Cyanuric acid is produced naturally as a contaminant in urea fertilizer, and it is used as a chlorine stabilizer in swimming pools. Cyanuric acid-degrading bacteria are used commercially in removing cyanuric acid from pool water when it exceeds desired levels. The total volume of cyanuric acid produced annually exceeds 200 million kilograms, most of which enters the natural environment. In this context, it is important to have a global understanding of cyanuric acid biodegradation by microbial communities in natural and engineered systems. Current knowledge of cyanuric acid metabolism largely derives from studies on the enzymes from a single model organism, Pseudomonas sp. ADP. In this study, we obtained and studied new microbes and discovered a previously unknown cyanuric acid degradation pathway. The new pathway identified here was found to be much more prevalent than the pathway previously established for Pseudomonas sp. ADP. In addition, the types of environment, taxonomic prevalences, and geospatial distributions of the different cyanuric acid degradation pathways are described here.
KEYWORDS: cyanuric acid, biuret, biodegradation, metabolic pathway, phenol red, high-throughput screen, enrichment culture, genomes
ABSTRACT
Cyanuric acid is an industrial chemical produced during the biodegradation of s-triazine pesticides. The biodegradation of cyanuric acid has been elucidated using a single model system, Pseudomonas sp. strain ADP, in which cyanuric acid hydrolase (AtzD) opens the s-triazine ring and AtzEG deaminates the ring-opened product. A significant question remains as to whether the metabolic pathway found in Pseudomonas sp. ADP is the exception or the rule in bacterial genomes globally. Here, we show that most bacteria utilize a different pathway, metabolizing cyanuric acid via biuret. The new pathway was determined by reconstituting the pathway in vitro with purified enzymes and by mining more than 250,000 genomes and metagenomes. We isolated soil bacteria that grow on cyanuric acid as a sole nitrogen source and showed that the genome from a Herbaspirillum strain had a canonical cyanuric acid hydrolase gene but different flanking genes. The flanking gene trtB encoded an enzyme that we show catalyzed the decarboxylation of the cyanuric acid hydrolase product, carboxybiuret. The reaction generated biuret, a pathway intermediate further transformed by biuret hydrolase (BiuH). The prevalence of the newly defined pathway was determined by cooccurrence analysis of cyanuric acid hydrolase genes and flanking genes. Here, we show the biuret pathway was more than 1 order of magnitude more prevalent than the original Pseudomonas sp. ADP pathway. Mining a database of over 40,000 bacterial isolates with precise geospatial metadata showed that bacteria with concurrent cyanuric acid and biuret hydrolase genes were distributed throughout the United States.
IMPORTANCE Cyanuric acid is produced naturally as a contaminant in urea fertilizer, and it is used as a chlorine stabilizer in swimming pools. Cyanuric acid-degrading bacteria are used commercially in removing cyanuric acid from pool water when it exceeds desired levels. The total volume of cyanuric acid produced annually exceeds 200 million kilograms, most of which enters the natural environment. In this context, it is important to have a global understanding of cyanuric acid biodegradation by microbial communities in natural and engineered systems. Current knowledge of cyanuric acid metabolism largely derives from studies on the enzymes from a single model organism, Pseudomonas sp. ADP. In this study, we obtained and studied new microbes and discovered a previously unknown cyanuric acid degradation pathway. The new pathway identified here was found to be much more prevalent than the pathway previously established for Pseudomonas sp. ADP. In addition, the types of environment, taxonomic prevalences, and geospatial distributions of the different cyanuric acid degradation pathways are described here.
INTRODUCTION
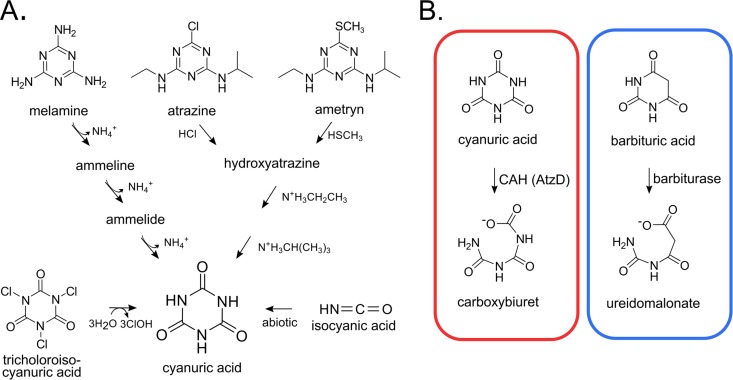
Cyanuric acid likely existed on prebiotic earth and is today a high-production-volume industrial chemical (1–3). It also derives from trichloroisocyanuric acid, which is used in chlorine disinfection, most notably for maintaining active chlorine in pools and spas (Fig. 1). Cyanuric acid is produced indirectly as a contaminant of urea fertilizer, typically comprising 0.2% of the mass (4). With an estimated 60 billion kilograms of urea fertilizer used per year worldwide (5), approximately 120 million kilograms of cyanuric acid is applied in agricultural fields as a contaminant. Cyanuric acid is also formed during the microbial biodegradation of widespread commercial s-triazine compounds, such as melamine, atrazine, and ametryn (6) (Fig. 1). The microbial metabolism of cyanuric acid impacts society in multiple ways: it is important for the bioremediation of recreational waters, the biodegradation of commercial s-triazine herbicides, and global nitrogen cycling. Recently, it has been proposed that cyanuric acid hydrolase (CAH) is an ancient enzyme undergoing a modern resurgence in response to the industrial production of s-triazine ring compounds (3).
FIG 1.
Environmental sources and enzymatic degradation of cyanuric acid. (A) Enzyme-catalyzed hydrolysis reactions transform industrially produced s-triazines into cyanuric acid. Trichloroisocyanuric acid and isocyanic acid react spontaneously to produce cyanuric acid. (B) The ring opening is catalyzed by CAH (AtzD). Barbiturase, a homolog of cyanuric acid hydrolase, catalyzes the analogous ring-opening reaction with barbituric acid.
Cyanuric acid is readily biodegraded in activated sludge and serves as a nitrogen source for some soil microorganisms (7, 8). Insights into the metabolic breakdown of cyanuric acid were derived as part of studies on the biodegradation of melamine and other s-triazine compounds (9). In 1991, the genes responsible for cyanuric acid degradation were identified and sequenced from a soil Pseudomonas strain (10, 11). In 1999, the first cyanuric acid ring-opening enzyme, TrzD, was purified and described as a hydrolase (12). Two years later, barbiturase, a homologous barbituric acid ring-opening enzyme, was purified and characterized (13, 14) (Fig. 1). The two enzyme activities comprise the majority of a small, readily identifiable protein family (3, 15, 16). Extensive biochemical characterization has revealed that the cyanuric acid hydrolases and barbiturases exhibit remarkably stringent substrate specificities, being reactive only with their specific substrates (14–18). The X-ray crystal structures of four cyanuric acid hydrolases and a barbiturase identified a unique protein fold with three protein domains defining a fairly symmetric active site (19–22). The cyanuric acid hydrolase active site is composed of three symmetric Ser-Lys-Arg triads that define the singular substrate specificity (20).
Cyanuric acid biodegradation and regulation have been most extensively studied in the atrazine-degrading bacterium Pseudomonas sp. strain ADP (23–30). The pathway proceeds sequentially via ring opening catalyzed by AtzD (17), deamination by AtzEG (29), decarboxylation by AtzH (28), and finally a hydrolytic reaction catalyzed by AtzF (31). Ultimately, the entire pathway transforms one molecule of cyanuric acid to yield three molecules of carbon dioxide and three molecules of ammonia. The ammonia can support growth of Pseudomonas sp. ADP. The genes are organized in an operon and are regulated by nitrogen availability and the presence of cyanuric acid (25, 26). Encoded in the operon is a high-affinity transport system that allows growth at low concentrations of cyanuric acid. Moreover, Pseudomonas sp. ADP is strongly chemotactic to atrazine and cyanuric acid (32). The comprehensive breadth of these studies has made Pseudomonas sp. ADP a paradigm for cyanuric acid metabolism. However, other pathways are plausible, and published data are suggestive of possible divergence in the metabolism of cyanuric acid (28, 33).
