Abstract
BACKGROUND
There is ongoing search for new noninvasive biomarkers to improve management of patients with hepatocellular carcinoma (HCC). Studies, mostly from the Asian-Pacific region, demonstrated differential expression of liver-specific microRNA-122 (miR-122) in tissue as well as in sera of patients with hepatitis B virus- and hepatitis C virus-induced HCC.
AIM
To evaluate prognostic value of miR-122 in patients with HCC in a European population and determine potential factors related to alteration of miR-122 in sera.
METHODS
Patients with confirmed HCC (n = 91) were included in the study over a two-year period. Patients were characterized according to Child-Pugh score, Barcelona clinic liver cancer (BCLC) staging system, etiology of liver disease, laboratory parameters and overall survival. MiR-122 was measured in sera using TaqMan assay normalized to spiked-in cel-miR-39.
RESULTS
Serum miR-122 quantity was independent of the Child-Pugh score, the BCLC stage or the underlying etiology. Significant positive correlation was found between miR-122 and alanine aminotransferase (P < 0.0001), aspartate aminotransferase (P = 0.0001), alpha-fetoprotein (AFP) (P = 0.0034) and hemoglobin concentration (P = 0.076). Negative correlation was observed between miR-122 level and creatinine concentration (P = 0.0028). AFP, Child-Pugh score and BCLC staging system were associated with survival differences. In overall cohort low miR-122 in sera was only associated with a trend for a better overall survival without reaching statistical significance. Subgroup analysis revealed that low miR-122 was significantly associated with better prognosis in patients with advanced cirrhosis (Child-Pugh class B/C), advanced tumor stage (BCLC B/C/D) and normal AFP (< 7 ng/mL).
CONCLUSION
Our results strongly support the value of miR-122 as potential biomarker of liver injury and probably prognosis. Nevertheless, the value of miR-122 in prediction of prognosis of HCC patients was limited to certain patients’ subgroups. Since circulating miR-122 may be influenced by impaired renal function, AFP and hemoglobin concentration, those factors need to be considered while interpreting miR-122 level.
Keywords: Hepatocellular carcinoma, MicroRNA, Prognosis, MicroRNA-122, Influencing factors
Core tip: Small non-coding RNAs are in focus of liver biomarker research. Here we confirm that the most abundant liver-specific microRNA-122 (miR-122) is a potential biomarker for liver injury and has potential value to predict the outcome of patients with hepatocellular carcinoma, but several influencing factors need to be taken into account while interpreting the miR-122 level. Besides clinical aspects, several coexisting factors like impairment of renal function, hemoglobin concentration, alpha-fetoprotein level and liver injury may strongly influence circulating miR-122 level and potential clinical translational application of miR-122.
INTRODUCTION
Hepatocellular carcinoma (HCC) is among the most common cancers with high mortality risk. The incidence is rising because of an increasing prevalence of chronic liver injury related to dietary and environmental factors[1,2]. Majority of HCC is developed in patients with liver cirrhosis. Prognosis of patients with HCC is strongly dependent on liver function as well as related complications of liver disease. Several different scores have been developed to estimate prognosis of HCC patients [e.g., Barcelona Clinic Liver Cancer (BCLC) staging system[3], Okuda staging system[4], CLIP score[5]]. Most widely used is the BCLC staging system[3], which estimates prognosis based on morphology of the tumor and the clinical presentation (liver function, portal vein thrombosis among others) without taking molecular biology into account. The BCLC staging system has been validated in multiple studies, but has limitations in the prognostic assessment of patients with intermediate or advanced HCC stages[6,7].
There is a need for biomarkers to optimize the prognostic assessment in HCC patients which would contribute to personalized management. So far, the only biomarker of HCC with world-wide clinical application is alpha-fetoprotein (AFP). AFP is broadly implemented for surveillance of patients at high-risk for developing HCC. Clear recommendations to applicability of AFP for prognostic assessment are still lacking[8]. Different molecules such as AFP, des-γ-carboxyprothrombin, Lens culinaris agglutinin-reactive AFP, Insulin-like growth factor-1, vascular endothelial growth factor, and Angiopoetin 2 were also evaluated regarding their prognostic value but have not made it into routine clinical management as individual parameters[9], although have been included into prognostic staging systems (e.g., CLIP score[5], ALBI grade[10], BALAD-2[11]).
MicroRNAs (miRNA) are still a relatively new class of molecules that show exceptional stability against degradation[12]. Alterations in miRNA expression pattern in liver tissue have been shown in various liver diseases and HCC[13-15]. Equally, variation of miRNA in serum and plasma were shown in different liver diseases[16-18]. MiR-122 is a liver-specific miRNA and with an average expression of 52% it is the most common miRNA in human liver tissue[19,20]. MiR-122 has been shown to play a crucial role in hepatitis C virus infection[21]. Chronic inflammation, for instance chronic hepatitis B virus, alcohol damage or non-alcoholic steatohepatitis, is associated with reduced miR-122 expression in hepatocytes[13,22,23]. Several studies suggested that the deregulation of miR-122 is associated with an aggressive type of HCC[24-26]. Overall, a reduced level of tissue miR-122 was shown in HCC compared to non-tumorous tissue[27-29]. However, opposite miR-122 behavior was described in plasma or sera of HCC patients compared to healthy people[17,30,31].
