Abstract
Both microwave (MW) ablation and radiofrequency (RF) ablation are widely used for hepatocellular carcinoma (HCC) treatments in clinic. However, it is still unclear if ablative methods could influence the recurrence-free survival (RFS) and overall survival (OS) of HCC patients. Therefore, we carried out this multi-center retrospective cohort study to investigate the differences of recurrence-free survival (RFS) and overall survival (OS) between MW ablation and RF ablation by survival analysis. From January 2014 to December 2016, patients who received thermal ablation surgery for HCC treatment were screened. Finally, 452 patients met the eligibility criteria and finished the follow-up. Univariable and multivariable regression analyses were used to identify independent predictive factors of the RFS and OS. Also, propensity score matching (PSM) was used to balance the bias between two groups. Finally, we found that before the PSM, the univariable and multivariable regression analyses revealed that there were no significant differences on the RFS between two groups. Same results were obtained for the OS. After PSM, 115 pairs of patients were created, and both the univariable and multivariable regression analyses suggested that there were still no significant differences on the RFS between two groups. Same results were obtained for the OS. In conclusion, our present study showed that there were no significant differences between MW ablation and RF ablation for HCC patients on the RFS or OS.
Introduction and background
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor and the third leading cause of cancer-related death worldwide [1,2]. Especially in Southeast Asia and Africa, the incidence rate and mortality rate of HCC are significantly higher than other areas [3]. In China, HCC is the third incidence rate and mortality rate for all cancers [4]. Though, in recent decades, it showed appreciable declines in rates of HCC in China, the mortality was still two- to five-fold higher than in most European countries and the Americas [5,6]. Related statistic data showed that China accounted for more than 50% of the deaths from liver cancer worldwide [7]. So, HCC and HCC treatments are still pressing problems in China.
Till now, diverse treatments have been applicated in overcoming HCC [8,9]. For example, hepatic resection, microwave ablation, radiofrequency ablation, biotherapy and transcatheter arterial chemoembolization. Surgical resection is currently the primary treatment for HCC. However, with the development of percutaneous ablation surgery, microwave (MW) ablation and radiofrequency (RF) ablation for curing HCC have been widely used in clinic. Compared with hepatic resection, MW ablation and RF ablation have some advantages such as speediness, lower injury, fast in recovery [10]. More important, MW ablation and RF ablation are more suitable for patients who had severe liver cirrhosis or locations of tumors were close to vessels.
Numerous studies have compared the efficacy and safety between MW ablation and RF ablation [11–15]. Some studies suggested that MW ablation was better than RF ablation and it seemed to lead a better prognosis for MW ablation. For example, a prospective study involved 111 patients who received MW ablation or RF ablation suggested that a lower incidence of local recurrence was observed in microwave group [11]. However, other studies revealed that there was no difference between MW ablation and RF ablation. A meta-analysis which involved 2062 patients showed that MW ablation and RF ablation had similar 1–5-year overall survival, disease-free survival, local recurrence rate, and adverse events [12]. It is currently unclear and lack of rigorous proof to recommend one ablative method. Therefore, we carried out this multi-center retrospective cohort study to investigate the differences of recurrence-free survival (RFS) and overall survival (OS) between MW ablation and RF ablation by survival analysis. We hypothesize that there are no significant differences between MW ablation and RF ablation for HCC patients’ prognosis.
Patients and methods
Patients selection
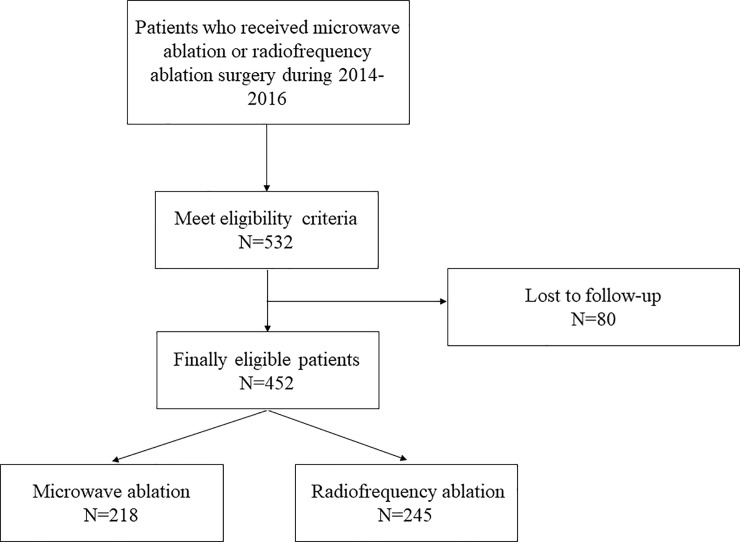
Patients who underwent MW ablation or RF ablation for HCC in Shenzhen University General Hospital, Hanzhong Central Hospital and The Second Affiliated Hospital of Kunming Medical University from January 2014 to December 2016 were screened. Finally, 532 patients accorded with the eligibility criteria and were enrolled into the study (Fig 1). This study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of the Shenzhen University General Hospital, and received approval from other two hospitals (the Ethics Committee of Hanzhong Central Hospital and the Ethics Committee of The Second Affiliated Hospital of Kunming Medical University). Also, a waiver of written consent was approved by the Clinical Research Ethics Committee of the Shenzhen University General Hospital.
