Abstract
Individuals with autism show a complex profile of differences in imitative ability, including a general deficit in precision of imitating another’s actions, and special difficulty imitating non-meaningful gestures relative to meaningful actions on objects. Given that they also show atypical patterns of visual attention when observing social stimuli, we investigated whether possible differences in visual attention when observing an action to be imitated may contribute to imitative difficulties in autism, in both non-meaningful gestures and meaningful actions on objects. Results indicated that 1) compared with a matched group of 13 typically-developing children, a group of 18 high functioning 8- to 15-year-olds with Autistic Disorder showed similar patterns of visual attention to the demonstrator’s action but decreased attention to his face when observing a model to be imitated; 2) non-meaningful gestures and meaningful actions on objects triggered distinct visual attention patterns that did not differ across groups, 3) the autism group demonstrated reduced imitative precision for both types of imitation, and 4) duration of visual attention to the demonstrator’s action was related to imitation precision for non-meaningful gestures in the autism group.
Keywords: IMITATION, VISUAL ATTENTION, AUTISM, ACTION UNDERSTANDING, EYE-TRACKING, GAZE PROCESSING
Imitation in Autism Spectrum Disorders
Individuals with autism spectrum disorders (ASD) show impaired development of social interaction and reciprocity, communication difficulties affecting both language and nonverbal skills, and restricted patterns of interests and activities (American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th edition, 1994; World Health Organization, 1990). An area of increasing interest to developmentalists has been that of imitative abilities in this population. Two comprehensive reviews of the literature suggest that individuals with ASD have difficulties imitating others that are specific to autism when compared with other developmental disorders (Rogers & Williams, 2006; Williams, Whiten & Singh, 2004).
Imitation abilities in typically-developing newborns have been found to predict later social responsiveness (Heimann, 1998, 2002), and concurrent positive relationships have been found between imitation and cognitive abilities (Strid, Tjus, Smith, Meltzoff & Heimann, 2006), language development (Bates, Thal, Whitesell & Oaks, 1989; Masur & Eichorst, 2002), sharing of affect (Uzgiris, 1999) and cooperation (Colombi et al., in preparation). Although the nature of these relationships is not fully understood, imitation appears to serve different cognitive and social functions and to be a powerful learning tool (Tomasello, 1999; Uzgiris, 1981). Imitation impairment in autism may lead to abnormal developmental trajectories involving multiple cognitive and social domains, with dramatic effects on the individual’s adaptive functioning and quality of life (DeMyer, Hintgen & Jackson, 1981; Hepburn & Stone, 2006; Rogers & Pennington, 1991). Improved insight into the nature of imitation impairment may lead to a better understanding of autism, as well as to the development of more effective strategies for dealing with these difficulties. Moreover, understanding why imitation is difficult for individuals with autism has far-reaching relevance: it provides a complementary viewpoint on the role of imitation in development and may elucidate the nature and role of imitation in typical development.
In the following analysis of imitation in ASD, we adopt the definition of imitation employed in the social learning literature (Tomasello, 2000; Want & Harris, 2002), which makes a crucial distinction between imitation, involving the appreciation and reproduction of both the goal and the specific actions that brought about that goal, and emulation, which involves the reproduction of the effects of observed actions without the reproduction of the means used by the demonstrator. Regarding the types of action that one can imitate, a crucial distinction opposes gestures (or movements, or intransitive actions, i.e., actions that do not involve objects) to actions on objects (transitive actions, Rogers, Cook & Meryl, 2005; Want & Harris, 2002).
Impairment in the imitation of gestures, specific to individuals with ASD, has been reported consistently across many studies using different stimuli, coding systems, and comparison groups, including different clinical populations. This impairment is common to a wide range of individuals with ASD; it has been attested in individuals with varying levels of IQ and language ability, and at different chronological ages (Aldridge, Stone, Sweeney, & Bower, 2000; Bennetto, 1999; Bernabei, Fenton, Fabrizi, Camaioni, & Perucchini, 2003; Bernier, Dawson, Webb, & Murias, 2007; Dawson, Meltzoff, Osterling, & Rinaldi, 1998; DeMyer et al., 1972, Otha, 1987; Rogers, Bennetto, McEvoy, & Pennington, 1996; Rogers, Stackhouse, Hepburn, & Wehner, 2003; Smith & Bryson, 1998). Following the definition provided by Williams, Whiten and Singh (2004) gestures can be classified as meaningful (gestures that have a semantic association, such as waving goodbye, or pretending to use a comb) or non-meaningful (gestures that can only be described in terms of postures, such as a hand moving across forehead). Most previous work employed non-meaningful gestures (Williams, Whiten & Singh, 2004).
Imitation of actions involving objects (transitive actions) appears to be less impaired than the imitation of intransitive gestures in autism. Although studies investigating this ability provide mixed findings (Dawson et al., 1998; Hammes & Langdell, 1981; Hobson & Lee, 1999; McDonough, Stahmer, Schreibman, & Thompson, 1997; Rogers, et al., 2003), a meta-analysis of the literature reports that imitation involving actions on objects is impaired in autism, but less so than imitation of gestures (Williams, Whiten, & Singh, 2004). Actions on objects can also be classified as meaningful (actions lead to an end state; e.g., a pencil is used to draw a line) or non-meaningful (actions that do not achieve an end-state; e.g., the demonstrator shakes a pencil). Most studies have employed meaningful actions on objects.
In summary, current evidence supports the existence of a graded imitation impairment in autism, involving a general decrement in precision of imitation, and increased difficulty with the imitation of non-meaningful gestures relative to meaningful actions on objects. Notably, young children with typical development also imitate actions on objects better than gestures (Killen & Uzgiris, 1981).
Nature of the imitation impairment in ASD
A series of studies has focused on identifying the cognitive operations and subcomponents that underlie imitation performance, and that may be impaired in autism. This component process approach requires an appreciation of the ingredients involved in accurate imitation performance. These components include (Rogers, 2006; Rothi, Ochipa & Heilmann, 1997; Smith & Bryson, 1994):
encoding phase: a) visual attention to the model, b) working memory
transformation/matching phase: mapping visual input onto body schema -- cross-modal transfer from visual to motor representation
execution phase: motor planning, execution, and evaluation/correction
With respect to the first component, the integrity of working memory for imitation tasks in ASD (e.g. the ability to maintain information “online” to guide action) has been tested in several studies (Bennetto, 1999; Rogers et al., 1996; Smith & Bryson, 1998). None of these studies reported group differences in participants’ ability to remember the observed action over time. The visual analysis aspect of the encoding phase will be discussed in detail below. The second component, involving body mapping and cross-modal matching, has also been examined by different authors (Bennetto, 1999; Smith & Bryson, 1998). No group differences were found with respect to the ability to map visual input from a model onto one’s own body in these studies.
In contrast, the literature suggests that motor difficulties related to the third component process may contribute to the imitation impairment observed in ASD. Motor abnormalities, involving both voluntary and involuntary movements, as well as a relative deficiency of motor skills are present in at least a subgroup of individuals with ASD (Canitano & Vivanti, 2006; Dewey, Cantell, & Crawford, 2007; Ghaziuddin & Butler, 1998; Green et al., 2002). Motor skill deficits, however, do not discriminate ASD from other clinical groups, whereas imitation impairment appears to be autism specific (Baranek, Parham & Bodfish, 2005; Rogers et al, 2003). Several studies have examined the role of motor difficulties in imitation with inconsistent results. The contribution of motor abilities to imitation in autism ranges from 0% to 80% across different studies (Bennetto, 1999; McDuffie, Yoder & Stone, 2005; Rogers et al., 2003; Smith & Bryson, 1998; Vanvuchelen, Roeyers & de Weerdt, 2007). One major issue in interpreting the relationship between motor and imitation abilities in experimental studies is that different studies assessed motor abilities using different motor tests, which, in turn have different imitative demands inherent in their instructions. Although several studies suggest that motor difficulties contribute to the imitation deficit in ASD, a direct causal link between motor and imitation impairment has not been established.