Several attempts to integrate miR-122 into various algorithms for HCC diagnosis have been made based on tissue[14,15,32] or blood analyses[16,17,30]. But it is clear that miRNA biogenesis follows its own cascade and it is crucial to characterize and identify potential influencing factors in order to implement miR-122 in clinical settings. Furthermore, systematic review of the literature revealed that there is a high heterogeneity of miRNA-biomarker studies related to technical, methodological aspects and quality reporting, which may affect the applicability and reproducibility of generated data[33].
Aim of our study was to evaluate the prognostic value of serum miR-122 in patients with HCC in a European cohort. In addition, we aimed to identify potential liver disease-, tumor-related or other factors that may influence circulating miR-122 level in HCC patients.
MATERIALS AND METHODS
Study design
We analyzed miRNA level in retrospectively collected serum samples (January 2009-April 2011, n = 91) from well characterized patients with histologically or clinically confirmed HCC. The study was performed according to the World Medical Association “Declaration of Helsinki – Ethical Principles for medical research involving human subjects” and approved by the local Institutional Review Board of Otto-von-Guericke University Magdeburg (Number: 99/10). All patients provided written informed consent prior inclusion in the primary study.
Description of the patients
Patient characteristics are presented in Table 1. In comparison to existing data, this cohort consisted of HCC patients with mostly alcohol-related liver damage (45.1%). After blood sampling, all patients were characterized with respect to clinical and laboratory parameters and Child-Pugh score and the BCLC stage were documented. We used survival data to evaluate the prognosis of HCC patients. The overall survival time was defined as the time between inclusion into our study (blood withdrawal) and death or the last documented contact to the patient.
Table 1.
Clinical and laboratory characteristics of the patients with hepatocellular carcinoma, n (%)
| Characteristics | Value |
| Patient number | 91 |
| Gender | |
| Women | 17 (18.7) |
| Men | 74 (81.3) |
| Age in yr, mean ± SD | 67.91 ± 8.98 |
| Etiology | |
| Alcohol abuse | 41 (45.1) |
| Viral hepatitis | 12 (13.2) |
| NASH | 13 (14.3) |
| Hemochromatosis | 6 (6.6) |
| Rare or other cause | 19 (20.8) |
| BCLC stage | |
| 0 | 0 (0.0) |
| A | 16 (17.6) |
| B | 37 (40.6) |
| C | 32 (35.2) |
| D | 6 (6.6) |
| Child-Pugh score | |
| No liver cirrhosis | 16 (17.6) |
| A | 45 (49.4) |
| B | 27 (29.7) |
| C | 3 (3.3) |
| Treatment | |
| Therapy naive | 26 (28.6) |
| Pretreated | 65 (71.4) |
NASH: Non-alcoholic steatohepatitis; BCLC: Barcelona clinic liver cancer.
Extraction of total RNA
After centrifugation and taking the supernatant serum samples were stored by ﹣80°C. Extraction of total RNA (including miRNA) was performed using miRNeasy Mini Kit (QIAGEN, Hilden, Germany) as previously described[34]. One hundred microliter of serum were added to 700 µL QIAzol Lysis Reagent and were homogenized in vortex mixer. Five μL of a 5 nmol/L cel-miR-39 (miR-39) were added for internal normalization. Following precipitation and washing steps, RNA was finally eluted in 30 µL RNase free water. UV-Spectrophotometry was used for analysis of RNA quality.
Reverse transcription and polymerase chain reaction
Reverse transcription was performed using TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, United States). Quantitative real time PCR (qPCR) (TaqMan® Universal Master Mix II, no UNG, Applied Biosystems, Foster City, CA, United States) was performed for miR-122 (Assay ID: 002245) and cel-miR-39 (Assay ID: 000200) according to the manufacturer’s instructions. The analyses were performed on the BioRad CFX Cycler System (BioRad, Hercules, CA, United States). Cel-miR-39 was used for normalization of miR-122 with method. All analysis were performed in duplicates and samples with known quantity were used for interplate normalization.
Statistical methods
GraphPad Prism® Version 6.0 (GraphPad Software, San Diego, CA, United States) was used for statistical analysis. Two-sided P value ≤ 0.05 was considered as significant. Based on the data distribution, we used nonparametric tests (Spearman correlation, Mann-Whitney test, Kruskal-Wallis test, Post-hoc Dunn’s test). The data are shown as boxplots with whiskers for the minimum and maximum, a lower and upper quartile and the median. Overall survival was analyzed using Kaplan-Meier survival curves and comparison was performed using nonparametric log-rank test.
