Abstract
The failure of a massive influx of tumor-infiltrating T lymphocytes to eradicate tumor cells in the tumor microenvironment is mainly due to the dysfunction of T cells hyporesponsive to tumors. T-cell exhaustion and senescence induced by malignant tumors are two important dysfunctional states that coexist in cancer patients, hindering effective antitumor immunity and immunotherapy and sustaining the suppressive tumor microenvironment. Although exhausted and senescent T cells share a similar dysfunctional role in antitumor immunity, they are distinctly different in terms of generation, development, and metabolic and molecular regulation during tumor progression. Here, we discuss the unique phenotypic and functional characteristics of these two types of dysfunctional T cells and their roles in tumor development and progression. In addition, we further discuss the potential molecular and metabolic signaling pathways responsible for the control of T-cell exhaustion and senescence in the suppressive tumor microenvironment. Understanding these critical and fundamental features should facilitate rethinking the unresponsiveness to current immunotherapies in clinical patients and lead to further development of novel and effective strategies that target different types of dysfunctional T cells to enhance cancer immunotherapy.
Keywords: T-cell dysfunction, Exhaustion, Senescence, Tumor microenvironment, Inhibitory receptor, Metabolism, Checkpoint blockade
Subject terms: Tumour immunology, Immune tolerance
Introduction
It is well established that the immunosuppressive tumor network with antagonistic cross talk between malignant cells and host immune cells poses a pivotal hurdle for successful tumor immunotherapy.1 Although large numbers of tumor-infiltrating lymphocytes (TILs), including CD4+ and CD8+ T cells, preferentially migrate to tumor sites to defend against malignancies, these cells display diminished antitumor effector functions in the tumor microenvironment (TME).2 Many studies have reported that high numbers of tumor-specific CD8+ T cells in the TME are converted into functionally hyporesponsive states and consequently fail to eliminate cancer cells and counteract tumor progression.3–5 The paradoxical coexistence of considerable numbers of TILs in the TME and tumor progression in cancer patients further suggests the presence of a dysfunctional state in T cells induced by the immunosuppressive TME, which becomes a challenging issue for antitumor immunity and immunotherapy.6,7 Therefore, a better understanding of the molecular mechanisms and regulatory processes of tumor-induced T-cell dysfunction in the TME is critical for the development of novel and effective strategies to improve tumor immunotherapy.
T-cell exhaustion and senescence are two dominant dysfunctional states that differ phenotypically and functionally from effector and memory states in certain pathological conditions, including in chronic infections and cancers.8–10 The definitions of “senescence” and “exhaustion” remain confusing because both states share overlapping characteristics and are associated with defective effector functions. However, they have distinct regulatory and molecular mechanisms governing their development and impaired antitumor functions.11–15 T-cell exhaustion was initially described in chronic viral infections with increased expression of a panel of inhibitory receptors, including programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte antigen-4 (CTLA-4), T-cell immunoglobulin, and mucin domain containing-3 (Tim-3), lymphocyte activation gene 3 (LAG-3), CD244 (2B4), and CD160.16,17 Other studies suggest that exhausted T cells exist in patients with various types of cancers and these cells show characteristics similar to those in chronic viral infections.18–21 Recent cancer immunotherapy clinical trials targeting these immune checkpoint molecules have shown promising benefits in certain types of cancer patients.22–24 However, the overall success rates for current immune checkpoint blockade therapies are still low, which suggests the existence of other interrupting tolerogenic pathways beyond simply the exhaustion of T cells in the TME. Cellular senescence was first described over five decades ago as a biological process in human diploid fibroblasts with a finite lifetime and low rate of proliferation after extensive serial passages in vitro.25 Recent studies have shown that senescence also occurs in human T cells in patients with chronic viral infections and various types of cancers, which can result in reduced vaccine efficacy and increased susceptibility to viral infections and malignant tumors.9,26–34 These studies strongly suggest that T-cell senescence is an alternative novel mechanism utilized by malignant tumors for immune evasion, which should be an emerging target for tumor immunotherapy.13–15,33,35,36 A better understanding of the molecular mechanisms involved in the generation and functional regulation of T-cell senescence and exhaustion in the TME should open new avenues for cancer immunotherapy. This review explores these two important T-cell dysfunctional states in cancer, focusing on their features, molecular regulation, crucial roles in the TME and therapeutic implications for cancer immunotherapy.
Features of exhausted and senescent T cells
It is widely recognized that exhausted and senescent T cells share several overlapping phenotypic and functional characteristics, such as defective proliferative activity, impaired cytotoxic activity, and increased cell cycle arrest. However, each state has unique molecular and developmental signatures, such as surface molecules, cytokines, and transcriptional profiles (summarized in Table 1).11,16,17,37
Table 1.
