Abstract
Summary
In the large community-based SCOOP trial, systematic fracture risk screening using FRAX® led to greater use of AOM and greater adherence, in women at high fracture risk, compared with usual care.
Purpose
In the SCreening of Older wOmen for Prevention of fracture’ (SCOOP) trial we investigated the effect of the screening intervention on subsequent long-term self-reported adherence to antiosteoporosis medications(AOM).
Methods
SCOOP was a primary care-based UK multi-centre trial of screening for fracture risk. 12,483 women (70-85years) were randomised to either usual NHS care, or assessment using the FRAX® tool +/- dual-energy X-ray absorptiometry(DXA), with medication recommended for those found to be at high risk of hip fracture. Self-reported AOM use was obtained by postal questionnaires at 6, 12, 24, 36, 48 and 60 months. Analysis was limited to those who initiated AOM during follow-up. Logistic regression was used to explore baseline determinants of adherence(good>=80%; poor<80%).
Results
The mean (SD) age of participants was 75.6 (4.2) years, with 6233 randomised to screening and 6250 to the control group. Of those participants identified at high fracture risk in the screening group, 38.2% of those on treatment at 6 months were still treated at 60 months; whereas the corresponding figure for the control group was 21.6%. Older age was associated with poorer adherence [OR per year increase in age 0.96 (95%CI: 0.93, 0.99), p=0.01], whereas history of parental hip fracture was associated with greater rates adherence [OR 1.67 (95%CI: 1.23, 2.26), p<0.01].
Conclusions
Systematic fracture risk screening using FRAX® leads to greater use of AOM and greater adherence, in women at high fracture risk, compared with usual care.
Keywords: Osteoporosis, epidemiology, adherence, medication, FRAX®, screening
Introduction
Osteoporosis risk assessment has advanced markedly in recent decades. The introduction of an operational definition of osteoporosis based on dual-energy X-ray absorptiometry (DXA) bone mineral density (BMD) by the World Health Organisation in the mid-1990s permitted identification of those at risk of fracture due to a reduced bone mass.[1] Recognition of the contribution of risk factors other than BMD, and the latter’s sub-optimal sensitivity for fracture prediction, led to the development of the FRAX® Fracture Risk Calculation tool. This uses a small number of intuitively reasonable and clinically readily available risk factors, together with femoral neck BMD if measured, to calculate an individualised 10-year probability of fracture, integrating risk of fracture with the competing hazard of death.[2]
There are around 120 guidelines internationally that use the FRAX® tool.[3] Whilst the majority of guidelines have suggested approaches based on opportunistic case finding (for example the earlier UK Royal College of Physicians Guidelines and subsequently the National Osteoporosis Guideline Group[4]), the effectiveness and cost effectiveness of systematic screening has recently been demonstrated in the SCOOP trial.[5–8] Although there was no effect on fractures overall, in this trial, identification in primary care of older women at high risk of fracture (using FRAX® probability of hip fracture and subsequent recommendation for treatment) led to a 28% reduction in hip fractures over 5 years compared with usual care.[6] Such advances must be viewed in the context of an international backdrop of declining medication use for both primary and secondary prevention for a variety of reasons but, critically, this makes interventions that optimise identification and treatment of patients at high fracture risk a global imperative.[9, 10]
Whilst the SCOOP trial demonstrated that the intervention was acceptable and associated with increased medication initiation, a key component of efficacy is adherence (proportion of prescribed doses taken).[11, 12] In this post hoc exploratory study, we used existing data from the trial to investigate whether the SCOOP screening intervention was associated with increased self-reported adherence to anti-osteoporosis medication, and explored the determinants thereof.