Visual attention and action understanding for imitation
A level of analysis that could elucidate the unique profile of imitation abilities in autism is that of action understanding during the encoding phase of imitation. Does the unique profile of imitation performance in ASD follow from atypical encoding or understanding of the observed action? A way to address this issue is to examine what children with autism look at during the demonstration of an action to be imitated. An individual’s pattern of visual attention during observation could provide crucial insights as to how the action to be imitated is encoded and represented, by revealing which aspects of the demonstration are considered (Carpenter & Call, 2007). Two previous studies that investigated visual attention during the demonstration of actions on objects indicate that typically-developing children, and children with mental retardation, look at both the object and the demonstrator’s face (Carpenter, Tomasello & Savage-Rumbaugh, 1995; Hobson & Hobson, 2007). In these studies, visual attention to aspects of the model was correlated with different aspects of imitative performance. Carpenter and colleagues found that visual attention to the demonstrator’s face was positively related to the reproduction of the means of an action, whereas visual attention to the object was positively correlated to the reproduction of both the means and end-state of the action. Hobson and Hobson found that a specific quality of visual attention to the demonstrator’s face, i.e., looks involving reciprocity and affective contact with the demonstrator, was positively correlated with imitative performance. In addition, Hobson and Hobson found a reduced tendency to look at the demonstrator’s face in a group of children with autism. However, the data reported in that study combine the amount of looks at the demonstrator’s face during both observation and execution of the action, so it remains unknown if reduced attention to the face occurred during observation/the encoding phase. In a recent study, the observation of a person performing a goal-directed movement facilitated imitation only if the observer could see the demonstrator’s gaze (Castiello, 2003). Interestingly, in a similar paradigm, participants with ASD did not gain any facilitation when they could look at the demonstrator’s gaze (Pierno, Mari, Glover, Georgiou, & Castiello 2002).
These findings point to the relevance of the processes underlying the observation of the modeled action. By observing the face, the imitator can gather information about the demonstrator’s intentions and emotions, which may influence how the imitator reproduces the action (or, in other words, the decision of what to imitate; Behne, Carpenter & Tomasello, 2006; Carpenter & Call, 2007). An appreciation of such information, and its integration with information related to the action itself (the kinematics) may be deficient in autism. A basic ability to encode the demonstrator’s actions by individuals with autism is supported by studies using recognition tests (Bennetto, 1999; Rogers et al., 1996; Smith & Bryson, 1998). In these studies participants with autism, like comparison participants, were able to discriminate the actions they had observed from an array of actions represented in pictures or videos. Although participants’ observation of the demonstrator’s action was adequate to permit later recognition, these studies leave open the question of differences in the focus and relative amount of visual attention during the demonstration.
In the present study we conducted a more fine-grained analysis of visual attention patterns in autism and typical development during the demonstration of an action to be imitated by employing eye-tracking technology. There is a growing body of research that utilizes eye-tracking methodology to study various aspects of attention and visual processing in ASD. A number of studies have found that individuals with ASD show atypical viewing patterns when presented with social scenes and faces (Dalton et al., 2005; Klin, Schultz, Volkmar & Cohen, 2002; Pelphrey et al., 2002; Speer, Cook, McMahon & Clark, 2007) as well as non-social scenes (Anderson, Colombo & Shaddy, 2006). Extending these results to situations of imitation, if children with ASD look at different aspects of the model than typically-developing participants, or if they look less at the model, this could affect their representation of the action to be imitated, and could result in differences in imitation performance. This raises the question: Are children with autism and typically developing children actually observing the same aspects of a scene when observing an action to be imitated? Furthermore, since children with autism, as well as typically developing children, diverge in their imitation of meaningful actions on objects versus non-meaningful gestures, do these two types of actions rely on different encoding processes? And, accordingly, do typically-developing children and children with autism attend to the same aspects of the scene when observing actions on objects versus gestures? We explicitly tested these questions in a sample of children with high-functioning autism and a developmentally and chronologically matched typically-developing comparison group. We focused our analysis of visual attention patterns on the two types of imitation that have received the most attention in literature: Meaningful actions on objects and non-meaningful gestures. In the rest of the paper we will use the term “autism” to refer to a sample of children who met full DSM-IV criteria for Autistic Disorder and have IQs and verbal abilities in the average range or above (children with high-functioning autism), and “comparison” to refer to a sample of typically-developing children.
Purpose of the present study
The aims of this study were threefold: 1) to determine whether visual attention to the model differs between children with autism and typically-developing children when observing actions for later imitation; 2) to assess whether visual attention to model differs with respect to the observation of meaningful actions on objects versus non-meaningful gestures; 3) to assess whether different visual attention patterns during the observation of the model contribute to individual differences in imitation performance.
We predicted that 1) children with autism will demonstrate less precise imitation than will typically-developing comparison subjects; 2) children with autism will demonstrate altered patterns of visual attention to the model than comparison subjects; 3) for children with autism, aspects of visual attention to the stimuli will be related to the precision of imitation performance (no specific prediction was made for typically developing children); and 4) since both children with autism and typically developing children (Killen & Uzgiris, 1981; Stone, Ousley & Littleford, 1997) diverge in their imitative performance of meaningful actions on objects vs. non-meaningful gestures, participants will show different visual fixation patterns while observing actions on objects versus gestures.
Method
Participants
Participants in the current study were 18 children and adolescents with high-functioning autism (16 male, 2 female) between the ages of 8 and 15 years, and 13 children and adolescents with typical development (11 male, 2 female) between the ages of 8 and 14 years. The groups did not differ significantly in terms of chronological age, language level, Performance IQ, or gender ratio. Participants were recruited through the M.I.N.D Institute research database and community outreach efforts in Sacramento, CA. This experiment was part of a 3-visit study approved by the UC Davis IRB. Participants received gift cards at the end of each visit. All participants obtained language standard scores above 85, as assessed by the Clinical Evaluation of Language Fundamentals Fourth Edition (CELF-4; Semel, Wiig, & Secord, 2003), which provides comprehensive assessment of both expressive and receptive language ability. Performance IQ was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) and adaptive functioning was assessed via the Daily Living Skills and Socialization subscales of the Vineland Adaptive Behavior Scales-II (VABS; Sparrow, Cicchetti & Balla, 2005). The Communication and Motor skills subscales of the Vineland were not administered because these domains were assessed using more detailed instruments (the CELF-4 for communication ability and the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition, described below). Parents of all participants completed the Social Communication Questionnaire-Lifetime (SCQ; Rutter, Bailey & Lord 2003) to verify group membership.
Current diagnosis of autism required scores above the ASD cutoffs on the Autism Diagnostic Observation Schedule-Module 3 (ADOS-3; Lord et al., 1999) and the SCQ. All participants met full criteria for DSM-IV Autistic Disorder (not Asperger’s Syndrome or PDD-NOS). Exclusion criteria for the autism group were the presence of a genetic or metabolic disorder known to cause autistic features (e.g., Fragile X syndrome or tuberous sclerosis), presence of major medical problems or physical disability, or language level below the average range (e.g., standard score < 85), as assessed by the CELF-4. Exclusion criteria for the typically-developing comparison group included the presence of psychiatric or major medical conditions, history of developmental problems or delay, presence of first- or second-degree relatives with an autism spectrum disorder, parent report of autistic symptoms as assessed with the SCQ, or language level below the average range, as assessed by the CELF-4. The decision to not include a clinical comparison group was based on the presence of similar and unimpaired levels of language and intellectual ability in both groups, making it unnecessary to control for possible effects of these differences on the group with autism. Moreover, previous findings indicate an autism-specific imitation impairment when other clinical groups were tested (Hobson & Lee, 1999; Rogers et al., 1996, 2003; Rogers, Cook & Meryl, 2005) as well as autism-specific differences with regard to visual attention (Hobson & Hobson, 2007). Sample characteristics are presented in Table 1.
Table 1.
Characteristics of High-Functioning Autism and Typically-Developing Participants
| High functioning autism (n=18) | Typically developing (n=13) | ||||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | t-test p value |
|
| CA | 11;4 years (22 months) | 8 to 15 | 11;1 years (24 months) | 8 to 14 | >.05 |
| Language level | 107 (13) | 91 to 134 | 116 (10) | 102 to 126 | >.05 |
| PIQ | 107 (18) | 67 to 133 | 110 (10) | 97 to 132 | >.05 |
| VABS II Social | 61 (7) | 48 to 71 | 101 (9) | 91 to 123 | <.05 |
| VABS II Daily Living | 68 (8) | 56 to 89 | 105 (10) | 95 to 123 | <.05 |
| SCQ | 27 (5) | 18 to 36 | 2 (3) | 0 to 7 | <.05 |
| ADOS - 3 | 17 met criteria for Autism, | N/A | |||
| 1 met criteria for ASD | |||||
| Gender | 16 male, 2 female | 11 male, 2 female | |||
| Ethnicity | 12 Caucasian, 1 Asian, | 13 Caucasian | |||
| 1 African-American/Caucasian | |||||
| 2 Asian/Caucasian | |||||
| 2 Asian/Latino | |||||
Note: there were no significant differences between groups with respect to CA, language level, or PIQ
Procedures
General procedures.
Participants visited the lab with a parent to learn about the study, sign consent and assent forms, and complete diagnostic, IQ, and language assessments. On a separate visit they participated in the motor assessment and eye-tracking imitation task, during a longer session of experimental testing.
Eye-tracking and imitation procedures.