Comparison of exhausted and senescent T-cell characteristics.
| Category | Exhaustion | Senescence | References |
|---|---|---|---|
| Cause | Continuous antigenic stimulation | Repetitive stimulation; DNA damage agents; stress signals | 13, 14, 38, 61 |
| Typical feature |
Proliferative activity ↓ Cell cycle arrest: p27, p15 ↑; cyclin E-Cdk2, Cdc25A ↓ |
Proliferative activity ↓ Cell cycle arrest: p16, p21, p53 ↑ DNA damage-associated molecules ↑ Telomere length, telomerase activity ↓ SA-β-gal activity ↑ |
13, 37, 95 |
| Surface marker | PD-1, CTLA-4, Tim-3, LAG-3, BTLA, TIGIT, CD244, CD160, CD39, 4-1BB ↑ |
CD27, CD28 ↓ CD57, KLRG1, Tim-3, TIGIT, CD45RA ↑ |
14, 20, 30, 38–45, 65, 67, 135, 136 |
| TCR signaling machinery | Lck, ZAP70 ↓ | Lck, ZAP70, DLG1, Lat, SLP-76 ↓ | 47, 72 |
| Cytokine profile |
Early stage: IL-2 ↓ Intermediate stage: TNF ↓ Terminal stage: IFN-γ, β-chemokines ↓ |
SASP, Proinflammatory cytokines: IL-6, IL-8, IFN-γ, TNF ↑ Inhibitory factors: IL-10, TGF-β ↑ |
14, 15, 21, 32, 70 |
| Transcriptional profile |
NFAT, Nr4a, Blimp-1, BATF, FoxP3 ↑ Progenitor subset: T-bethigh Eomeslow PD-1int Terminal subset: T-betlow Eomeshigh PD-1high |
FoxP3 ↑ | 26, 48, 54, 55, 58, 59 |
| Epigenetic change | Exhaustion-associated DNA methylation programs | SAHF ↑ | 56, 57, 137 |
| Metabolic alternation |
Glycolysis ↓ Mitochondrial biogenesis ↓ Reactive oxygen species ↑ |
Glycolysis ↑ Mitochondrial biogenesis ↓ Reactive oxygen species ↑ |
112, 126 |
| Functional alteration |
Cytotoxic activity ↓ Effector molecule: GzmB ↓ |
Cytotoxic activity ↓ Suppressive functions ↑ Effector molecules: perforin, GzmB ↓ |
8, 33, 37, 71 |
SA-β-gal senescence-associated β-galactosidase, SAHF senescence-associated heterochromatin foci, SASP senescence-associated secretory phenotype, PD-1 programmed cell death protein 1, CTLA-4 cytotoxic T-lymphocyte antigen-4, Tim-3 T-cell immunoglobulin and mucin domain containing-3, LAG-3 lymphocyte activation gene 3, BTLA B- and T-lymphocyte attenuator, TIGIT T-cell immunoreceptor with Ig and ITIM domains, KLRG1 killer cell lectin-like receptor G1, Lck lymphocyte-specific protein tyrosine kinase, ZAP70 zeta-chain-associated protein kinase 70, DLG1 disks large homolog 1, Lat linker for activation of T cells, SLP-76 SH2 domain-containing leukocyte protein of 76 kD, GzmB granzyme B, NFAT nuclear factor of activated T cell, BATF basic leucine transcription factor, Blimp-1 B lymphocyte-induced maturation protein-1, T-bet T-box transcription factor, Eomes eomesodermin, FoxP3 forkhead box P3, Cdk2 cyclin-dependent kinase 2
Symbols: ↑, increased; ↓, decreased; int, intermediate