Materials and methods
Study Design
The ‘Screening of older women for prevention of fracture’ (SCOOP) study was a pragmatic, unblinded, two group, parallel randomised controlled trial to assess the effectiveness of screening to prevent fractures in older women. Details of the study have been published:[5] in brief, women aged 70-85 years were invited from primary care lists within seven UK centres; those responding were randomly assigned (1:1) to either a screening arm or a control arm. Randomisation was completed using an online, web-based system, and was set up by an independent database programme from the Norwich Clinical Trials Unit. In the screening arm, the FRAX® risk algorithm was used to determine baseline fracture risk (10-year probability of hip fracture) and those participants identified as being at moderate or high risk of fracture (using an age-dependent threshold, equivalent to the 10-year probability consequent to the presence of a previous fracture) had a DXA scan to obtain femoral neck bone mineral density (BMD). Their 10-year hip fracture probability was then recalculated including BMD. Those in the control arm received usual UK NHS care (opportunistic discussion of osteoporosis). In the screening arm, anti-osteoporosis medication was recommended to those participants found to be at high risk of fracture after inclusion of the BMD measurement in FRAX®. If required anti-osteoporosis mediation was issued by the study participants’ Primary Care physicians, in accordance with national guidance from the United Kingdom Royal College of Physicians and National Osteoporosis Guideline Group.[4]
Data collection
Self-reported anti-osteoporosis medication (AOM) use was obtained by postal questionnaire at 6, 12, 24, 36, 48 and 60 months after randomisation for both study arms. Since it was not possible to assess whether medicines were actually taken, prescription adherence was assessed over the full 60-month study duration, and calculated as the percentage of subsequent time points at which the participants reported taking anti-osteoporosis medication, following a positive report of medication use at the 6 month (or subsequent) questionnaire.
Statistical analysis
Characteristics of participants were described using means and standard deviations (SD) for normally distributed continuous variables, and using medians and inter-quartile ranges for skewed variables. Frequencies and percentages were used to summarise binary and categorical variables. Study participants were then grouped into two adherence groups,[13] good adherence (defined as medication adherence 80% or more) or poor adherence (defined as less than 80% adherence). Logistic regression was used to investigate whether FRAX® probability or FRAX® component clinical risk factors at baseline were associated with adherence. Since some patients may have been commenced on treatment as a result of experiencing a fracture during follow-up rather than as a direct result of the screening, we also examined initiation and adherence firstly amongst those who did not experience an incident (post-baseline) fracture before initiation of treatment, and secondly amongst the group who did experience an incident fracture before commencing medication. Given that information on fractures and medication was obtained at the follow-up questionnaires, it was not possible to establish the order of such events prior to 6 months, and so analysis of initiation at 6 months assumes no prior fracture between baseline and this time point. The analysis based on medication initiation after an incident fracture thus only used follow-up from 12 months onwards. All analyses were undertaken using Stata 14.[14]
