Abstract
The corticospinal and rubrospinal tracts are the predominant tracts for controlling skilled hand function. Injuries to these tracts impair grasping but not gross motor functions such as overground locomotion. The aim of the present study was to determine whether or not, after damage to both the corticospinal and rubrospinal tracts, other spared subcortical motor pathway can mediate the recovery of skilled hand function. Adult rats received a bilateral injury to the corticospinal tract at the level of the medullar pyramids and a bilateral ablation of the rubrospinal axons at C4. One group of rats received, acutely after injury, two injections of chondroitinase-ABC at C7, and starting at 7 days post-injury were enrolled in daily reaching and grasping rehabilitation (CHASE group, n = 5). A second group of rats received analogous injections of ubiquitous penicillinase, and did not undergo rehabilitation (PEN group, n = 5). Compared to rats in the PEN group, CHASE rats gradually recovered the ability to reach and grasp over 42 days after injury. Overground locomotion was mildly affected after injury and both groups followed similar recovery. Since the reticulospinal tract plays a predominant role in motor control, we further investigated whether or not plasticity of this pathway could contribute to the animal’s recovery. Reticulospinal axons were anterogradely traced in both groups of rats. The density of reticulospinal processes in both the normal and ectopic areas of the grey ventral matter of the caudal segments of the cervical spinal cord was greater in the CHASE than PEN group. The results indicate that after damage to spinal tracts that normally mediate the control of reaching and grasping in rats other complementary spinal tracts can acquire the role of those damaged tracts and promote task-specific recovery.
Keywords: Reticulospinal, Corticospinal, Reaching and grasping, Chondroitinase-ABC, Spinal cord injury, Plasticity
Introduction
Among the spinal cord motor pathways, the corticospinal tract (CST), and to a lesser extent the rubrospinal tract (RbST), play a major role in controlling manual dexterity (Lawrence and Kuypers, 1968). When these tracts are individually (Morris et al., 2011; Whishaw et al., 1993) or collectively (Girgis et al., 2007; Kanagal and Muir, 2009) damaged, forelimb skilled movements such as grasping, but not other gross motor functions such as reaching or ground locomotion, are impaired. Animal studies have consistently reported the limited capacities of CST axons to spontaneously regenerate, leading to chronic disruption of the direct motor cortex to spinal cord connections. An alternative strategy to axon regeneration is for undamaged axons to sprout and make new synaptic contacts with spinal neurons. This anatomical reorganization has been proposed to have contributed to motor recovery under different spinal cord injury conditions (Blesch and Tuszynski, 2009).
Anatomical and functional studies have demonstrated that the motor cortex is connected to the spinal cord not only via the CST but also indirectly via extrapyramidal routes (Bolzoni et al., 2013; Canedo, 1997). It is hypothesized that following CST damage the brainstem motor pathways “by pass” the direct corticospinal connections, by conducting the information indirectly from the cortex to the spinal cord. It has been shown that sprouting of sub-cortical, uninjured projecting axons in the spinal cord can occur in mice after a unilateral cortical stroke (Bachmann et al., 2014), and in rats after a spinal lateral hemisection (Filli et al., 2014). In both of these studies, the anatomical plasticity observed was thought to contribute to the spontaneous locomotor recovery achieved by the animals. It remains uncertain, however, whether or not plasticity of these sub-cortical pathways could be responsible for the recovery of skilled motor functions, such as reaching and grasping.
In the present study, we hypothesize that rats with complete CST and RbST injuries can recover the ability to reach and grasp by remodeling the cervical spinal circuitry. Rats received complete CST and RbST tract injuries and receive injections of either chondroitinase-ABC or penicillinase (as a control) into the caudal cervical spinal cord segments which host the spinal interneurons and motoneurons innervating the distal forelimb muscles important for grasping (McKenna et al., 2000). The removal of spinal chondroitin sulfate proteoglycans (CSPGs) via chondroitinase-ABC administration creates an environment permissive to axonal sprouting and synapse formation. It is important to note, however, that these same rats were enrolled in intensive reaching and grasping rehabilitation, that in itself, has been shown to actively strengthen newly formed functional connections and improve coordinated performance of a motor task (Girgis et al., 2007). These interventions were integrated into our CHASE intervention given our previously reported synergistic effects of chondroitinase-ABC treatment and rehabilitation on the recovery of reaching and grasping after a dorsal funiculi crush (Garcia-Alias et al., 2009). Since the reticulospinal tract (RET) plays a prominent role in motor control and is malleable to drive functional recovery (Ballermann and Fouad, 2006), the distribution of RET axons within the gray matter of the cervical spinal cord was analyzed and compared between the treated and untreated groups.
The results obtained show a significant recovery of reaching and grasping in the rats that received both interventions. The recovery was accompanied by increased density and expansion of RST projections into the areas of the gray matter that were vacated of CST and RbST input.
Material & methods
All experimental procedures were performed in compliance with the University of California Los Angeles Chancellor’s Animal Research Committee and complied with the guidelines of the National Institutes of Health.