Pseudomonas sp. ADP initiates cyanuric acid biodegradation via a hydrolytic ring cleavage reaction catalyzed by AtzD, a homolog of TrzD. TrzD was reported to make biuret directly, but AtzD was shown by nuclear magnetic resonance (NMR) to make carboxybiuret (15). Carboxybiuret spontaneously decarboxylates within minutes to yield biuret, so it is unclear if the studies on TrzD would have detected carboxybiuret, if formed. However, it is plausible that some cyanuric acid hydrolase enzymes catalyze both the ring-opening hydrolysis reaction and subsequent decarboxylation to form biuret directly. Some indirect data in support of an enzyme-assisted decarboxylation of cyanuric acid come from X-ray structure data of TrzD, in which a carbon dioxide molecule was found in the proposed active-site exit channel (21). A recent study on biuret hydrolase enzymes (BiuH) in microorganisms examined genes in proximity to biuH (33). Ten percent of bacteria contain an identifiable cyanuric acid hydrolase gene within seven genes upstream or downstream of biuH. This gene structure begs the question of whether some cyanuric acid hydrolases might catalyze a hydrolytic ring opening and a subsequent decarboxylation reaction with cyanuric acid. While Pseudomonas sp. ADP was reported in 2001 to catabolize cyanuric acid via biuret (24), more recent studies have clearly demonstrated a pathway not involving biuret (28, 29).
In light of the outstanding questions and the commercial significance of cyanuric acid degradation, it was important to expand our understanding of cyanuric acid metabolism beyond what is known from the model organism, Pseudomonas sp. ADP. To begin, we sampled diverse environments for organisms growing on cyanuric acid. Pure cultures thus obtained were subjected to complete genome sequencing. Genes for a new catabolic pathway were identified, enzymes were purified, and the pathway was reconstituted in vitro. Bioinformatic analysis of more than 250,000 microbial genomes indicated the new pathway that proceeds via biuret to be more prevalent than that previously demonstrated in Pseudomonas sp. ADP.
RESULTS AND DISCUSSION
High-throughput screen, enrichment, and isolations.
We sought here to study cyanuric acid degradation broadly. To accomplish that, a high-throughput screen was developed based on pH increase from released ammonia (34, 35), bacteria were isolated and characterized in detail, and genomic data sets were used to examine pathways for cyanuric acid metabolism. Initially, soil and water were inoculated into 96 cultures in deep-well microtiter plates with the pH indicator phenol red. Those that showed a deep-purple color were presumed to be rapidly degrading cyanuric acid that had been supplied as the sole nitrogen source (see Fig. S1 in the supplemental material). The cultures that degraded cyanuric acid most rapidly were streaked onto cyanuric acid-phenol red agar plates, pure cultures were obtained, cyanuric acid removal was confirmed using analytical methods, and characterizations of isolates were carried out as described below.
Taxonomy, growth characteristics, and genome sequences of three isolates.
Three isolates that grew most rapidly with cyanuric acid as the sole nitrogen source were characterized further. They were clearly distinct, belonging to different genera, Pseudomonas, Comamonas, and Herbaspirillum. The closest species are Pseudomonas plecoglossicida, Comamonas aquatica, and Herbaspirillum aquaticum, with 100%, 100%, and 99.8% sequence identity, respectively, as determined using the BLAST algorithm and alignments of 16S rRNA gene sequences. The genome of each strain was sequenced and annotated, and the growth of each isolate on relevant substrates was determined.
The compiled data for the three isolated strains and the model cyanuric acid degrader Pseudomonas sp. ADP are presented in Table S1 in the supplemental material. The genome sizes of the isolates ranged from 4.0 to 5.9 Mb, all of which are significantly smaller than the previously sequenced, 7.3-Mb Pseudomonas sp. ADP genome (27). The coding densities ranged from 89 to 95%, which is typical of fast-growing soil bacteria. The new isolates grew rapidly with cyanuric acid, with a doubling time of 1.9 to 2.8 h compared to 4.1 h for Pseudomonas sp. ADP. Two of the three strains, the Comamonas and Herbaspirillum species, grew as fast on cyanuric acid as with ammonium chloride as the sole nitrogen source.
All three isolates had a canonical cyanuric acid hydrolase (atzD) gene. To date, all the genes identified as encoding a cyanuric acid ring-opening enzyme are homologous to atzD in the cyanuric acid hydrolase/barbiturase family, and the results here are consistent with previous findings (14, 15). Prior to this work, no AtzD had been identified in Herbaspirillum. The closest relative in the NCBI database, sharing 62% identity, is from Bradyrhizobium diazoefficiens USDA122. Cyanuric acid hydrolases had previously been identified in one Comamonas and multiple Pseudomonas species. The AtzD from Comamonas sp. strain CAH-2 isolated in this study is 82% identical to the AtzD found in Comamonas serinivorans, and the AtzD from Pseudomonas sp. strain CAH-1 is 100% identical to that previously identified in multiple strains of Pseudomonas aeruginosa. There are transposon-related sequences near the atzD gene in both the Herbaspirillum and Comamonas isolates from this study, suggesting that these gene regions may have undergone horizontal transfer on a mobile element. In addition, the cyanuric acid hydrolase gene in Herbaspirillum exists on a contig with two times higher sequencing coverage than the other large contigs, suggesting atzD may exist on a plasmid in the strain.
The three isolates did not contain identifiable atrazine-degrading genes, unlike Pseudomonas sp. ADP, but Herbaspirillum sp. strain CAH-3 did contain putative melamine-metabolizing genes that would produce cyanuric acid as an intermediate. The translated melamine deaminase from that strain shared 99.5% amino acid sequence identity to the melamine deaminase of Melaminivora alkalimesophila. Melamine deaminase yields ammeline. Further deaminations yield ammelide and cyanuric acid (9). We tested for growth on melamine, ammeline, and ammelide individually (Table 1). As predicted from the bioinformatic analysis, the Herbaspirillum strain grew well on melamine, but the other two strains did not. The Herbaspirillum strain grew weakly on ammeline and ammelide. It had been shown previously that guanine deaminase is capable of catalyzing the deamination of ammeline, which is structurally analogous to guanine (36).
TABLE 1.
Growth characteristics of fast-growing, cyanuric acid-degrading isolates
| Bacterium | Growth on s-triazinesa |
Growth on biureta | ||
|---|---|---|---|---|
| Melamine | Ammeline | Ammelide | ||
| Pseudomonas sp. CAH-1 | 0.07 ± 0.01 | 0.08 ± 0.03 | 0.06 ± 0.01 | 0.08 ± 0.01 |
| Comamonas sp. CAH-2 | 0.058 ± 0.003 | 0.017 ± 0.006 | 0.013 ± 0.006 | 0.08 ± 0.01 |
| Herbaspirillum sp. CAH-3b | 0.75 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.02 | 0.8 ± 0.1 |
| Pseudomonas sp. ADP | 0.02 ± 0.01 | 0.06 ± 0.04 | 0.039 ± 0.003 | 0.11 ± 0.01 |
OD600 after 16 h at 28°C.
At 37°C.
AtzD gene regions in isolates.