Participants were seated in a comfortable chair at a table, approximately 20 inches from an 18 inch computer monitor. The experimenters demonstrated how to wear the light-weight headgear of the eye-tracking system (Model H6, Applied Science Laboratories, Inc., Bedford, MA), and explained its function to the participant before starting the experimental procedure. To increase motivation and maintain interest in the procedure, the eye-tracker was referred to as a “space helmet,” the testing room was decorated with pictures of planets and stars, and the space theme was extended to a calibration screen that participants were asked to look at. Compliance with the procedures generally did not differ between the two groups and the eye-tracking device was a strong motivator for many participants with autism. In fact a number of participants came up with their own playful ideas about the wearing the eye-tracker, e.g. “It’s like a spy helmet.” Participants had free head, hand and body movement while wearing the eye-tracker; a magnetic head-tracking system (Ascension Technology Co., Burlington, VT) was used in combination with the eye-tracker to correct the eye movement data for head movements. For participants with autism who required additional motivational supports to remain engaged, reinforcements (e.g. favorite objects, bubbles, snacks, gift card) were promised and provided at the end of the task.
The next step in the procedure was a brief calibration routine, where the participant was required to keep his or her head still and look at a series of nine points to achieve an accurate track of eye movement. In some cases assistance from a parent or experimenter was required to help the participant keep his or her head still. Once calibration was successful the imitation task was presented using these verbal instructions: You will see some video clips showing a person performing an action. Watch the screen carefully. At the end of each clip, after the action is done and the screen turns black, you will imitate what you saw the person do in the clip. Three practice trials were given to ensure comprehension of the task requirements. The practice trials included feedback from the experimenter regarding observance of the task demands (i.e. encouragement for attempting to imitate the action at the appropriate time, or repetition of instructions if necessary). All participants demonstrated understanding of the task and imitation of the model after the practice trials and so continued into the experiment.
The experimental stimuli, 12 video clips which ranged in length from 7 to 19 seconds and depicted the same demonstrator performing different actions, were presented at approximately 22° of visual angle via GazeTracker software (EyeResponse Technologies, Charlottesville, VA). While participants watched the screen their eye movements were recorded at 60 Hz by the monocular eye-tracking system. Following each demonstration the screen turned black and participants imitated the observed action while seated at the table. Objects necessary for the imitation of actions on objects were placed on the table just before the presentation of the video involving that specific object. Their imitative performance was recorded by a concealed video camera for later scoring.
The imitation battery was developed by Rogers, Cook and Greiss-Heiss (2005, unpublished), based in part on previous research (Hobson & Lee, 1999; Kimura & Archibald, 1974; Rogers et al, 1996). Each participant saw two types of stimuli: meaningful actions on objects (e.g. drawing a line with a pencil on paper, striking a xylophone), and non-meaningful gestures (e.g. arm flexing at elbow, hand moving across forehead; see Table 2 for detailed descriptions). For the sake of simplicity we will use the terms ‘meaningful’ actions and ‘non-meaningful’ gestures to refer to these two types of stimuli throughout the rest of the paper. Definition of these types of stimuli follows that of Williams, Whiten and Sing (2004) and Rogers, Meryl and Cook (2005). In all the meaningful stimuli, the demonstrator’s action on an object was instrumental to an end-state. These actions can be also defined as goal-directed (Zibetti & Tijus, 2005) or teleological (Csibra & Gergely, 1998, 2007). In all the non-meaningful stimuli, the gestures performed by the demonstrator were not instrumental to an end-state. These gestures can only be described in terms of changes in posture; they were not goal directed or teleological (e.g., they did not produce any effect) and did not have semantic associations or conventional communicative meanings. The “non-meaningful gestures” battery was developed by Kimura and Archibald (1974) as a test for apraxia, and has been used in or adapted for several imitation studies (e.g., Bernier, et al., 2007; Rogers et al., 1996)
Table 2.
Imitation Items
| Non-meaningful gestures (Kimura & Archibald, 1974) | Meaningful Actions on Objects (Rogers, Cook & Greiss-Heiss, 2005) |
|---|---|
| Hand slaps arm | Draw a line |
| Arm flexes at elbow | Brush arm with lint brush |
| Hand/fist on table | Strike a xylophone gently |
| Hand moves across forehead | Flatten dough with rolling pin |
| Arm across chest | Stamp on ink pad |
| Hand from shoulder to front | Strike a xylophone with force |
We decided to compare these two types of stimuli because they have received the most attention in the literature on imitation in autism (Williams, Whiten & Sing, 2004), and because practical constraints limited the number of trials we could successfully run with this population. Adding a category of meaningful gestures, which encompasses communicative gestures such as waving goodbye, would introduce the potentially confounding factor of familiarity due to social experience, or lack thereof. Moreover, children with autism spontaneously use less descriptive, meaningful gestures than typically developing children (Camaioni, Perucchini, Muratori, & Milone, 1997); consequently a difference in the mastery of gestural communication (both expressive and receptive) might influence imitation performance for this type of action. Given this, a fully crossed design of meaningfulness by presence or absence of object was not feasible, so we decided not include a condition examining non-meaningful actions on objects and instead to focus on the two types of stimuli that are most often contrasted: meaningful actions on objects and non-meaningful gestures.
In all video clips the same demonstrator (a 30-year-old male) was seated at a table, hands and arms on the table, and performed the action with a neutral emotional expression, without speaking. He was recorded by a camera directly in front that framed the upper half of his body, from the table to the top of his head. No other stimuli appeared in the background or foreground (see figures 1 and 2). Actions were carried out slowly and distinctly. In the meaningful action on object stimuli, the demonstrator looked at the object on which he performed the action throughout the video clip. In the non-meaningful gesture stimuli, the demonstrator’s gaze was directed straight ahead at the camera, as it would have been odd to look at the empty table or in any other direction.
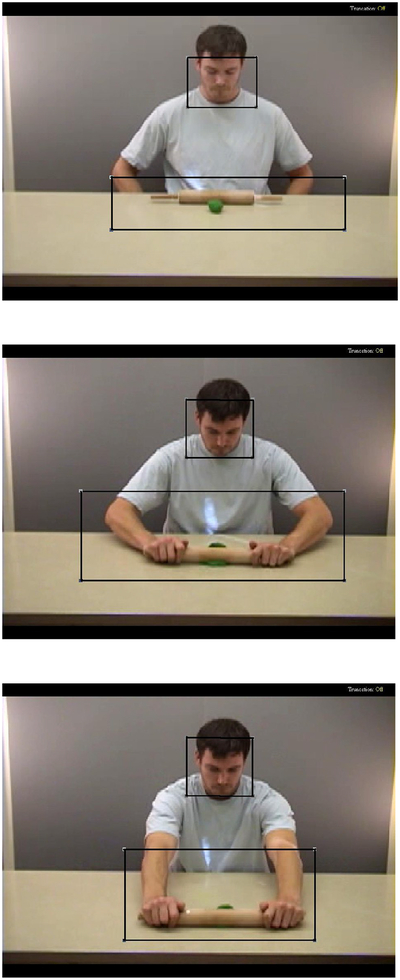
Figure 1.
Face and Action regions of interest shown on sample “meaningful action on object” item. The top image displays the start point, middle image displays a midpoint, and bottom image displays the end point of an action that was presented in a continuous video clip.
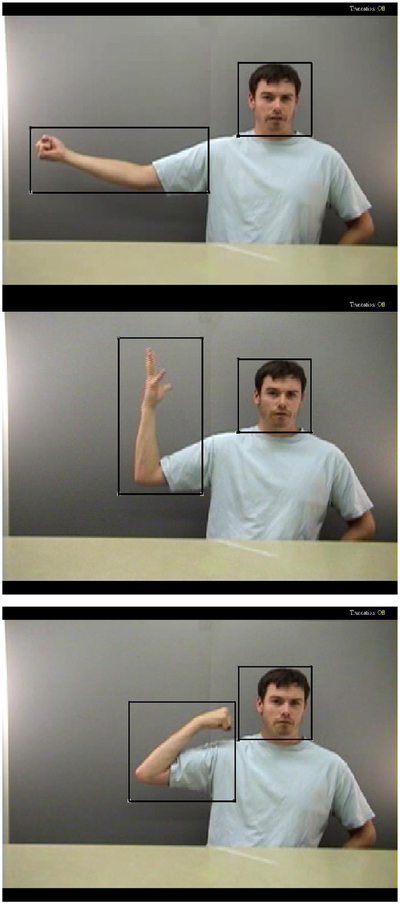
Figure 2.
Face and Action regions of interest shown on sample non-meaningful gesture item. The top image displays the start point, middle image displays a midpoint, and bottom image displays the end point of an action that was presented in a continuous video clip.