Fig 1. Flow chart showing how the cohort including 532 patients was generated for analysis in this study.
Inclusion and exclusion criteria
Eligible patients who met the inclusion and exclusion criteria would be enrolled, and related data would be collected by trained researchers (Table 1). The diagnoses of HCC were confirmed by postoperative histopathology. Severe organ failure mainly includes liver failure (Child-Pugh C degree), heart failure (NYHA Ⅲ-Ⅳ), and renal failure (serum creatinine>442 μmol/L). Severe immune system disease mainly includes immunodeficiency disease, systemic lupus erythematosus, rheumatoid arthritis and ankylosing spondylitis.
Table 1. Study eligibility criteria.
| Inclusion criteria • Age: 18–75 years • Race: all racial groups • American Society of Anesthesiologists degree: Ⅰ-Ⅲ • Primary liver cancer • Child-Pugh degree: A or B • No macrovascular vessels were invaded by tumor • No distal lymph node or extrahepatic metastasis • Patients who received microwave ablation or radiofrequency ablation surgery as the first choice after liver cancer was found Exclusion criteria • A history of liver surgery • A combined surgery of liver resection surgery and microwave ablation / radiofrequency ablation surgery • Had severe organ failure or immune system disease • The sum of the long diameter of individual hepatocellular carcinoma or multiple hepatocellular carcinoma> 5 cm • Metastatic liver cancer • Had severe postoperative complications: massive haemorrhage (>200 ml), infection, diaphragmatic injury and biliary tract injury. • A history of any kinds of cancers or currently associated with any kinds of cancers |
Follow-up
All patients were reexamined using serum alpha fetoprotein (AFP), ultrasound or CT, and chest X-ray at 1 month after surgery. Then, patients were followed-up at a 2-monthly interval for the first 6 months and at a 3-monthly interval thereafter. Tumor recurrence was defined as new appearance of intra- or extrahepatic tumor nodule. The clinical practice guidelines of EASL-EORTC was used for the diagnosis of tumor recurrence [16]. Patients with tumor recurrence were actively treated with percutaneous ablative, hepatic resection, transcatheter arterial chemoembolization (TACE), radiotherapy or conservative treatment.
The follow-up began from January, 2014 and ended at March, 2018. All data used for analysis was collected from digital medical history or paper medical records, and all data were fully anonymized before access. In every center, two trained researchers were in charge of follow-up and all data were entered using “Epidata”.
Variables and outcomes
In this study, 22 variables were collected and analyzed. All variables could be divided as patient characteristics (age, sex, ASA score, hypertension, diabetes and cardiopathy), liver function variables (cirrhosis, HBV/HCV infection, child-pugh stage, AFP, TBiL, ALB, ALT, and AST), operative variables (tumor number, tumor size, anesthetic methods and adjuvant chemoradiotherapy) and follow-up information (dates of operation, inpatient days, dates of tumor recurrence and dates of death). All data were collected and entered with the same way as the follow-up data. In every center, two trained researchers were in charge of collecting data.
The main outcome of this study was RFS and the second outcome was OS. The RFS was defined from the date of the surgery to the date of first recurrence. If the recurrence of tumor was not recorded, the RFS was defined as the time between the date of surgery and the date of last follow-up. The OS was calculated from the date of the surgery to either the date of death or the date of the last follow-up visit.
Propensity score matching
Because patients were not randomly allocated to the MW ablation group and RF ablation group, and variables in two groups were imbalanced. We decided to use the propensity score matching as described before [17] to eliminate the imbalance between two groups. This method consisted of ordering the case and control subjects, then selecting the first case subject and finding the control subject with the closest propensity score [18]. A logistic regression model was built given the covariates of tumor number, cirrhosis, HBV/HCV infection, Child-Pugh stage, AFP, ALB, TBIL, anesthetic methods, tumor size, adjuvant chemoradiotherapy and hypertension, and the dependent variable of ablation methods. We applied 1:1 nearest neighbor matching without replacement to ensure that conditional bias was minimized. For each patient having MW ablation, a patient having RF ablation with a minimum in distance of propensity scores was matched. The caliper width was 0.05 for propensity score matching. Propensity score matching was carried out using IBM SPSS Statistics 23.0 version.