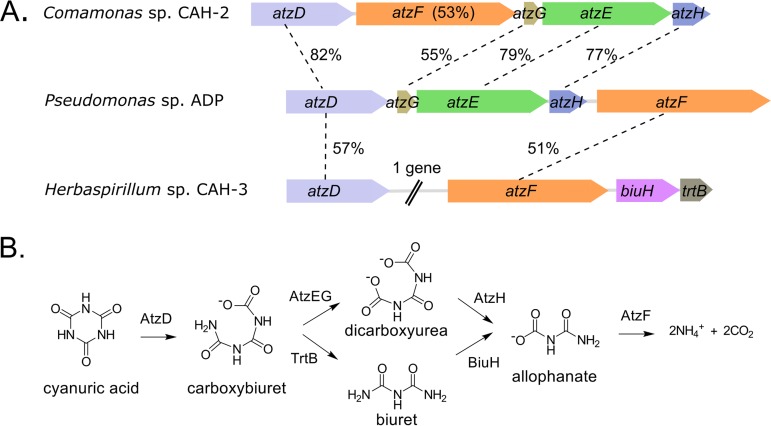
Genome sequencing revealed the presence of genes encoding likely cyanuric acid hydrolase and allophanate hydrolases (AtzF) in all three isolates, although the sequences were divergent (Fig. 2A). Amino acid sequence identities in pairwise comparisons to Pseudomonas sp. ADP proteins ranged from 48% to 82%. Two of the isolates, Pseudomonas sp. CAH-1 and Comamonas sp. CAH-2, have atzEGH genes, analogous to Pseudomonas sp. ADP (Fig. 2A). Cyanuric acid degradation in these strains is predicted to proceed through the upper pathway depicted in Fig. 2B. The third isolate, Herbaspirillum sp. CAH-3, does not have atzEGH genes. Instead, the atzD gene region contains a gene that when translated shows high amino acid sequence identity to hundreds of previously identified biuret hydrolase (BiuH) enzymes (33) (Fig. 2A). Sequence relatedness to the known biuret hydrolases ranged from 75 to 93%. The genomic context of the atzD of Herbaspirillum sp. CAH-3 suggests a previously unrecognized pathway for cyanuric acid degradation that proceeds through biuret (Fig. 2B). Consistent with the gene localization and sequence homology, the Herbaspirillum strain grew very well on biuret as the sole nitrogen source, whereas the other isolates and Pseudomonas sp. ADP, which lack biuH, did not (Table 1). These data further support the presence of a second cyanuric acid degradation pathway capable of conferring the ability to grow rapidly on cyanuric acid as a sole nitrogen source.
FIG 2.
Identification of two degradation pathways for cyanuric acid. (A) Gene regions for atzD in cyanuric acid-degrading isolates, obtained via complete genomic sequencing. The gene synteny in Pseudomonas sp. CAH-1 is identical to that of Comamonas sp. CAH-2. The percentages of identity of atzDEFGH gene products from the new isolates are compared to those of Pseudomonas sp. ADP. The Pseudomonas sp. CAH-1 atzD gene is 48% identical to that of Pseudomonas sp. ADP. Herbaspirillum sp. CAH-3 lacks the atzEGH genes but contains biuH and trtB genes. (B) Schematic of two metabolic routes for cyanuric acid. After carboxybiuret formation by AtzD enzymes, metabolism can proceed via one of two pathways, through 1,3-dicarboxyurea (top) or biuret (bottom). The pathways converge again at allophanate.
The gene region for Herbaspirillum sp. CAH-3 also contains a small gene recently proposed to encode a carboxybiuret decarboxylase (TrtB) (37). TrtB is predicted to decarboxylate carboxybiuret to form biuret, which is hydrolyzed into allophanate by the biuret hydrolase (Fig. 2B, bottom pathway). This functional assignment for TrtB activity was confirmed as described below.
TrtB purification and demonstrated role in the cyanuric acid pathway.
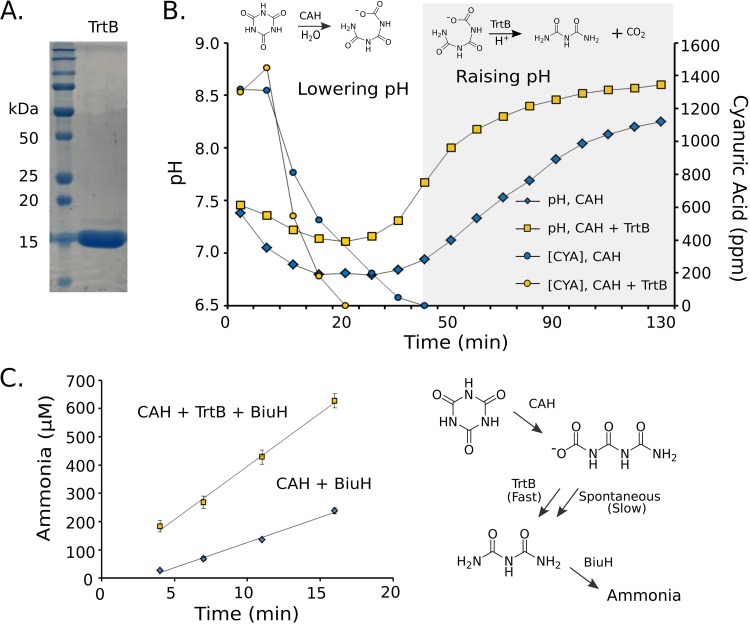
The trtB gene was expressed, the protein was purified, and the protein’s reaction was demonstrated in vitro. The TrtB protein was expressed well in Escherichia coli and was purified with only a very minor contaminating band observable at slightly higher molecular weight (Fig. 3A). The predicted TrtB substrate, carboxybiuret, is known to decarboxylate rapidly and therefore was generated for assays in situ using a purified cyanuric acid hydrolase known to produce carboxybiuret.
FIG 3.
Herbaspirillum sp. CAH-3 TrtB increases the rate of carboxybiuret decarboxylation. (A) Gel with purified TrtB. (B) Cyanuric acid (CYA) concentrations (circles) and pH profiles (squares) of unbuffered reactions containing cyanuric acid with M. thermoacetica cyanuric acid hydrolase with (yellow) and without (blue) TrtB. Chemical reactions contributing to pH change are indicated above the graph of a representative reaction. (C) Ammonia released from triplicate reactions containing cyanuric acid with M. thermoacetica cyanuric acid hydrolase, with (squares) or without (blue diamonds) Herbaspirillum sp. CAH-3 TrtB, and with R. leguminosarum BiuH. A reaction omitting BiuH released 15 μM ammonia. Reactions resulting in release of ammonia from cyanuric acid are shown.
The first experimental demonstration of activity was based on expected pH changes. The ring-opening reaction transformed a relatively mild acid (cyanuric acid) into a stronger acid (carboxybiuret), leading to an initial lowering of pH (Fig. 3B). Subsequent carboxybiuret decarboxylation produced a neutral species, biuret, and carbon dioxide, leading to a rise in pH. In the presence of TrtB, the pH decrease was shallower and the rise happened more quickly, indicating that TrtB accelerates the decarboxylation reaction (Fig. 3B). Furthermore, TrtB increased the rate of cyanuric acid disappearance. The decarboxylation of carboxybiuret is expected to pull the equilibrium of the cyanuric acid hydrolase reaction toward the ring-opened form. The equilibrium constant of the cyanuric acid hydrolase reaction cannot be experimentally determined, due to the instability of carboxybiuret. However, the equilibrium constant of the analogous ring-opening reaction with barbituric acid is 0.16 (14). In light of this result, one would expect that TrtB would accelerate the cyanuric acid hydrolysis reaction by removing the reaction product, carboxybiuret, in a decarboxylation reaction that is largely irreversible.
As a further demonstration of TrtB activity, cyanuric acid was again hydrolyzed with purified cyanuric acid hydrolase with or without biuret hydrolase (BiuH). TrtB activity was observed as an increased rate of ammonia release from biuret, the TrtB reaction product, in the presence of BiuH (Fig. 3C). Previously, 1H NMR experiments had shown that carboxybiuret decarboxylated nonenzymatically over a time course of 1 to 20 min, explaining why some ammonia is observed even in the absence of TrtB (38).
Lastly, we reconstituted the newly discovered TrtB- and BiuH-dependent cyanuric acid degradation pathway by incubating cyanuric acid hydrolase, carboxybiuret decarboxylase, biuret hydrolase, and allophanate hydrolase with cyanuric acid (Fig. 2B, lower pathway). The complete pathway of cyanuric acid degradation transforms one cyanuric acid molecule to three molecules of ammonia. In an experiment with CAH, TrtB, BiuH, and AtzF in one reaction mixture, 1 mM cyanuric acid was metabolized in vitro to 3.2 ± 0.2 mM ammonia.