RESULTS
Serum miR-122 is independent of Child-Pugh score, etiology and BCLC stage
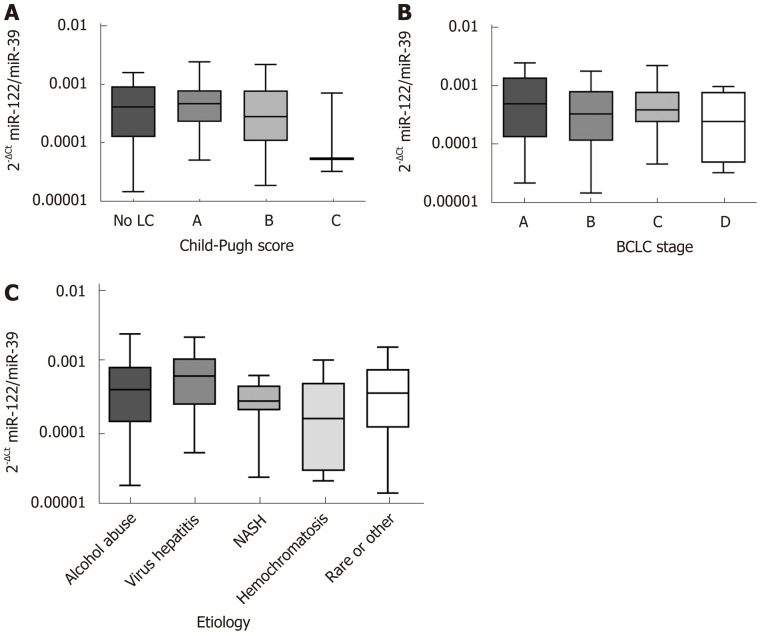
Liver function, etiology of liver disease and accordingly tumor stage may be important factors that could impact miR-122 quantity in serum. Analysis of sera samples from patients with different Child-Pugh scores revealed no significant differences of miR-122 between different stages (P = 0.3060) (Figure 1A). In similar manner, we observed no significant differences of the serum miR-122 level with regard to BCLC staging system (P = 0.5289) or underlying etiology of liver disease (P = 0.2456) (Figure 1B and C).
Figure 1.
Serum microRNA-122 level in correlation to Child-Pugh score, Barcelona clinic liver cancer stage and underlying etiology. A: Serum microRNA-122 (miR-122) level in relation to Child-Pugh score: No liver cirrhosis (n = 16), class A (n = 45), class B (n = 27), and class C (n = 3); B: Serum miR-122 level in relation to Barcelona clinic liver cancer staging system: Stage A (n = 16), stage B (n = 37), stage C (n = 32), and stage D (n = 6); C: Serum miR-122 level in relation to underlying etiology of the hepatocellular carcinoma: Alcohol abuse (n = 41), viral hepatitis (n = 12), non-alcoholic steatohepatitis (n = 13), hemochromatosis (n = 6), rare or other (n = 19). Kruskal-Wallis test and post-hoc Dunn’s test were used for statistical analysis. LC: Liver cirrhosis; NASH: Non-alcoholic steatohepatitis; BCLC: Barcelona clinic liver cancer.
Correlation between serum miR-122 and AFP, hepatocellular damage, renal function and hemoglobin level
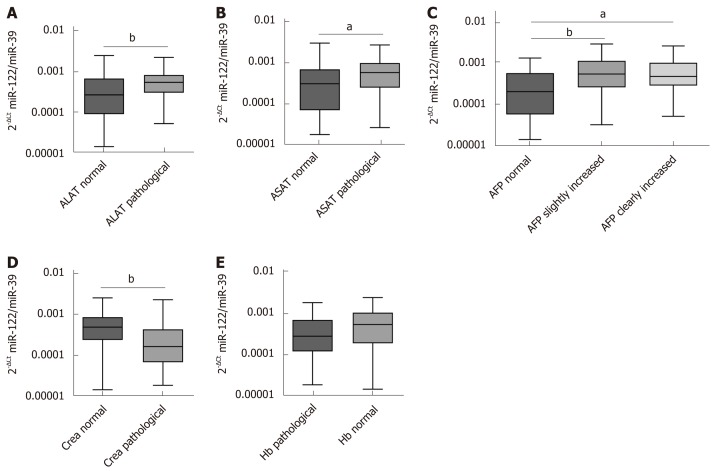
To estimate possible influencing factors that may impact miR-122 concentration in sera, we analyzed various laboratory parameters in correlation to miR-122. Our analysis revealed positive association between miR-122 and alanine aminotransferase (ALAT) (r = 0.4731, P < 0.0001) and aspartate aminotransferase (ASAT) (r = 0.3937, P = 0.0001). In both cases miR-122 level was higher in the group with pathologically elevated transaminases (ALAT: P = 0.0050, ASAT: P = 0.0214) (Figure 2A and B). Next, in patients with HCC elevated AFP was associated with elevated miR-122 concentration (r = 0.3043, P = 0.0034). After subdividing patients into a group with normal (< 7 ng/mL), with slightly increased (7 ng/mL ≤ AFP ≤ 400 ng/mL) and with strongly increased AFP (> 400 ng/mL), we observed significant differences between the group with normal AFP compared to both groups with increased AFP values (P = 0.0071 and P = 0.0144), while no difference was observed between both AFP-elevated groups (Figure 2C). Impairment of the renal function is a frequent consequence of the chronic advanced liver disease. Negative association was observed between miR-122 and creatinine levels (r = ﹣0.3100, P =0.0028), where patients with pathological creatinine value had lower miR-122 levels (P = 0.0027) (Figure 2D). Also, an anemia is a common event in patients with cancer. Despite positive association between miR-122 and hemoglobin levels (r = 0.2783, P = 0.0076), there was only a non-significant trend for lower miR-122 in patients with anemia compared to subjects with normal hemoglobin values (P = 0.0618) (Figure 2E). Supplemental Tables 1 and 2 show analysis of additional parameters that did not show significant differences.