Surgical and drug injection procedures
All survival surgical procedures were performed under aseptic conditions. Ten adult female Long Evans rats (280–300 g) were anesthetized with 1.5–2.5% isoflurane in 0.4% O2. A ventral skin incision of the neck was made and the underlying muscles, trachea, and esophagus were displaced. A small piece of the ventrocaudal part of the occipital bone was removed and the medullary pyramids were exposed. The pyramids were incised bilaterally with a sharp scalpel blade. The esophagus, trachea, and muscles were repositioned, and the skin was sutured (Kanagal and Muir, 2009). A partial laminectomy at spinal segments C4–C5 was performed to expose the spinal cord. The dura was cut and an ~1.5 mm deep incision was made in the right and left lateral funiculi and the tissue displaced laterally using a sharp scalpel blade. This procedure damaged the most dorsal aspect of the dorsolateral funiculi. A laminectomy then was performed at C7 and two injections, each containing 1 μl of chondroitinase-ABC at 100 U/ml (Seikagaku) (CHASE group, n = 5) or Penicillinase (Sigma) (PEN group, n = 5), were made into the most rostral and caudal edges of the exposed spinal segment. A glass micropipette was lowered ~1 mm into the gray matter and the drug delivered over a period of 10 min (Garcia-Alias et al., 2011). The muscles and skin were sutured. The rats were allowed to fully recover in an incubator (37 °C) before being returned to their home cages. To evaluate the extension of CSPG digestion in the cervical spinal cord, three additional rats received a spinal cord injury followed by chondroitinase-ABC injection and were perfused the following day. Their tissue was processed as described below.
Training and testing of reaching and grasping
The rats were placed individually inside a clear plastic box (18 cm × 15 cm × 31 cm) with a small opening in the front wall (3 cm × 1.5 cm) and trained to reach and grasp for chocolate pellets (45 mg, Bio-Serv) placed on a platform positioned 1 cm outside the window. The rats had to extend their preferred forelimb to reach and grasp the pellets. During each testing session, 20 pellets were presented to the rats and the ratio of the number of pellets eaten to the number of attempts was calculated (Whishaw et al., 2008). The rats were tested prior to the injury and weekly for 6 weeks post-injury. The rats were video recorded at 400 frames/s (Casio EX-FH25) and the movement elements during each attempt were analyzed following Whishaw’s score (Piecharka et al., 2005). Five reaches with each limb by each rat were rated for qualitative features of the movement. A score of “0” was given if the movement was performed normally. A score of “2” was given if the movement was abnormal. A score of “1” was given in cases where there was some ambiguity concerning the normality of the movement. Seven component movements of a reach were rated: 1) advance: the limb is advanced directly through the slot toward the food target; 2) digits extend: during the advance, the digits extend so that the digit tips are pointing toward the target; 3) arpeggio: when the paw is over the target, the paw pronates from digit 5 (the outer digit) through digit 2 and, at the same time, the digits open; 4) grasp: the digits close and flex around the food, with the paw remaining in place, and the wrist is slightly extended to lift the food; 5) supination I: as the paw is withdrawn, the paw supinates by almost 90°; 6) supination II: once the paw is withdrawn from the slot to the mouth the paw further supinates by about 45° to place the food in the mouth; and 7) release: the paw contacts the mouth and the paw opens to release the food.
Grip strength
At 6 weeks post-injury, the grip strength of each rat was measured with a custom designed grip strength meter. Rats were allowed to grip a horizontal bar connected to a force transducer with both forepaws and then pulled away until the grip was released. The force exerted on the bar at the time of release was measured in three consecutive trials (Garcia-Alias et al., 2009). The maximum force for the right and left forepaws was calculated and compared with a group of three intact, age and weight matched, control rats.
Assessment of overground locomotor ability
Overground locomotor ability was tested prior to the injury and at 1 and 6 weeks post-injury. The forepaws and hindpaws were inked with different colors. The footprints were recorded on paper as the rats walked across a runway (8 cm wide and 100 cm long). The footprints then were used to determine mean stride length. The stride length was measured between the central pads of two consecutive prints on each side for the right and left forelimbs and hindlimbs. For each animal, the stride length was calculated as the average distance of 3–4 consecutive steps (Garcia-Alias et al., 2011).
Reaching and grasping rehabilitation
Starting at 1 week post-surgery, rats in the CHASE group were subjected to reaching and grasping rehabilitation. Groups of 2–3 rats were placed in a cage with a plastic grid with square openings on the floor for 1 h/day, 5 days/wk. Seeds were placed in the square openings and the rats had to retrieve the seeds by extending their paws and grasping the seeds (Garcia-Alias et al., 2009). Rats in the PEN group did not undergo any rehabilitation regime; instead, the rats were placed in an empty cage without a grid on the floor, and with no seeds, for the same amount of time as the rats in the CHASE group were performing rehabilitation.