Frequencies of the AtzEG and BiuH pathways.
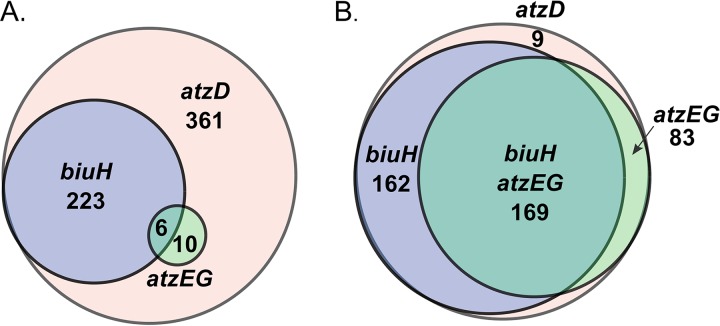
Prior to the discovery of TrtB, it had been considered that some cyanuric acid hydrolases might catalyze the decarboxylation of carboxybiuret, thus producing biuret. However, carboxybiuret is transformed by the AtzEG enzymes (29) or by TrtB as described here (Fig. 3), suggesting that most or all cyanuric acid hydrolases generate carboxybiuret. Investigation of atzD genomes revealed that trtB cooccurence with biuH is relatively rare (14%). This finding suggests that alternative enzymes may catalyze decarboxylation in genomes without trtB, since decarboxylases are common. In support of this hypothesis, a cupin family gene shown to encode a decarboxylase is found in place of trtB in 22% of atzD genomes (see Fig. S2 in the supplemental material) (37). Therefore, it was relevant to ask whether AtzEG- or BiuH-mediated pathways are more prevalent in nature.
To begin to address that question, genomes were searched for cyanuric acid hydrolases that initiate both pathways. Six hundred unique sequences were identified in the NCBI nonredundant database as cyanuric acid hydrolases. Next, the eight genes upstream and downstream of atzD were searched computationally for the prevalence of biuH and atzEG genes. In the 600 eight-gene windows represented by the Venn diagram in Fig. 4A, the atzD gene is clustered with a biuH gene 14 times more frequently than with atzEG genes. We searched for the genes anywhere in the genomes for a set of 423 genomes (Fig. 4B). Again, the biuH genes predominated; however, this analysis is less reliable, since there are multiple enzymes homologous to BiuH and AtzE.
FIG 4.
Prevalence of biuH and atzEG within eight genes of atzD (A) and anywhere in genome assemblies (B) from the NCBI database. The largest circle represents total genomes with atzD, and each inner circle represents a subset of atzD genomes or gene regions with biuH and/or atzEG.
Taxonomic abundance of cyanuric acid hydrolase and associated metabolic genes.
The atzD-containing microorganism genomes were compiled from assemblies in the NCBI database, in a proprietary environmental database to which access was provided by AgBiome, and in NCBI metagenomes (Table 2). The cyanuric acid hydrolases in the NCBI database are largely confined to bacteria and fungi. Within bacteria, Proteobacteria and Cyanobacteria are the most highly represented taxa. In contrast, no cyanuric acid hydrolase genes were identified in 7,317 Bacteroidetes genome assemblies, and only 1 of 3,334 archaeal genomes, that of a Thaumarchaeota archaeon, was found to encode a cyanuric acid hydrolase. Previously, no biuret hydrolase genes had been found in Bacteroidetes, and only one was found in archaea, Nitrososphaera (33), consistent with the low number of cyanuric acid hydrolases in those phyla. A cyanuric acid hydrolase gene was also identified in one protist, Emiliania huxleyi.
TABLE 2.
Taxonomic abundance of atzD
| Taxon | NCBIa |
AgBiomeb |
Metagenomesc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AtzD | No. of assemblies | %d | AtzD | No. of assemblies | % | AtzD | No. of assemblies | % | |
| Proteobacteria | 587 | 102,045 | 0.6 | 350 | 11,853 | 3.0 | 29 | 2,841 | 1.0 |
| Firmicutes | 62 | 51,654 | 0.1 | 128 | 26,132 | 0.5 | 11 | 2,131 | 0.5 |
| Actinobacteria | 48 | 18,721 | 0.3 | 55 | 3,357 | 1.6 | 8 | 443 | 1.8 |
| Unclassified bacteria | 34 | 10,087 | 0.3 | 772 | |||||
| Fungi | 34 | 4,823 | 0.7 | 2 | 295 | 0.7 | |||
| Cyanobacteria | 14 | 1,512 | 0.9 | 30 | 2 | 95 | 2.1 | ||
| Archaea | 1 | 3,334 | 0.0 | 615 | |||||
| Tenericutes | 1 | 854 | 0.1 | 1 | 38 | ||||
| Protists | 1 | 298 | 0.3 | ||||||
| Chloroflexi | 449 | 3 | 256 | 1.2 | |||||
| Nitrospinae | 46 | 2 | 12 | 0.2 | |||||
| Bacteroidetes | 7,371 | 1 | 627 | 0.2 | 1 | 1,772 | 0.1 | ||
| Acidobacteria | 165 | 1 | 52 | 1.9 | |||||
| Total | 782 | 201,359 | 0.4 | 536 | 42,295 | 1.3 | 57 | 9,027 | 0.6 |
In the AgBiome collection of environmental isolates from the United States and Uganda, the taxonomic abundance of atzD largely matched that observed in the public data set (Table 2). In both, Proteobacteria is the major phylum represented, and the percentage of Firmicutes with atzD is 1/6 that of Proteobacteria. Importantly, more than half of the isolates in the proprietary environmental collection are Firmicutes. Firmicutes is a major phylum of bacteria found in the environment, and many members (e.g., Bacillus) are hardy and easy to cultivate.
The assembled genomes of the NCBI and AgBiome data sets are inherently biased toward culturable microbes; therefore, analysis of metagenomic data was done to provide a more representative picture of the taxonomic distribution of atzD. Parks et al. have contributed over 10,000 newly assembled bacterial and archaeal genomes from metagenomic data available in public databases (39–41). The new genomes expanded the phylogenetic diversity of bacterial and archaeal clades by more than 30%. The taxonomic distribution of the atzD-containing strains in the metagenomic data set is consistent with that of the data sets for whole-genome sequences (Table 2). One notable difference is the high frequency of atzD found in Actinobacteria in both the metagenomic data and the AgBiome collection, indicating that Actinobacteria are also a rich source of atzD. In addition, closer inspection of the distribution of atzD in Proteobacteria in all three databases revealed differences (see Table S2 in the supplemental material). The atzD-containing genomes were found primarily in Alphaproteobacteria in the public database, whereas the Beta- and Gammaproteobacteria, respectively, were more highly represented in the other two data sets.
There are a wide variety of genomic contexts and gene orders surrounding atzD. A few patterns in specific genera or phyla were identified upon comparison of the syntenies (see Fig. S2). All Paenibacillus and almost all Rhizobium, Agrobacterium, and Variovorax strains analyzed have biuH near atzD. The majority of Pseudomonas and Actinobacteria strains have biuH, as well. The strains with atzE are a small subset of Proteobacteria. For example, the majority of Pseudomonas strains have biuH, but Pseudomonas sp. ADP and the Pseudomonas sp. CAH-1 isolates in this study have atzEG near atzD. All the atzD contexts with the rarer linkage to atzE are provided in Fig. S2B. Bradyrhizobium, Cyanobacteria, fungi, and most Firmicutes do not have biuH or atzE genes in the eight-gene window (see Fig. S2). These isolates constitute the largest group of atzD-containing isolates and reflect the existence of another cyanuric acid metabolic pathway, incomplete pathways, nonoperon gene structure, or the inability of current methods to identify genes of interest.
Relatedness of AtzD sequences.