Six items of each type of imitation were presented in a fixed random order across two blocks of stimuli (each block contained 3 trials of each type). This allowed for a short break in between blocks when the eye-tracker could be recalibrated if necessary.
Motor skills assessment.
To assess participants’ motor abilities and to examine the potential influence of motor difficulties on imitation performance, a standardized test of motor proficiency, the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition – Short form (BOT-2; Bruininks & Bruininks, 2006) was administered to all participants. It includes 8 subscales and has been standardized for children from 4.5 to 14.5 years of age. Test-retest reliability of this assessment has been shown to be satisfactory (r = .86), and previous work has found the BOT-2 to be sensitive to motor differences in autism (Dewey, Cantwell & Crawford, 2007). Instructions for this assessment are provided verbally (e.g., “Fold this corner of the paper on the line,” or “Hop up and down on one leg until I tell you to stop”) rather than by demonstration, so imitative abilities are not required.
Results of the BOT-2 were scored according to the guidelines provided by the manual. The main variable of interest used in this study was the overall motor skills T score, which incorporates performance on 8 subtests: fine motor precision, fine motor integration, manual dexterity, bilateral coordination, balance, running speed and agility, upper limb coordination and strength. Scores are age- and gender-normed. The mean value of a T score is 50 with a standard deviation of 10 (average range = 40 to 60).
Data coding and analysis
Imitation precision.
Imitation precision was coded using the scoring scheme developed by Rogers, Cook and Greiss-Heiss (2005, unpublished), which is based in part on those developed by Hobson and Lee, (1999), Kimura and Archibald (1974), and Rogers et al. (1996). Accuracy is coded by rating imitation precision on an ordinal scale, where higher scores designate more accurate reproductions of the modeled action. Standardization was achieved through the use of a detailed rating sheet which outlined the criteria for accurate reproduction of each of the presented actions. Two examples of the scoring criteria are presented in Table 3. Scoring criteria for meaningful actions on objects consisted of three non-overlapping categories: Tool grip, Movement, and Tool use, each scored 0 or 1, for a maximum possible score of 3 per item and 18 total score. Categories considered when scoring non-meaningful gestures were: Start position, Plane of movement, Posture change, End position, each coded 0, 1, or 2, for a maximum possible score of 8 per item and 48 total score.
Table 3.
Sample Scoring Criteria for a Meaningful Action on Object Item (above) and a Non-Meaningful Gesture Item (below) from Rogers, Cook and Greiss-Heiss (2005, unpublished)
| Type: Meaningful action on object |
|---|
| Item: Stamp on ink pad |
Scoring criteria
|
| Type: Non-meaningful gesture |
| Item: Arm across chest |
Scoring criteria
|
Three-fourths of participants’ performance data was coded from video records of the testing session by a trained rater who was blind to diagnosis and hypotheses, and the remaining quarter was coded by the first author. In addition, 20% of the entire data set was coded by both raters to calculate inter-rater reliability. Inter-rater reliability for the coding of performance data, using Intra-class correlation, was 0.829 for meaningful and 0.774 for non-meaningful actions.
Visual attention measures.
While they viewed videos of the action to be imitated, participants’ eye movements were digitally recorded by the ASL eye-tracking system, interfaced with GazeTracker software. Region of interest analyses were performed on the visual fixation data with GazeTracker software. GazeTracker allows for analysis based on moving regions of interest, as is required for dynamic video stimuli. Two regions of interest were manually defined for each stimulus item: the region where the action was performed (Action) and the face of the demonstrator performing the action (Face). The Face region covered the entire face of the demonstrator, which remained in relatively the same location throughout each action, so the Face region of interest box did not need to be moved to capture its location (see Figure 1). The average area covered by the Face region was 30,188 square pixels (SD=11,209). There was no significant difference in the size of the Face region between the two types of imitation. The Action region covered the area of the screen where the action was occurring and was updated on a frame-by-frame basis to accurately capture movement (see Figure 1). The Action region covered an average area of 84,430 square pixels (SD=52,591). The average area covered by the Action region was significantly greater for meaningful compared with non-meaningful actions, therefore we controlled for the size of the Action region when analyzing visual attention to this region. The two regions partially overlapped in just one stimulus item, where Face and Action regions overlap for 2 seconds. However, data from this specific item did not differ significantly from that obtained from other items so it was retained in analyses.
Computer generated data provided the percentage of time each participant spent fixating (1) the Action region, (2) the Face region, and (3) anywhere outside these regions (Non-relevant region), for each stimulus video. Participants’ average percentage of looking time for the Action region and Face region for both meaningful and non-meaningful stimuli were computed for further analysis.
Results
Preliminary analyses
In the analyses reported below group means are followed by standard deviations in parentheses.
The distribution of group data was examined for 1) imitation precision for meaningful actions on objects vs. non-meaningful gestures, 2) visual attention to the Action region when observing meaningful actions on objects vs. non-meaningful gestures, and 3) visual attention to the Face region when observing meaningful actions on objects vs. non-meaningful gestures. In most cases, the data were not normally distributed, for either group. For this reason the nonparametric Mann-Whitney U test was performed for all group comparisons and is presented in Tables 4, 5, and 6 below. In the text describing the results of the imitation experiment, main and interaction effects from Anovas are also reported as the non-parametric test results did not differ from the ANOVA results for group.
Table 4.
Percentage of Imitation Precision
| Type of imitation | Group | Mann-Whitney U | Effect size | |
|---|---|---|---|---|
| HFA (n=17) | TYP (n=13) | |||
| Meaningful actions on objects | 93.46 (8.15) | 98.71 (2.43) | 60.00* | d = 0.87 |
| Non-meaningful gestures | 81.37 (13.05) | 95.29 (6.93) | 45.50** | d =1.33 |
Note. HFA= high-functioning autism, TYP= typically-developing
p < .05.
p < .01.
Table 5.
Percentage of Time Spent Looking at the Action Region
| Type of imitation | Group | Mann-Whitney U | Effect size | |
|---|---|---|---|---|
| HFA (n=18) | TYP (n=13) | |||
| Meaningful actions on objects | 62.14 (11.14) | 61.54 (15.77) | 111.00 | d = 0.04 |
| Non-meaningful gestures | 41.79 (12.86) | 37.22 (10.46) | 95.00 | d = 0.38 |
Note. HFA= high-functioning autism, TYP= typically-developing
Table 6.
Percentage of Time Spent Looking at the Face Region
| Type of imitation | Group | Mann-Whitney U | Effect Size | |
|---|---|---|---|---|
| HFA (n=18) | TYP (n=13) | |||
| Meaningful actions on objects | 6.83 (5.41) | 15.02 (8.95) | 49.00** | d = 1.1 |
| Non-meaningful gestures | 13.52 (9.92) | 26.35 (12.84) | 51.00** | d = 1.1 |
Note. HFA= high-functioning autism, TYP= typically-developing
p < .01.
Though there was no significant difference between the groups in terms of language level, we also computed each analysis using CELF-4 core language scores as a covariate to ensure that performance could not be attributed to differences in language ability. All of the group differences reported below remain when language level is controlled for.
Motor skills assessment
Motor assessment data were not available for 1 participant in the comparison group, resulting in an n of 18 for the autism group and 12 for the comparison group. The two groups significantly differed in their performance on the BOT-2 motor skills assessment: the autism group had a mean standard score of 41.6 (5.0) and their distribution was negatively skewed, while the comparison group had a mean standard score of 56 (5.5) and a positively skewed distribution, U=7.00, p<.001. The effect size for this difference (d= 2.7) qualifies as a large effect (Cohen, 1988). The potential contribution of this difference in motor skills to imitation performance is addressed below.
Imitation precision
Within each type of imitation, a total score was calculated by summing the precision scores for each item. Then we calculated the percentage of precision for each type of imitation (meaningful actions on objects and non-meaningful gestures). Percentages were used given the different scoring systems employed for the two types of action (see Method and Table 3). Due to mechanical error with the video recording device, performance data was missing for one participant in the autism group, resulting in an n of 17 for the autism group and 13 for the comparison group. Precision scores were submitted to a 2 (group) X 2 (type of imitation) ANOVA. There was a main effect of group, whereby the autism group was less accurate in their imitation than the comparison group, F(1, 28) = 13.03, p<.005, r = .65. There was also a main effect of type of imitation, F(1,28) = 16.23, p<.001, r = .61; both groups had higher percentages of precision for meaningful actions on objects than non-meaningful gestures imitation. In addition, there was a significant interaction between group and type of imitation: the autism group showed a greater increase in precision for meaningful actions on objects relative to non-meaningful gestures than the comparison group: F(1,28) = 5.07, p<.05, r = .39. Table 4 shows the group means for percentage of precision for each type of imitation.