Histological analyses
At the end of the functional evaluation, the rats received a unilateral stereotaxic injection of 1 μl of 10% dextran amine conjugated to 555-Alexafluoro in the gigantocellular nucleus of the reticular formation ipsilateral (coordinates: AP: −11.5 cm; DL: 0.15 cm; depth: 0.95 cm) to the preferred paw to reach and grasp (Paxinos and Watson, 1998). Ten days after the injection, the rats were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer solution (PBS) The brainstems and spinal cords were removed and stored in a 30% sucrose solution. Coronal sections (30 μm thick) were cut in a cryostat. Two sections per spinal segment were selected and used to determine CST preservation and RST fiber density, respectively. To evaluate the extent of the injury, serial transverse sections at the site of injury were stained with cresyl violet. The images were magnified (10×) and the area of the dorso-lateral funiculi injuries was quantified using ImageJ software. To evaluate the localization of the injury the contour of the spinal cord and injury area was outlined. To verify the CST injury, 5 coronal sections per spinal segment were incubated overnight at 4 °C with rabbit polyclonal antibodies against γPKC (1:500, Santa Cruz, CA). After several washes, the sections were incubated with the secondary antibody goat anti-rabbit conjugated to Alexa Fluor 488 (1:500; Jackson Immunoresearch, West Grove, PA, USA) overnight at 4 °C. Following additional washes, the sections were mounted on gelatin-coated slides. For evaluating antibody specificity, some sections were processed as described, but primary antibodies were not added. To evaluate the digestion of extracellular CSPG by the injected chondroitinase-ABC, serial transverse sections were incubated 72 h at 4 °C with mouse monoclonal antibodies against CS-56 (1:200, Sigma). After several washes, the sections were incubated with the secondary biotinylated antibody goat anti-mouse IgM (Jackson 1:500) and amplified with TSA + kit (1:500; Perkin-Elmer) and followed the procedures aforementioned. For determining RST fiber density, magnified images (10×) of serial coronal spinal cord sections were taken. A grid was overlaid on each image and the traced axons were tagged following the inclusion criteria of Rosenzweig et al. (2010). Tagged coordinate points were imported to Microsoft Excel that translated the coordinate points into a spreadsheet grid that represented the distribution of axons in the segment when divided into a 45 × 45 frame grid. Grid values indicate the number of axons counted in that particular frame. A color gradient was applied based on the value in each grid frame. A heat map color gradient was adjusted for each segment according to its highest value. A diagram of the laminae distribution in each segment was overlaid on each heat map to quantify the number of axons found in each lamina for each spinal segment between C2 and T1. Finally, the number of quantified axons per in each laminae was normalized with the total number of axons quantified in the white matter of the most rostral section of the same spinal cord segment.
Statistical analyses
The data are reported as mean ± SEM. Comparisons in the behavioral parameters among groups were made using repeated measures ANOVAs followed by Bonferroni post-hoc analyses. Histological data were compared using two tailed Student t-tests. Differences were considered statistically significant at P < 0.05.
Results
CST and RbST tract injuries
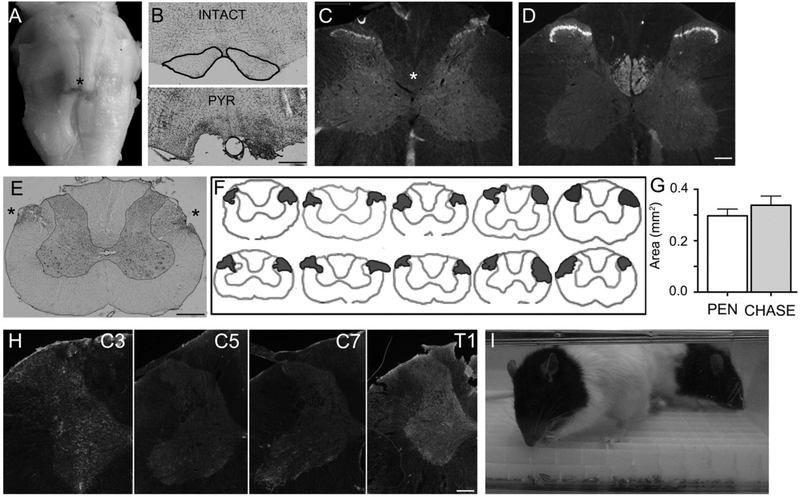
The rats received a bilateral pyramidotomy in the brainstem’s medulla (Figs. 1A,B) that damaged the CST. The ablation of the CST was corroborated by the absence of γPKC staining in the cervical spinal cord segments of injured (Fig. 1C) compared to uninjured spinal cords (Fig. 1D). The same rats received a bilateral ablation of the dorsolateral funiculi at C4 (Figs. 1E,F) that damaged the RbST tracts. At the site of injury, there was no difference in the extension of the dorso-lateral funiculi injury areas between the CHASE and PEN groups (Fig. 1g). Importantly, the depth of the injury only affected the dorsal aspect of the lateral funculi, without affecting the medial nor the ventral part of the lateral white matter, that would have robustly impaired forepaw mobility. Overall, these anatomical results indicate that the CST and RbST injuries were not different between groups.
Fig. 1.
(A) Ventral view of a brainstem with a bilateral injury to the pyramids (asterisk). (B) Transverse sections of the medullar pyramids of an intact (outlined in black) and pyramidotomized (PYR) rat. (C) Transverse sections from the C4 spinal segment of a pyramidotomized rat immunostained with γPKC, showing the absence of CST labeling (asterisk) compared to an uninjured spinal cord (D). Note the staining of neurons in Rexed laminae 1–3 in both the intact and injured rat, excluding the possibility of false negative staining. (E) Cresyl violet stained transverse section from the C4 spinal segment with a bilateral injury of the dorso-lateral funiculi (asterisks). (F) Illustration showing the contour of C4 spinal cord sections with the localization and extension of the bilateral dorsolateral funiculi injuries in individual rats. The top illustrations belong to CHASE animals and the bottom to PEN animals. (G) Bar graphs showing the mean (±SEM) transverse extension area of the bilateral dorsolateral funiculi injury for 5 rats in each group. (H) Transverse section of cervical spinal segments immunostained against CSPGs sugars with CS-56 antibody. The gray matter is outlined in orange. The tissues were processed in parallel and the images were taken under the same optical conditions. (I) Photograph of rats from the CHASE group inside a cage with a grid on the bottom filled with sunflower seeds undergoing reaching and grasping rehabilitation. Scale bars in B and E = 500 μm; in C, D, and H = 200 μm.