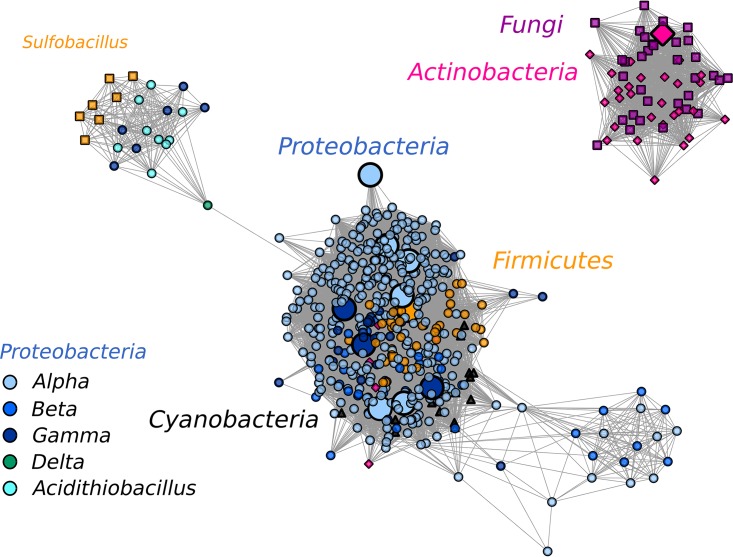
AtzD sequence relatedness was generally correlated with taxonomy, with some crossover suggestive of horizontal gene transfer, supporting the conclusions of Seffernick and Wackett (3). The sequence similarity network shown in Fig. 5 shows a major cluster comprised of sequences from Proteobacteria, with additional sequences from Firmicutes and Cyanobacteria. Eleven members of this cluster have been characterized biochemically to exhibit cyanuric acid hydrolase activity (12, 15–18). Actinobacterial sequences largely cluster with those from fungi in a separate group. One member of each phylum from this cluster has been characterized biochemically, and a second actinobacterial sequence has been characterized genetically (16, 42, 43). A third cluster is joined to the main cluster by one sequence, the single AtzD from a deltaproteobacterium. This third cluster includes Acidithiobacillus, Sulfobacillus (a subset of Firmicutes), and six gammaproteobacteria. The highly conserved gene context of atzD homologs in these organisms suggests that the sequences found in the cluster may not have cyanuric acid hydrolase activity (see Fig. S2B). The genome context contains two genes not typically found in atzD gene regions, encoding proteins annotated as XdhC Rossman domain proteins and ureidoglycolate lyase. This possibility remains to be tested, as Peat et al. reported that two sequences from the cluster, the atzD-like genes from Sulfobacillus acidophilus DSM 10332 and Acidithiobacillus ferrooxidans ATCC 53993, failed to produce soluble proteins (16).
FIG 5.
SSN of AtzD sequences from NCBI with a BLAST E value threshold of 10−85. The sequences are represented by 547 nodes sharing 100% sequence identity. Sequences of barbiturases are not included. Sequences from subclasses of Proteobacteria are shown in circles with shades of blue and green. The remaining sequences are from fungi (purple squares), Actinobacteria (pink diamonds), Firmicutes (orange circles, with the Sulfobacillus subset shown as squares), and Cyanobacteria (black triangles). The nodes with active cyanuric acid hydrolases characterized in vitro are enlarged. The sequence from the protist E. huxleyi did not cluster at the threshold shown.
Geospatial distribution of cyanuric acid degradation pathways.
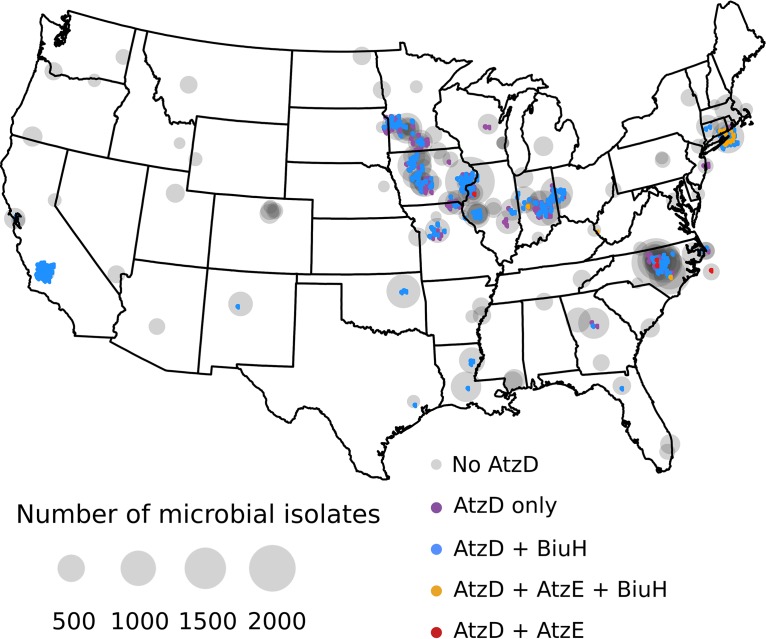
In the present study, we analyzed more than 42,000 environmental genome assemblies paired with extensive metadata to look for geospatial patterns in the distributions of the two cyanuric acid metabolic pathways (Fig. 6). All of the atzD-containing isolates in the AgBiome database were found within the contiguous United States (Fig. 6). They were absent in the samples obtained from the northwestern United States, Alaska, Hawaii, and Uganda. (see Fig. S3 in the supplemental material). Despite being a rare sequence in genomes—0.4% of NCBI genomes (Table 2)—at least one isolate with atzD was found in 28% of the sites sampled. Of the genomes with atzD, the most abundant pathway of cyanuric acid degradation observed in the AgBiome data set was via biuret as an intermediate (66%), and the second most prevalent genome context of atzD was one with neither biuH nor atzE (31%). Together, these contexts accounted for 97% of the isolates with atzD. These two categories accounted for the same percentage of atzD isolates in the NCBI data set (Fig. 4); however, in the environmental data set, the biuH-containing isolates outnumbered the isolates with atzD alone. Notably, isolates with atzD and biuH existed in nearly every location where an atzD-containing isolate was found, whereas the isolates with atzD alone were found almost exclusively in the midwestern United States. The isolates with genes encoding the dicarboxyurea and biuret pathways (3%) simultaneously or the dicarboxyurea pathway alone (<1%) were rare and were found in a few diverse locations, including soil from Long Island, NY, and seawater off the coast of North Carolina.
FIG 6.
Geospatial distribution of atzD-containing strains with biuH- and atzE-mediated cyanuric acid metabolic pathways within the proprietary data set. The colored dots indicate the locations of atzD-containing isolates (jittered to allow visualization of all data points) and are colored according the biuH or atzE presence within 10 genes of atzD, as indicated. The sizes of the gray circles, centered on each sampling site, indicate the sampling density. No atzD-containing genomes were found in three locations: Alaska, Hawaii, and Uganda (see Fig. S3).
Conclusions.
Overall, we defined and investigated the relative prevalences of two metabolic pathways for the degradation of cyanuric acid. One pathway was initially characterized in Pseudomonas sp. ADP. A newly identified cyanuric acid degradation pathway was shown to proceed via biuret as an intermediate. A method was developed to isolate highly efficient cyanuric acid-degrading bacteria, and three Proteobacteria isolates were characterized by genome sequencing and laboratory experiments. A canonical cyanuric acid hydrolase gene (atzD) was present in each isolate. One isolate harbored a biuret hydrolase gene and an uncharacterized gene near the atzD gene. Purification of the uncharacterized gene’s product revealed a new enzyme, carboxybiuret decarboxylase (TrtB), establishing a new cyanuric acid degradation pathway in the bacterium Herbaspirillum sp. CAH-3. Bioinformatic investigation of NCBI and AgBiome databases showed that the atzD gene is most common in Proteobacteria and that the newly characterized pathway using biuret hydrolase (BiuH) is more prevalent among microbes than the dicarboxyurea (AtzEG) pathway.
MATERIALS AND METHODS
High-throughput screen for cyanuric acid-degrading microbes.