Full ethics approval was obtained from the North Western – Haydock Research Ethics Committee of England in September 2007 (REC 07/H1010/70). The trial was registered on the International Standard Randomised Controlled Trial Register in June 2007 (ISRCTN55814835). All participants gave written, informed consent.
Results
Participant characteristics
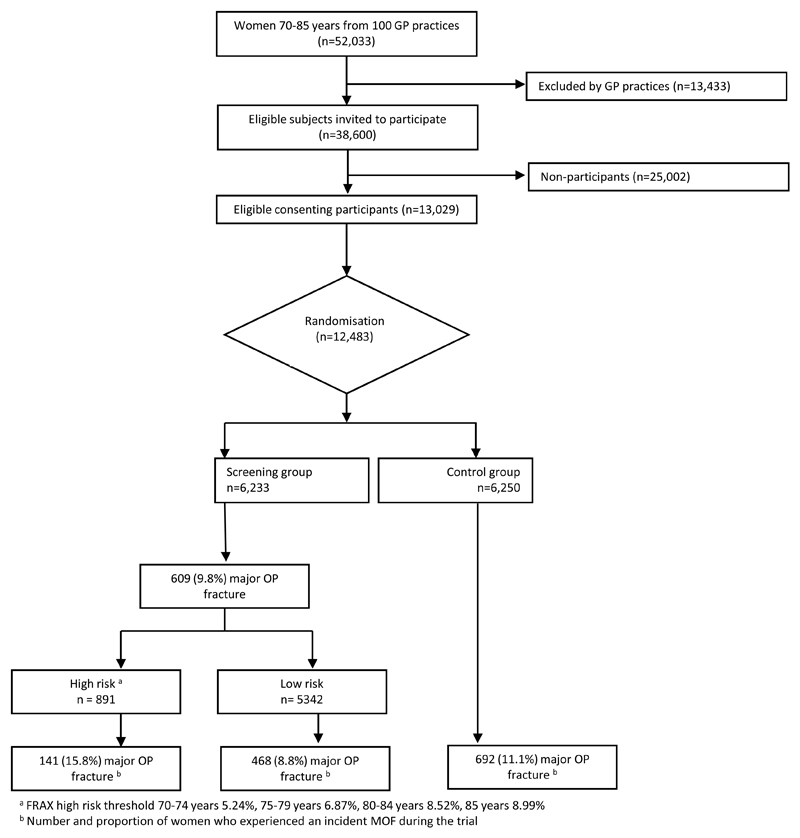
A total of 12,483 participants were randomised: 6,233 women to the screening arm and 6,250 to the control arm. Overall, the mean age was 75.6 years and the median body mass index (BMI) 26kg/m2. At baseline, the median FRAX® hip fracture probability of all participants calculated without BMD was 6.3% and of those with BMD measured the mean T-score was -1.7. Just under 5% of participants reported smoking at baseline, 3.6% drank more than 3 units of alcohol a day and 10% of participants reported a parental history of hip fracture. Characteristics of all study participants are presented by randomisation group in Table 1, demonstrating that the baseline characteristics were well balanced between the two groups. Of those in the screening arm, 14.3% were classified at high risk of fracture based on FRAX® 10-year hip fracture probability (Figure 1). Over the 60 month study duration, 15.7% reported an incident fracture.
Table 1. Participant characteristics at baseline assessment.
| Screening arm | Control arm | |||||
|---|---|---|---|---|---|---|
| Characteristic | n | Mean | SD | n | Mean | SD |
| Age (years) | 6233 | 75.5 | 4.2 | 6250 | 75.6 | 4.1 |
| Height (cm) | 6233 | 160.7 | 6.3 | 6250 | 160.9 | 6.4 |
| T-Score | 2818 | -1.7 | 1.0 | - | - | - |
| n | Median | Inter-quartile range | n | Median | Inter-quartile range | |
| BMI (kg/m2) | 6233 | 26.0 | 23.4-29.3 | 6250 | 26.1 | 23.4-29.2 |
| Weight (kg) | 6233 | 67.1 | 60.3-76.2 | 6250 | 67.6 | 60.3-76.2 |
| FRAX® Probability (hip without BMD) | 6233 | 6.3 | 3.8-10.5 | 6250 | 6.3 | 3.8-10.5 |
| n | % | n | % | |||
| Parental history of hip fracture | 585 | 9.4 | 577 | 9.2 | ||
| Incident fracture (post baseline) | 956 | 15.3 | 1010 | 16.2 | ||
| Prior fracture b | 1399 | 22.7 | 1463 | 23.6 | ||
| Smoker | 291 | 4.7 | 290 | 4.6 | ||
| Taken corticosteroids for more than a few weeks | 316 | 5.1 | 312 | 5.0 | ||
| Rheumatoid arthritis | 426 | 6.8 | 410 | 6.6 | ||
| > 3 units of alcohol a day | 219 | 3.5 | 225 | 3.6 | ||
| Risk category a | ||||||
| Low | 5342 | 85.7 | - | - | ||
| High | 891 | 14.3 | - | - | ||
Risk categorisation undertaken in intervention arm only; FRAX® high risk threshold 70-74 years 5.24%, 75-79 years 6.87%, 80-84 years 8.52%, 85 years 8.99%
Broken bone since age of 50 years
Figure 1.
Consort diagram
Medication initiation by time and group
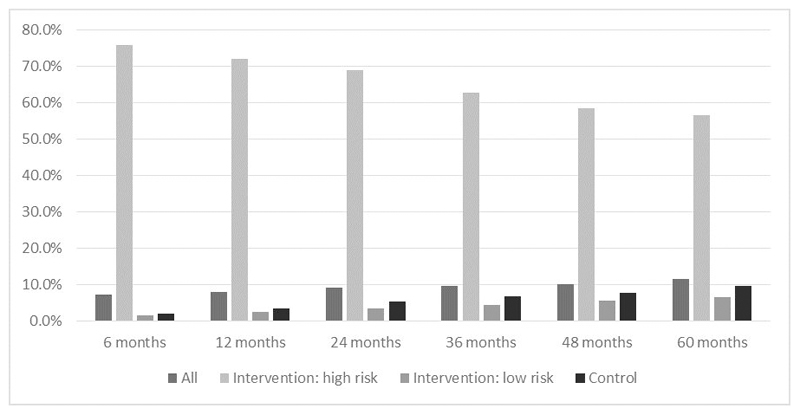
At six months, 7.2% of the whole study population reported using anti-osteoporosis medication (AOM)(Figure 2). Of those study participants in the screening arm identified to be at high risk of fracture, 75.8% were taking AOM compared with only 2.0% in the control arm overall. By 60 months, 11.5% of all study population were taking an AOM, with 56.6% of those identified as at high risk of fracture reporting taking medication, compared with 9.7% in the control arm overall.