Statistical analysis
Statistical analyses were carried out using the IBM SPSS Statistics 23.0 (SPSS Inc., Armonk, NY, USA). Categorical variables were reported as number (n) or proportion (%) and continuous variables were expressed as mean ± standard deviation (SD) or median (range). The Student’s t test was used for comparisons of continuous variables. Otherwise, the Mann-Whitney U test was applied. Categorical variables were compared with the χ2 test with the Yates correction or the Fisher’s exact test, as appropriate. To identify independent predictive factors of prognosis, univariable and multivariable regression analyses were used. The RFS and OS rates were compared between the MW ablation and RF ablation groups before and after propensity matching using the Kaplan-Meier regression analyses or univariable Cox regression analyses. Multivariable Cox proportional hazard regression analyses were then performed to adjust for other prognostic factors which were associated with OS and RFS [19]. All statistical tests were 2 sided, and P values <0.05 were considered statistically significant.
Result
532 patients who underwent MW or RF ablation met the eligibility criteria, and finally 452 patients finished follow-up. Patients were divided into two groups: the MW ablation group (N = 218, 48.2%), and the RF ablation group (N = 234, 51.8%). The comparisons of patients’ characteristics and other variables between two groups in the entire cohort are illustrated in Table 2. Patients’ characteristics including Hypertension, Tumor size and Anesthetic methods are significantly different between two groups (P<0.05). The follow-up time was at a range of 1.25- to 4.25-year, and the average follow-up time was 2.34-year.
Table 2. Comparisons of patients’ characteristics and other variables between MW ablation group and RF ablation group in the entire cohort.
| Variables | Before PSM* | After PSM | ||||
|---|---|---|---|---|---|---|
| MW Group | RF Group | P value | MW Group | RF Group | P value | |
| PS score | 0.29 (0.22) | 0.43 (0.21) | 0.000 | 0.46 (0.21) | 0.48 (0.21) | 0.641 |
| Sex | ||||||
| male | 173 (79.4%) | 192 (82.1%) | 0.468 | 92 (80.0%) | 88 (76.5%) | 0.525 |
| female | 45 (20.6%) | 42 (17.9%) | 23 (20.0%) | 27 (23.5%) | ||
| Age | 56.4±10.3 | 57.3±9.3 | 0.323 | 56.3±10.0 | 57.5±9.4 | 0.315 |
| ≤60 | 137 (62.8%) | 143 (61.1%) | 0.705 | 74 (64.3%) | 69 (60.0%) | 0.499 |
| >60 | 81 (37.2%) | 91 (38.9%) | 41 (35.7%) | 46 (40.0%) | ||
| ASA score* | ||||||
| Ⅱ | 169 (77.5%) | 195 (83.3%) | 0.119 | 88 (76.5%) | 87 (75.7%) | 0.878 |
| Ⅲ | 49 (22.5%) | 39 (16.7%) | 27 (23.5%) | 28 (24.3%) | ||
| Hypertension (Yes/No) | 41/177 | 68/166 | 0.011 | 26/89 | 37/78 | 0.105 |
| Diabetes (Yes/No) | 31/187 | 31/203 | 0.198 | 17/98 | 19/96 | 0.718 |
| Cardiopathy** (Yes/No) | 5/213 | 14/220 | 0.051 | 2/113 | 7/108 | 0.171 |
| Tumor number | ||||||
| 1 | 177 (81.2%) | 202 (86.3%) | 0.333 | 96 (83.5%) | 95 (82.6%) | 0.983 |
| 2 | 37 (17.0%) | 29 (12.4%) | 17 (14.8%) | 18 (15.7%) | ||
| 3 | 4 (1.8%) | 3 (1.3%) | 2 (1.7%) | 2 (1.7) | ||