Approximately 200 soil and water samples were collected from rural and urban settings within 350 miles of St. Paul, MN, USA. Environments sampled included public lawns, soccer fields, golf courses, parks, fountains, compost, and swimming pools. Microbes from water samples (1 liter, unless there was a heavy sediment load) were concentrated using a 2-μm filter. Samples collected at ambient temperatures were stored at 4°C. Samples collected from compost at temperatures ranging from 50 to 80°C were stored at room temperature for less than 3 days before screening; 2 to 5 mg of soil or 100 μl of concentrated water samples was used to inoculate 1 ml minimal medium, with cyanuric acid as the sole nitrogen source. Two different sets of carbon sources were used at 20 mM each. One combination was acetate and citrate, and the other was glycerol and ethanol. The minimal medium contained, per liter deionized water, 0.13 g cyanuric acid, 5.45 g K2HPO4, 1.36 g KH2PO4, 0.2 g MgSO4·7H2O, 0.1 g NaCl, 1.5 g sodium acetate, 0.5 g sodium citrate (or 20 ml glycerol and 10 ml ethanol), and 20 ml (each) of salt and vitamin stock solutions previously described by Mandelbaum et al. (44).
Samples were screened in deep 96-well plate format at both 28°C and 37°C with shaking at 210 to 225 rpm. The pH change of the medium was monitored with 15 mM phenol red. Cells were passaged with 10-fold dilutions in fresh medium every 24 h for 2 or 3 days and every 8 to 16 h for another 2 days. Colonies were isolated on LB from wells that showed a rapid change in pH after passaging. Cyanuric acid hydrolase activity was confirmed on agar plates using the minimal medium described above with both phenol red and excess cyanuric acid crystallized in the plate. Municipal and agricultural compost samples taken from piles ranging in temperature from 50 to 80°C were tested with both carbon source combinations at 55 and 65°C.
Genomic DNA was extracted from all candidates grown in LB with a DNeasy blood and tissue kit (Qiagen, Hilden, Germany). atzD and the 16S rRNA gene were PCR amplified and sequenced (ACGT, Wheeling, IL, USA) with degenerate primers for atzD (forward, TSAGTTCNGGCGGCAC, and reverse, GGNCCCTGRTGYTCGGC) and 27F (AGAGTTTGATCMTGGCTCAG) and 1492R (TACGGYTACCTTGTTACGACTT) for the 16S rRNA gene.
Growth tests for new atzD-containing isolates.
Doubling times were determined in quadruplicate in 96-well plates (250 μl) using 1 mM cyanuric acid and 3 mM NH4Cl in minimal medium. Growth of the isolated strains was followed by measuring the optical density at 600 nm (OD600) every 20 min for 24 h at 28°C or 37°C using a Tecan shaker (Tecan Trading AG, Switzerland). Growth on melamine and biuret as the sole nitrogen source was tested in minimal medium with each compound at 1 mM. For testing growth on ammeline and ammelide, each compound was suspended at 10 mg/ml in methanol; 250 μl of each methanol stock was added as the sole nitrogen source to 5 ml minimal medium. All the cultures were inoculated from a single colony of each cyanuric acid-degrading isolate or Pseudomonas sp. ADP. The cultures were grown overnight at 28°C, with the exception of the Herbaspirillum sp. CAH-3 strain, which was grown at 37°C.
Genome sequencing and assembly.
Genomic DNA was provided to the University of Minnesota Genomics Center (UMGC) (St. Paul, MN, USA) for Illumina Nextera XT library preparation and sequencing on a MiSeq with V3 chemistry and 300-bp paired-end reads. More than 2 million reads each were generated for the three strains. Adapters and low-quality bases were trimmed from raw reads with Trimmomatic v. 0.36. De novo assembly was performed using SPAdes v. 3.13.0 (45). Pseudomonas sp. CAH-1, Comamonas sp. CAH-2, and Herbaspirillum sp. CAH-3 had 156, 88, and 47 contigs of >500 bp each, and the N50s, defined as the minimum contig length needed to cover 50% of the sequenced genome, were 86,429, 94,697, and 271,710 bp, respectively. Initial annotation was performed with Prokka v. 1.12, and the genes of interest, biuH, trtB, and atzDEFGH, were manually annotated using hidden Markov models (HMMs) described in genome mining (46).
Herbaspirillum sp. CAH-3 TrtB expression and activity.
A synthetic Herb_3840 gene with E. coli codon optimization and appropriate homologous arms was purchased from Integrated DNA Technologies (Coralville, IA). The gene was introduced into pET28b+ using NdeI and HindIII sites to create an N-terminally tagged gene under the control of the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible T7 promoter using Gibson assembly. A 1-liter LB culture with 50 μg/ml kanamycin was grown at 37°C and cooled to 14°C at an OD600 of 0.25 prior to overnight induction with 1 mM IPTG. The protein was purified using nickel affinity chromatography in 20 mM phosphate buffer, 0.5 M NaCl, pH 7.4; washed at 20% imidazole; and eluted on a gradient up to 0.25 M imidazole. The purified protein was buffer exchanged into 20 mM phosphate, 0.2 M NaCl, pH 7.4, to remove the imidazole prior to activity assays. The activity of TrtB was assessed by following the pH of 10- ml reaction mixtures containing 10 mM cyanuric acid and 0.1 μM Moorella thermoacetica CAH with and without 1 μM Herbaspirillum sp. CAH-3 TrtB. The cyanuric acid concentration was determined with a melamine precipitation assay by measuring turbidity (OD600) (47). The activity of TrtB was also determined by following the release of ammonia from 1 mM cyanuric acid in the presence of 0.5 μM M. thermoacetica CAH and 0.1 μM Rhizobium leguminosarum BiuH (38) in 105 mM phosphate buffer, pH 8. The ammonia concentration was determined from reactions with and without Herbaspirillum sp. CAH-3 TrtB (2.6 μM) with the Berthelot assay (38). As a negative control, a reaction without BiuH was included. To determine the stoichiometry of nitrogen released from 1 mM cyanuric acid with the complete cyanuric acid mineralization pathway reconstituted, 0.1 μM Enterobacter cloacae strain 99 allophanate hydrolase (48) was combined with the enzymes and concentrations used in the previous experiment. The reaction mixture was incubated for 1 h prior to measuring ammonia with the Berthelot assay.
Genome mining.
The top 1,000 BLAST hits for Pseudomonas sp. ADP AtzD (NP_862537.1) were retrieved from the NCBI nonredundant protein sequence database (https://www.ncbi.nlm.nih.gov/ [accessed 23 October 2018]). The lowest identity was ∼40%. After trimming truncated and redundant sequences and manually sorting based on the presence of two 100% conserved arginines in cyanuric acid hydrolases that are not present in barbiturases (R194 and R324; Pseudomonas sp. ADP numbering) (15), 600 sequences remained. Total genome counts were retrieved from NCBI on 13 March 2019. RODEO was used to retrieve the 8-gene window for atzD (49). The following HMMs were used: atzD, atzE, and atzF were identified using existing TIGRFAM HMMs (50); atzG genes were identified with a PFAM HMM (DUF4089) (51); and HMMs were created with HMMER v 3.1b2 to identify biuH, trtB, and atzH (37, 52). The BLAST hits (40% sequence identity cutoff) for Pseudomonas sp. ADP AtzD, Herbaspirillum sp. CAH-3 trtB, and a cupin domain protein (WP_020047892.1) were retrieved from 423 atzD-containing genomes for which FASTA files could be retrieved. All AtzD and atzD genome accession files and HMMs have been uploaded to https://github.com/serina-robinson/AtzD. A sequence similarity network (SSN) was created using EFI-Enzyme Similarity Tool with AtzD sequences from the NCBI data set using a pairwise BLAST E value of 10−85 (53). The accession numbers for AtzD sequences confirmed biochemically to hydrolyze cyanuric acid are WP_012760697.1, WP_011654380.1, WP_009028459.1, WP_003459769.1, WP_013675305.1, WP_011090016.1, WP_031302833.1, WP_011117191.1, WP_011393610.1, WP_012333591.1, and BAF89201.1. The SSN was visualized with Cytoscape (54).