Figure 2.
Anti-osteoporosis medication use over the duration of the SCOOP trial by randomisation group [screening (intervention) vs usual care (control)].
Medication adherence
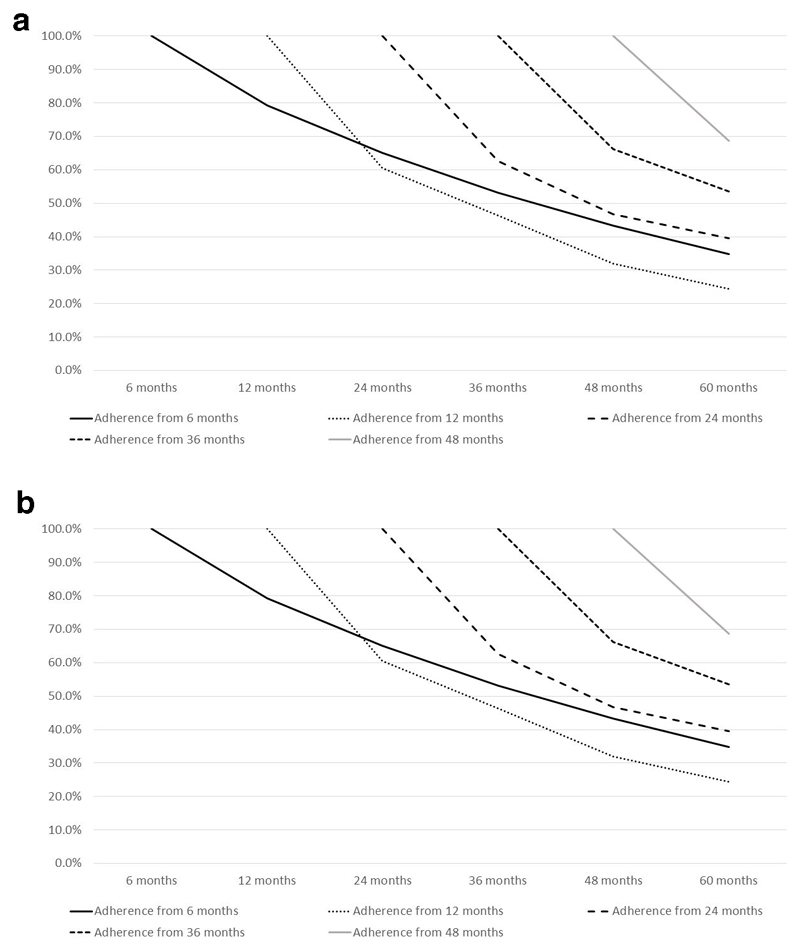
Of the 823 SCOOP participants who self-reported AOM use at 6 months (and assumed not to have experienced a fracture between the baseline assessment and the 6 month questionnaire), 79.2% (n=652) remained on treatment at 12 months, 65.0% (n=535) at 24 months and 34.9% (n=287) remained on treatment for the entire 60 month duration of follow-up. Similar patterns of treatment decay were seen when study participants commenced medication at later study time points (restricted to those individuals who had not experienced a fracture between baseline assessment and treatment commencement, demonstrated graphically in Figure 3a). Of the 628 study participants who were identified at high risk of fracture in the screening arm and reported treatment at 6 months, 38.2% (n=240) remained on treatment for the 60 month duration; the respective figure for the control group was 21.6% (n=25).