| Cirrhosis (Yes/No) | 176/42 | 185/49 | 0.657 | 85/30 | 87/28 | 0.763 |
| HBV/HCV* infection (Yes/No) | 190/28 | 213/32 | 0.945 | 98/17 | 97/18 | 0.855 |
| Adjuvant chemoradiotherapy*** | ||||||
| (Yes/No) | 28/190 | 90/144 | 0.000 | 19/96 | 26/89 | 0.247 |
| Child-Pugh stage | ||||||
| A | 200 (91.7%) | 216 (92.3%) | 0.825 | 107 (93.0%) | 105 (91.3%) | 0.625 |
| B | 18 (8.3%) | 18 (7.7%) | 8 (7.0%) | 10 (8.7%) | ||
| Tumor size | 2.9±1.2 | 2.4±1.0 | 0.000 | 2.4±1.1 | 2.6±1.1 | 0.465 |
| <3cm | 110 (50.5%) | 166 (70.9%) | 0.000 | 80 (69.6%) | 74 (64.3%) | 0.400 |
| ≥3cm | 108 (49.5%) | 68 (29.1%) | 35 (30.4%) | 41 (35.7%) | ||
| Anesthetic methods | ||||||
| General anesthesia | 56 (25.7%) | 155 (66.2%) | 0.000 | 31 (27.0%) | 40 (34.8%) | 0.201 |
| Local anesthesia | 162 (74.3%) | 79 (33.8%) | 84 (73.0%) | 75 (65.2%) | ||
| AFP* | ||||||
| <400ng/ml | 179 (82.1%) | 201 (85.9%) | 0.272 | 101 (87.8%) | 98 (85.2%) | 0.564 |
| ≥400ng/ml | 39 (17.9%) | 33 (14.1%) | 14 (12.2%) | 17 (14.8%) | ||
| TBIL* | ||||||
| <34mmol/L | 201 (92.2%) | 211 (90.2%) | 0.447 | 106 (92.2%) | 103 (89.6%) | 0.494 |
| ≥34mmol/L | 17 (7.8%) | 23 (9.8%) | 9 (7.8%) | 12 (10.4%) | ||
| ALB* | ||||||
| ≤35g/L | 32 (14.7%) | 36 (15.4%) | 0.834 | 11 (9.6%) | 14 (12.2%) | 0.527 |
| >35g/L | 186 (85.3%) | 198 (84.6%) | 104 (90.4%) | 101 (87.8%) | ||
| ALT* | ||||||
| <40U/L | 128 (58.7%) | 144 (61.5%) | 0.540 | 72 (62.6%) | 63 (54.8%) | 0.230 |
| ≥40U/L | 90 (41.3%) | 90 (38.5%) | 43 (37.4%) | 52 (45.2%) | ||
| AST* | ||||||
| <40U/L | 134 (61.5%) | 133 (56.8%) | 0.317 | 75 (65.2%) | 65 (50.4%) | 0.177 |
| ≥40U/L | 84 (38.5%) | 101 (43.2%) | 40 (34.8%) | 50 (49.6%) | ||
| Inpatient days# | ||||||
| ≤5 days | 213 (97.7%) | 222 (94.9%) | 0.113 | 113 (98.3%) | 107 (93.0%) | 0.102 |
| >5 days | 5 (2.3%) | 12 (5.1%) | 2 (1.7%) | 8 (7.0%) | ||
*ASA, American Society of Anesthesiologists; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha-fetoprotein; TBIL, total bilirubin; ALB, serum albumin; ALT, Alanine transaminase; AST, aspartate aminotransferase; PSM, propensity score matching.
** Cardiopathy illnesses include coronary heart disease, heart failure (NYHA Ⅰ-Ⅱ), arrhythmia, myocardiopathy and valvulopathy.
*** Adjuvant chemoradiotherapy includes transcatheter arterial chemoembolization (TACE) and radioactive seed implantation. Adjuvant chemoradiotherapy is defined as patients received TACE or radioactive seed implantation during the hospital admission for first treatment.
#Inpatient days is defined as the period from operation finished to hospital discharge.
In our retrospective study, all patients received ablation surgery by percutaneous approach. Data regarding the complete response, differentiation between local and distant recurrence, need for repeat ablations, postoperative complications and Edmandson grade were showed in Table 3. No statistical differences were observed between two groups.
Table 3. Data of the complete response, recurrence, repeat ablations, postoperative complications and Edmandson grade.
