Abstract
Stearoyl-CoA desaturase (SCD) generates monounsaturated fatty acids (MUFAs) which contribute to cell growth, survival, differentiation, metabolic regulation and signal transduction. Overexpression of SCD is evident and implicated in metabolic diseases such as diabetes and non-alcoholic fatty liver disease. SCD also stimulates canonical Wnt pathway and YAP activation in support of stemness and tumorigenesis. SCD facilitates metabolic reprogramming in cancer which is mediated, at least in part, by regulation of AKT, AMPK, and NF-kB via MUFAs. Our research has revealed the novel positive loop to amplify Wnt signaling through stabilization of LRP5/6 in both hepatic stellate cells and liver tumor-initiating stem cell-like cells. As such, this loop is pivotal in promoting liver fibrosis and liver tumor development. This review summarizes the mechanisms of SCD-mediated tumor promotion described by recent studies and discusses the future prospect for SCD-mediated signaling crosstalk as a potential therapeutic target for cancer.
Keywords: Hepatic stellate cells, Wnt, β-catenin, YAP
Introduction
1. Stearoyl-CoA desaturase
Stearoyl-CoA desaturase (SCD) is a delta-9 fatty acid desaturase localized in the endoplasmic reticulum (ER) membrane, which forms a carbon-carbon double bond at the 9th-10th position from the COOH (C)-terminus of saturated fatty acids (SFAs), palmitic acid and stearic acid to generate monounsaturated fatty acids (MUFAs), palmitoleic acid and oleic acid, respectively. MUFAs are required for biosynthesis of polyunsaturated fatty acids, phospholipids (PL), triglycerides (TG), cholesterol esters (CE), diacylglycerols and wax esters, which are fundamental components of the cellular membrane and essential for cell growth, survival, differentiation, metabolic energy generation, and signal transduction.
SCD has its own isoforms. These isoforms are different based on the species, developmental stage, and tissue localization. For example, four isoforms of Scd1-4 are present in chromosome 19 in mice. Scd1 is ubiquitously expressed with dominant expression in tissues with active lipogenesis such as liver, adipose tissue, meibomian gland, Harderian gland, and preputial gland of adult mice. Scd2 is also ubiquitously expressed except for adult liver. In contrast, Scd2 is expressed in embryonic or neonatal liver in place of Scd1. Scd3 is expressed in the skin. In adult mouse brain, SCD5 is responsible for most delta-9 fatty acid desaturase activity. In human, two isoforms of SCD1 and SCD5 have been identified. SCD1, which is classified as SCD as an official nomenclature, is localized in chromosome 10 while SCD5 is in chromosome 4. SCD shares ~85% amino acid homology with the mouse four isoforms. SCD is ubiquitously expressed but most abundant in liver and adipose while SCD5 expression is high in brain and pancreas [1].
SCD1 protein consists of four transmembrane domains with both NH2(N) and C-termini directed to cytosol. It contains 8 histidine (His) residues. They form three His regions, one of which is located in C-terminus and the other two in the cytoplasmatic loop. These regions collectively configure a His box, which serves to form a prosthetic group by binding nonheme iron as the catalytic center of SCD1. SCD1 activity relies on ER-bound cytochrome b5 reductase which accepts an electron from NADH and donate it to cytochrome b5 and then to the SCD1 prosthetic iron for its reduction. In the presence of oxygen, reduced SCD1 introduces a single double bond at the delta-9, 10 position of long-chain acyl-CoAs.
Scd1 is both transcriptionally and post-transcriptionally regulated. Scd1 transcription is induced by binding of liver X receptor (LXR) to LXR response element (LXRE) and that of sterol regulatory-element binding protein-1c (SREBP-1c) to the SREBP element (SRE). As LXR activates Srebp-1c transcription, LXR is capable of activating Scd1 directly and indirectly. Dietary carbohydrate such as glucose, fructose and sucrose, increases the expression of liver Scd1 in both SREBP-1c dependent and independent manners [2, 3] and the latter is caused by carbohydrate-induced LXR and the activation of carbohydrate response-element binding protein (ChREBP) [4, 5]. Melanocortin receptor agonist MT II and leptin suppress the liver Scd1 independently of insulin and SREBP-1c [6, 7]. Scd1 is upregulated by activation of the xenobiotic nuclear receptor pregnane-X-receptor (PXR), but this regulation may be mediated by the ability of PXR agonists to activate both PXR and LXR and to induce CD36 which takes up native lipoproteins, oxidized LDL, oxidized phospholipids, and long-chain fatty acids (FA) [8, 9]. Toll-like receptor 2 (TLR2)-NF-κB activation transcriptionally induces SCD1 in human sebocytes and this underlies antimicrobial defense facilitated by MUFA in skin [10]. SCD1 protein is a short-lived protein with a half-life of 2-4 hours and is stabilized by the PPAR agonist clofibric acid, which also stimulates Scd1 transcription [11, 12]. N-terminus of mouse SCD1 has the domain involved in the ubiquitin-proteasome-dependent degradation and a 70kD plasminogen-like protein rapidly and selectively degrades SCD1 [13, 14]. Human SCD1/5 may gain the stability by forming dimers and oligomers [15].