Regression analyses demonstrated that motor skills did not contribute to the variance in imitation precision for meaningful actions on objects, for either group (Autism group: F(1, 15) = 2.11, p=.17, f2 = .12; Comparison group: F(1, 10) = .001, p=.98, f2 < .00). No significant correlation was found, in either group, between imitation of meaningful actions on objects and language level or Performance IQ. Similarly for imitation of non-meaningful gestures we did not find a significant relationship between motor skills and imitation precision for either group (Autism: F(1, 15) = .01, p=.90, f2 < .001; Comparison group: F(1,10)=.007, p=.93, f2 < .001). Language level contributed to imitative performance for non-meaningful gestures in the comparison group only (R= .48, p<.05, f2 =.23), while Performance IQ was significantly correlated with non-meaningful gesture performance in the Autism group only (R = .67, p=.001, f2 = .08).
Visual attention to the Action region
Each participant’s average percentage of looking time to the Action region was submitted to a 2 (group) X 2 (type of imitation) ANOVA. There was no main effect of group, F(1, 29) = .64, p = .43, r = .15); both children with autism and typically-developing children looked to the Action region for a similar amount of time. However, there was a main effect of type of imitation: both groups looked more to the Action region when observing meaningful actions on objects than when observing non-meaningful gestures, F(1,29) = 46.68, p<.001, r = .79. A multiple regression analysis showed that the size of the Action region was not a significant predictor of the percentage of looking time, F(1, 9) = 1.98, p=.19, f2 = .21 Finally, there was no group by type of imitation interaction: both groups showed a similar tendency to increase visual attention to the Action region when observing meaningful actions on objects compared to non-meaningful gestures, F(1,29) = .37, p=.55, r = .11. Group means for percentage of time spent looking at the Action region are presented in Table 5.
Visual attention to the Face region
In the same manner, each participant’s average percentage of looking time to the Face region was submitted to a 2 (group) X 2 (type of imitation) ANOVA. There was a main effect of group, F(1, 29) = 11.07, p<.01, r = .52; the autism group looked to the Face region approximately half as much as the comparison group did. There was also a main effect of type of imitation: both groups looked more to the Face region when observing non-meaningful gestures than meaningful actions on objects, F(1,29) = 47.76, p<.001, r = .79. No significant group by type of imitation interaction was found F(1,29) = 3.17, p=.09, r = .31. No correlation was found between percentage of visual attention to the Face region and to the Action region in either group for both meaningful (r= .155; p=.41) and non-meaningful actions (r=.067; p=.72). Group means for percentage of time spent looking at the Face region are presented in Table 6.
Visual attention and Imitation Precision
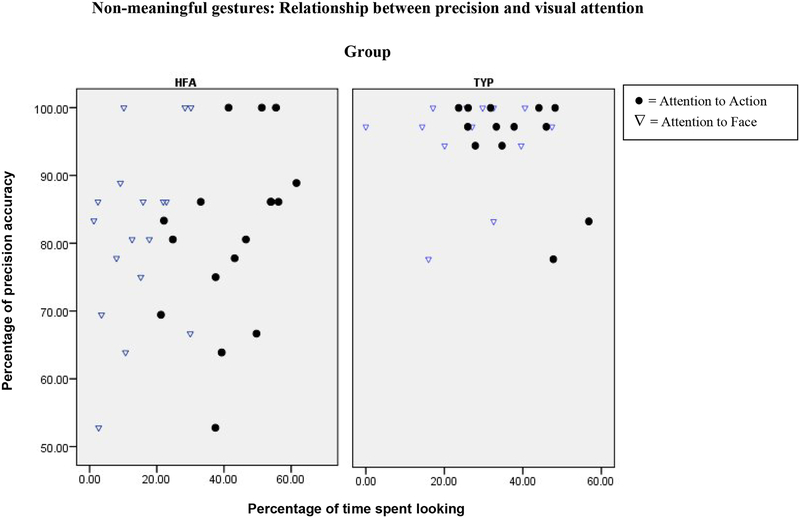
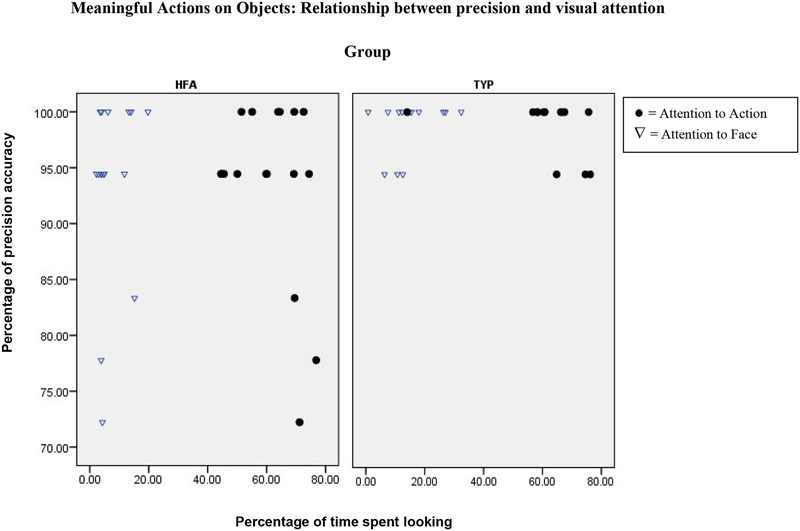
Our last hypothesis concerned the relationship between visual attention and imitation precision. Scatterplots of these results are shown in Figure 3 for non-meaningful gestures, and in Figure 4 for meaningful actions on objects.
Figure 3.
Scatterplot of the Percentage of time spent looking at regions of interest (x axis) and the Percentage of precision accuracy (y axis) for non-meaningful gesture stimuli. HFA= high-functioning autism, TYP= typically-developing.
Figure 4.
Scatterplot of the Percentage of time spent looking at regions of interest (x axis) and the Percentage of precision accuracy (y axis) for meaningful action on objects stimuli. HFA= high-functioning autism, TYP= typically-developing.
Visual attention to the Action region was correlated with imitation precision for non-meaningful gestures in the autism group (τ = .38, p<.05) but not in the comparison group. No other significant relationships were found between visual attention and imitation precision, for either type of imitation stimuli. We return to this finding in the discussion section.
Discussion
In this study we investigated whether 1) children with high-functioning autism show diminished or different visual attention to a model to be imitated compared with typically-developing children, 2) whether different types of imitation elicit different patterns of visual attention behavior, and 3) whether visual attention differences influence imitation performance.
Do children with autism have problems imitating because they pay less attention to the action to be imitated? Our data unequivocally show that this is not the case – the autism group was virtually indistinguishable from the comparison group with respect to visual attention to the Action region. However, our findings do show reliable differences between children with autism and comparison children in their overall patterns of viewing the model to be imitated, with respect to looks to the demonstrator’s face.
Our eye-tracking data are consistent with previous reports of children’s visual attention patterns during imitation tasks (Carpenter, Tomasello & Savage-Rumbaugh, 1995; Hobson & Hobson, 2007). In our study, observation of the model by typically-developing participants involved a significant amount of time spent looking at the face of the demonstrator (15% of the time on average when observing meaningful actions on objects, and 26% of the time on average when observing non-meaningful gestures). Looking at faces may play a role in the action encoding process that underlies imitation in typical development. Why watch the demonstrator’s face when imitating his actions? Meltzoff (2004; Meltzoff & Gopnik, 1993) suggested that the informativity and interpretability of another’s actions are constrained by the observer’s ability to detect similarities between self and the other performing the action. This functional analysis enables the distinction of “like-me” from “non-like-me” entities. Actions performed by entities that behave “like-me” are accorded a special status and are more likely to be imitated (Meltzoff, 2002). The visual attention to the face that we observed could provide the input for this specific “social coding” of the action to be imitated. However, this account cannot explain why participants look at the demonstrator’s face more when the action to be imitated is non-meaningful. In many cases, looking at the demonstrator’s face may provide relevant clues to the meaning of the performed action; knowing where the demonstrator is looking or what emotional expression accompanies his action can provide important information regarding the relevant aspects of the action (Carpenter & Call, 2007). This explanation fits with our finding of different visual attention responses to meaningful actions on objects versus non-meaningful gestures, to which we will return below.