Figure 2.
Relation between serum microRNA-122 level and other laboratory parameters. A: Differences of serum microRNA-122 (miR-122) in relation to alanine aminotransferase (ALAT) [ALAT normal: ≤ 0.58 (w) / 0.83 (m) μmol/Ls, ALAT pathological: > 0.58 (w) / 0.83 (m) μmol/Ls]; B: Differences of serum miR-122 in relation to aspartate aminotransferase (ASAT) [ASAT normal: ≤ 0.58 (w) / 0.83 (m) μmol/Ls, ASAT pathological: > 0.58 (w) / 0.83 (m) μmol/Ls]; C: Differences of serum miR-122 in relation to alpha-fetoprotein (AFP) (AFP normal: AFP < 7 ng/mL, AFP slightly increased: 7 ng/mL ≤ AFP ≤ 400 ng/mL, AFP clearly increased: AFP > 400 ng/mL); D: Differences of serum miR-122 in relation to creatinine [Crea normal: ≤ 84 (w) / 104 (m) µmol/L, Crea pathological: > 84 (w) / 104 (m) µmol/L]; E: Differences of serum miR-122 in relation to hemoglobin [Hb normal: ≥ 7.4 (w) / 8.6 (m) mmol/L, Hb pathological: < 7.4 (w) / 8.6 (m) mmol/L]. Mann-Whitney test, Kruskal-Wallis test and post-hoc Dunn’s test were used for statistical analysis. aP < 0.05 and bP < 0.01 vs normal group, not significant- not shown. ASAT: Aspartate aminotransferase; ALAT: Alanine aminotransferase; AFP: Alpha-fetoprotein; Crea: creatinine.
Survival analysis and prognostic value of serum miR-122
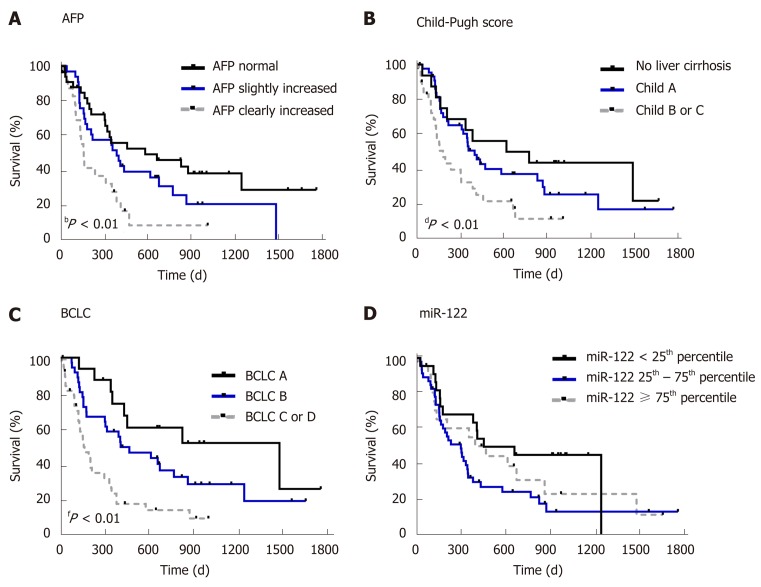
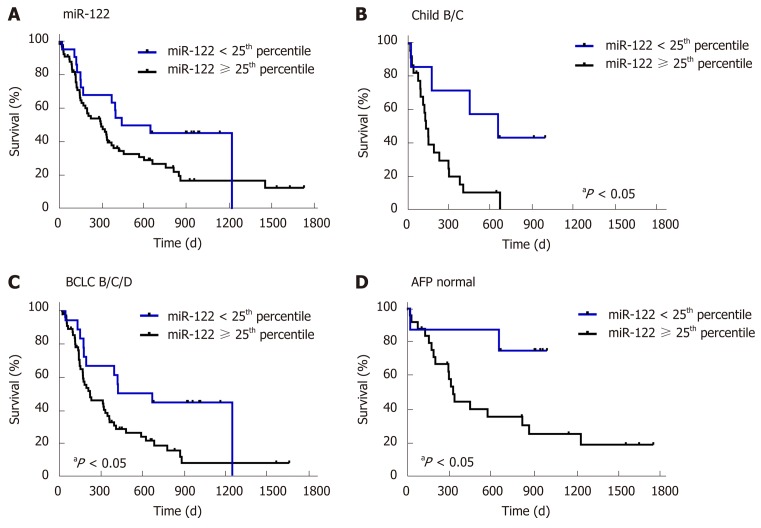
First, to confirm the suitability of survival data in our cohort, we evaluated the impact of known prognostic parameters on survival of HCC patients. As expected, higher AFP level (P = 0.0038), higher Child-Pugh score (P = 0.0129) and higher BCLC stage (P = 0.0001) were all associated with worse overall survival of patients with HCC (Figure 3). To evaluate the prognostic potential of miR-122 in sera, we subdivided our study cohort into three groups by taking the 25th and the 75th percentile which would allow better subdivision. As shown in Figure 3, only a non-significant trend was observed between the groups (P = 0.1019). Based on this observation, we hypothesized that low miR-122, but not intermediate or high, may be of the greatest prognostic value, and therefore, we used 25th percentile as cut-off value for the subsequent analysis. By applying this subgrouping of patients the statistical trend was improved but with P = 0.0610 remain non-significant suggesting that additional factors may influence the performance of miR-122 as prognostic biomarkers (Figure 4A). To address this issue, we performed subgroup analyses based on the Child-Pugh score, BCLC staging and AFP. Interestingly, low miR-122 level was associated with better overall survival in patients with advanced cirrhosis (Child-Pugh B/C) (P = 0.0129) (Figure 4B). In similar fashion, low miR-122 level in patients with BCLC B-D was also associated with better overall survival (P = 0.0157) (Figure 4C). In subgroup of patients with normal AFP, low miR-122 level was also associated with better prognosis (P = 0.0353) (Figure 4D). The results of the subgroup analysis support the potential of miR-122 as potential prognostic biomarker, however, critical attention and consideration of confounding factors need to be considered.