| MW Group | RF Group | P value | |
|---|---|---|---|
| Complete response | |||
| Yes | 193 (88.5%) | 200 (85.5%) | 0.334 |
| No | 25 (11.5%) | 34 (14.5%) | |
| Recurrence | |||
| Local | 106 (89.8%) | 120 (89.5%) | 0.942 |
| Distant | 12 (10.2%) | 14 (10.5%) | |
| Repeat ablations (Yes/No) | 91/27 | 111/23 | 0.256 |
| Postoperative complications | 0.618 | ||
| Fever | 7 | 10 | |
| Seroperitoneum | 1 | 2 | |
| Pain | 13 | 13 | |
| Skin burn | 3 | 1 | |
| Edmandson grade | 0.214 | ||
| Ⅰ | 28 | 32 | |
| Ⅱ | 122 | 147 | |
| Ⅲ | 65 | 50 | |
| Ⅳ | 3 | 5 |
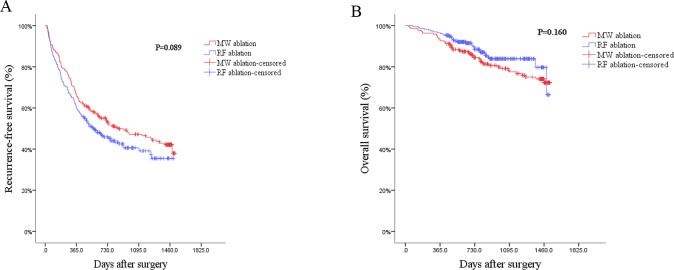
First, we used Kaplan-Meier analysis or univariable Cox regression model analysis to screen variables which had significant influence on the RFS and OS. From the Table 4 showed that ASA score, Hypertension, Tumor number, Cirrhosis, Adjuvant chemoradiotherapy, AFP, Tumor size and anesthetic methods would significantly influence patients’ RFS (P<0.05). However, we found that the Ablation methods had no observable influence on the RFS by log-rank test (Fig 2A, P = 0.089). Also, results suggested that Age, ALB, Child-Pugh stage, Tumor size and anesthetic methods could remarkably influence the OS. But the ablation methods had no significant influence on the OS by log-rank test, too (Fig 2B, P = 0.160).
Table 4. Results of univariable Cox regression model analysis on RFS and OS.
| Univariable Cox regression | HR (95% CI)* | P value |
|---|---|---|
| RFS* | ||
| ASA score* | 0.619 (0.463–0.826) | 0.001 |
| Hypertension | 0.704 (0.534–0.927) | 0.013 |
| Tumor number | 0.504 (0.389–0.652) | 0.000 |
| Cirrhosis | 0.680 (0.486–0.950) | 0.024 |
| Adjuvant chemoradiotherapy | 0.563 (0.430–0.736) | 0.000 |
| AFP* | 0.716 (0.519–0.989) | 0.043 |
| Tumor size | 0.532 (0.414–0.682) | 0.000 |
| Anesthesia methods | 0.640 (0.498–0.822) | 0.000 |
| Ablation methods | 1.241 (0.967–1.593) | 0.089 |
| OS* | ||
| Age | 0.617 (0.396–0.959) | 0.032 |
| ALB* | 0.465 (0.282–0.766) | 0.003 |
| Child-Pugh stage | 0.449 (0.237–0.850) | 0.014 |
| Tumor size | 0.445 (0.285–0.693) | 0.000 |
| Anesthesia methods | 0.632 (0.402–0.993) | 0.046 |
| Ablation methods | 0.723 (0.459–1.139) | 0.160 |
*RFS, recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiologists; AFP, alpha-fetoprotein; ALB, serum albumin.
Fig 2.
2A: Log-rank test showed on difference between the two groups in recurrence-free survival; 2B: Log-rank test showed on difference between the two groups in overall survival.
Significant variables (P<0.05) as shown in Table 4 were entered into multivariable Cox regression model analysis. As the Table 5 showed, the ablation methods had no significant influence on the RFS and OS (both P>0.05). It suggested that different ablation methods would not influence the RFS or OS for HCC patients.
Table 5. Multivariable Cox regression model analysis of RFS and OS.
| Independent predictive factor | HR (95% CI) | P value |
|---|---|---|
| RFS | ||
| ASA score | 0.722 (0.531–0.982) | 0.038 |
| Tumor size | 0.653 (0.491–0.870) | 0.004 |
| Adjuvant chemoradiotherapy | 0.694 (0.514–0.937) | 0.017 |
| Hypertension | 0.743 (0.555–0.996) | 0.047 |
| AFP | 0.720 (0.519–1.000) | 0.050 |
| Tumor number | 0.611 (0.464–0.809) | 0.000 |
| Ablation methods | 0.872 (0.638–1.192) | 0.390 |
| OS | ||
| Age | 0.603 (0.386–0.943) | 0.026 |
| Tumor size | 0.526 (0.328–0.841) | 0.007 |
| Ablation methods | 0.672 (0.400–1.129) | 0.133 |
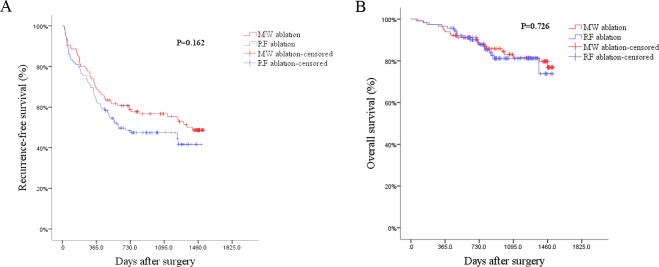
PSM analysis was carried out as illustrated above and finally created 115 pairs of patients. After the PSM, there no significant differences of variables and PS score between two groups (Table 2). Comparisons of patients’ RFS and OS between two groups after PSM were shown in Table 6. It suggested again that different ablation methods would not influence the RFS (Fig 3A, P = 0.162) or OS of HCC patients (Fig 3B, P = 0.726). Results in multivariable Cox regression model analysis showed that there were no differences on the RFS and OS after PSM (Table 7).
