Main Text
Kinesin-1 is a motor protein that carries cargoes along microtubules in neurons and other cells. It is one of the best understood molecular motors from the physical, chemical, and structural points of view. Kinesin takes 8-nm steps, moving from one tubulin dimer to the next toward the plus-, or fast-growing end, of a microtubule. The steps are driven by the hydrolysis of ATP with kinesin’s two motor domains moving in a coordinated, hand-over-hand manner. In the absence of load, kinesin takes hundreds of steps before detaching from the microtubule; it is highly processive. Under loading forces that oppose forward motion, however, kinesin detaches more quickly, and as the load force approaches the stall force of ∼6 pN, kinesin takes only a few steps before dissociating (1). Herein lies a biological problem. If kinesin detaches at high loads, then ensembles of kinesins are not expected to be able to generate forces much larger than 6 pN because the detachment of one kinesin will lead to a cascade of motor detachment as the remaining motors experience higher and higher forces (2, 3, 4). Yet, teams of kinesin can generate forces as high as 100 pN (5). Furthermore, two kinesins can generate more force than one in cells (6), can clear organellar traffic jams in axons (7), and can pull against other motors such as dynein (8). The paper by Pyrpassopoulos et al. in this issue of Biophysical Journal (9) offers an unexpected solution to this problem.
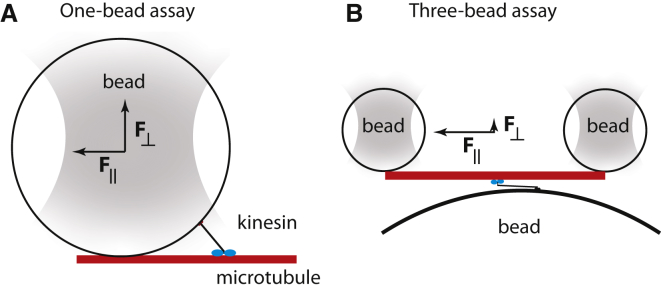
The forces generated by motor proteins such as kinesin, myosin, and dynein have been measured in several ways. Forces can be exerted using flexible fibers, optical tweezers, solution viscosity, and filament buckling (10). The most common assay for measuring processive motors like kinesin is the traditional one-bead assay; motors are bound to a micron-sized glass or plastic bead, which is held in an optical trap. By titrating the kinesin density on the bead surface, the behavior of single motors can be studied. Bead assays have shown that the processivity of kinesin is decreased by force; as the force component directed toward the minus end of the microtubule increases, both the distance and duration of kinesin runs along the microtubule decrease (1). This suggests that kinesin has a slip-bond character; load force promotes detachment. If this is the case, then assisting forces (i.e., forces directed toward the plus-end of the microtubule) should slow detachment and increase processivity. This is not found, however Andreasson et al. (1) showed that assisting forces also promote detachment. Khataee and Howard (3) argued that this symmetric effect of force was due to detachment by the vertical component of the force, which is the same whether the horizontal component is loading or assisting. The vertical component arises because the optical force acts at the center of the bead, and both the force and torque are balanced; because the bead is much larger than the motor, the vertical and horizontal components are of similar magnitude (Fig. 1 A). Furthermore, Khataee and Howard (3) hypothesized that the deviation from symmetry measured by Andreasson et al. (1) accorded with hindering load forces decreasing (not increasing) kinesin’s detachment rate; in other words, kinesin makes a catch (rather than slip) bond. This hypothesis can account for the large kinesin forces measured in ensemble measurements; the higher the force, the less likely to let go. The key test of this hypothesis is to measure the processivity of kinesin under forces that are directed exclusively along the axis of the microtubule (without a vertical component). This is the experiment that Pyrpassopoulos et al. performed (9).
Figure 1.
Kinesin-bead force assays. (A) In the one-bead assay, the vertical and horizontal forces have similar magnitudes. (B) In the three-bead assay, the force is primarily horizontal. To see this figure in color, go online.
In this issue, Pyrpassopoulos and colleagues (9) investigate the roles of axial and vertical forces on the processivity of kinesin-1. They used a three-bead assay in which beads are attached to each end of a microtubule and held in two optical traps, while the filament is manipulated to interact with kinesins sparsely arranged on a larger third bead attached to the glass coverslip (Fig. 1 B). Thus, instead of the motor being subjected to vertical forces inherent in the one-bead geometry, trapping forces are predominantly aligned along the axis of the microtubule. By comparing the duration of events in which the motor steps up to its stall force and waits there before detaching, they found that the three-bead geometry results in threefold longer interaction times and a higher fraction of beads reaching stall. This implies that in the traditional one-bead geometry, vertical forces are contributing to motor detachment. To further test this idea, the one-bead assay was repeated using larger beads that produce larger vertical forces. As predicted, attached durations were shorter and fewer motors reached stall with these larger beads. Together, these data convincingly argue that when forces are directed exclusively parallel to the microtubule, the detachment rates of kinesin-1 motors are much less sensitive to load than previously thought.
These results have implications for understanding both in vitro and in vivo motor investigations. Force experiments using one-bead assays have usually assumed that the relevant forces are axial. These experiments will need to be reevaluated. Multi-motor investigations using one-bead assays have generally concluded that teams of kinesin-1 motors do not effectively sum their forces (11,12), which contrasts with large multimotor forces measured in the gliding assay geometry (5). This conflict can now likely be accounted for by the different geometries of the two assays (3). In cells, besides the viscous and elastic loads inherent in pulling a vesicle through the cytoplasm, many if not most vesicles also have attached dyneins that may compete in a tug of war against the kinesins (13). A recent modeling study that used kinesin and dynein parameters taken from published bead assays found that the most important determinant of the net velocity and directionality of bidirectional cargo is the load-dependent motor detachment rate (14). Thus, if kinesin has catch-bond behavior that allows it to resist axial loads, then it is expected to be a more competitive antagonist to dynein. There are hints of this in published in vitro work in which one kinesin-1 and one dynein-dynactin-BicD2 complex were connected through a DNA adaptor (15). The kinesin-dynein-dynactin-BicD2 complexes moved slowly and smoothly for tens of seconds, which is at odds with the subsecond run times against loads measured in force-clamp optical tweezer experiments (1). As dynein has been shown to have catch-bond characteristics under some conditions (16), this persistence of kinesin against axial loads now puts the motors on more equal footing.
