidman J.G. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 2007;117:1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sleiman N.H., McFarland T.P., Jones L.R., Cala S.E. Transitions of protein traffic from cardiac ER to junctional SR. J. Mol. Cell. Cardiol. 2015;81:34–45. doi: 10.1016/j.yjmcc.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priori S.G., Chen S.R. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Györke I., Hester N., Jones L.R., Györke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern M.D., Song L.S., Cheng H., Sham J.S.K., Yang H.T., Boheler K.R., Ríos E. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J. Gen. Physiol. 1999;113:469–489. doi: 10.1085/jgp.113.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Györke S. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2009;6:123–129. doi: 10.1016/j.hrthm.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Kučerová D., Baba H.A., Bokník P., Fabritz L., Heinick A., Mát’uš M., Müller F.U., Neumann J., Schmitz W., Kirchhefer U. Modulation of SR Ca2+ release by the triadin-to-calsequestrin ratio in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2008–H2017. doi: 10.1152/ajpheart.00457.2011. [DOI] [PubMed] [Google Scholar]
- 31.Knollmann B.C. New roles of calsequestrin and triadin in cardiac muscle. J. Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chopra N., Knollmann B.C. Triadin regulates cardiac muscle couplon structure and microdomain Ca(2+) signalling: a path towards ventricular arrhythmias. Cardiovasc. Res. 2013;98:187–191. doi: 10.1093/cvr/cvt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones L.R., Suzuki Y.J., Wang W., Kobayashi Y.M., Ramesh V., Franzini-Armstrong C., Cleemann L., Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J. Clin. Invest. 1998;101:1385–1393. doi: 10.1172/JCI1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato Y., Ferguson D.G., Sako H., Dorn G.W., 2nd, Kadambi V.J., Yatani A., Hoit B.D., Walsh R.A., Kranias E.G. Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J. Biol. Chem. 1998;273:28470–28477. doi: 10.1074/jbc.273.43.28470. [DOI] [PubMed] [Google Scholar]
- 35.Lodola F., Morone D., Denegri M., Bongianino R., Nakahama H., Rutigliano L., Gosetti R., Rizzo G., Vollero A., Buonocore M. Adeno-associated virus-mediated CASQ2 delivery rescues phenotypic alterations in a patient-specific model of recessive catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 2016;7:e2393. doi: 10.1038/cddis.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naso M.F., Tomkowicz B., Perry W.L., 3rd, Strohl W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pacak C.A., Byrne B.J. AAV vectors for cardiac gene transfer: experimental tools and clinical opportunities. Mol. Ther. 2011;19:1582–1590. doi: 10.1038/mt.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marty I., Robert M., Villaz M., De Jongh K., Lai Y., Catterall W.A., Ronjat M. Biochemical evidence for a complex involving dihydropyridine receptor and ryanodine receptor in triad junctions of skeletal muscle. Proc. Natl. Acad. Sci. USA. 1994;91:2270–2274. doi: 10.1073/pnas.91.6.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marty I., Robert M., Ronjat M., Bally I., Arlaud G., Villaz M. Localization of the N-terminal and C-terminal ends of triadin with respect to the sarcoplasmic reticulum membrane of rabbit skeletal muscle. Biochem. J. 1995;307:769–774. doi: 10.1042/bj3070769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vassilopoulos S., Thevenon D., Rezgui S.S., Brocard J., Chapel A., Lacampagne A., Lunardi J., Dewaard M., Marty I. Triadins are not triad-specific proteins: two new skeletal muscle triadins possibly involved in the architecture of sarcoplasmic reticulum. J. Biol. Chem. 2005;280:28601–28609. doi: 10.1074/jbc.M501484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fauconnier J., Thireau J., Reiken S., Cassan C., Richard S., Matecki S., Marks A.R., Lacampagne A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker M.J., Curtis M.J., Hearse D.J., Campbell R.W., Janse M.J., Yellon D.M., Cobbe S.M., Coker S.J., Harness J.B., Harron D.W. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc. Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 44.Thireau J., Karam S., Roberge S., Roussel J., Aimond F., Cassan C., Gac A., Babuty D., Le Guennec J.Y., Lacampagne A. Β-adrenergic blockade combined with subcutaneous B-type natriuretic peptide: a promising approach to reduce ventricular arrhythmia in heart failure? Heart. 2014;100:833–841. doi: 10.1136/heartjnl-2013-305167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.