Table 6. Results of univariable Cox regression model analysis on RFS and OS after PSM.
| Univariable Cox regression | HR (95%CI) | P value |
|---|---|---|
| RFS | ||
| ASA | 0.578 (0.390–0.857) | 0.006 |
| Hypertension | 0.651 (0.441–0.960) | 0.030 |
| Diabetes | 0.518 (0.332–0.808) | 0.004 |
| Tumor number | 0.403 (0.265–0.612) | 0.000 |
| Cirrhosis | 0.591 (0.368–0.948) | 0.029 |
| Adjuvant chemoradiotherapy | 0.641 (0.415–0.989) | 0.045 |
| AFP | 0.520 (0.324–0.835) | 0.007 |
| Tumor size | 0.446 (0.307–0.649) | 0.000 |
| Anesthesia method | 0.617 (0.420–0.907) | 0.014 |
| Ablation method | 0.769 (0.532–1.111) | 0.162 |
| OS | ||
| Age | 0.407 (0.216–0.768) | 0.006 |
| Tumor size | 0.360 (0.189–0.687) | 0.002 |
| Anesthesia method | 0.576 (0.413–0.804) | 0.001 |
| Inpatient day | 0.347 (0.123–0.983) | 0.046 |
| Ablation method | 0.892 (0.470–1.691) | 0.726 |
Fig 3.
3A: Log-rank test showed on difference between the two groups in recurrence-free survival after PSM. 3B: Log-rank test showed on difference between the two groups in overall survival after PSM.
Table 7. Multivariable Cox regression model analysis of RFS and OS after PSM.
| Multivariable Cox regression | HR (95%CI) | P value |
|---|---|---|
| RFS | ||
| Tumor number | 0.521 (0.330–0.823) | 0.005 |
| AFP | 0.496 (0.307–0.802) | 0.004 |
| Tumor size | 0.565 (0.338–0.945) | 0.030 |
| Ablation method | 0.890 (0.603–1.313) | 0.558 |
| OS | ||
| Age | 0.390 (0.205–0.741) | 0.004 |
| Anesthesia method | 0.422 (0.195–0.912) | 0.028 |
| Ablation method | 1.169 (0.596–2.291) | 0.65l |
Before the PSM, the Kaplan–Meier survival rate estimates and 95% CIs at landmark follow-up times showed that the 1-, 2-, 3- and 4-year RFS rates of tumor in MW ablation group were 66.5% (95%CI, 60.2%-72.8%), 52.8% (95%CI, 46.1%-59.5%), 47.2% (95%CI, 40.3%-54.1%) and 42.1% (95%CI, 34.8%-49.4%) respectively; In RF ablation group, the 1-, 2-, 3- and 4-year RFS rates of tumor were 60.3% (95%CI, 54.0%-66.6%), 45.9% (95%CI, 39.4%-52.4%), 40.6% (95%CI, 33.7%-47.5%) and 35.5% (95%CI, 27.5%-43.5%) respectively (Table 8). The log-rank test showed that there was no statistic difference on the RFS rates between two groups (Fig 2A). Same conclusion could be affirmed again for the OS rates between MW ablation and RF ablation groups (Table 8 and Fig 2B).
Table 8. The RFS rates and survival rates of patients before PSM.