Why do children with autism look at faces less than typically-developing children do? Three possible explanations for this finding are that children with autism look less at faces (1) due to decreased social interest or reward (Dawson, Webb & McPartland, 2005; Grelotti, Gauthier & Schulz, 2002); (2) because they perceive social stimuli as threatening and hyper-arousing (as a consequence of atypical amygdala activation; Dalton et al., 2005), or (3) because they have general difficulty in shifting attention between different stimuli (Landry & Bryson, 2004), possibly related to oculomotor deficits (Luna, Doll, Hegedus, Minshew & Sweeney, 2007; Minshew, Luna & Sweeney, 1999; Takarae, Minshew, Luna & Sweeney. 2004). The first explanation supports an increasingly popular model in which decreased social behavior leads to less social experience, which in turn affects “social specialization” of brain areas, social learning and social-cognitive development (Dawson, Webb & McPartland, 2005; Klin, Jones, Schultz & Volkmar, 2003; Mundy & Neal, 2001). Our finding of decreased attention to the Face region in the autism group is consistent with this explanation, however it does not account for the effects of type of imitation on the autism group. In terms of the second explanation, the fact that children with autism looked to the face more often when observing non-meaningful gestures, where the eyes of the demonstrator were more salient, is not consistent with an explanation based on avoidance due to discomfort with social stimuli. The third explanation is based on the finding that children with autism have difficulties disengaging their attention from a stimulus and shifting attention between two stimuli even when the stimuli are not social in nature (Landry & Bryson, 2004; Townsend, Courchesne & Egaas, 1996). This account does not fit with our findings of different visual attention patterns across types of stimuli. Thus, none of these models accounts for our findings that visual attention to the Face region significantly increases in children with autism when the action to be imitated is a non-meaningful gesture.
In this study, the type of imitation stimuli influenced attention to the demonstrator’s face and increased attention to his action, in both groups. This potentially indicates different action understanding processes for meaningful actions on objects versus non-meaningful gestures. Why should the type of imitation trigger different ways of viewing the model? It has been proposed that from infancy observed actions are either interpreted as goal-directed or as referential (Csibra, 2003; Gergely & Csibra, 2003). In the first case the observer attaches a goal (and, thus, a meaning) to actions that are instrumental to an end state. In the second, the demonstrator’s behavior is interpreted as referring to aspects of the environment which communicate what elements of the environment are relevant to understanding the action. Our data may reflect, at a behavioral level, the observers’ tendency to use a goal-directed (or teleological) interpretation with object acts and a referential interpretation of an action involving non-meaningful gestures. However, an alternative explanation involves the fact that the demonstrator’s direction of gaze also differed between these two types of stimuli – gaze was directed downward towards the object in the meaningful action stimuli whereas it was directed ahead in the non-meaningful gesture stimuli. We need to consider whether different patterns of visual attention to the Face region were driven by the demonstrator’s gaze direction, rather than by distinct action understanding processes. Such an account would not explain why a difference in attention to the Action region was found between types of imitation stimuli, as attention to the Face region was not inversely related to attention to the Action region in either group; attention to the Face and Action regions were independent in our data. Given this, we believe that teleological versus referential interpretation processes, described in detail below, account best for our findings.
In the case of the meaningful actions on objects we presented (e.g., drawing a line, striking a xylophone and so on), the actions themselves, and possibly the objects involved, provide all the elements necessary for a goal-directed interpretation. In other words, these actions are self-explaining, and both groups, when observing them, tended to focus on the Action region and less on other regions. The non-meaningful gestures we presented are ambiguous; they are not “self-explaining,” they do not have an obvious end-state or goal. When observing them, typically-developing children tended to focus much more on the face of the demonstrator, potentially searching for additional information to explain the action, as if asking “Why is he doing this?” Consistent with the notion of top-down, “rational” action understanding, participants shifted their gaze from the Action region to other locations when more elements were needed to interpret the action. A “semantic” coding, as opposed to “kinematic” or “motor” coding, would result in a decreased demand on information processing resources (Gattis, Bekkering & Wohlschlager, 2002) and more efficient imitative performance. Following this account, we hypothesize that increased attention to the Face region in an ambiguous situation, where interpretation requires searching for more information beyond the action given, would be related to imitation precision. We did not find evidence of this particular relationship in either group, perhaps because in our stimuli the demonstrator had a neutral expression. We predict that a relationship between attention to the Face region during non-meaningful gestures and imitation precision would be observed when information conveyed by the face is needed to understand the action, and we plan to test this hypothesis in future studies.
Our data show that typically-developing children look at faces for a small amount of time even when observing meaningful actions on objects, in which the action itself and the object involved provide all the elements necessary for a goal-directed interpretation. If the action is self-explaining, why do these children still look to the demonstrator’s face? As discussed above, humans may be innately biased to search for justification for action (Csibra & Gergely, 2007), which would explain why they look more at faces when observing non-meaningful gestures. They may also have a tendency to assume some identification with other human beings when observing their behavior (Hobson & Meyer, 2006; Meltzoff, 2002), and this would explain why a certain amount of visual attention to the face is present even during observation of meaningful, self-explaining actions. These processes were present but reduced in high functioning children with autism.
Interestingly, our participants with autism demonstrated the same patterns as comparison participants to look both less at the Action region and more at the Face region when the model to be imitated was not self-explaining. What differentiated the two groups was only the quantity of face-looking. This suggests that rather than being qualitatively different, processes of action understanding are inefficient in this population. It is important to note that our stimuli involved video presentation of a non-interactive model, which has been shown to facilitate imitative performance (Nielsen, Simcock & Jenkins, in press) and performance on executive function tasks (Ozonoff & Strayer, 2001) in autism. Greater differences between groups may be found when a live, interactive model is used. However, even using a video paradigm that may enhance performance in autism, we found the hypothesized group differences and reduced attention to the Face region in our autism group.
The ability to rely on information from faces when interpreting ambiguous stimuli is crucial in a number of social domains other than imitation. For instance, in an eye-tracking study investigating language comprehension, typically-developing kindergarteners looked to their adult partner’s face when given a verbal instruction that could refer to more than one object in sight (Nadig & Sedivy, 2002). Looking to an adult’s face to interpret or “frame” a situation is also the basis of the phenomenon of social referencing, in which emotional meanings about an ambiguous object or situation are conveyed from adult to infant (Sorce, Emde, Campos, & Klinnert, 1985). Both behaviors, based on the tendency to use social information to frame an otherwise ambiguous stimulus, are exhibited less by individuals with ASD (Nadig & Ozonoff, in preparation; Klin et al., 2003).
Finally, children with autism who looked more at the Action region had more precise imitation for the non-meaningful gesture stimuli. This relationship was not found in the typically-developing comparison group, which may have been due to the near-ceiling level of imitation precision in this group. It is tempting to speculate that imitation accuracy in children with autism may depend on the reproduction of the kinematics of the demonstrator’s action, rather than on the understanding of the meaning/goal of the action that is often (but not in our stimuli) conveyed by the demonstrator’s face. It is possible that this relationship between duration of looking and accuracy of imitation was not found with respect to meaningful actions on objects because the affordances of the object may constrain degrees of freedom, simplifying the task and supporting more accurate reproductions of the demonstrator’s actions. Further research is necessary to clarify how mechanisms of action understanding, as reflected in visual attention behavior, are related to imitation performance both in autism and in typical development.
Limitations of this study include the possibility that characteristics other than the meaningfulness of the actions distinguish the two types of imitation presented (i.e., the presence of objects itself, complexity of the actions, or the direction of the demonstrator’s gaze). However the group differences we observed extend beyond situations involving objects; we found that children with autism looked less at faces than comparison participants even when observing intransitive gestures without objects. The direction of the demonstrator’s gaze may have had some influence on visual attention to the Face region, but it cannot explain why differences in attention to the Action region were found between meaningful and non-meaningful stimuli in both groups. This study represents a first step at investigating visual attention patterns and the action understanding processes they reflect across different types of imitation stimuli; we hope that future research will build on these findings by examining the contribution of each of these stimuli characteristics in turn. Another limitation is that our participants with autism represented only a very select and high functioning subgroup of older children with Autism Spectrum Disorders. We believe that studying imitation in this subgroup yields a conservative bias, if any, regarding group differences between typical development and ASD. Yet it is possible that more fundamental abnormalities in both imitation and visual attention are masked by experience and education in older children with high functioning autism. Future work should address these questions in a more representative sample, and possibly focus on younger children. The study of clinical comparison groups with specific impairments in attention or motor skills would be productive in investigating questions of specificity left unresolved by this study. Finally, the role of motor skills in imitation performance may have been underestimated in this study because the assessment we used (the BOT-2) incorporates some abilities (i.e. strength, balance, speed) that are unrelated to the motor demands required by the imitation task we employed.
This study supports the idea that when an imitator observes a demonstrator performing an action, information other than the action itself is considered; in particular, the demonstrator’s face is attended to. Our findings suggest that this source of information is referenced more often when the actions to be imitated are ambiguous, by both typically-developing children and children with high functioning autism. Overall, children with high-functioning autism displayed qualitatively similar patterns of visual attention to matched typically-developing children: they looked to the Action region for the same amount of time, and had a decreased amount but similar pattern of visual attention to the Face region. Past studies have found a relationship between motor impairments and imitative skill. Our findings indicate differences in the encoding phase that might play a role in the imitation impairment in autism. Having autism interferes with many processes important for development (Klin et al., 2003); this may well include multiple aspects of understanding, and therefore of imitating, others’ actions.