Figure 3.
Survival analysis in relation to alpha-fetoprotein, Child-Pugh score, Barcelona clinic liver cancer stage and serum microRNA-122 level. A: Survival analysis in hepatocellular carcinoma (HCC) patients in relation to alpha-fetoprotein (AFP) [divided into three groups: patients with regular AFP (< 7 ng/mL, n = 33), patients with slightly (7 ng/mL ≤ AFP ≤ 400 ng/mL, n = 33) and patients with clearly increased AFP (> 400 ng/mL, n = 25)]; B: Survival analysis in HCC patients in relation to Child-Pugh score [divided into three groups: patients without cirrhosis (n = 16), patients with Child-Pugh class A (n = 45), and patients with Child-Pugh class B or C (n = 30), class B and class C were summarized, because only a few patients were in the more severe class (n = 3)]; C: Survival analysis in HCC patients in relation to Barcelona clinic liver cancer (BCLC) staging system [divided into three groups: patients with BCLC A (n = 16), with BCLC B (n = 37), with BCLC C or D (n = 38), BCLC C and BCLC D were summarized, because only a few patients were in the more severe stage (n = 6)]; D: Survival analysis in HCC patients in relation to serum miR-122 level [divided into three groups: < 25th percentile (n = 22), 25th-75th percentile (n = 46), ≥ 75th percentile (n = 23)]. Nonparametric log-rank test was used for statistical analysis. bP < 0.01: AFP normal vs AFP slightly increased vs AFP clearly increased; dP < 0.01 No liver cirrhosis vs Child A vs Child B or C; fP < 0.01 BCLC A vs BCLC B vs BCLC C or D. BCLC: Barcelona clinic liver cancer; AFP: Alpha-fetoprotein.
Figure 4.
Survival analysis of subgroups of patients depending on serum microRNA-122 level. A: Survival analysis in relation to serum miR-122 in all patients [divided into two groups: < 25th percentile (n = 22), ≥ 25th percentile (n = 69)]; B: Survival analysis in relation to serum miR-122 in patients with Child-Pugh class B or C [divided into two groups: < 25th percentile (n = 7), ≥ 25% percentile (n = 23)]; C: Survival analysis in relation to serum miR-122 in patients with Barcelona clinic liver cancer stage B/C/D [divided into two groups: < 25th percentile (n = 18), ≥ 25th percentile (n = 57)]; D: Survival analysis in relation to serum miR-122 in patients with normal alpha-fetoprotein (AFP) (AFP < 7 ng/mL) [divided into two groups: < 25th percentile (n = 8), ≥ 25th percentile (n = 25)]. Nonparametric log-rank test was used for statistical analysis. P < 0.05 miR-122 < 25th percentile vs miR-122 ≥ 25th percentile. BCLC: Barcelona clinic liver cancer; AFP: Alpha-fetoprotein.
DISCUSSION
Deregulation of miR-122 has been reported in several studies for patients with HCC; however, translational and clinicopathological value of serum miR-122 in real-life setting is still unknown. In this study, we systematically characterized the prognostic value of serum miR-122 in HCC patients in a European cohort. Although, we observed only a trend for a better prognosis in patients with low miR-122 level in total cohort, we identified several valuable tumor- and liver disease-related factors that may influence miR-122 biogenesis or its biomarker performance. In particular, our data demonstrate strong positive correlation between miR-122 level and biomarkers of liver injury (transaminases ALAT and ASAT, but not liver function), AFP and hemoglobin and negative correlation with renal function.
An association between miR-122 and unspecific liver injury has been previously suggested but exact mechanism remains poorly understood[18,35,36]. A release of miR-122 during hepatocellular damage into blood because of the extraordinary high expression in liver tissue may be the best possible explanation[19,20]. Our results support the assumption showing positive correlation between miR-122 levels and elevated transaminases and potential value of miR-122 as biomarker of hepatocellular damage is also supported by others[35,36]. From another point of view, two previous publications suggested miR-122 as a biomarker of residual liver function in patients with cirrhosis and HCC[37,38] even though we observed no correlation to liver function.
AFP is among the most recognized diagnostic and prognostic biomarkers for HCC[9]. The potential link between AFP and miR-122 has been suggested in a mouse model[23]. Our data also strongly support this positive interaction between serum miR-122 and serum AFP levels. Since negative correlation between AFP and miR-122 has been described in HCC tissue[26], we conclude that the link between AFP and circulating miR-122 may be rather indirect and reflect general liver injury and not HCC-specific alterations.