A large geospatial data set of sequenced environmental isolates with rich metadata was obtained through collaboration with AgBiome, a private agricultural research company in Research Triangle Park, NC. The data set includes 42,295 microbial strains from the United States and Uganda. Homologs of Pseudomonas sp. ADP AtzD (WP_011117191.1) and AtzE (WP_011117192.1) and R. leguminosarum bv. viciae BiuH (WP_011654379) with at least 40% sequence identity were retrieved from the data set using blastp. Truncated sequences and noncyanuric acid hydrolase sequences were removed as described for the NCBI data set. The presence of biuH and atzE within a 10-gene window of atzD was determined manually using the genome position of each homolog in the atzD-containing isolates. The geospatial distribution of isolates was mapped using the R package ggmap. Colored data points for atzD-containing isolates were jittered 50 km unless there were fewer than 6 points, in which case the jitter was 20 km (where "jitter" refers to random noise sampled from a uniform distribution to prevent overlapping data points).
A custom database of 10,000 genomes from the Uncultured Bacteria and Archaea (UBA) collection in PATRIC was used to retrieve the top BLAST hits for Bradyrhizobium diazoefficiens USDA 110 AtzD with at least 40% sequence identity (https://www.patricbrc.org/ [accessed 20 May 2019]) (39–41, 55). Truncated sequences and non-cyanuric acid hydrolase sequences were removed as with the other two data sets.
Accession number(s).
The Pseudomonas sp. CAH-1, Comamonas sp. CAH-2, and Herbaspirillum sp. CAH-3 assemblies and raw sequencing reads have been deposited in NCBI under BioProject PRJNA561581 and BioSample SAMN12620835, SAMN12620871, and SAMN12620971.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the University of Minnesota Genomics Center and Jon Badalamenti for genome sequencing and annotation; Eric Ward, Jake Trimble, James Kremer, and Matt Biggs from AgBiome for the generous use of their environmental genome data set; Carl Rosen and Galen Bergquist for soil samples collected from University of Minnesota research fields; Will Harcombe and Beth Adamowicz for the use of the Tecan shaker; and Tony Dodge for technical expertise and enzyme preparation.
S.L.R. is supported by a National Science Foundation (NSF) Graduate Research Fellowship (grant no. 00039202). This work was supported by funding from the MnDRIVE Initiative at the University of Minnesota and by the USDA National Institute of Food and Agriculture, Agricultural and Food Research Initiative Competitive Program, Ecosystem Services and Agro-Ecosystem Management, grant no. 2019-67019-29403.
L.P.W. owns equity in and is entitled to royalties from Minnepura Technologies, Inc., a company involved in the development, commercialization, and marketing of patented encapsulated biological platforms for water treatment. The University of Minnesota also has equity and royalty interest in Minnepura. These interests have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hayatsu R, Studier MH, Oda A, Fuse K, Anders E. 1968. Origin of organic matter in early solar system. II. Nitrogen compounds. Geochim Cosmochim Acta 32:175–190. doi: 10.1016/S0016-7037(68)80003-1. [DOI] [Google Scholar]
- 2.Cafferty BJ, Gállego I, Chen MC, Farley KI, Eritja R, Hud NV. 2013. Efficient self-assembly in water of long noncovalent polymers by nucleobase analogues. J Am Chem Soc 135:2447–2450. doi: 10.1021/ja312155v. [DOI] [PubMed] [Google Scholar]
- 3.Seffernick JL, Wackett LP. 2016. Ancient evolution and recent evolution converge for the biodegradation of cyanuric acid and related triazines. Appl Environ Microbiol 82:1638–1645. doi: 10.1128/AEM.03594-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu H, Wang Y, Sun H, Kannan K. 2019. Fertilizers as a source of melamine and cyanuric acid in soils: a nationwide survey in China. Environ Sci Technol Lett 6:55–61. doi: 10.1021/acs.estlett.8b00711. [DOI] [Google Scholar]
- 5.Mayo R, Batello C. 2015. World fertilizer trends and outlook to 2018. Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- 6.Wackett LP, Sadowsky MJ, Martinez B, Shapir N. 2002. Biodegradation of atrazine and related s-triazine compounds: from enzymes to field studies. Appl Microbiol Biotechnol 58:39–45. doi: 10.1007/s00253-001-0862-y. [DOI] [PubMed] [Google Scholar]
- 7.Saldick J. 1974. Biodegradation of cyanuric acid. Appl Microbiol 28:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myśków W, Lasota T, Stachyra A. 1983. Cyanuric acid: a s-triazine derivative as a nitrogen source for some soil microorganisms. Acta Microbiol Pol 32:177–183. [PubMed] [Google Scholar]
- 9.Jutzi K, Cook AM, Hütter R. 1982. The degradative pathway of the s-triazine melamine. The steps to ring cleavage. Biochem J 208:679–684. doi: 10.1042/bj2080679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton RW, Karns JS. 1991. Cloning and analysis of s-triazine catabolic genes from Pseudomonas sp. strain NRRLB-12227. J Bacteriol 173:1215–1222. doi: 10.1128/jb.173.3.1215-1222.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton RW, Karns JS. 1991. Cloning and comparison of the DNA encoding ammelide aminohydrolase and cyanuric acid amidohydrolase from three s-triazine-degrading bacterial strains. J Bacteriol 173:1363–1366. doi: 10.1128/jb.173.3.1363-1366.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karns JS. 1999. Gene sequence and properties of an s-triazine ring-cleavage enzyme from Pseudomonas sp. strain NRRLB-12227. Appl Environ Microbiol 65:3512–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soong CL, Ogawa J, Shimizu S. 2001. Novel amidohydrolytic reactions in oxidative pyrimidine metabolism: analysis of the barbiturase reaction and discovery of a novel enzyme, ureidomalonase. Biochem Biophys Res Commun 286:222–226. doi: 10.1006/bbrc.2001.5356. [DOI] [PubMed] [Google Scholar]
- 14.Soong CL, Ogawa J, Sakuradani E, Shimizu S. 2002. Barbiturase, a novel zinc-containing amidohydrolase involved in oxidative pyrimidine metabolism. J Biol Chem 277:7051–7058. doi: 10.1074/jbc.M110784200. [DOI] [PubMed] [Google Scholar]
- 15.Seffernick JL, Erickson JS, Cameron SM, Cho S, Dodge AG, Richman JE, Sadowsky MJ, Wackett LP. 2012. Defining sequence space and reaction products within the cyanuric acid hydrolase (AtzD)/barbiturase protein family. J Bacteriol 194:4579–4588. doi: 10.1128/JB.00791-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peat TS, Balotra S, Wilding M, Hartley CJ, Newman J, Scott C. 2017. High-resolution X-ray structures of two functionally distinct members of the cyclic amide hydrolase family of toblerone fold enzymes. Appl Environ Microbiol 83:e03365-16. doi: 10.1128/AEM.03365-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruchey I, Shapir N, Sadowsky MJ, Wackett LP. 2003. On the origins of cyanuric acid hydrolase: purification, substrates, and prevalence of AtzD from Pseudomonas sp. strain ADP. Appl Environ Microbiol 69:3653–3657. doi: 10.1128/aem.69.6.3653-3657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Seffernick JL, Sadowsky MJ, Wackett LP. 2009. Thermostable cyanuric acid hydrolase from Moorella thermoacetica ATCC 39073. Appl Environ Microbiol 75:6986–6991. doi: 10.1128/AEM.01605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peat TS, Balotra S, Wilding M, French NG, Briggs LJ, Panjikar S, Cowieson N, Newman J, Scott C. 2013. Cyanuric acid hydrolase: evolutionary innovation by structural concatenation. Mol Microbiol 88:1149–1163. doi: 10.1111/mmi.