2. SCD in metabolic diseases and cancer
Global Scd1 knockout (KO) mice are protected from adiposity, insulin resistance and fatty liver induced by high carbohydrate diet (HCD), high fat diet (HFD), and SFA [16, 17]. These effects appear to be mediated, at least in part, by insulin sensitization and increased glucose oxidation by skeletal muscle and heart [18, 19]. As Scd1 expression is highly induced in liver and in response to HCD [20], these results suggest the role of hepatic Scd1 in the pathogenesis of obesity and fatty liver associated with HCD. In fact, liver specific SCD1 deficiency protects against these metabolic consequences [1]. However, HFD-induced adiposity and fatty liver are not ameliorated in this conditional KO mice. In contrast, skin specific Scd1 KO mice are protected against HFD-induced obesity, insulin resistance, and fatty liver due to hypermetabolic state as shown in global Scd1 KO mice [16]. These findings highlight tissue-specific functionality of SCD1 for metabolic regulation and suggest MUFAs produced in different sites may have diverse signaling outcomes.
SCD is overexpressed in malignancies, including cancer of lung, breast, colorectum, esophagus, bladder, and liver. SCD may be used as a biomarker for the prognosis of bladder cancer because the SCD overexpression is associated with progressive and metastatic cancer and SCD mRNA level inversely correlates with the survival rate [21]. However, the precise mechanisms by which SCD potentially promotes carcinogenesis, are currently unknown.
Because SCD is implicated in metabolic diseases, it is not surprising that SCD upregulation is associated with and implicated in non-alcoholic fatty liver disease (NAFLD), the liver phenotype of the metabolic syndrome. NAFLD progresses to non-alcoholic steatohepatitis (NASH) which, is characterized by inflammation and perisinusoidal and pericellular fibrosis commonly called “chicken-wire fibrosis”. We need to address how SCD and MUFA generated by SCD mechanistically promote these pathologic cellular phenotypes, namely, fatty hepatocytes, M1 macrophage activation, activated hepatic stellate cells (HSCs). In fact, SCD may have selective effects on different pathologic phases of chronic liver disease, ranging from hepatic steatosis, inflammation, fibrosis, and cancer by targeting the different cell types involved in each of these pathologic spectra. The overexpression of SCD in human may be causally linked to hypertriglyceridemia, atherosclerosis, and diabetes, and other important phenotypes of metabolic syndrome. These diseases may have hereditary influences, and the activity of SCD-1 is recently shown increased in the muscle in familial combined hyperlipidemia [22].
3. SCD and Lipid metabolic reprogramming in cancer
SCD is implicated in cancer, and this potential link may involve β-catenin, the effector of the canonical Wnt pathway, which is known to participate in carcinogenesis including liver cancer. It acts as a transcriptional co-activator to enhance cell growth and survival by mediating the expression of cell-cycle and growth promoting genes in hepatocyte-derived transformed cells or liver tumor-initiating cells (TICs).
Wnt proteins belong to a family of secreted signaling glycoproteins, which has the critical role in tissue development and cell fate regulation in embryogenesis. The level of nuclear β-catenin is maintained low when Wnt signal is off. Upon activation of canonical Wnt pathway, β-catenin is stabilized and translocated to nucleus to bind the T cell factor (TCF) family of transcription factor, activating Wnt target genes. SCD-generated palmitoleate is utilized for lipidation of Wnt ligands catalyzed by the Porcupine acyltransferase for their release into the extracellular space, supporting paracrine or autocrine Wnt action. This mechanism may signify concomitant overexpression of SCD and β-catenin commonly observed in cancer cells.
Metabolic reprogramming is an integral driving force to promote growth and metastasis of cancer cells. The most known example is the higher rate of aerobic glycolysis called the Warburg effect, which produces the excessive amounts of lactate but also citrate and glycerol, which stimulate the de novo synthesis of lipids via induced fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC). The PL content dramatically increases accompanying a large quantity of MUFAs produced by overexpressed SCD in neoplastic cells. Most MUFAs made by SCD are thought to be utilized for PL biosynthesis partly because the continuous membrane biogenesis of dividing cancer cells requires PLs [23]. FA and lipids are also actively taken up by cancer cells. The simultaneous activation of glycolysis and FA synthesis in cancer leads to the generation of MUFAs by SCD in cancer cells. As a consequence, the concentration of MUFAs increases in cancer cells [23–26].
SCD also increases the expression of other enzymes in FA biosynthesis. SCD allosterically upregulates ACC which produces malonyl-CoA from acetyl-CoA. When SCD is repressed, increased palmitate and stearate suppress ACC [27]. Conversely, induced SCD in cancer cells, turns SFAs into MUFAs and promotes sustainable lipid synthesis by suppressing this negative regulation.