Acknowledgments
Support for this research was provided by a doctoral fellowship from the University of Siena to Giacomo Vivanti, by the National Institute of Deafness and Communication Disorders through NRSA F32-DC007297 to Aparna Nadig, by the National Institute of Mental Health through T32 MH T32 073124-3 and the National Institute of Child and Human Development HD U19 035468-10 to Sally Rogers, and by the MIND Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association; (1994). Diagnostic and statistical manual of mental disorders, 4th edition Washington, DC: American Psychiatric Press, Inc. [Google Scholar]
- Aldridge MA, Stone KR, Sweeney MH, & Bower TGR (2000). Preverbal children with autism understand the intentions of others. Developmental Science, 3, 294–301. [Google Scholar]
- Anderson CJ, Colombo J, & Jill Shaddy D (2006). Visual scanning and pupillary responses in young children with Autism Spectrum Disorder. Journal of Clinical Experimental Neuropsychology, 28, 1238–56. [DOI] [PubMed] [Google Scholar]
- Baraneck G, Parham D, & Bodfish J (2005). Sensory and motor features in autism: assessment and intervention Volkmar F, Paul R, Klin A, Cohen D (Eds), Handbook of autism and pervasive developmental disorders, 3th edition ( 312–355). New York, Wiley. [Google Scholar]
- Bates E, Thal D, Whitesell K, & Oaks L (1989). Integrating language and gesture in infancy. Developmental Psychology, 25, 1004–1019. [Google Scholar]
- Behne T, Carpenter M, & Tomasello M (2006). From Attention to Intention: 18-month-olds use Others’ Focus of Attention for Action Interpretation. Paper presented at the annual meeting of the XVth Biennial International Conference on Infant Studies, Westin Miyako, Kyoto, Japan [Google Scholar]
- Bennetto L (1999). A componential approach to imitation and movement deficits in autism. Dissertation abstracts international, 60(2-B), 0819. [Google Scholar]
- Bernabei P, Fenton G, Fabrizi A, Camaioni L, & Perucchini P (2003). Profiles of sensorimotor development in children with autism and with developmental delay. Perceptual and Motor Skills, 96, 1107–16. [DOI] [PubMed] [Google Scholar]
- Bernier R, Dawson G, Webb S, & Murias M (2007). EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition, 64, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruininks R, & Bruininks B (2006). Bruininks-Oseretsky Test of Motor Proficiency -Second Edition (BOT-2). Circle Pines, MN: AGS Publishing. [Google Scholar]
- Camaioni L, Perucchini P, Muratori F, Parrini B, & Cesari A (2003). The communicative use of pointing in autism: developmental profile and factors related to change. European Psychiatry, 18, 6–12. [DOI] [PubMed] [Google Scholar]
- Canitano R, & Vivanti G. (2006). Tics and Tourette syndrome in Autistic Spectrum Disorders. Autism, the International Journal of Research and Practice, 11, 19–28. [DOI] [PubMed] [Google Scholar]
- Carpenter M, & Call J (2007). The question of ‘what to imitate’: Inferring goals and intentions from demonstrations In Dautenhahn K & Nehaniv C (Eds), Imitation and social learning in robots, humans and animals: Behavioural, social and communicative dimensions ( 135–151). Cambridge: Cambridge University Press. [Google Scholar]
- Carpenter M, Tomasello M, Savage-Rumbaugh S. (1995). Joint attention and imitative learning in children, chimpanzees, and enculturated chimpanzees. Social Development, 4, 217–237. [PubMed] [Google Scholar]
- Castiello U (2003). Understanding other people’s actions: intention and attention. Journal of Experimental Psychology: Human Perception and Performance, 29, 416–430. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed). Hillsdale, NJ: Lawrence Earlbaum Associates.. [Google Scholar]
- Colombi C, Liebal K, Tomasello M, Young G, Warneken F, & Rogers SJ (in preparation). Cooperation, Intentions, and Shared Intentions. What is Missing in Autism? [Google Scholar]
- Csibra G (2003). Teleological and referential understanding of action in infancy. Philosophical Transaction of the Royal Society of London, 29, 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G, & Gergely G (1998). The teleological origins of mentalistic action explanations: A developmental hypothesis. Developmental Science, 1, 255–259 [Google Scholar]
- Csibra G, & Gergely G (2007). ‘Obsessed with goals’: Functions and mechanisms of teleological interpretation of actions in humans. Acta Psychologica, 124, 60–78. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, & Davidson RJ (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience 8, 519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, & Rinaldi J (1998). Neuropsychological correlates of early symptoms of autism. Child development, 69, 1276–1285 [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb S, & McPartland J (2005). Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology, 27, 403–424. [DOI] [PubMed] [Google Scholar]
- DeMyer MK, Alpern GD, Barton S, DeMyer WE, Churchill DW, Hingtgen JN, Bryson CQ, Pontius W, & Kimberlin C (1972). Imitation in autistic, early schizophrenic, and non-psychotic subnormal children. Journal of Autism and Child Schizophrenia, 2, 264–87. [DOI] [PubMed] [Google Scholar]
- DeMyer MJ, Hintgen JN, & Jackson RK (1981). Infantile autism reviewed: A decade of research. Schizophrenia Bulletin, 7, 388–451 [DOI] [PubMed] [Google Scholar]
- Dewey D, Cantell M, & Crawford SG (2007). Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society, 13, 246–256 [DOI] [PubMed] [Google Scholar]
- Gattis M, Bekkering H, & Wohlschlager A (2002). Goal-directed imitation Meltzoff A & Prinz W, (Eds), The Imitative Mind ( 183–205). New York: Cambridge University Press [Google Scholar]
- Ghaziuddin M, & Butler E (1998). Clumsiness in autism and Asperger syndrome: A further report. Journal of Intellectual Disability Research, 42, 43–48. [DOI] [PubMed] [Google Scholar]
- Gergely G, & Csibra G (2003). Teleological reasoning in infancy: the naive theory of rational action. Trends in Cognitive Science 7, 287–292. [DOI] [PubMed] [Google Scholar]
- Green D, Baird G, Barnett AL, Henderson L, Huber J, & Henderson SE (2002). The severity and nature of motor impairment in Asperger’s syndrome: a comparison with specific developmental disorder of motor function. Journal of Child Psychology and Psychiatry, 43, 655–668 [DOI] [PubMed] [Google Scholar]
- Grelotti D, Gauthier I, & Schultz RT (2002). Social interest and the development of cortical face specialization: What autism teaches us about face processing. Developmental Psychobiology, 40, 213–225. [DOI] [PubMed] [Google Scholar]
- Hammes JGW, & Langdell T (1981). Precursor of symbol formation and childhood autism. Journal of Autism and Developmental Disorders, 11, 331–346. [DOI] [PubMed] [Google Scholar]
- Heimann M (1998). Imitation in neonates, in older infants and in children with autism: feedback to theory Braten S (Ed), Intersubjective communication and emotion in early ontogeny ( 89–104). Cambridge: Cambridge University Press. [Google Scholar]
- Heimann M (2002). Notes on individual differences and the assumed elusiveness of neonatal imitation Meltzoff A & Prinz W, (Eds), The Imitative Mind ( 74–85). New York: Cambridge University Press. [Google Scholar]
- Hepburn SL, & Stone WL (2006). Longitudinal research on motor imitation in autism In Rogers SJ & Williams JHG (Eds), Imitation and the social mind: Autism and Typical Development (pp.310–329). New York: Guilford Press. [Google Scholar]
- Hobson RP, & Lee A (1999). Imitation and identification in autism. Journal of Child Psychology and Psychiatry, 40, 649–660 [PubMed] [Google Scholar]
- Hobson JA, & Hobson RP (2007). Identification: The missing link between joint attention and imitation? Development and Psychopathology, 19, 411–431. [DOI] [PubMed] [Google Scholar]
- Hobson P, & Meyer J (2006). Imitation, Identification, and the Shaping of Mind: Insights from autism Rogers SJ & Williams JHG (Eds), Imitation and the social mind: Autism and Typical Development ( 198–225). New York: Guilford Press [Google Scholar]
- Klin A, Jones W, Schultz R, & Volkmar FR (2003). The enactive mind, or from action to cognition: lessons from autism. Philosophical transactions of the Royal Society of London – Biological Sciences, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar FR, & Cohen DJ (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism, Archives generals of Psychiatry, 59, 809–816 [DOI] [PubMed] [Google Scholar]
- Killen M, & Uzgiris IC (1981). Imitation of actions with objects: The role of social meaning. Journal of Genetic Psychology, 138, 219–229. [Google Scholar]
- Kimura Y, & Archibald D (1974). Motor functions of the left hemisphere. Brain, 97: 337–350 [DOI] [PubMed] [Google Scholar]
- Landry R, & Bryson SE (2004). Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry, 45(6):1115–22 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, & Risi S (1999). Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, & Sweeney JA (2007). Maturation of executive function in autism. Biological Psychiatry 5, 474–81. [DOI] [PubMed] [Google Scholar]
- Masur EF, & Eichorst DL (2002). Infants’ spontaneous imitation of novel versus familiar words: Relations to observational and maternal report measures of their lexicons. Merrill-Palmer Quarterly, 48, 405–426. [Google Scholar]
- McDonough L, Stahmer A, Schreibman L, & Thompson SJ (1997). Deficits, delays, and distractions: an evaluation of symbolic play and memory in children with autism. Development and Psychopathology, 9, 17–41 [DOI] [PubMed] [Google Scholar]
- McDuffie A, Yoder P, & Stone W (2005). Prelinguistic predictors of vocabulary in Young Children With Autism Spectrum Disorders. Journal of Speech, Language, and Hearing Research, 48, 1080–1097 [DOI] [PubMed] [Google Scholar]
- Meltzoff A (2002). Elements of a developmental theory of imitation Meltzoff A & Prinz W (Eds), The Imitative Mind ( 19–42). New York: Cambridge University Press [Google Scholar]
- Meltzoff A (2004). The case for a developmental cognitive science: Theories of people and things Bremner G, & Slater A (Eds), Theories of Infant Development ( 145–173). Malden, MA: Blackwell [Google Scholar]
- Meltzoff A, Gopnik A (1993). The role of imitation: Understanding persons and developing a theory of mind In Baron-Cohen S, Tager-Flusberg H & Cohen D (Eds), Understanding other minds ( 335–366). New York: Oxford University Press [Google Scholar]
- Minshew NJ, Luna B, & Sweeney JA (1999). Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology 23; 917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, & Neal RA (2001). Neural plasticity, joint attention, and a transactional social-orienting model of autism Glidden LM (Ed), International review of research in mental retardation: Autism. ( 139–168). San Diego, CA: Academic Press. [Google Scholar]
- Nadig A, & Ozonoff S (in preparation). Visual attention during referential communication in high functioning autism.