Acutely after injury, rats in the CHASE group received two intraparenchymal injections of chondroitinase-ABC at C7. As shown in Fig. 1H, spinal cords with chondroitinase-ABC injection and processed 1 day after the injury had decreased CS-56 immunoreactivity at the site of injection but not at the most rostral and caudal cervical segments, corroborating the specificity of the location of the catalytic activity of the delivered chondroitinase-ABC. In addition, starting at 7 days post-injury, rats in the CHASE group underwent daily reaching and grasping rehabilitation (Fig. 1I).
Behavioral recovery
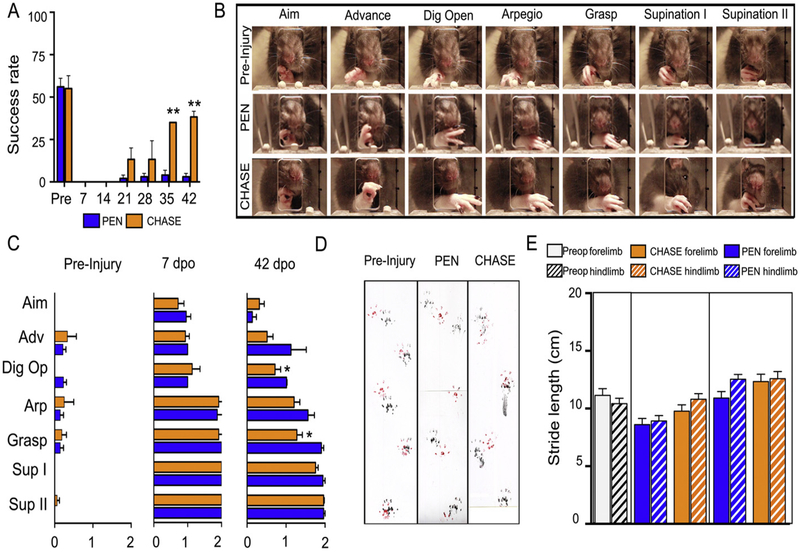
Prior to the injury, the rats were trained to reach and grasp pellets. Only those rats (n = 10) obtaining a pre-injury success rate of ≥ 50% were included in the experiment (Fig. 2A). Reaching and grasping were composed of a stereotypical sequence of forelimb movements, starting with lifting of the paw and its protraction through the window towards the pellet. Along the trajectory, the paw was gradually pronated and the digits were extended. Once the paw was on top of the pellet, the digits were maximally opened (arpeggio) and abruptly flexed to precisely grasp the pellet. The paw was retracted by gradual supination of the forelimb until the pellets touched the mouth (Fig. 2B). At 7 days post-injury, the rats from both injured groups were able to stand and to use their forelimbs to ambulate, but were severely impaired when attempting to reach and grasp (Fig. 2A). The rats protracted the paw out the box, but failed to grasp the pellets. Their digits were only partially extended and the rats were unable to pronate and grip the pellet (Fig. 2C). At 42 days post-injury, rats in the PEN group slightly improved their grasping performance, obtaining a success rate of ~5% compared to over 60% pre-injury (Fig. 2A). Detailed movement analyses showed no improvement between 1 and 6 weeks post-injury and the ability to extend the digits, pronate the paw, and precisely grip the pellets remained impaired (Figs. 2B,C). In contrast, rats in the CHASE group progressively improved their grasping performance and at 35 and 42 days post-injury their success rate was ~50% (P < 0.001 vs. PEN) (Fig. 2A). Compared to pre-injury performance, the rats in the CHASE group displayed marginally effective forelimb movements, similar to those observed in the rats in the PEN group, characterized by an incomplete pronation and retrieval of the pellet to the mouth. The rats in the CHASE group were able to open the digits (P < 0.01 vs. PEN) and grasp the pellet (P < 0.01 vs. PEN) more precisely without displacing or releasing the pellet during the grasping and retrieval to the mouth (Figs. 2B,C). Grip strength was measured to determine the degree of forepaw rigidity or flaccidity, either of which could affect paw mobility to reach and grasp. At 6 weeks post-injury, the grip strength was 223 ± 47, and 332 ± 35 g for CHASE and PEN rats respectively, compared to 397 ± 101 g of uninjured age and weight matched control animals. Differences were found between the injured groups and the control (PEN vs CNT p < 0.05 and CHASE vs CNT p < 0.01), but not between the injured groups.
Fig. 2.