(*Point-wise 95% CI).
| Time | MW ablation group (n = 218) | RF ablation group (n = 234) | ||||||
|---|---|---|---|---|---|---|---|---|
| RFS rates (95% CI*) | No. events | No. censored | No. left | RFS rates (95% CI*) | No. events | No. censored | No. left | |
| At treatment | 100 | 218 | 100 | 234 | ||||
| 1 yr | 66.5 (60.2–72.8) | 73 | 0 | 145 | 60.3 (54.0–66.6) | 93 | 0 | 141 |
| 2 yr | 52.8 (46.1–59.5) | 101 | 24 | 93 | 45.9 (39.4–52.4) | 124 | 34 | 76 |
| 3 yr | 47.2 (40.3–54.1) | 110 | 41 | 67 | 40.6 (33.7–47.5) | 131 | 60 | 43 |
| 4 yr | 42.1 (34.8–49.4) | 117 | 72 | 29 | 35.5 (27.5–43.5) | 134 | 97 | 3 |
| OS rates (95% CI*) | No. events | No. censored | No. left | OS rates (95% CI*) | No. events | No. censored | No. left | |
| At treatment | 100 | 218 | 100 | 234 | ||||
| 1 yr | 92.7 (89.2–96.2) | 16 | 0 | 202 | 96.6 (94.2–99.0) | 8 | 0 | 226 |
| 2 yr | 84.5 (79.6–89.4) | 32 | 47 | 139 | 88.5 (84.0–93.0) | 24 | 69 | 141 |
| 3 yr | 77.5 (71.2–83.8) | 42 | 81 | 95 | 83.9 (78.4–89.4) | 30 | 148 | 56 |
| 4 yr | 72.3 (64.9–79.7) | 47 | 132 | 39 | 79.7 (70.1–89.3) | 31 | 196 | 7 |
Discussion
According to this multi-center retrospective cohort study, we found that there were no significant differences between MW ablation and RF ablation for HCC patients on the RFS or OS. Though liver resection is the first-line curative treatment for patients with HCC, several studies had verified that hepatic resection contributed to a higher rate of complications and surgical mortality [20,21]. At the same time, more studies also found that ablation surgery was equivalent to surgical resection for overall survival [22,23]. In conclusion, ablation surgery is a kind of effective and less invasive method for tumor treatments.
However, with the development of MW ablation and RF ablation, researchers focus on the differences between two methods. Some studies [11,24,25] reported that MW ablation seemed to have a lower rate of local recurrence of tumor. It could be explained that MW ablation has an improved convection profile, higher intratumoral temperatures, faster ablation time, larger ablation volume, and less susceptibility to heat-sink effect [26,27]. But still other studies found that no significant differences on the RFS or OS were observed between MW ablation group and RF ablation group [28,29]. In our study, we further verified that there were similar RFS and OS for HCC patients in MW ablation group and RF ablation group. In a word, RF ablation and MW ablation have same clinical value in treating HCC conforming to the Milan criteria, and these two methods are both safe and effective techniques for HCC as clinical applications.
In this study, more than 20 related variables were collected and analyzed. After PSM, results of multiple Cox regression analysis showed that AFP, Tumor size and Tumor number were independent risk factors for the RFS, and the Anesthetic methods and Age were independent risk factors for the OS. In this study, we found that the local anesthesia was better than general anesthesia, and the OS in local anesthesia group was longer than the general anesthesia group. This finding could be verified by related studies [30,31].
This study has several limitations. First, it is a retrospective cohort study rather a randomized controlled trail. But we had used the PSM and multiple Cox regression analyses to minimize the bias between two groups. Second, the follow-up time could be longer. In the further study, we would prolong the follow-up period to obtain more information about the RFS and OS. Third, the sample capacity could be larger and more hospitals are needed. Fourth, some variables could be collected with more details. For example, because different hospitals have diverse medical record software, some data was missing during the replacement of software. And some data about the dosage of anesthetic drugs and adjuvant chemotherapeutic drugs were not recorded with details, so we could not collect relevant data intactly.