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho S, Shi K, Seffernick JL, Dodge AG, Wackett LP, Aihara H. 2014. Cyanuric acid hydrolase from Azorhizobium caulinodans ORS 571: crystal structure and insights into a new class of Ser-Lys dyad proteins. PLoS One 9:e99349. doi: 10.1371/journal.pone.0099349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bera AK, Aukema KG, Elias M, Wackett LP. 2017. Structure of the cyanuric acid hydrolase TrzD reveals product exit channel. Sci Rep 7:45277. doi: 10.1038/srep45277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi K, Cho S, Aukema KG, Lee T, Bera AK, Seffernick JL, Wackett LP, Aihara H. 2019. Crystal structures of Moorella thermoacetica cyanuric acid hydrolase reveal conformational flexibility and asymmetry important for catalysis. PLoS One 14:e0216979. doi: 10.1371/journal.pone.0216979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandelbaum RT, Allan DL, Wackett LP. 1995. Isolation and characterization of a Pseudomonas sp. that mineralizes the s-triazine herbicide atrazine. Appl Environ Microbiol 61:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez B, Tomkins J, Wackett LP, Wing R, Sadowsky MJ. 2001. Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J Bacteriol 183:5684–5697. doi: 10.1128/JB.183.19.5684-5697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-González V, Govantes F, Porrúa O, Santero E. 2005. Regulation of the Pseudomonas sp. strain ADP cyanuric acid degradation operon. J Bacteriol 187:155–167. doi: 10.1128/JB.187.1.155-167.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govantes F, García-González V, Porrúa O, Platero AI, Jiménez-Fernández A, Santero E. 2010. Regulation of the atrazine-degradative genes in Pseudomonas sp. strain ADP. FEMS Microbiol Lett 310:1–8. doi: 10.1111/j.1574-6968.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 27.Devers-Lamrani M, Spor A, Mounier A, Martin-Laurent F. 2016. Draft genome sequence of Pseudomonas sp. strain ADP, a bacterial model for studying the degradation of the herbicide atrazine. Genome Announc 4:e01733-15. doi: 10.1128/genomeA.01733-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esquirol L, Peat TS, Wilding M, Hartley CJ, Newman J, Scott C. 2018. A novel decarboxylating amidohydrolase involved in avoiding metabolic dead ends during cyanuric acid catabolism in Pseudomonas sp. strain ADP. PLoS One 13:e0206949. doi: 10.1371/journal.pone.0206949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esquirol L, Peat TS, Wilding M, Liu JW, French NG, Hartley CJ, Onagi H, Nebl T, Easton CJ, Newman J, Scott C. 2018. An unexpected vestigial protein complex reveals the evolutionary origins of an s-triazine catabolic enzyme. J Biol Chem 293:7880–7891. doi: 10.1074/jbc.RA118.001996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platero AI, Santero E, Govantes F. 2014. Genetic evidence of a high-affinity cyanuric acid transport system in Pseudomonas sp. ADP. FEMS Microbiol Lett 352:150–156. doi: 10.1111/1574-6968.12392. [DOI] [PubMed] [Google Scholar]
- 31.Shapir N, Sadowsky MJ, Wackett LP. 2005. Purification and characterization of allophanate hydrolase (AtzF) from Pseudomonas sp. strain ADP. J Bacteriol 187:3731–3738. doi: 10.1128/JB.187.11.3731-3738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Parales RE. 2009. Bacterial chemotaxis to atrazine and related s-triazines. Appl Environ Microbiol 75:5481–5488. doi: 10.1128/AEM.01030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson SL, Badalamenti JP, Dodge AG, Tassoulas LJ, Wackett LP. 2018. Microbial biodegradation of biuret: defining biuret hydrolases within the isochorismatase superfamily. Environ Microbiol 20:2099–2111. doi: 10.1111/1462-2920.14094. [DOI] [PubMed] [Google Scholar]
- 34.Rustigian R, Stuart CA. 1941. Decomposition of urea by proteus. Proc Soc Exp Biol Med 47:108–112. doi: 10.3181/00379727-47-13054. [DOI] [Google Scholar]
- 35.Christensen WB. 1946. Urea decomposition as a means of differentiating proteus and paracolon cultures from each other and from Salmonella and Shigella types. J Bacteriol 52:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seffernick JL, Dodge AG, Sadowsky MJ, Bumpus JA, Wackett LP. 2010. Bacterial ammeline metabolism via guanine deaminase. J Bacteriol 192:1106–1112. doi: 10.1128/JB.01243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tassoulas L. 2019. Novel discrimination of biuret and triuret degradation by enzymatic deamination: regulation and significance for slow-release nitrogen fertilizers. Master’s thesis. University of Minnesota, St. Paul, MN. [Google Scholar]
- 38.Cameron SM, Durchschein K, Richman JE, Sadowsky MJ, Wackett LP. 2011. New family of biuret hydrolases involved in s-triazine ring metabolism. ACS Catal 2011:1075–1082. doi: 10.1021/cs200295n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parks DH, Rinke C, Chuvochina M, Chaumeil PA, Woodcroft BJ, Evans PN, Hugenholtz P, Tyson GW. 2017. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol 2:1533–1542. doi: 10.1038/s41564-017-0012-7. [DOI] [PubMed] [Google Scholar]
- 40.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil P-A, Hugenholtz P. 2018. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36:996. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 41.Parks DH, Rinke C, Chuvochina M, Chaumeil PA, Woodcroft BJ, Evans PN, Hugenholtz P, Tyson GW. 2018. Author correction: Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol 3:253. doi: 10.1038/s41564-017-0083-5. [DOI] [PubMed] [Google Scholar]
- 42.Dodge AG, Preiner CS, Wackett LP. 2013. Expanding the cyanuric acid hydrolase protein family to the fungal kingdom. J Bacteriol 195:5233–5241. doi: 10.1128/JB.00965-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dodge AG, Wackett LP, Sadowsky MJ. 2012. Plasmid localization and organization of melamine degradation genes in Rhodococcus sp. strain Mel. Appl Environ Microbiol 78:1397–1403. doi: 10.1128/AEM.06468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandelbaum RT, Wackett LP, Allan DL. 1993. Mineralization of the s-triazine ring of atrazine by stable bacterial mixed cultures. Appl Environ Microbiol 59:1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 47.Downes CJ, Mitchell JW, Viotto ES, Eggers NJ. 1984. Determination of cyanuric acid levels in swimming pool waters by UV absorbance, HPLC and melamine cyanurate precipitation. Water Res 18:277–280. doi: 10.1016/0043-1354(84)90100-3. [DOI] [Google Scholar]
- 48.Shapir N, Cheng G, Sadowsky MJ, Wackett LP. 2006. Purification and characterization of TrzF: biuret hydrolysis by allophanate hydrolase supports growth. Appl Environ Microbiol 72:2491–2495. doi: 10.1128/AEM.72.4.2491-2495.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tietz JI, Schwalen CJ, Patel PS, Maxson T, Blair PM, Tai HC, Zakai UI, Mitchell DA. 2017. A new genome-mining tool redefines the lasso peptide biosynthetic landscape. Nat Chem Biol 13:470–478. doi: 10.1038/nchembio.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haft DH, Loftus BJ, Richardson DL, Yang F, Eisen JA, Paulsen IT, White O. 2001. TIGRFAMs: a protein family resource for the functional identification of proteins. Nucleic Acids Res 29:41–43. doi: 10.1093/nar/29.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, Sonnhammer ELL, Hirsh L, Paladin L, Piovesan D, Tosatto SCE, Finn RD. 2019. The Pfam protein families database in 2019. Nucleic Acids Res 47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol 7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerlt JA, Bouvier JT, Davidson DB, Imker HJ, Sadkhin B, Slater DR, Whalen KL. 2015. Enzyme function initiative-enzyme similarity tool (EFI-EST): a web tool for generating protein sequence similarity networks. Biochim Biophys Acta 1854:1019–1037. doi: 10.1016/j.bbapap.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, Gerdes S, Henry CS, Kenyon RW, Machi D, Mao C, Nordberg EK, Olsen GJ, Murphy-Olson DE, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Vonstein V, Warren A, Xia F, Yoo H, Stevens RL. 2017. Improvements to PATRIC, the all-bacterial Bioinformatics database and analysis resource center. Nucleic Acids Res 45:D535–D542. doi: 10.1093/nar/gkw1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.