Figure 3.
a: Anti-osteoporosis medication (AOM) adherence over the 5 year duration of the SCOOP trial in study participants who initiated treatment, and who had not experienced a fracture between baseline and commencement of medication. (Calculated as the percent study participants who remained on AOM at each subsequent timepoint having initiated treatment at each index timepoint.)
b: Anti-osteoporosis medication (AOM) adherence over the 5 year duration of the SCOOP trial in study participants who initiated treatment after the occurrence of a fracture post-baseline. (Calculated as the percent study participants who remained on AOM at each subsequent timepoint having initiated treatment at each index timepoint.)
Medication adherence following initiation after an incident fracture
Figure 3b demonstrates the decay in adherence following initiation of medication after an incident fracture. At 12 months, 30 participants had initiated treatment following a post-baseline fracture prior to this assessment. 96.7% (n=29) were still adherent at 24 months and 36.7% (n=11) at 60 months. Patterns of adherence decay were similar with treatment initiation at later time points.
Baseline characteristics associated with 60 month adherence
As expected, the components of the FRAX® score were associated with initiation of treatment, and on univariate modelling, on average the odds of having good adherence to AOM reduced with each year higher age[OR 0.96 (95%CI: 0.93, 0.99), p<0.01], whereas the odds of having good adherence to AOM over the five-year study duration was higher in those with a history of a parental hip fracture [OR 1.67 (95%CI: 1.23, 2.26), p<0.01] (Table 2). In the screening arm, participants who underwent a DXA assessment had odds nearly twice as high as those without a DXA assessment for reporting good adherence to AOM [OR 1.89 (95%CI: 1.33, 2.68), p<0.01] and participants who were identified at high fracture risk after inclusion of the BMD measurement in FRAX® had higher odds of good adherence [OR 2.80 (95%CI: 1.21, 6.50), p=0.02].
Table 2. Univariate associations between participant characteristics (at baseline) and adherence to anti-osteoporosis medication (AOM) over the 5 year follow-up period (Logistic regression).
| Adherence during follow-up | |||
|---|---|---|---|
| Odds ratio | 95% CI | p-value | |
| Age (years)a | 0.96 | (0.93,0.99) | 0.01 |
| Weight [log(kg)]b | 1.15 | (0.52,2.51) | 0.74 |
| Height (cm)c | 1.01 | (0.99,1.03) | 0.42 |
| Prior fracture (Y/N) | 0.89 | (0.67,1.17) | 0.40 |
| Parent broken hip (Y/N) | 1.67 | (1.23,2.26) | <0.01 |
| Smoker (Y/N) | 1.00 | (0.61,1.64) | 1.00 |
| Taken corticosteroids (Y/N) | 0.81 | (0.54,1.23) | 0.32 |
| Rheumatoid arthritis (Y/N) | 0.80 | (0.50,1.27) | 0.34 |
| Alcohol consumption (Y/N) | 0.57 | (0.32,1.03) | 0.06 |
| DXA Scan (Y/N) | 1.89 | (1.33, 2.68) | <0.01 |
| Total hip BMD T-score (SD) | 1.02 | (0.82,1.27) | 0.85 |
| Incident fracture (post baseline) (Y/N) | 1.03 | (0.76,1.40) | 0.86 |
| FRAX® risk category (High/Low)d | 2.80 | (1.21,6.50) | 0.02 |
OR for each year higher in age
OR for each log(kg) increase in weight
OR for each cm increase in height
in a subgroup of study participants with a FRAX® category using BMD
Discussion
In this pragmatic randomised trial of systematic screening for fracture risk in older women, using FRAX® in primary care, the screening intervention was associated with greater rates of AOM prescription, and self-reported medication adherence, than that those observed with usual NHS care. Furthermore, greater adherence was associated with younger age and a history of parental hip fracture. Since further routine FRAX® calculation and BMD scanning were not part of the protocol, our findings highlight the importance of the initial screening assessment.