Among various studied influential factors, kidney function may deserve a key attention potentially affecting miRNA biogenesis. Negative association between total small RNA level and creatinine has been previously described in patients with severe kidney injury[39]. Here we showed that HCC patients with renal impairment have significant lower miR-122 values in serum. Similar result has been shown for patients with liver cirrhosis[38] and in a cohort of critically ill patients[35]. From one side, alterations in liver and renal function may lead to relative dilution of miR-122. From another side, either excretion of miRNAs or stability and degradation of miRNAs in exosomes or protein-bond miRNAs may be affected. In similar fashion, we showed that miR-122 correlates positively with hemoglobin. It is important to mention that secretion of miR-122 has been recently linked to development of inflammation-induced anemia[40]. Simply, isotonic hyperhydration, frequently observed in liver cirrhosis patients, could be another possible explanation for our result.
Having shown the impact of various factors on miR-122 in serum one may question the validity of miR-122 for prognostic assessment. Our data do not support the use of miR-122 as general biomarker for HCC prognosis. However, the subgroup analyses still highlight the potential value in predefined groups, but the knowledge of confounding factors and understanding of related mechanism is crucial. This may explain why several studies have reported the prognostic role of serum miR-122 in HCC patients with contrary results[37,41]. Supporting our results, Liu et al[41] showed that low miR-122 was associated with better prognosis in a cohort with large portion of HCC patients with BCLC B and BCLC C stage.
There is an increasing evidence for the decoupling of miR-122 in tissue and blood[23,35]. Reduced miR-122 expression is described for HCC tissue in human samples[20,27,29]. Higher miR-122 blood values are measured in patients with hepatitis or HCC compared to healthy people[30,31,36]. Following these results, miR-122 level in serum does not allow any statement related to miR-122 expression in liver tissue, where lower expression is associated with worse prognosis[24,42]. Interestingly, preoperative and postoperative miR-122 revealed significant reduction of miR-122 in patients with HCC[31]. This may be related to the reduced liver volume, although the HCC may still be a potential source of circulating miRNAs.
With an increasing number of studies related to miRNAs as biomarkers, our work highlight additional aspects to be considered in the future. There are strong patient-independent factors that may impact comparability between studies[33]. Some of those factors may be related to miRNA extraction and qPCR methods. Also, appropriate normalization is still a great limitation in miRNA-based research. In particular, miR-16 has been used in various studies[31,37,43,44], but it is prone to strong bias in HCC[45] and from our point of view it needs to be abandoned. Nevertheless, our work has several limitations to be mentioned as well. The number of samples may be still too low and larger studies with prospective study design would be needed. Even though our data are close to the real-life setting, the role of miR-122 in HCC patients’ needs to be evaluated in larger cohort of patients with at different diseases stages if possible in prospective manner.
In summary, out data support the unique biomarker value of miR-122 in liver-related diseases and specifically in HCC. Although low miR-122 was associated only with a trend for better prognosis in total cohort of patients with HCC, the prognostic output could be improved by consideration of various factors in subgroup analyses. This work clearly emphasizes the need for accurate assessment of potential systemic cofactors in miRNA-based biomarker research. Serum miR-122 level may be strongly affected by various patient-related conditions including AFP level, liver injury, renal function and anemia. Therefore, future studies with careful and systematic characterization of those potential cofactors are urgently needed.
ARTICLE HIGHLIGHTS
Research background
Hepatocellular carcinoma (HCC) is among the deadliest conditions worldwide. One of the challenges related to HCC is the identification of specific and sensitive diagnostic and prognostic biomarkers. MicroRNAs have been shown to be deregulate in HCC and microRNA-122 (miR-122) is among the most promising liver-specific molecules.
Research motivation
miR-122 has been studied in Asian-Pacific regions but only limited knowledge is available from European population. At present the role of miR-122 as prognostic is not independently validated. Most importantly, it is still less known about potential factors that may influence miR-122 level in blood samples.
Research objectives
Circulating miR-122 may be influenced by impaired renal function, alpha-fetoprotein (AFP) and hemoglobin concentration. Those factors may strongly influence the performance of miR-122 a potential biomarker in HCC.
Research methods
A cohort of well characterized patients with HCC were included in this study. Quantitative TaqMan assay was used to analyze miR-122 in serum and the results were normalized to spiked-in cel-miR-39. The data were stratified based on individual characteristics including liver disease, liver biochemistry, tumor staging and overall survival.
Research results
Overall miR-122 was shown to be independent to Child-Pugh score, Barcelona clinic liver cancer tumor staging classification or etiology of liver disease. Among the studied factors, we identified alanine aminotransferase, aspartate aminotransferase, AFP and renal function (creatinine) as factors that may influence miR-122 level in serum. According to our results, low miR-122 may be associated with lower overall survival, however, only if certain conditions including cirrhosis score, tumor stage or APF are considered.
Research conclusions
The results from this study strongly suggest that renal function and liver inflammation may impact microRNA biomarkers. In particular, a liver-specific miR-122 may be strongly influenced by intrahepatic inflammation which may create potential bias. Our results support the use of miR-122 as a marker for liver inflammation. The value of miR-122 in prediction of overall survival is, however, limited due to the co-existing factors. Further studies will need to determine the mechanism responsible for the influence, but also find a way of controlling the influencing factors in biomarker studies.