Exhausted T cells have been identified to accumulate in patients with chronic infections and cancers. The principal feature of exhausted T cells in the TME is the elevated expression of a panel of inhibitory receptors, including PD-1, CTLA-4, Tim-3, LAG-3, B- and T-lymphocyte attenuator (BTLA), T-cell immunoreceptor with Ig and ITIM domains (TIGIT), the natural killer cell receptor 2B4 (also called CD244), and the glycoprotein CD160.38–43 Furthermore, high expression levels of immunomodulatory E-NTPDase1 (CD39) and the costimulatory molecule 4-1BB have also been shown in exhausted tumor-infiltrating CD8+ T cells.44,45 However, 4-1BB-mediated stimulation and signaling for the proliferation and expansion of exhausted T cells are suppressed due to the lack of 4-1BB ligand in the suppressive TME.46 In addition, PD-1 expression can directly antagonize T-cell receptor (TCR) signaling by preventing the lymphocyte-specific protein tyrosine kinase (Lck)-mediated phosphorylation of zeta-chain-associated protein kinase 70 (ZAP70) (Fig. 1).47 In addition to suppressed T-cell proliferation, the other functional characteristics of exhausted T cells are impaired cytotoxicity and effector cytokine production, including IL-2, TNF, and IFN-γ.21 The development of T-cell exhaustion is dominated by both transcriptional and epigenetic regulations. The transcription factor nuclear factor of activated T cell (NFAT), nuclear receptor Nr4a, and HMG-box transcription factor TOX specifically activate the exhaustion-associated transcriptional program that drives T-cell exhaustion.48–51 Furthermore, the zinc-finger transcription factor Gata-3 is another major driver of dysfunctional CD8+ TILs in cancers.52,53 During chronic LCMV infection, the T-box transcription factor T-bet can be replaced by another T-box protein, Eomesodermin (Eomes), in response to continuous antigenic stimulation in conjunction with high PD-1 expression, resulting in T-cell exhaustion.48,54,55 In addition, exhausted CD8+ TILs progressively acquire DNA methyltransferase 3A (Dnmt3a)-mediated DNA methylation programming, which functions as a cell-intrinsic obstacle in the response to PD-1 blockade therapy.56,57 Recent studies have revealed that exhausted T cells have phenotypic and functional heterogeneity, which allows these cells to be classified into progenitor exhausted T (T-bethighEomeslowPD-1int) and terminally exhausted T (T-betlowEomeshighPD-1high) subsets, based on their distinct functional, epigenetic, and transcriptional states.54,58,59 Progenitor exhausted T-cell subpopulations possess stem-like characteristics allowing them to undergo self-renewal and exclusively provide the proliferative response to PD-1 blockade. Terminally exhausted T cells are differentiated from the progenitor subset by superior cytotoxicity, but they have decreased long-term survival and are unable to respond to anti-PD-1 checkpoint blockade therapy.59 Therefore, a better understanding of the characteristics and functions of different exhausted T-cell subpopulations is critical for improving checkpoint blockade therapy.
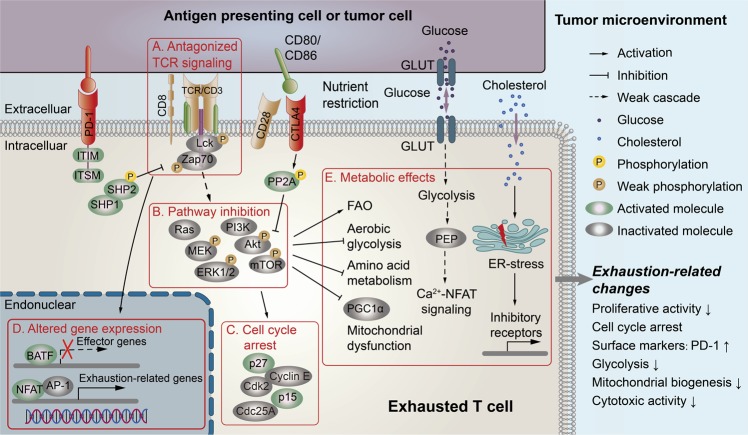
Fig. 1. PD-1 and CTLA4 signaling pathways and metabolic regulations involved in T-cell exhaustion in the tumor microenvironment.