What are the implications for in vivo vesicle transport? Kinesins transport cargo ranging from ∼30-nm vesicles to ∼3 micron mitochondria. For small vesicles, in which the coiled-coil tether is on the order of the vesicle diameter, the motors are expected to organize with their tails pulling normal to the vesicle surface and the motor forces aligned nearly parallel to the microtubule axis. Similarly, for large mitochondria, motor forces will be aligned parallel to the microtubule axis, similar to a gliding assay. In contrast, for spherical vesicles considerably larger than the motor tether, the geometry will much more closely resemble the single-bead assay and forces in the z direction are expected to be substantial. The mechanical picture is complicated, however. Inherent in vesicle transport is the possibility that the motor tails slip in the plane of the membrane to take on preferred orientations. And it is possible that motor forces can deform spherical vesicles—kinesins attached to the surface of giant unilamellar vesicles in vitro are able to pull out narrow membrane tethers, resulting in the motor forces being more closely aligned parallel to the microtubule (17).
Thus, kinesin potentially having catch bond-like behavior makes sense of many in vitro and in vivo experiments. It begs the question: where in kinesin’s ATPase cycle would the catch bond reside? It is tempting to think that the key transition is the lifting of the rear head, which puts the motor into a one head-bound state. During unloaded stepping, the leading head will exert force in the direction of stepping, accelerating the detachment of the trailing head. Rearward tension on the motor stalk should counter this force, causing the motor to spend a longer time in a two-head-bound state that resists detachment. This will need to be carefully investigated in assays in which the direction of the force is carefully controlled.
Editor: David Sept.
Contributor Information
Jonathon Howard, Email: joe.howard@yale.edu.
William O. Hancock, Email: woh1@psu.edu.
References
- 1.Andreasson J.O., Milic B., Block S.M. Examining kinesin processivity within a general gating framework. eLife. 2015;4:e07403. doi: 10.7554/eLife.07403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grill S.W., Kruse K., Jülicher F. Theory of mitotic spindle oscillations. Phys. Rev. Lett. 2005;94:108104. doi: 10.1103/PhysRevLett.94.108104. [DOI] [PubMed] [Google Scholar]
- 3.Khataee H., Howard J. Force generated by two kinesin motors depends on the load direction and intermolecular coupling. Phys. Rev. Lett. 2019;122:188101. doi: 10.1103/PhysRevLett.122.188101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller M.J., Klumpp S., Lipowsky R. Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc. Natl. Acad. Sci. USA. 2008;105:4609–4614. doi: 10.1073/pnas.0706825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bormuth V., Jannasch A., Schäffer E. Optical trapping of coated microspheres. Opt. Express. 2008;16:13831–13844. doi: 10.1364/oe.16.013831. [DOI] [PubMed] [Google Scholar]
- 6.Shubeita G.T., Tran S.L., Gross S.P. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–1107. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurd D.D., Saxton W.M. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welte M.A., Gross S.P., Wieschaus E.F. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell. 1998;92:547–557. doi: 10.1016/s0092-8674(00)80947-2. [DOI] [PubMed] [Google Scholar]
- 9.Pyrpassopoulos S., Shuman H., Michael Ostap E. Modulation of kinesin’s load-bearing capacity by force geometry and the microtubule track. Biophys. J. 2020;118:243–253. doi: 10.1016/j.bpj.2019.10.045. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard J. Sinauer Associates; Sunderland, MA: 2001. Mechanics of Motor Proteins and the Cytoskeleton. [Google Scholar]
- 11.Furuta K., Furuta A., Kojima H. Measuring collective transport by defined numbers of processive and nonprocessive kinesin motors. Proc. Natl. Acad. Sci. USA. 2013;110:501–506. doi: 10.1073/pnas.1201390110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamison D.K., Driver J.W., Diehl M.R. Two kinesins transport cargo primarily via the action of one motor: implications for intracellular transport. Biophys. J. 2010;99:2967–2977. doi: 10.1016/j.bpj.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock W.O. Bidirectional cargo transport: moving beyond tug of war. Nat. Rev. Mol. Cell Biol. 2014;15:615–628. doi: 10.1038/nrm3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi K.G., Han L., Hancock W.O. Load-dependent detachment kinetics plays a key role in bidirectional cargo transport by kinesin and dynein. Traffic. 2019;20:284–294. doi: 10.1111/tra.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belyy V., Schlager M.A., Yildiz A. The mammalian dynein-dynactin complex is a strong opponent to kinesin in a tug-of-war competition. Nat. Cell Biol. 2016;18:1018–1024. doi: 10.1038/ncb3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunwar A., Tripathy S.K., Gross S.P. Mechanical stochastic tug-of-war models cannot explain bidirectional lipid-droplet transport. Proc. Natl. Acad. Sci. USA. 2011;108:18960–18965. doi: 10.1073/pnas.1107841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leduc C., Campàs O., Prost J. Cooperative extraction of membrane nanotubes by molecular motors. Proc. Natl. Acad. Sci. USA. 2004;101:17096–17101. doi: 10.1073/pnas.0406598101. [DOI] [PMC free article] [PubMed] [Google Scholar]