To our knowledge, this is the first study to demonstrate the benefit of systematic screening on osteoporosis medication adherence. Good adherence to osteoporosis medications is clearly essential, demonstrated by the reduced efficacy of medications such as alendronic acid when prescription regimens are poorly followed.[15, 16] Similar to the prevention of many other common chronic non-communicable diseases, adherence to bisphosphonates is generally sub-optimal, with reported rates as low as 40%.[11, 17, 18] Reasons for poor adherence are not well understood, but there is evidence from a large, international cohort study of 60,000 older women, that appreciation of individual risk is variable, and the majority of women underestimate their risk of fracture, even having experienced a prior fracture.[19, 20] In recent years, there has been concern over rare serious side effects of long-term antiresorptive treatment.[9, 10] These have often been excessively reported in the media (and sometimes also in the scientific press); in particular, communication of the appropriate balance between risk and benefit has usually not occurred, especially in the context of the global media.[21, 22] It is notable that rates of medication use for both primary and secondary prevention appear to be falling over recent years.[10, 21, 23–25]
Previous investigations have explored a variety of methods to improve medication adherence. These have included measurement of bone turnover markers, BMD, nurse/practitioner review, and educational programmes, with the aim of providing positive feedback and monitoring of progress.[11, 16, 26] However, there are clear resource implications for such interventions, and the value of specific measures such as bone turnover markers over and above simple contact with a health professional is not certain.[11] It is therefore notable that the present screening intervention, undertaken in primary care using the FRAX® tool, led not just to increased uptake of medication but also to improved adherence compared with those individuals prescribed medication in the usual care group.
That family history of hip fracture was associated with better adherence is easily comprehensible, although the lack of association with prior fracture is perhaps counterintuitive, albeit consistent with findings from the GLOW study.[19] Increased adherence in those who underwent BMD testing may simply reflect collinearity with other risk variables, since these individuals were by definition at moderate to high fracture risk, or potentially a positive effect of the DXA scan on adherence. Our analysis explored adherence amongst those individuals who had (or had not) experienced a fracture after the baseline assessment, but before initiation of medication, and suggested perhaps greater adherence in the first 12 months after initiation for the post-incident fracture group commencing medication at one year. However, the percent adherence was similar at the end of the study, and the numbers were not large enough to permit a logistic regression analysis based on incident fracture. We were unable to reliably assess the temporal relationships between medication initiation and fracture occurrence in the first 6 months of follow-up, and so assumed that medication initiation preceded a fracture event during this period. The validity of this assumption cannot be tested, but given the findings in relation to medication adherence for initiation following a fracture in our subsequent analyses, it is possible that the balance of fractures either side of the 6 month questionnaire could have influenced our results.
Our finding of lower adherence at older ages may reflect a higher burden of comorbidity and associated medications. Indeed, key perceptions that influence older women with regard to adherence to such medications was investigated in a qualitative study, nested within the SCOOP trial[27]. In this investigation of 30 women aged 70-85 years who were offered anti-osteoporosis medication, there were no overall predictors of adherence across two years of assessment. The women’s perceptions and motivations related to persistence with medication were influenced by factors such as their understanding of adherence/non-adherence, motivations and self-care, appraising/prioritising risk, anticipating/managing side effects and issues relating to problems of understanding and decision-making. Importantly, those engaged with supportive professionals better tolerated/overcame potential barriers posed by side effects.[27] The present results complement these detailed findings from interviews in a small group of women, by elucidating overarching predictors of adherence across the whole trial population.
We studied a unique multicentre, primary care-based UK randomised controlled trial with comprehensive assessment of medication use. However, there are some limitations that could should be considered in the interpretation of our findings. Firstly, medication use was obtained by self-report questionnaire at specific time points, and was not validated by semi-objective measures such as pill counts. It is possible that transient use was therefore underestimated, though if anything this would tend to reduce the chances of observing differences between the groups. Furthermore, self-report may lead to over-estimation of adherence compared with pill counts, but it is likely, given the context of participation in a trial for the prevention of osteoporotic fracture, that participants were motivated to take treatment[28]. The self-report question within the SCOOP postal questionnaire asked study participants whether they were currently taking AOM, and no detail of the types of medication were captured at this time. AOM were prescribed by General Practitioners and so the vast majority are likely to be oral bisphonates; however in this study we are unable to assess whether the type of medication impacted the level of adherence: for example we could not readily assess any influence of annual intravenous zoledronate prescription on our findings. Additionally, we lacked information relating to new prescriptions of corticosteroids. Secondly, we have limited capacity to explore psychosocial aspects related to adherence, but these have been investigated previously in subsets of the trial.[27, 29] Thirdly, the study population consisted of older women, limiting the generalisability of these findings to younger women and to men, and we had limited information about aspects of clinical care in the control group, for example, use of DXA scanning. Finally, it is possible that trial participants were somewhat healthier than the general population. This “healthy selection effect” may limit generalisability, but should not materially influence differences between the two groups, since participants were randomly allocated to screening or usual care. Further studies in a population of men and women, in which in-depth analysis examining whether different AOM medications had significantly differing levels of adherence and treatment adherence captured using more sophisticated methods would be warranted.