(A) Mean (±SEM) success rate for reaching and grasping performance for rats in the PEN and CHASE groups over 42 days post-injury. (B) Sequence of video frames showing each movement component during a reaching and grasping attempt previous to the injury and at 42 days post-injury in a representative rat in the PEN and CHASE groups (see Methods for description of each component). (C) Detailed behavioral analyses of the reaching and grasping components pre-injury and at 7 and 42 days post-injury (dpo). (D) Imprints of the forepaws (red) and hindpaws (black) of a rat pre-injury and from a representative rat in the PEN and CHASE groups at 42 days post-injury when stepping along a corridor. (E) Histograms showing the mean (±SEM) stride length bilaterally pre-injury and 7 and 42 days post-injury for all rats in the PEN and CHASE groups. ** P < 0.001 and *P < 0.01 vs. PEN.
Since the reticular formation is involved in the generation and control of locomotion, we determined how the interventions affected the locomotor ability of the injured rats. The right and left forelimb and hindlimb paws were inked and the stride length and forepaw toe spreading were determined from the footprints as the rats walked along a corridor (Fig. 2D). At all time points, the rats plantar stepped with the both the forelimbs and hindlimbs. At one week post-injury, forelimb stride length was slightly shorter in both groups of injured rats compared to pre-injury values, although there were no significant differences between groups. By 6 weeks post-injury, forelimb stride length was not different from pre-injury values (Fig. 2E).
Reticulospinal plasticity
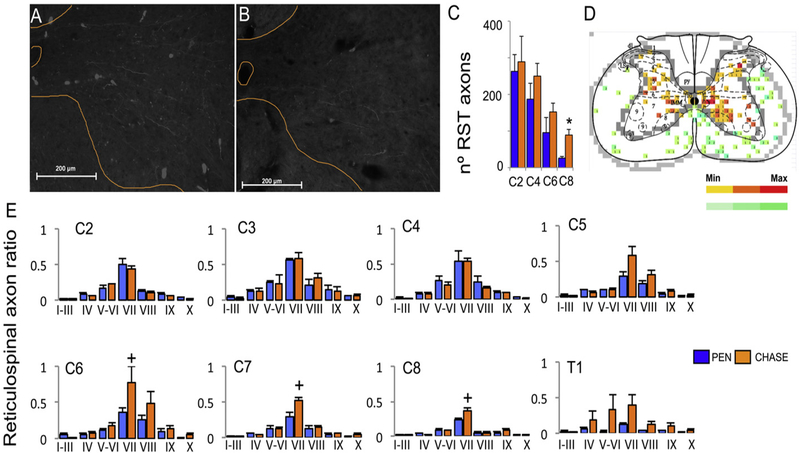
To evaluate RET axonal plasticity, the rats were injected with 1 μl of 555 Alexa conjugated Dextran tracer in the reticular formation ipsilateral to the preferred paw to reach and grasp (Paxinos and Watson, 1998). Labeled reticulospinal axons were found descending ipsilaterally along the lateral, ventrolateral, and ventral white matter funiculi and mostly penetrated the gray matter in the ventral and medial horns in the rats in both the CHASE (Fig. 3A) and PEN groups (Fig. 3B). The number of RET axons in the gray matter progressively decreased from C2 to C8 (Fig. 3C). This pattern was similar in both injured groups and there were no differences at any spinal segment except for C8 (P < 0.05). RET axons were quantified in both the white and gray matter of each cervical segment and plotted as heat maps for each spinal segment (Fig. 3D). For all spinal segments in both injured groups, the density of RET processes (normalized as the number of processes in the gray matter/number of axons in the white matter of the same spinal segment) was highest in Rexed laminae V-VIII. In the C6, C7, and C8 spinal segments, the rats in the CHASE group showed a trend for a higher density of RET processes in laminae V–VI than rats in the Pen group (P < 0.1) This trend was observed in the most caudal spinal segments where the chondroitinase-ABC was injected and diffused. In addition, RET axons in the CHASE group spread into adjacent dorsal and ventral spinal laminae (Fig. 3E)
Fig. 3.
Anterogradely traced reticulospinal processes in the ventral gray matter of the C7 spinal segment from a representative rat in the CHASE (A) and PEN (B) groups. (C) Mean (±SEM) total number of reticulospinal processes in the gray matter ispilateral to the injection side. (D) The number of processes was determined in 100 μm2 square grids and plotted as heat maps for each spinal segment. (E) Mean (±SEM) number of reticulospinal processes in each Rexed lamina of the spinal gray matter normalized to the number of reticulospinal axons in the white mater on the ispilateral side of the same spinal segment. +, P < 0.1 vs. PEN.* p < 0.05 vs PEN.
Discussion
The results show that in the absence of CST and RbST axons, which are the major tracts associated with skilled hand function, rats can regain the ability to grasp objects if the extracellular matrix in the cervical spinal cord is digested using chondroitinase-ABC and the rats receive intensive sensorimotor rehabilitation training for reaching and grasping. The anatomical analyses show that at least one mechanism involved in mediating recovery is the sprouting of RET axons into the medial and ventral laminae in the caudal segments of the cervical spinal cord segments. Thus the results indicate that with appropriate interventions sprouting within tracts that are less involved with a specific motor task can complement for the loss of the pathways that are normally associated with the performance of that task.