Research perspectives
This study clearly highlights the need for better understanding of microRNAs biogenesis in circulation. Since large number of studies focus on microRNAs as potential biomarkers, we urge for better characterization of co-existing factors to identify potential individual influencing factors. Future studies related to miR-122 need to consider also renal function and liver biochemistry.
ACKNOWLEDGEMENTS
We would like to thank Ursula Stolz (from the GI Research Laboratory of the Department of Gastroenterology, Hepatology and Infectious Diseases) for technical support.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the ethical board of the Otto-von-Guericke University.
Informed consent statement: In this study we used retrospectively collected anonymized samples from a previous study. No additional informed consent other than from the primary study was obtained. Ethical committee approved the study protocol.
Conflict-of-interest statement: Alexander Link is principle investigator of the “LiLife”-Project supported by the funds of European Commission through the “Europäischer Fond für regionale Entwicklung” (EFRE) as well as by the regional Ministry of Economy, Science and Digitalization as part of the “Autonomie im Alter” research group. Other authors declare that they have no potential conflicts of interest.
Peer-review started: September 2, 2019
First decision: October 14, 2019
Article in press: December 13, 2019
P-Reviewer: Luo GH, Niu ZS, Zhu F S-Editor: Tang JZ L-Editor: A E-Editor: Zhang YL
Contributor Information
Martin Franck, Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University Magdeburg, Magdeburg 39120, Germany; Department of Gastroenterology, Hepatology and Endocrinology, Hannover Medical School, Hannover 30625, Germany.
Kerstin Schütte, Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University Magdeburg, Magdeburg 39120, Germany; Department of Internal Medicine and Gastroenterology, Niels-Stensen-Kliniken Marienhospital, Osnabrück 49074, Germany.
Peter Malfertheiner, Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University Magdeburg, Magdeburg 39120, Germany.
Alexander Link, Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University Magdeburg, Magdeburg 39120, Germany. alexander.link@med.ovgu.de.
Data sharing statement
Technical appendix and dataset available from the corresponding author at alexander.link@med.ovgu.de. Participants gave informed consent for data analysis and publication. Since no patients consent to data sharing was obtained, no additional data will be made available. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.The Cancer of the Liver Italian Program (Clip) Investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 6.Huitzil-Melendez FD, Capanu M, O'Reilly EM, Duffy A, Gansukh B, Saltz LL, Abou-Alfa GK. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28:2889–2895. doi: 10.1200/JCO.2009.25.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 8.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Schütte K, Schulz C, Link A, Malfertheiner P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis. World J Hepatol. 2015;7:139–149. doi: 10.4254/wjh.v7.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox R, Berhane S, Teng M, Cox T, Tada T, Toyoda H, Kumada T, Kagebayashi C, Satomura S, Johnson PJ. Biomarker-based prognosis in hepatocellular carcinoma: validation and extension of the BALAD model. Br J Cancer. 2014;110:2090–2098. doi: 10.1038/bjc.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Link A, Goel A. MicroRNA in gastrointestinal cancer: a step closer to reality. Adv Clin Chem. 2013;62:221–268. doi: 10.1016/b978-0-12-800096-0.00006-8. [DOI] [PubMed] [Google Scholar]
- 13.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 15.Ura S, Honda M, Yamashita T, Ueda T, Takatori H, Nishino R, Sunakozaka H, Sakai Y, Horimoto K, Kaneko S. Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology. 2009;49:1098–1112. doi: 10.1002/hep.22749. [DOI] [PubMed] [Google Scholar]
- 16.Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, Shen HB, Zhang CY, Zen K. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798–9807. doi: 10.1158/0008-5472.CAN-10-1001. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, Wang JF, Zhang Z, Lu S, Huang X, Wang Z, Qiu S, Wang X, Yang G, Sun H, Tang Z, Wu Y, Zhu H, Fan J. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–4788. doi: 10.1200/JCO.2011.38.2697. [DOI] [PubMed] [Google Scholar]
- 18.Ward J, Kanchagar C, Veksler-Lublinsky I, Lee RC, McGill MR, Jaeschke H, Curry SC, Ambros VR. Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proc Natl Acad Sci USA. 2014;111:12169–12174. doi: 10.1073/pnas.1412608111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y, Tian W, Zhang Q, Wang C, Zhang Q, Zhuang S-M, Zheng L, Liang A, Tao W, Cao X. Identification of miRNomes in Human Liver and Hepatocellular Carcinoma Reveals miR-199a/b-3p as Therapeutic Target for Hepatocellular Carcinoma. Cancer Cell. 2011;19:232–243. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Henke JI, Goergen D, Zheng J, Song Y, Schüttler CG, Fehr C, Jünemann C, Niepmann M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Qiu L, Yan X, Jin W, Wang Y, Chen L, Wu E, Ye X, Gao GF, Wang F, Chen Y, Duan Z, Meng S. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1) -modulated P53 activity. Hepatology. 2012;55:730–741. doi: 10.1002/hep.24809. [DOI] [PubMed] [Google Scholar]