In conclusion, systematic screening for fracture risk using FRAX® in primary care led to increased use of, and adherence to, anti-osteoporosis medications, compared with usual care. Taken with recent evidence that this intervention results in a reduction in risk of hip fracture, the present findings further support the use of systematic screening approaches for fracture prevention.
Acknowledgements
This study was jointly funded by Arthritis Research UK (formerly the Arthritis Research Campaign) and the UK Medical Research Council. NMR’s time is supported by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care West (NIHR CLAHRC West) at University Hospitals Bristol NHS Foundation Trust. The SCOOP study was designed and done with substantial input from the Norwich Clinical Trials Unit, UK, particularly the construction of the study database and provision of online randomisation (completed by Tony Dyer). We thank Margaret McWilliams and Ann Pulford, the study’s public and patient involvement representatives, for invaluable advice and support, and our trial steering committee and data monitoring committee. CP and NH are joint first author.
Footnotes
Disclosures
CC has received consultancy fees and honoraria from Amgen, Danone, Eli Lilly, GlaxoSmithKline, Medtronic, Merck, Nestlé, Novartis, Pfzer, Roche, Servier, Shire, Takeda, and UCB. NH has received consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare, and Internis Pharma. JK has held grants from Amgen, Lilly, Unigene, and Radius Health; has received non-fnancial support from Medimaps, Asahi, and AgNovos; and is the architect of FRAX®, but has no financial interest. EM has been, or currently is, an adviser or speaker for and has received research support from ActiveSignal, Amgen, AstraZeneca, Consilient Healthcare, GlaxoSmithKline, Hologic, Internis, Eli Lilly, Medtronic, Merck, Novartis, Pfzer, Roche, Sanof-Aventis, Servier, Synexus, Tethys, UCB, and Warner Chilcott; and has received research support from I3 Innovus, International Osteoporosis Foundation, and Unilever. All other authors declare no competing interests.
References
- 1.World Health Organisation. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO; Geneva: 1994. [PubMed] [Google Scholar]
- 2.Kanis JA. WHO Scientific Group Technical Report. World Health Organization; Geneva: 2007. Assessment of osteoporosis at the primary health care level. [Google Scholar]
- 3.Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV. A systematic review of intervention thresholds based on FRAX : A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Archives of osteoporosis. 2016;11:25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Archives of osteoporosis. 2017;12:43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepstone L, Fordham R, Lenaghan E, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int. 2012;23:2507–2515. doi: 10.1007/s00198-011-1876-7. [DOI] [PubMed] [Google Scholar]
- 6.Shepstone L, Lenaghan E, Cooper C, et al. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet. 2017 doi: 10.1016/S0140-6736(17)32640-5. [DOI] [PubMed] [Google Scholar]
- 7.Turner DA, Khioe RFS, Shepstone L, et al. The Cost-Effectiveness of Screening in the Community to Reduce Osteoporotic Fractures in Older Women in the UK: Economic Evaluation of the SCOOP Study. J Bone Miner Res. 2018;33:845–851. doi: 10.1002/jbmr.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCloskey E, Johansson H, Harvey NC, et al. Management of Patients With High Baseline Hip Fracture Risk by FRAX Reduces Hip Fractures-A Post Hoc Analysis of the SCOOP Study. J Bone Miner Res. 2018;33:1020–1026. doi: 10.1002/jbmr.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanis JA, Svedbom A, Harvey N, McCloskey EV. The osteoporosis treatment gap. J Bone Miner Res. 2014;29:1926–1928. doi: 10.1002/jbmr.2301. [DOI] [PubMed] [Google Scholar]