SCD regulates signaling pathways to promote metabolic reprogramming. While cancer cells activate lipid synthesis during cell proliferation, they inhibit catabolic pathways such as FA β-oxidation. AKT and AMPK are both the targets of SCD and thought to integrate signals, which have the opposing regulatory effects on lipid metabolism, cell proliferation, survival and tumorigenesis. AKT is an inducer of glucose-mediated lipid synthesis in cancer cells and catalytically stimulates enzymes involved in glycolysis and lipogenesis. SCD stimulates the synthesis of phosphatidylinositols, which generate PI(3,4,5)P3, an activator of AKT [28]. AKT then phosphorylates Glycogen Synthase Kinase 3β (GSK3β) at S9 and by doing so inactivates it [29]. GSK3β is the key component of the β-catenin degradation complex, which phosphorylates β-catenin for subsequent ubiquitination and degradation. In SCD deficient cancer cells, S9 phosphorylation and inhibition of GSK3β are suppressed, β-catenin reduced, and the β-catenin-target gene Cyclin D1, is repressed [30, 31]. On the other hand, AMPK inhibits lipogenesis and stimulates FA oxidation. When SCD is inhibited, the activation of AMPK by phosphorylation of itsα-subunit causes the downregulation of ACC by phosphorylating its serine residues. [32] Conversely, overexpressed SCD inhibits AMPK phosphorylation and upregulates ACC, de novo FA synthesis, cancer cell growth and survival.
4. Wnt-Scd-Lrp5/6 positive loop in liver fibrosis and cancer
The Wnt pathway contributes to activation of HSCs and liver fibrosis [33]. Activated HSCs (aHSCs) support tumorigenesis via mechanisms, which may involve the release of growth factors [34] and matrix remodeling [35]. However, whether and how β-catenin activates HSCs and promotes liver tumor development, are not known. In fact, what target genes are upregulated by β-catenin in aHSCs, were not even investigated. To address this question, we have used a gene expression profiling approach on HSCs treated with the three inhibitors of the Wnt pathway: adenovirus-expressing Dickkopf-1 (DKK-1), ICG-001, and FJ9 [36]. DKK-1 forms a complex with LRP5/6 and Kremen1/2 and prevents activation of Wnt pathway [37]. ICG-001 antagonizesβ-catenin/TCF-mediated transcription by binding to cyclic AMP response element-binding protein (CBP) and preventing its interaction with β-catenin [38]. FJ9 binds the PDZ domain of Dishevelled (Dvl) and interferes its association with the Wnt receptor frizzled (Fz) [39]. This study has identified, Scd1/2 as a putative Wnt target gene in aHSCs. Scd1/2 transcription is promoted by the interaction of β-catenin with SREBP-1c bound to the “novel” SRE sites [40]. Scd2 is the predominant isoform expressed in HSCs while Scd1 is a major form expressed in hepatocytes. Scd2 expression is induced in activation of HSCs in culture and in rodent models of liver fibrosis. Ablation of the Scd2 gene or pharmacologic inhibition of SCD, inhibits HSC activation in culture and attenuates liver fibrosis in mouse models [36]. Interestingly, Scd2 is upregulated in TICs as in embryonic hepatoblasts, and Scd2 knockdown suppresses self-renewal, stemness gene expression, and tumor-initiating activity of TICs. Thus, SCD appears to serve to promote both liver fibrosis and cancer. Indeed, SCD is overexpressed in aHSCs in liver fibrosis and hepatocellular carcinoma (HCC) cells in patients, and SCD expression correlates with the clinical outcome of HCC.
Then, how does Wnt- β-catenin activate HSCs and cancer cells via SCD? Higher expressions of Wnt5a and Fz2 are observed in aHSCs than in quiescent HSCs [41]. Wnt5a is overexpressed in fibrotic liver, and the activation of HSCs is suppressed by reducing it [42, 43]. In addition, the activation of canonical Wnt pathway by SCD increases the expression of Wnt5a, activates HSCs, and promotes fibrogenesis. Our finding reveals that MUFAs, which are produced by Wnt-dependent SCD, play an important role in establishing the positive forward loop by suppressing HuR translocation to nucleus via TNPO1-Ran1 and promoting HuR-mediated stabilization of Lrp6 mRNA leading to the expression of LRP5/6 protein. In fact, SCD activity is required for the expression of LRP5/6, which facilitates Wnt-induced Dvl phosphorylation, GSK3β phospho-inhibition, and stabilization of β-catenin. This MUFA-regulation of HuR has further implications as it may stabilize many other genes involved in liver fibrogenesis and tumorigenesis, which are targets of both HuR and β-catenin. Unsurprisingly, this positive loop is also evident in TICs and HCC cell lines. Further, the disruption of the loop by Scd2 conditional knockout in aHSCs, not only suppresses liver fibrosis but also largely abrogates the development of liver tumor induced by diethyl nitrosamine and promoted by feeding alcohol-containing Western diet [36]. This finding implies that activation of HSCs driven by the Wnt-Scd-Lrp5/6 positive loop, participates in either tumor initiation or promotion possibly by disseminating the oncogenic positive loop to tumor microenvironment.