In summary, after using PSM analyses and multivariable Cox regression analyses, our present study showed that there were no significant differences between MW ablation and RF ablation for HCC patients on the RFS or OS. Both MW ablation and RF ablation were effective and safe for patients who suffered HCC.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study received financial support from National Natural Science Foundation of China (81471140), and Shenzhen university general hospital science and technology personnel booster program (85706-0000040537)
References
- 1.Allemani C, Matsuda T, Di CV, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018; 391(10125): 1023–1075. 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68(1): 7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018; 69(3): 718–735. 10.1016/j.jhep.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66(2): 115–132. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 5.Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017; 67(2): 302–309. 10.1016/j.jhep.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 6.Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016; 27(5): 926–933. 10.1093/annonc/mdw027 [DOI] [PubMed] [Google Scholar]
- 7.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014; 60(6): 2099–2108. 10.1002/hep.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangiovanni A, Triolo M, Iavarone M, Forzenigo LV, Nicolini A, Rossi G, et al. Multimodality treatment of hepatocellular carcinoma: How field practice complies with international recommendations. Liver Int. 2018; 38(9): 1624–1634. 10.1111/liv.13888 [DOI] [PubMed] [Google Scholar]
- 9.Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018; 68(4): 783–797. 10.1016/j.jhep.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 10.Vogl TJ, Farshid P, Naguib NN, Zangos S, Bodelle B, Paul J, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging. 2015; 40(6): 1829–1837. 10.1007/s00261-015-0355-6 [DOI] [PubMed] [Google Scholar]
- 11.Abdelaziz A, Elbaz T, Shousha HI, Mahmoud S, Ibrahim M, Abdelmaksoud A, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014; 28(12): 3429–3434. 10.1007/s00464-014-3617-4 [DOI] [PubMed] [Google Scholar]
- 12.Huo YR, Eslick GD. Microwave Ablation Compared to Radiofrequency Ablation for Hepatic Lesions: A Meta-Analysis. J Vasc Interv Radiol. 2015; 26(8): 1139–1146.e2. 10.1016/j.jvir.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 13.Potretzke TA, Ziemlewicz TJ, Hinshaw JL, Lubner MG, Wells SA, Brace CL, et al. Microwave versus Radiofrequency Ablation Treatment for Hepatocellular Carcinoma: A Comparison of Efficacy at a Single Center. J Vasc Interv Radiol. 2016; 27(5): 631–638. 10.1016/j.jvir.2016.01.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol. 2009; 24(2): 223–227. 10.1111/j.1440-1746.2008.05596.x [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One. 2013; 8(10): e76119 10.1371/journal.pone.0076119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56(4): 908–943. 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 17.Baek S, Park SH, Won E, Park YR, Kim HJ. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol. 2015; 16(2): 286–296. 10.3348/kjr.2015.16.2.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang T, Lu JH, Lau WY, Zhang TY, Zhang H, Shen YN, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: A Propensity Score Matching Analysis. J Hepatol. 2016; 64(3): 583–593. 10.1016/j.jhep.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 19.Boffa DJ, Kosinski AS, Furnary AP, Kim S, Onaitis MW, Tong BC, et al. Minimally Invasive Lung Cancer Surgery Performed by Thoracic Surgeons as Effective as Thoracotomy. J Clin Oncol. 2018; 36(23): 2378–2385. 10.1200/JCO.2018.77.8977 [DOI] [PubMed] [Google Scholar]
- 20.Dong W, Zhang T, Wang ZG, Liu H. Clinical outcome of small hepatocellular carcinoma after different treatments: a meta-analysis. World J Gastroenterol. 2014; 20(29): 10174–10182. 10.3748/wjg.v20.i29.10174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaibori M, Matsui Y, Hijikawa T, Uchida Y, Kwon AH, Kamiyama Y. Comparison of limited and anatomic hepatic resection for hepatocellular carcinoma with hepatitis C. Surgery. 2006; 139(3): 385–394. 10.1016/j.surg.2005.08.035 [DOI] [PubMed] [Google Scholar]
- 22.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012; 57(4): 794–802. 10.1016/j.jhep.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 23.Kagawa T, Koizumi J, Kojima S, Nagata N, Numata M, Watanabe N, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010; 116(15): 3638–3644. 10.1002/cncr.25142 [DOI] [PubMed] [Google Scholar]
- 24.Lee KF, Wong J, Hui JW, Cheung YS, Chong CC, Fong AK, et al. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: A retrospective comparative study. Asian J Surg. 2017; 40(4): 301–308. 10.1016/j.asjsur.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 25.Correa-Gallego C, Fong Y, Gonen M, D'Angelica MI, Allen PJ, DeMatteo RP, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014; 21(13): 4278–4283. 10.1245/s10434-014-3817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrafiello G, Laganà D, Mangini M, Fontana F, Dionigi G, Boni L, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008; 6 Suppl 1: S65–69. [DOI] [PubMed] [Google Scholar]
- 27.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008; 19(7): 1087–1092. 10.1016/j.jvir.2008.03.023 [DOI] [PubMed] [Google Scholar]
- 28.Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013; 82(9): 1379–1384. 10.1016/j.ejrad.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi H, Seki S, Tsuji K, Teramoto K, Suzuki M, Kioka K, et al. Endoscopic thermal ablation therapies for hepatocellular carcinoma: a multi-center study. Hepatol Res. 2009; 39(1): 47–52. 10.1111/j.1872-034X.2008.00410.x [DOI] [PubMed] [Google Scholar]
- 30.Lin L, Liu C, Tan H, Ouyang H, Zhang Y, Zeng W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. Br J Anaesth. 2011; 106(6): 814–822. 10.1093/bja/aer055 [DOI] [PubMed] [Google Scholar]
- 31.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008; 109(2): 180–187. 10.1097/ALN.0b013e31817f5b73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.