We previously reported the beneficial effects of combining chondroitinase-ABC treatment and task-specific rehabilitation in promoting the recovery of skilled hand function in rats after a dorsal funiculi crush (Garcia-Alias et al., 2009). Treatment with chondroitinase-ABC promoted sprouting of CST axons located in the lateral funiculi and task-specific rehabilitation selectively strengthened the newly formed functional connections (Fawcett and Curt, 2009). In the present study we excluded the possibility of CST axonal regeneration or sprouting into the caudal cervical spinal cord by ablating the CST axons at the level of the medulla in the brainstem. In addition, we also damaged the RbST axons that are involved in fine motor function of the paw (Morris et al., 2011) and whose plasticity has been shown to promote reaching and grasping recovery after a spinal cord injury (Raineteau et al., 2001). Furthermore, chondroitinase-ABC was injected caudally, and not at the injury site as done previously (Garcia-Alias et al., 2009), to promote sprouting of undamaged axons rather than regeneration of the damaged axons (Soleman et al., 2012). In effect, the plasticity of undamaged spinal descending tracts must have driven the recovery of hand function in the present study.
Role of sub-cortical descending pathways on motor recovery
We hypothesized that rats with a complete CST and RbST injuries can recover the ability to reach and grasp by remodeling the circuitry within the cervical spinal cord. In this anatomical and functional reorganization sub-cortical descending motor pathways must have played a role by acting as a bypass between the pyramidal cell in the cortex and the cervical motoneurons. Our anatomical study has focused on the RET system given the robust evidence of its plasticity and role in inducing motor recovery. Nevertheless, we cannot exclude the role of other brainstem (Bachmann et al., 2014) and/or propriospinal (Bareyre et al., 2004) systems for the observed functional recovery.
The RET system is a viable candidate to mediate recovery after a spinal cord injury. It is phylogenetically one of the oldest systems preserved among vertebrate species (ten Donkelaar, 2000). The RET system acts over a wide population of spinal neurons, playing an important role in multiple sensorimotor functions. Importantly, after injury, RET axons possess some intrinsic malleability that allow them to regenerate (Deumens et al., 2005; Vavrek et al., 2007) or sprout (Ballermann et al., 2006) and combined with other factors may be responsible for spontaneous recovery of locomotor function after a spinal cord injury (Ballermann et al., 2006).
The RET system also is a functional substrate for hand control (Baker, 2011; Zhou et al., 2014), as RET axons innervate distal, as well as proximal, arm and intrinsic hand muscles (Riddle et al., 2009). Extracellular recordings from the pontomedullar reticular formation show evidence of reticular neuronal activity during primate reaching (Buford and Davidson, 2004). In hemi-decorticated pups, the cortico-reticular pathway spontaneously reorganizes to develop skilled forelimb function, demonstrating the plasticity and functional malleability of the system (Umeda et al., 2010). However, spontaneous RET plasticity alone may be limited in its ability to restore skilled hand function, e.g., rodents (Whishaw et al., 1993), felines (Alstermark et al., 1989), and primates (Lawrence and Kuypers, 1968) with CST injury do not successfully recover the ability to precisely grasp objects. In the present study, sprouting of RET axons was observed only in the caudal cervical cord, i.e., where the chondroitinase-ABC was injected, suggesting a role of the RET system in the recovery of reaching and grasping post-injury.
Since the RST system is involved in the control of locomotion, we tested if the interventions may have interfered with the recovery of locomotor ability. Injury to the RbST and/or CST exerts only mild motor deficits in gross locomotion (Garcia-Alias et al., 2009; Webb and Muir, 2003). Rats in both the CHASE and PEN groups were able to ambulate soon after the injury and showed some impairment when stepping on a treadmill. Whereas discrete injuries to the dorsal or lateral areas of the spinal cord specifically impair fine motor tasks, injuries to the ventral spinal cord affect gross posture and locomotion (Schucht et al., 2002).
Reticulospinal axon plasticity
We determined the density of RET axonal processes in the laminae of the gray matter from C2 to T1 spinal segments to quantify the extent of RET plasticity post-injury. There was a trend for an unexpected decrease in the number of RET processes along the longitudinal spinal cord axes in both experimental groups. Although Weishaupt et al. (2013) reported a retraction of RET processes in the gray matter in the segments below a dorsolateral quadrant injury, the injury model employed in our study minimally damaged the lateral and ventrolateral RET axons.
RET axon density was similar along the spinal gray matter laminae in the rostral cervical spinal segments (including C2 to C4) and the RET processes were confined predominantly in laminae VI–VIII. In contrast, RET axons were present in spinal Rexed VI–VIII laminae below C5 in the injured groups, with the densities being higher in the CHASE than PEN group. Importantly, this pattern was observed in the spinal cord segments treated with chondroitinase-ABC, which had decreased CS-56 immunoreactivity as a consequence of the removal of CSPGs from the extracellular matrix. Combined, these results suggest that in the absence of the spinal parenchyma extracellular matrix, RET axons may have innervated some denervated CST and RbST target spinal interneurons in the gray matter of the spinal cord. Following a spinal cord injury, digestion of the spinal CSPG has shown to promote plasticity of several ascending and descending spinal axons (Bradbury and Carter, 2011). Injuries compromising a complete transection or hemisection of the spinal cord have irrefutably shown that the behavioral recovery was mediated by the regenerated axons, which formed functional contacts with spinal cord neurons and lead to the recovery of breathing (Alilain et al., 2011), motor (Houle et al., 2006) and urinary function (Lee et al., 2013). These reports indicate that in the present study, the observed reaching and grasping recovery may at least rely in the observed reticulospinal axon malleability mediated by the chondroitinase treatment. Alternatively, chondroitinase-ABC treatment may have reduced the number of RET processes that are commonly withdrawn after injury. These possibilities are not exclusive and may explain the high density of preserved RET processes in laminae VI to VIII in addition to the presence of some RET axons in adjacent laminae.