5. Multifaceted tumor promotion mechanisms of SCD
SCD promotes the stemness of TICs commonly called cancer stem cells. TICs are capable of self-renewal and initiating tumor development in vivo. They have the resistance to common cancer therapies and cause the recurrence and metastasis after chemotherapy. SCD1 induces aldehyde dehydrogenase (ALDH), which is one of TIC markers. Lipogenesis is also important in TICs. For example, inhibition of FAS suppresses the growth of TICs in breast cancer [44]. PPARγ pathway may upregulate the genes involved in lipogenesis, energy metabolism, proliferation and tumor progression and maintains TICs by promoting de novo lipogenesis via the activation of SCD [45, 46]. Abundance of intracellular lipid droplets is a distinct marker of TICs in colorectal cancer and also correlates with Wnt pathway activity [47].
In TICs of ovarian cancer, another positive forward loop was identified [48]. Unsaturated FAs made by de novo FA synthesis activate NF-κB, which in turn transcriptionally upregulates SCD1. As a result, the concentration of MUFAs increases, leading to further activation of NF-κB. The suppression of SCD1 decreases the stemness of the cancer by downregulation of NF-κB activity.
SCD also plays a causal role in metastasis, which is driven by epithelial to mesenchymal transition (EMT) of cancer cells. Cancer cells weaken the adhesion between the cells and degrade basal membrane matrix components by releasing matrix-degrading enzymes. Epithelial cancer cells for the most part, maintain the adhesion between the cells via E-cadherin, but the cells at the invasive and growing front, lose E-cadherin expression and gain the mesenchymal characteristics with a shift to mesenchymal gene expression induced by transcription factors such as TWIST, SNAIL, TCF-4 [49, 50]. Circulating tumor cells, which have the potential to colonize distant organs by invading vessels, express both epithelial and mesenchymal markers simultaneously and exhibit EMT activity [51]. SCD and EMT markers are commonly co-expressed and SCD suppresses the expression of E-cadherin and promotes that of vimentin, the mesenchymal marker, suggesting that SCD promotes EMT [52]. However, the precise molecular mechanism of SCD-mediated EMT promotion is still elusive.
Kinases in the tumor suppressor Hippo pathway (MIST1, LATS1/2), are frequently inactivated in cancers, suppressing phosphorylation of Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) and promoting their stabilization and nuclear accumulation leading to cancer cell growth [53]. The Hippo kinase inhibition may be caused by increase generation of geranylgeranyl diphosphate (GGPP), the source of protein prenylation, which activates Rho GTPase and consequently inhibits LATS1/2 [54, 55]. These lipid metabolites are produced via lanosterol/cholesterol synthetic pathway, which is often activated in cancer. They promote the tumorigenesis by activating YAP/TAZ, reprograming cancer cells into TICs, and promoting tumor initiation, development and metastasis [53]. SCD is often co-upregulated with β-catenin, YAP/TAZ, and their target genes such as BIRC5 and CTGF in lung cancer, suggesting the potential link of the β-catenin-SCD1 loop to inhibition of the Hippo pathway [54].
SCD residing in ER, has an intimate relationship with ER stress. SCD1 deficiency in hepatocytes induces hepatic ER stress and inflammation in mice fed lipogenic high-carbohydrate diet, and these phenotypes are rescued by oleate supplementation, suggesting the protective role of SCD and SCD-derived MUFAs in this model [56]. In contrast, SCD1 upregulated by PPAR-γ agonist attenuates ER stress induced by palmitic acid [57]. In glioblastoma, ER sensor IRE1 increases the expression of SCD by activating SREBP-1 and promotes the cell survival under ER stress, suggesting the role of SCD in tumorigenesis via this protective mechanism [58].
6. Potential role of SCD in Wnt-YAP interactions in cancer.
SCD has emerged as a tumor promoter in recent years, and several plausible mechanisms have been proposed for its tumor promoting actions as summarized in Table 1. As a mechanism related to canonical Wnt pathway, increased SCD1-dependent MUFA generation causes stabilization of LRP5/6 and inhibition of the β-catenin degradation complex, leading to the nuclear translocation of β-catenin and tumor promotion [52]. SCD, which amplifies Wnt- β-catenin pathway via this positive loop, may also promote YAP/TAZ activation via inhibition of the Hippo pathway [54], which is known to inhibit canonical Wnt pathway. In fact, there are several layers of cross-regulation between Wnt and YAP/TAZ as depicted in a summarized figure (Figure 1). TRIB2, a target of β-catenin, causes YAP stabilization and activation by interacting with βTrCP and preventing it from binding to phosphorylated ‘degron’ motif for ubiquitination and by relieving C/EBPα-mediated inhibition of YAP transcriptional activation [59]. There is also an alternative Wnt-YAP/TAZ signaling axis, in which the Wnt binding to Fz/ROR activates Gα12/13 and Rho GTPase, leading to LATS1/2 inhibition and YAP activation [60]. As SCD provides the Wnt-β-catenin amplifying loop, it may potentially upregulate these Wnt-YAP/TAZ interactions and promote cancers. However, it should be noted that an antagonism also exists between β-catenin and YAP. Dvl mediates the post-receptor Wnt signaling by releasing β-catenin from the degradation complex. Interestingly, Dvl is also involved in cytosolic translocation of phosphorylated YAP in the manner dependent on the p53-LATS2 and LKB1-AMPK tumor suppressor axis [61]. Then, cytoplasmic p-YAP/TAZ binds β-catenin and prevents its nuclear translocation [62]. How SCD interfaces with this regulation in tumorigenesis is yet to be investigated.