- 10.Harvey NC, McCloskey EV, Mitchell PJ, Dawson-Hughes B, Pierroz DD, Reginster JY, Rizzoli R, Cooper C, Kanis JA. Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int. 2017;28:1507–1529. doi: 10.1007/s00198-016-3894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diez-Perez A, Naylor KE, Abrahamsen B, et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int. 2017;28:767–774. doi: 10.1007/s00198-017-3906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmett CL, Redmond NM, Peters TJ, Clarke S, Shepstone L, Lenaghan E, Shaw AR. Acceptability of screening to prevent osteoporotic fractures: a qualitative study with older women. Fam Pract. 2012;29:235–242. doi: 10.1093/fampra/cmr069. [DOI] [PubMed] [Google Scholar]
- 13.Cauley JA, Chlebowski RT, Wactawski-Wende J, Robbins JA, Rodabough RJ, Chen Z, Johnson KC, O'Sullivan MJ, Jackson RD, Manson JE. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women's Health Initiative. Journal of women's health (2002) 2013;22:915–929. doi: 10.1089/jwh.2013.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stata Statistical Software. Texas: 2015. 2015 S. [Google Scholar]
- 15.Hiligsmann M, Rabenda V, Gathon HJ, Ethgen O, Reginster JY. Potential clinical and economic impact of nonadherence with osteoporosis medications. Calcif Tissue Int. 2010;86:202–210. doi: 10.1007/s00223-009-9329-4. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R. Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int. 2011;22:2565–2573. doi: 10.1007/s00198-011-1668-0. [DOI] [PubMed] [Google Scholar]
- 17.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 18.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 19.Siris ES, Gehlbach S, Adachi JD, et al. Failure to perceive increased risk of fracture in women 55 years and older: the Global Longitudinal Study of Osteoporosis in Women (GLOW) Osteoporos Int. 2011;22:27–35. doi: 10.1007/s00198-010-1211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litwic AE, Compston JE, Wyman A, et al. Self-perception of fracture risk: what can it tell us? Osteoporos Int. 2017 doi: 10.1007/s00198-017-4200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in Media Reports, Oral Bisphosphonate Prescriptions, and Hip Fractures 1996-2012: An Ecological Analysis. J Bone Miner Res. 2015;30:2179–2187. doi: 10.1002/jbmr.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peeters G, Tett SE, Duncan EL, Mishra GD, Dobson AJ. Osteoporosis medication dispensing for older Australian women from 2002 to 2010: influences of publications, guidelines, marketing activities and policy. Pharmacoepidemiology and drug safety. 2014;23:1303–1311. doi: 10.1002/pds.3703. [DOI] [PubMed] [Google Scholar]
- 23.van der Velde RY, Wyers CE, Curtis EM, Geusens PP, van den Bergh JP, de Vries F, Cooper C, van Staa TP, Harvey NC. Secular trends in fracture incidence in the UK between 1990 and 2012. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, Javaid MK, Judge A. Anti-Osteoporosis Medication Prescriptions and Incidence of Subsequent Fracture Among Primary Hip Fracture Patients in England and Wales: An Interrupted Time-Series Analysis. J Bone Miner Res. 2016;31:2008–2015. doi: 10.1002/jbmr.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden : A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Archives of osteoporosis. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiligsmann M, Salas M, Hughes DA, Manias E, Gwadry-Sridhar FH, Linck P, Cowell W. Interventions to improve osteoporosis medication adherence and persistence: a systematic review and literature appraisal by the ISPOR Medication Adherence & Persistence Special Interest Group. Osteoporos Int. 2013;24:2907–2918. doi: 10.1007/s00198-013-2364-z. [DOI] [PubMed] [Google Scholar]
- 27.Salter C, McDaid L, Bhattacharya D, Holland R, Marshall T, Howe A. Abandoned acid? Understanding adherence to bisphosphonate medications for the prevention of osteoporosis among older women: a qualitative longitudinal study. PloS one. 2014;9:e83552. doi: 10.1371/journal.pone.0083552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82:268–279. doi: 10.1111/bcp.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salter CI, Howe A, McDaid L, Blacklock J, Lenaghan E, Shepstone L. Risk, significance and biomedicalisation of a new population: older women's experience of osteoporosis screening. Social science & medicine (1982) 2011;73:808–815. doi: 10.1016/j.socscimed.2011.06.030. [DOI] [PubMed] [Google Scholar]