A limitation of the study is that we cannot confirm a complete ablation of the CST. If as little as 10% of the CST is preserved, the ability to reach and grasp using stereotyped movements similar to the used previous to the injury is retained (Piecharka et al., 2005). Several behavioral and anatomical observations, however, suggest that there was near complete damage to both the CST and RbST. For example, in both groups post-injury the success rate for the reaching and grasping task dropped to zero and the rats exhibited compensatory movements when opening the digits, pronating the paw, and griping the pellets. The absence of γPKC immunostaining in the ventral aspect of the dorsal funiculi also is consistent with complete damage of the CST (Starkey et al., 2011). Furthermore, the grip strength and plantar stepping results indicate that the severity of the injury was similar in both groups. All rats were able to place the plantar surface of the forepaw when walking and did not show symptoms of forepaw muscle rigidity or flaccidity. Consequently, the greater recovery of function in the rats in the CHASE compared to the PEN group cannot be attributed to differences in severity of the injury or the amount of CST preservation, but must be associated with the imposed interventions. In addition, the lack of rubrospinal axon tracing that prevents us from verifying that all RbST fibers have been interrupted and leaves the possibility that some fibers were spared and played a role in the recovery of the reaching and grasping task. In rodents, however, the rubrospinal axons run bilaterally in a small area of the dorsolateral funiculi (Raineteau et al., 2001) and in the present study the histological analysis of the injury site shows that both the location and the size of the dorso-lateral funiculi injuries were extensive and most likely compromised all or at least a large proportion of these axons.
The results are consistent with our original hypothesis, by which, specific pharmacological and activity dependent interventions have promoted plasticity of the reticulospinal axons to complement the loss of corticospinal and rubrospinal tracts for the performance of skilled hand function. However, since the effects of each individual treatment were not tested, at this time we cannot determine their relative importance on the animals recovery and neither compare their possible additive or adverse effects when combined together. Studies combining chondroitinase ABC and rehabilitation have not reported adverse effects, although in some cases the anatomical plasticity enhanced by the CSPG digestion was not followed by a greater behavioral recovery compared to the untreated animals (Alluin et al., 2014). Although the mechanisms by which CSPG digestion and rehabilitation promote recovery after injury are different (Garcia-Alias and Fawcett, 2012) the location of the injury and motor task trained may strongly influence the outcome.
Conclusions
The present results indicate that after damaging the most important spinal tracts involved in the control of manual dexterity (CST and RbST) the animals can regain the ability to grasp objects. The observed remodeling of the RET projections in the caudal cervical spinal cord suggests that this is one mechanism for the functional recovery. These results have important implication for restoring upper limb function after a spinal cord injury as they demonstrate that an extrapyramidal tract that normally is presumed to have a minor role in hand function control can complement that function if the CST and RbST are damaged severely. Additionally, the RET has a unique distribution within the spinal cord, i.e., traversing along the ventral and ventro-lateral areas of the white matter in both humans (Nathan et al., 1996) and rats (Du Beau et al., 2012). Consequently, in the absence of CST regeneration, tapping into the neuroplastic potential of the RET might offer new possibilities for the recovery of skilled hand function after a debilitating injury.
Acknowledgments
The authors thank Dr. Michelle Starkey for her advice in performing the bilateral pyramidotomies, and to Miss Rana Khankan for performing the CS-56 immunohistochemistry. This work was supported by a grant from Wings for Life, Spinal Cord Foundation (WFL-US-004/11) to GGA, the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number U01EB015521 and the Christopher & Dana Reeve Foundation (VEC-2013(2)).