Table 1:
Summary of potential tumor-promoting mechanisms of SCD
| Effector/Pathway | Potential Mechanisms |
|---|---|
| Wnt | MUFA stabilizes LRP5/6 via HuR [36] |
| YAP/TAZ | Activates YAP/TAZ by inhibition of Hippo pathway through Rho activation [55] |
| AKT | Activates AKT via phosphatidylinositol generation, inhibits GSK3β and stabilize β-catenin [28, 29] |
| AMPK | Inhibits AMPK and upregulates lipogenesis via ACC induction [32] |
| NF-κB | MUFA activates NF-κB which transcriptionally upregulates SCD [48] |
| EMT | Suppresses E-cadherin and induces vimentin [52] |
| Metabolic reprogramming | Links glycolysis to lipogenesis |
| ER stress | Provides cytoprotection against palmitic acid-induced ER stress [57] |
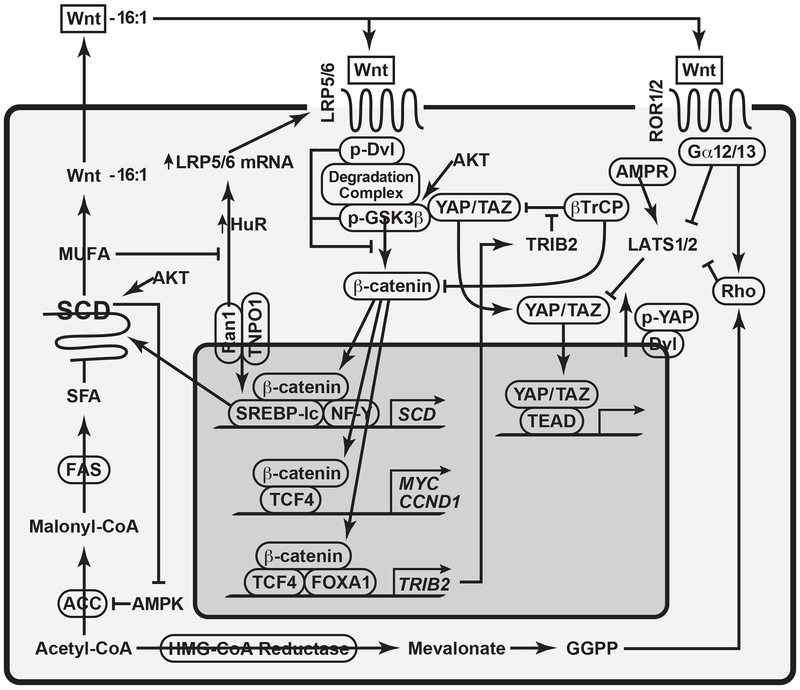
Figure 1:
This figure depicts how SCD establishes tumor-promoting pathways. SCD produces MUFAs such as palmitoleate (16:1) which is used to lipidate Wnt proteins via Porcupine acyltransferase for their extracellular release. Scd1/2 is a Wnt-target gene and its transcription is amplified by association of β-catenin with SREBP-1 and NF-Y bound to the respective elements in the Scd1/2 proximal promoter. MUFAs produced by SCD inhibit HuR nuclear translocation via impairment of TNPO1-Ran1 association. Increased HuR in cytosol stabilizes Lrp5/6 mRNA via its binging to their AU-rich elements. LRP 5/6 are functional co-receptors of Wnt pathway and their expression, optimal Wnt pathway, and β-catenin/TCF-dependent gene transcription are dependent on SCD activity. Wnt-YAP positive crosstalk may be mediated by Wnt binding to Fz and ROR 1/2, the G-protein-coupled receptor, activating the catalytic Gα12/13 subunit, which inhibits LATS 1/2 via activation of Rho GTPase, causing stabilization and nuclear translocation of YAP/TAZ. Rho is also activated by geranylgeranyl diphosphate (GGPP), lipid metabolites in lanosterol-cholesterol synthetic pathway via prenylation of Rho. Wnt-YAP positive crosstalk is also mediated by TRIB2, a Wnt target gene which activates YAP by binding βTrCP and interfering YAP degradation. Wnt-YAP reciprocal regulation may also take place. Dvl is the key adaptor protein for Wnt pathway, phosphorylation of Dvl attracts the β-catenin destruction complex and causes inhibitory phosphorylation of GSK3β for stabilization of β-catenin. Dvl with the nuclear export signal, causes p-YAP cytosolic translocation and localization, which are stimulated by the tumor suppressor p53-LATS2 and LKB1-AMPK axes. Thus, SCD, which establishes and amplifies Wnt pathway activation loop, likely establishes crosstalk with these interactive pathways with YAP to promote tumor development and these crosstalk interfaces may serve as potential therapeutic targets.