- Nadig A, & Sedivy J (2002). Evidence of perspective-taking constraints in children’s online reference resolution. Psychological Science, 13, 329–336. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Simcock G, & Jenkins L (in press). The effect of social engagement on 24-month-olds’ imitation from live and televised models. Developmental Science. [DOI] [PubMed] [Google Scholar]
- Ohta M (1987). Cognitive disorders of infantile autism: a study employing the WISC, spatial relationship, conceptualization, and gestural imitation. Journal of autism and developmental disorders, 17, 45–62. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, & Strayer DL (2001). Further evidence of intact working memory in autism. Journal of Autism and Developmental Disorders, 31, 257–26. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, & Piven J (2002). Visual scanning of faces in autism. Journal of autism and developmental disorders, 32, 249–61. [DOI] [PubMed] [Google Scholar]
- Pierno AC, Mari M, Glover S, Georgiou I, & Castiello U (2006). Failure to Read Motor Intentions from Gaze in Children with Autism. Neuropsychologia, 44, 1483–1488. [DOI] [PubMed] [Google Scholar]
- Rogers SJ (2006). Imitation Difficulties in Autism: Findings and Mechanisms. Presentation at UCLA, October 6. [Google Scholar]
- Rogers SJ, Bennetto L, McEvoy R, & Pennington BF (1996). Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Development; 67, 2060–73. [PubMed] [Google Scholar]
- Rogers SJ, Cook I, & Greiss Heiss L (unpublished). Mature imitation manual – The M.I.N.D. Institute, Sacramento, CA. [Google Scholar]
- Rogers SJ, Cook I, & Meryl A (2005). Imitation and play in Autism Volkmar F, Paul R, Klin A., Cohen D(Eds) Handbook of autism and pervasive developmental disorders, 3th edition, ( 382–405). New York: Wiley. [Google Scholar]
- Rogers SJ, & Pennington BF (1991). A theoretical approach to the deficits in infantile autism. Development and Psychopathology, 3, 137–162. [Google Scholar]
- Rogers SJ, Stackhouse T, Hepburn SL, & Wehner EA (2003). Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 44, 763–781. [DOI] [PubMed] [Google Scholar]
- Rogers SJ & Williams JHG (2006). Imitation in Autism Rogers SJ & Williams JHG (Eds), Imitation and the social mind: Autism and Typical Development ( 277–310). New York: Guilford Press. [Google Scholar]
- Rothi LJG, Ochipa C, & Heilman KM (1997). A cognitive neuropsychological model of limb praxis and apraxia In Rothi LJG & Heilman KM (Eds.), Apraxia: the neurology of action. (pp. 29–50). East Sussex, UK: Psychology Press. [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). Social Communication Questionnaire (SCQ). Los Angeles: Western Psychological Services. [Google Scholar]
- Semel EM, Wiig EH, & Secord WA (2004). Clinical Evaluation of Language Fundamentals 4 (CELF-4). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Smith IM, & Bryson SE (1994). Imitation and action in autism: a critical review. Psychological Bulletin, 116, 259–73. [DOI] [PubMed] [Google Scholar]
- Smith IM, & Bryson SE (1998). Gesture imitation in autism: nonsymbolic postures and sequences. Cognitive Neuropsychology, 15, 747–770. [DOI] [PubMed] [Google Scholar]
- Sorce J, Emde R, Campos J, & Klinnert M (1985). Maternal emotional signaling: Its effect on the visual cliff behavior of 1-year-olds. Developmental Psychology, 21, 195–200. [Google Scholar]
- Sparrow S, Balla D & Cicchetti D (2005). Vineland Adaptive Behaviour Scales, second edition Circle Pines: AGS Publishing [Google Scholar]
- Speer LL, Cook AE, McMahon WM, & Clark E (2007). Face processing in children with autism: Effects of stimulus contents and type. Autism, 11, 265–77. [DOI] [PubMed] [Google Scholar]
- Stone WL, Ousley OY, & Littleford CL (1997). Motor imitation in young children with autism: What’s the object? Journal of Abnormal Child Psychology, 25, 475–485. [DOI] [PubMed] [Google Scholar]
- Strid K, Tjus T, Smith L, Meltzoff A, & Heimann M (2006). Infant recall memory and communication predicts later cognitive development. Infant Behavior and Development 29, 545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA (2004). Oculomotor abnormalities parallel cerebellar histopathology in autism. Journal of Neurology, Neurosurgery, and Psychiatry, 75, 1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M (1999). The Cultural Origins of Human Cognition. Cambridge, MA: Harvard University Press. [Google Scholar]
- Tomasello M (2000). Culture and cognitive development. Currrent Directions in Psychological Science, 9, 37–40. [Google Scholar]
- Townsend J, Courchesne E, Egaas B (1996). Slowed orienting of covert visual-spatial attention in autism: Specific deficits associated with cerebellar and parietal abnormality. Development and Psychopathology, 8, 563–584. [Google Scholar]
- Uzgiris IC (1981). Two functions of imitation during infancy. International Journal of Behavioral Developmental, 4, 1–12. [Google Scholar]
- Uzgiris IC (1999). Imitation as activity: Its developmental aspects In Nadel J & Butterworth G (Eds.), Imitation in Infancy (pp.186–206). Cambridge, MA: Cambridge University Press. [Google Scholar]
- Vanvuchelen M, Roeyers H, & De Weerdt W (2007). Nature of motor imitation problems in school-aged boys with autism: A motor or a cognitive problem? Autism, 11, 225–240. [DOI] [PubMed] [Google Scholar]
- Want SC, & Harris PL (2002). How do children ape? Applying concepts from the study of non-human primates to the developmental study of ‘imitation’ in children. Developmental Science, 5, 1–4 [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX:Harcourt Assessment. [Google Scholar]
- Williams J, Whiten A, & Singh T (2004). A systematic review of action imitation in autistic spectrum disorder. Journal of autism and developmental disorders, 34, 285–296 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (1990). International classification of Diseases, 10th ed. [Google Scholar]
- Zibetti E, & Tijus C (2005). Understanding Actions: Contextual Dimensions and Heuristics Dey A, Kokonov B, Lake D, Turner R(Eds), Lectures Notes in Artificial Intelligence, Modelling and Using Context (pp. 542–555). New-York: Springer. [Google Scholar]