References
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J, 2011. Functional regeneration of respiratory pathways after spinal cord injury. Nature 475, 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alluin O, Delivet-Mongrain H, Gauthier MK, Fehlings MG, Rossignol S, Karimi-Abdolrezaee S, 2014. Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics. PLoS One 9, e111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Lundberg A, Pettersson LG, Tantisira B, 1989. The effect of low pyramidal lesions on forelimb movements in the cat. Neurosci. Res 7, 71–75. [DOI] [PubMed] [Google Scholar]
- Bachmann LC, Lindau NT, Felder P, Schwab ME, 2014. Sprouting of brainstem-spinal tracts in response to unilateral motor cortex stroke in mice. J. Neurosci 34, 3378–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, 2011. The primate reticulospinal tract, hand function and functional recovery. J. Physiol 589, 5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann M, Fouad K, 2006. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur. J. Neurosci 23, 1988–1996. [DOI] [PubMed] [Google Scholar]
- Ballermann M, Tse AD, Misiaszek JE, Fouad K, 2006. Adaptations in the walking pattern of spinal cord injured rats. J. Neurotrauma 23, 897–907. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME, 2004. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci 7, 269–277. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH, 2009. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 32, 41–47. [DOI] [PubMed] [Google Scholar]
- Bolzoni F, Pettersson LG, Jankowska E, 2013. Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat. J. Physiol 591, 3381–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM, 2011. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull 84, 306–316. [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG, 2004. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp. Brain Res 159, 284–300. [DOI] [PubMed] [Google Scholar]
- Canedo A, 1997. Primary motor cortex influences on the descending and ascending systems. Prog. Neurobiol 51, 287–335. [DOI] [PubMed] [Google Scholar]
- Deumens R, Koopmans GC, Joosten EA, 2005. Regeneration of descending axon tracts after spinal cord injury. Prog. Neurobiol 77, 57–89. [DOI] [PubMed] [Google Scholar]
- Du Beau A, Shakya Shrestha S, Bannatyne BA, Jalicy SM, Linnen S, Maxwell DJ, 2012. Neurotransmitter phenotypes of descending systems in the rat lumbar spinal cord. Neuroscience 227, 67–79. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, 2009. Damage control in the nervous system: rehabilitation in a plastic environment. Nat. Med 15, 735–736. [DOI] [PubMed] [Google Scholar]
- Filli L, Engmann AK, Zorner B, Weinmann O, Moraitis T, Gullo M, Kasper H, Schneider R, Schwab ME, 2014. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J. Neurosci 34, 13399–13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alias G, Fawcett JW, 2012. Training and anti-CSPG combination therapy for spinal cord injury. Exp. Neurol 235, 26–32. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW, 2009. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci 12, 1145–1151. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Petrosyan HA, Schnell L, Horner PJ, Bowers WJ, Mendell LM, Fawcett JW, Arvanian VL, 2011. Chondroitinase ABC combined with neurotrophin NT-3 secretion and NR2D expression promotes axonal plasticity and functional recovery in rats with lateral hemisection of the spinal cord. J. Neurosci 31, 17788–17799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K, 2007. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain 130, 2993–3003. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J, 2006. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J. Neurosci 26, 7405–7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagal SG, Muir GD, 2009. Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp. Neurol 216, 193–206. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG, 1968. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain 91, 15–36. [DOI] [PubMed] [Google Scholar]
- Lee YS, Lin CY, Jiang HH, Depaul M, Lin VW, Silver J, 2013. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci 33, 10591–10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ, 2000. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J. Comp. Neurol 419, 286–296. [DOI] [PubMed] [Google Scholar]
- Morris R, Tosolini AP, Goldstein JD, Whishaw IQ, 2011. Impaired arpeggio movement in skilled reaching by rubrospinal tract lesions in the rat: a behavioral/anatomical fractionation. J. Neurotrauma 28, 2439–2451. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith M, Deacon P, 1996. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain 119 (Pt 6), 1809–1833. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The Rat Brain in Stereotaxic Coordinates. Academic Press, New York. [Google Scholar]
- Piecharka DM, Kleim JA, Whishaw IQ, 2005. Limits on recovery in the corticospinal tract of the rat: partial lesions impair skilled reaching and the topographic representation of the forelimb in motor cortex. Brain Res. Bull 66, 203–211. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Noth P, Thallmair M, Schwab ME, 2001. Functional switch between motor tracts in the presence of the mAb IN-1 in the adult rat. Proc. Natl. Acad. Sci. U. S. A 98, 6929–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN, 2009. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J. Neurosci 29, 4993–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH, 2010. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat. Neurosci 13, 1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K, 2002. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol 176, 143–153. [DOI] [PubMed] [Google Scholar]
- Soleman S, Yip PK, Duricki DA, Moon LD, 2012. Delayed treatment with chondroitinase ABC promotes sensorimotor recovery and plasticity after stroke in aged rats. Brain 135, 1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey ML, Bleul C, Maier IC, Schwab ME, 2011. Rehabilitative training following unilateral pyramidotomy in adult rats improves forelimb function in a non-task-specific way. Exp. Neurol 232, 81–89. [DOI] [PubMed] [Google Scholar]
- ten Donkelaar H, 2000. Development and Regenerative Capacity of Descending Supraspinal Pathways in Tetrapods: A Comparative Approach. [DOI] [PubMed]
- Umeda T, Takahashi M, Isa K, Isa T, 2010. Formation of descending pathways mediating cortical command to forelimb motoneurons in neonatally hemidecorticated rats. J. Neurophysiol 104, 1707–1716. [DOI] [PubMed] [Google Scholar]
- Vavrek R, Pearse DD, Fouad K, 2007. Neuronal populations capable of regeneration following a combined treatment in rats with spinal cord transection. J. Neurotrauma 24, 1667–1673. [DOI] [PubMed] [Google Scholar]
- Webb AA, Muir GD, 2003. Unilateral dorsal column and rubrospinal tract injuries affect overground locomotion in the unrestrained rat. Eur. J. Neurosci 18, 412–422. [DOI] [PubMed] [Google Scholar]
- Weishaupt N, Hurd C, Wei DZ, Fouad K, 2013. Reticulospinal plasticity after cervical spinal cord injury in the rat involves withdrawal of projections below the injury. Exp. Neurol 247, 241–249. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny B, Kolb B, Tetzlaff W, 1993. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res 56, 59–76. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Whishaw P, Gorny B, 2008. August 8 The structure of skilled forelimb reaching in the rat: a movement rating scale. J. Vis. Exp 18 pii: 816 10.3791/816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Wolpert DM, De Zeeuw CI, 2014. Motor systems: reaching out and grasping the molecular tools. Curr. Biol 24, R269–R271. [DOI] [PubMed] [Google Scholar]