7. Future therapeutic prospect for targeting SCD-driven tumor promotion
MUFAs supplied by overexpressed SCD appear to render metabolic reprogramming, which is not only tumor-promoting but also essential for chemoresistance and tumor recurrence caused by TICs. The increased concentrations of MUFAs are evident in TICs of pancreatic cancer, leukemia and ovarian cancer [48], suggesting that FA desaturation ratio may also be considered as a novel TIC marker and those with high desaturation ratio may therapeutically benefit most from SCD inhibition. In addition to this prognostic value, SCD-mediated tumor promotion involving β-catenin and YAP, may be considered as plausible therapeutic targets. Approaching this potential prospect, however, requires careful consideration of spatial-temporal issues. For instance, SCD activity in normal cells is essential for their cellular homeostasis and its inhibition may promote excessive ER stress, inflammation, and consequently tumorigenesis as in the case of SCD-1-deficient intestinal epithelial cells [63]. However, once TICs or tumor cells arise, SCD inhibition may be specifically targeted to these cells to abrogate its tumor promoting and cytoprotective effects. Recently, sapienate biosynthesis catalyzed by delta-6 desaturase of palmitate, has been identified as another tumor-promoting FA desaturation pathway, which allows cancer cells to by-pass SCD [64]. This may explain some cancers are not responsive to SCD inhibition and raises a potential prospect for a dual inhibition approach for both desaturation pathways.
Highlights.
Stearoyl-CoA desaturase, which produces monounsaturated fatty acids, is increasingly recognized to promote malignancies of different organs and this review article discusses potential mechanisms underlying this link.
Acknowledgements
The manuscript preparation and our own studies described in this review article were supported by NIH grants (P50AA011999, R24AA012885, U01AA018663, U01AA027681) and the Department of Veterans Affairs (I01BX001991 and IK6BX004205). Kohtaro Kikuchi’s research fellowship as a visiting scholar from the Tokyo Medical and Dental University, was supported in part by the Japan Student Services Association. The authors thank Felicia Owusu, MPA for her support for the manuscript preparation and submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.AM AL, Syed DN, and Ntambi JM, Insights into Stearoyl-CoA Desaturase-1 Regulation of Systemic Metabolism. Trends Endocrinol Metab, 2017. 28(12): p. 831–842. 10.1016/j.tem.2017.10.003. 29089222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyazaki M, Kim YC, and Ntambi JM, A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res, 2001. 42(7): p. 1018–24. 11441127. [PubMed] [Google Scholar]
- 3.Miyazaki M, et al. , Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem, 2004. 279(24): p. 25164–71. 10.1074/jbc.M402781200. 15066988. [DOI] [PubMed] [Google Scholar]
- 4.Mitro N, et al. , The nuclear receptor LXR is a glucose sensor. Nature, 2007. 445(7124): p. 219–23. 10.1038/nature05449. 17187055. [DOI] [PubMed] [Google Scholar]
- 5.Iizuka K, et al. , Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A, 2004. 101(19): p. 7281–6. 10.1073/pnas.0401516101. 15118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J, et al. , CNS melanocortin and leptin effects on stearoyl-CoA desaturase-1 and resistin expression. Biochem Biophys Res Commun, 2003. 311(2): p. 324–8. 10.1016/j.bbrc.2003.10.004. 14592417. [DOI] [PubMed] [Google Scholar]
- 7.Biddinger SB, et al. , Leptin suppresses stearoyl-CoA desaturase 1 by mechanisms independent of insulin and sterol regulatory element-binding protein-1c. Diabetes, 2006. 55(7): p. 2032–41. 10.2337/db05-0742. 16804073. [DOI] [PubMed] [Google Scholar]
- 8.Mitro N, et al. , T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett, 2007. 581(9): p. 1721–6. 10.1016/j.febslet.2007.03.047. 17418145. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, et al. , A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem, 2006. 281(21): p. 15013–20. 10.1074/jbc.M511116200. 16556603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgel P, et al. , A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun, 2005. 73(8): p. 4512–21. 10.1128/IAI.73.8.4512-4521.2005. 16040962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyama T, et al. , Stearoyl-CoA desaturase activity is elevated by the suppression of its degradation by clofibric acid in the liver of rats. J Pharmacol Sci, 2007. 103(4): p. 383–90. 17409633. [DOI] [PubMed] [Google Scholar]
- 12.Miller CW and Ntambi JM, Peroxisome proliferators induce mouse liver stearoyl-CoA desaturase 1 gene expression. Proc Natl Acad Sci U S A, 1996. 93(18): p. 9443–8. 10.1073/pnas.93.18.9443. 8790349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato H, Sakaki K, and Mihara K, Ubiquitin-proteasome-dependent degradation of mammalian ER stearoyl-CoA desaturase. J Cell Sci, 2006. 119(Pt 11): p. 2342–53. 10.1242/jcs.02951. 16723740. [DOI] [PubMed] [Google Scholar]