- 23.Ambade A, Satishchandran A, Szabo G. Alcoholic hepatitis accelerates early hepatobiliary cancer by increasing stemness and miR-122-mediated HIF-1α activation. Sci Rep. 2016;6:21340. doi: 10.1038/srep21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–3536. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, Hsu MT, Hsiao M, Huang HD, Tsou AP. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–1582. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- 26.Kojima K, Takata A, Vadnais C, Otsuka M, Yoshikawa T, Akanuma M, Kondo Y, Kang YJ, Kishikawa T, Kato N, Xie Z, Zhang WJ, Yoshida H, Omata M, Nepveu A, Koike K. MicroRNA122 is a key regulator of α-fetoprotein expression and influences the aggressiveness of hepatocellular carcinoma. Nat Commun. 2011;2:338. doi: 10.1038/ncomms1345. [DOI] [PubMed] [Google Scholar]
- 27.Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E, Grazi GL, Croce CM, Bolondi L, Negrini M. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–6099. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- 28.Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H, Yadav A, Nuovo G, Kumar P, Ghoshal K. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284:32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, Jacob ST, Ghoshal K. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99:671–678. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C, Lin D. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136–142. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- 31.Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6:e28486. doi: 10.1371/journal.pone.0028486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Xie L, He X, Li J, Tu K, Wei L, Wu J, Guo Y, Ma X, Zhang P, Pan Z, Hu X, Zhao Y, Xie H, Jiang G, Chen T, Wang J, Zheng S, Cheng J, Wan D, Yang S, Li Y, Gu J. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616–1622. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 33.Link A, Kupcinskas J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: Current insights and future perspectives. World J Gastroenterol. 2018;24:3313–3329. doi: 10.3748/wjg.v24.i30.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schönauen K, Le N, von Arnim U, Schulz C, Malfertheiner P, Link A. Circulating and Fecal microRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2018;24:1547–1557. doi: 10.1093/ibd/izy046. [DOI] [PubMed] [Google Scholar]
- 35.Roderburg C, Benz F, Vargas Cardenas D, Koch A, Janssen J, Vucur M, Gautheron J, Schneider AT, Koppe C, Kreggenwinkel K, Zimmermann HW, Luedde M, Trautwein C, Tacke F, Luedde T. Elevated miR-122 serum levels are an independent marker of liver injury in inflammatory diseases. Liver Int. 2015;35:1172–1184. doi: 10.1111/liv.12627. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, Fei M, Sun S. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010;56:1830–1838. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 37.Köberle V, Kronenberger B, Pleli T, Trojan J, Imelmann E, Peveling-Oberhag J, Welker MW, Elhendawy M, Zeuzem S, Piiper A, Waidmann O. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur J Cancer. 2013;49:3442–3449. doi: 10.1016/j.ejca.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Waidmann O, Köberle V, Brunner F, Zeuzem S, Piiper A, Kronenberger B. Serum microRNA-122 predicts survival in patients with liver cirrhosis. PLoS One. 2012;7:e45652. doi: 10.1371/journal.pone.0045652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neal CS, Michael MZ, Pimlott LK, Yong TY, Li JY, Gleadle JM. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3794–3802. doi: 10.1093/ndt/gfr485. [DOI] [PubMed] [Google Scholar]
- 40.Rivkin M, Simerzin A, Zorde-Khvalevsky E, Chai C, Yuval JB, Rosenberg N, Harari-Steinfeld R, Schneider R, Amir G, Condiotti R, Heikenwalder M, Weber A, Schramm C, Wege H, Kluwe J, Galun E, Giladi H. Inflammation-Induced Expression and Secretion of MicroRNA 122 Leads to Reduced Blood Levels of Kidney-Derived Erythropoietin and Anemia. Gastroenterology. 2016;151:999–1010. doi: 10.1053/j.gastro.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 41.Liu M, Liu J, Wang L, Wu H, Zhou C, Zhu H, Xu N, Xie Y. Association of serum microRNA expression in hepatocellular carcinomas treated with transarterial chemoembolization and patient survival. PLoS One. 2014;9:e109347. doi: 10.1371/journal.pone.0109347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM, Tavolari S, Chieco P, Negrini M, Bolondi L. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Bu X, Dai C, Shang C. High serum microRNA-122 level is independently associated with higher overall survival rate in hepatocellular carcinoma patients. Tumour Biol. 2015;36:4773–4776. doi: 10.1007/s13277-015-3128-5. [DOI] [PubMed] [Google Scholar]
- 44.Cho HJ, Kim JK, Nam JS, Wang HJ, Lee JH, Kim BW, Kim SS, Noh CK, Shin SJ, Lee KM, Cho SW, Cheong JY. High circulating microRNA-122 expression is a poor prognostic marker in patients with hepatitis B virus-related hepatocellular carcinoma who undergo radiofrequency ablation. Clin Biochem. 2015;48:1073–1078. doi: 10.1016/j.clinbiochem.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Qu KZ, Zhang K, Li H, Afdhal NH, Albitar M. Circulating microRNAs as biomarkers for hepatocellular carcinoma. J Clin Gastroenterol. 2011;45:355–360. doi: 10.1097/MCG.0b013e3181f18ac2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix and dataset available from the corresponding author at alexander.link@med.ovgu.de. Participants gave informed consent for data analysis and publication. Since no patients consent to data sharing was obtained, no additional data will be made available. The data that support the findings of this study are available from the corresponding author upon reasonable request.