- 14.Heinemann FS, Korza G, and Ozols J, A plasminogen-like protein selectively degrades stearoyl-CoA desaturase in liver microsomes. J Biol Chem, 2003. 278(44): p. 42966–75. 10.1074/jbc.M306240200. 12928439. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Yang Y, and Shi Y, Characterization of human SCD2, an oligomeric desaturase with improved stability and enzyme activity by cross-linking in intact cells. Biochem J, 2005. 388(Pt 1): p. 135–42. 10.1042/BJ20041554. 15610069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampath H, et al. , Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem, 2009. 284(30): p. 19961–73. 10.1074/jbc.M109.014225. 19429677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ntambi JM, et al. , Loss of stearoyl-CoA desaturase-1 function protects mice agains adiposity Proc Natl Acad Sci U S A 200299(17): p. 11482–6. 10.1073/pnas.132384699. 12177411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman SM, et al. , Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci U S A, 2003. 100(19): p. 11110–5. 10.1073/pnas.1934571100. 12960377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobrzyn P, et al. , Loss of stearoyl-CoA desaturase 1 inhibits fatty acid oxidation and increases glucose utilization in the heart. Am J Physiol Endocrinol Metab, 2008. 294(2): p. E357–64. 10.1152/ajpendo.00471.2007. 18042664. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki M, Gomez FE, and Ntambi JM, Lack of stearoyl-CoA desaturase-1 function induces a palmitoyl-CoA Delta6 desaturase and represses the stearoyl-CoA desaturase-3 gene in the preputial glands of the mouse. J Lipid Res, 2002. 43(12): p. 2146–54. 10.1194/jlr.m200271-jlr200. 12454277. [DOI] [PubMed] [Google Scholar]
- 21.Presler M, et al. , Increased expression of the gene encoding stearoyl-CoA desaturase 1 in human bladder cancer. Mol Cell Biochem, 2018. 447(1-2): p. 217–224. 10.1007/s11010-018-3306-z. 29396722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mar-Heyming R, et al. , Association of stearoyl-CoA desaturase 1 activity with familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol, 2008. 28(6): p. 1193–9. 10.1161/ATVBAHA.107.160150. 18340007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scaglia N, Chisholm JW, and Igal RA, Inhibition of stearoylCoA desaturase-1 inactivates acetyl-CoA carboxylase and impairs proliferation in cancer cells: role of AMPK. PLoS One, 2009. 4(8): p. e6812 10.1371/journal.pone.0006812. 19710915.2728543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggieri S, Roblin R, and Black PH, Lipids of whole cells and plasma membrane fractions from Balb/c3T3, SV3T3, and concanavalin A-selected revertant cells. J Lipid Res, 1979. 20(6): p. 760–71. 226641. [PubMed] [Google Scholar]
- 25.Bougnoux P, et al. , Prognostic significance of tumor phosphatidylcholine stearic acid level in breast carcinoma. Breast Cancer Res Treat, 1992. 20(3): p. 185–94. 1571571. [DOI] [PubMed] [Google Scholar]
- 26.Horie Y, et al. , Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest, 2004. 113(12): p. 1774–83. 10.1172/JCI20513. 15199412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scaglia N and Igal RA, Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells. J Biol Chem, 2005. 280(27): p. 25339–49. 10.1074/jbc.M501159200. 15851470. [DOI] [PubMed] [Google Scholar]
- 28.Fritz V, et al. , Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther, 2010. 9(6): p. 1740–54. 10.1158/1535-7163.MCT-09-1064. 20530718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doble BW and Woodgett JR, GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci, 2003. 116(Pt 7): p. 1175–86. 10.1242/jcs.00384. 12615961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diehl JA, et al. , Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev, 1998. 12(22): p. 3499–511. 10.1101/gad.12.22.3499. 9832503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yost C, et al. , The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev, 1996. 10(12): p. 1443–54. 10.1101/gad.10.12.1443. 8666229. [DOI] [PubMed] [Google Scholar]
- 32.Hawley SA, et al. , Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem, 1996. 271(44): p. 27879–87. 10.1074/jbc.271.44.27879. 8910387. [DOI] [PubMed] [Google Scholar]
- 33.Monga SP, beta-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology, 2015. 148(7): p. 1294–310. 10.1053/j.gastro.2015.02.056. 25747274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson AI, Conroy KP, and Henderson NC, Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol, 2015. 15: p. 63 10.1186/s12876-015-0291-5. 26013123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coulouarn C, Factor VM, and Thorgeirsson SS, Transforming growth factor-beta gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology, 2008. 47(6): p. 2059–67. 10.1002/hep.22283. 18506891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai KKY, et al. , Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology, 2017. 152(6): p. 1477–1491. 10.1053/j.gastro.2017.01.021. 28143772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boudin E, et al. , The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin Arthritis Rheum, 2013. 43(2): p. 220–40. 10.1016/j.semarthrit.2013.01.004. 23433961. [DOI] [PubMed] [Google Scholar]
- 38.Emami KH, et al. , A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci U S A, 2004. 101(34): p. 12682–7. 10.1073/pnas.0404875101. 15314234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujii N, et al. , An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res, 2007. 67(2): p. 573–579. 67/2/573 [pii]; 10.1158/0008-5472.CAN-06-2726 [doi]. [DOI] [PubMed] [Google Scholar]
- 40.Tabor DE, et al. , Identification of conserved cis-elements and transcription factors required for sterol-regulated transcription of stearoyl-CoA desaturase 1 and 2. J Biol Chem, 1999. 274(29): p. 20603–10. 10400691 [DOI] [PubMed] [Google Scholar]
- 41.Jiang F, Parsons CJ, and Stefanovic B, Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J.Hepatol, 2006. 45(3): p. 401–409. [DOI] [PubMed] [Google Scholar]
- 42.Rashid ST, et al. , Proteomic analysis of extracellular matrix from the hepatic stellate cell line LX-2 identifies CYR61 and Wnt-5a as novel constituents of fibrotic liver. J Proteome Res, 201211(8): p. 4052–64. 10.1021/pr3000927. 22694338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong WJ, et al. , Wnt5a participates in hepatic stellate cell activation observed by gene expression profile and functional assays. World J Gastroenterol, 2012. 18(15): p. 1745–52. 10.3748/wjg.v18.i15.1745. 22553398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandey PR, et al. , Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat, 2011. 130(2): p. 387–98. 10.1007/s10549-010-1300-6. 21188630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, et al. , PPARgamma maintains ERBB2-positive breast cancer stem cells. Oncogene, 2013. 32(49): p. 5512–21. 10.1038/onc.2013.217. 23770845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi HB, et al. , Peroxisome proliferator-activated receptor-gamma stimulates the synthesis of monounsaturated fatty acids in dairy goat mammary epithelial cells via the control of stearoyl-coenzyme A desaturase. J Dairy Sci, 2013. 96(12): p. 7844–53. 10.3168/jds.2013-7105. 24119817. [DOI] [PubMed] [Google Scholar]
- 47.Tirinato L, et al. , Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells, 2015. 33(1): p. 35–44. 10.1002/stem.1837. 25186497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, et al. , Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell, 2017. 20(3): p. 303–314 e5 10.1016/j.stem.2016.11.004. 28041894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye X, et al. , Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature, 2015. 525(7568): p. 256–60. 10.1038/nature14897. 26331542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puram SV, et al. , Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell, 2017. 171(7): p. 1611–1624 e24 10.1016/j.cell.2017.10.044. 29198524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu M, et al. , Circulating breast tumor cells exhibit dynamic changes in epithelia and mesenchymal composition. Science, 2013. 339(6119): p. 580–4. 10.1126/science.1228522. 23372014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ran H, et al. , Stearoyl-CoA desaturase-1 promotes colorectal cancer metastasis in response to glucose by suppressing PTEN. J Exp Clin Cancer Res, 2018. 37(1): p. 54 10.1186/s13046-018-0711-9. 29530061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccolo S, Dupont S, and Cordenonsi M, The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev, 2014. 94(4): p. 1287–312. 10.1152/physrev.00005.2014. 25287865. [DOI] [PubMed] [Google Scholar]
- 54.Zhang K, et al. , YAP and TAZ Take Center Stage in Cancer. Biochemistry, 2015. 54(43): p. 6555–66. 10.1021/acs.biochem.5b01014. 26465056. [DOI] [PubMed] [Google Scholar]
- 55.Noto A, et al. , Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene, 2017. 36(32): p. 4573–4584. 10.1038/onc.2017.75. 28368399. [DOI] [PubMed] [Google Scholar]
- 56.Liu X, et al. , Hepatic oleate regulates liver stress response partially through PGC-1alpha during high-carbohydrate feeding. J Hepatol, 2016. 65(1): p. 103–112. 10.1016/j.jhep.2016.03.001. 26976120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikeda J, et al. , PPARgamma Agonists Attenuate Palmitate-Induced ER Stress through Up-Regulation of SCD-1 in Macrophages. PLoS One, 2015. 10(6): p. e0128546 10.1371/journal.pone.0128546. 26061913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinkham K, et al. , Stearoyl CoA Desaturase Is Essential for Regulation of Endoplasmic Reticulum Homeostasis and Tumor Growth in Glioblastoma Cancer Stem Cells. Stem Cell Reports, 2019. 12(4): p. 712–727. 10.1016/j.stemcr.2019.02.012. 30930246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, et al. , TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPalpha function. Mol Cell, 2013. 51(2): p. 211–25. 10.1016/j.molcel.2013.05.013. 23769673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park HW, et al. , Alternative Wnt Signaling Activates YAP/TAZ. Cell, 2015. 162(4): p. 780–94. 10.1016/j.cell.2015.07.013. 26276632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee Y, et al. , Dishevelled has a YAP nuclear export function in a tumor suppressor context-dependent manner. Nat Commun, 2018. 9(1): p. 2301 10.1038/s41467-018-04757-w. 29895829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imajo M, et al. , A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J, 2012. 31(5): p. 1109–22. 10.1038/emboj.2011.487. 22234184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ducheix S, et al. , Deletion of Stearoyl-CoA Desaturase-1 From the Intestinal Epithelium Promotes Inflammation and Tumorigenesis, Reversed by Dietary Oleate. Gastroenterology, 2018. 155(5): p. 1524–1538 e9 10.1053/j.gastro.2018.07.032. 30063922. [DOI] [PubMed] [Google Scholar]
- 64.Vriens K, et al. , Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature, 2019. 566(7744): p. 403–406. 10.1038/s41586-019-0904-1. 30728499. [DOI] [PMC free article] [PubMed] [Google Scholar]