Exhausted T cells in the TME are characterized by the coexpression of multiple inhibitory molecules, including PD-1 and CTLA4. There are several signaling pathways potentially involved in the development of T-cell exhaustion within the TME: a PD-1/PDL1 signaling can antagonize TCR signaling by preventing the phosphorylation of the TCR-proximal signaling molecules Lck and ZAP70. b, c PD-1/PD-L1 signaling inhibits the TCR/CD28-mediated activation of the PI3K/Akt/mTOR and Ras/MEK/ERK pathways to induce cell cycle arrest through p27- and p15-mediated cascades. CTLA-4 binding to CD80/86 activates the phosphatase PP2A, directly inhibits Akt/mTOR signaling and promotes cell cycle arrest in T cells. d PD-1/PDL1 signaling induces the transcription of exhaustion-related molecules and suppresses the transcription of effector molecules regulated by the transcription factors NFAT and BATF, respectively. e The inhibition of PI3K/Akt/mTOR signaling mediated by either PD-1/PD-L1 or CTLA4 results in decreases in glycolysis and amino acid metabolism and suppresses the metabolic regulator PGC1α and mitochondrial biogenesis. Furthermore, glucose deprivation decreases the concentration of the glycolytic metabolite PEP and subsequently inactivates the Ca2+-NFAT signaling pathway to suppress T-cell effector functions. In addition, cholesterol accumulation in the TME induces T-cell exhaustion via the molecular regulation of ER stress. PD-1 programmed cell death protein 1, PD-L1 PD ligand 1, CTLA-4 cytotoxic T-lymphocyte antigen-4, GLUT glucose transporter, TCR T-cell receptor, Lck lymphocyte-specific protein tyrosine kinase, ZAP70 zeta-chain-associated protein kinase 70, ITSM immunoreceptor tyrosine-based switch motif, ITIM immunoreceptor tyrosine-based inhibitory motif, SHP-1/SHP-2 SH2-domain containing tyrosine phosphatases 1/2, NFAT nuclear factor of activated T cell, BATF basic leucine transcription factor, AP-1 activator protein 1, PP2A protein phosphatase 2A, PI3K phosphoinositide 3-kinase, Akt protein kinase B, mTOR mammalian target of rapamycin, MEK mitogen-activated protein/extracellular signal-regulated kinase kinase, ERK extracellular signal-regulated kinase, Cdk2 cyclin-dependent kinase 2, CDC25A cell division 25A, PGC1α PPAR-gamma coactivator 1α, PEP phosphoenolpyruvate, FAO fatty acid β-oxidation, ER endoplasmic reticulum.
Increased senescent T-cell numbers have been found in elderly individuals, causing age-associated dysregulation of the immune system.9,60 Furthermore, the accumulation of senescent CD8+ T cells has also been found in relatively young patients with chronic viral infection, as well as in patients with certain types of cancers.26–31 Cellular senescence processes are triggered by telomere shortening or erosion (termed telomere-dependent senescence or replicative senescence) and/or “damage” signals (termed premature senescence), including oxidative stress, cell culture stress, DNA-damaging chemotherapeutic agents, and mitogenic oncogenes.61 More recent studies suggest that both naturally occurring regulatory T cells (Tregs) and tumor-derived Treg cells can strongly suppress naive/effector T cells through the induction of responder T-cell senescence.13–15,62 In addition, different types of tumor cells can directly convert normal immune cells into senescent T cells.30,32,33 Mechanistically, both tumor cells and Treg cells induce DNA damage responses in responder T cells, resulting in responder cell cycle arrest and senescence13,14,32,33,62 (Fig. 2). These senescent T cells have altered phenotypes, including high expression of senescence-associated-β-galactosidase (SA-β-Gal),14,15,63 dramatically downregulated expression of the costimulatory molecules CD27 and CD28,14,64 and high expression of additional senescence-associated markers, including Tim-3, CD57, CD45RA, and killer cell lectin-like receptor subfamily G member 1 (KLRG-1) (Table 1).65–69 Among changes in these surface markers, significant loss of the costimulatory molecules CD27 and CD28 is the most typical phenotypic change in senescent T cells.13,14,30 Unlike exhausted T cells, senescent T cells acquire a unique senescence-associated secretory phenotype (SASP), producing high amounts of proinflammatory cytokines, such as IL-2, IL-6, IL-8, TNF, and IFN-γ, and the suppressive cytokines IL-10 and TGF-β.14,15,33 In addition, senescent T cells have reduced expression of the effector molecules perforin and granzyme B (GzmB) and possess strong suppressive activity that potently amplifies the immune suppression within the TME.13,14,32,33,62,70,71 Similar to exhausted T cells, senescent T cells do not proliferate after TCR stimulation due to the loss of several key molecules involved in the TCR signaling machinery, including Lck, ZAP70, disks large homolog 1 (DLG1), linker for activation of T cells (Lat), and SH2 domain-containing leukocyte protein of 76 kD (SLP-76).72 Furthermore, senescent T cells have upregulated expression of cell cycle regulatory genes, p16, p21, and p53, and display cell cycle arrest.14,15,33 SASP secretion and growth arrest in senescent T cells are maintained by persistent molecular processes, termed “DNA segments with chromatin alterations reinforcing senescence” (DNA-SCARS).73,74 The roles of exhausted T cells in antitumor immunity and immunotherapy have been well studied in the past several years. However, limited information is known about the functional role of senescent T cells in the TME. Increasing evidence indicates that senescent T cells are critical players in immune suppression and tumor development and progression promotion.14,15,30,32,33 Exploring the functional role of senescent T cells in antitumor immunity and developing novel strategies to target senescent T cells for cancer immunotherapy are urgently needed.
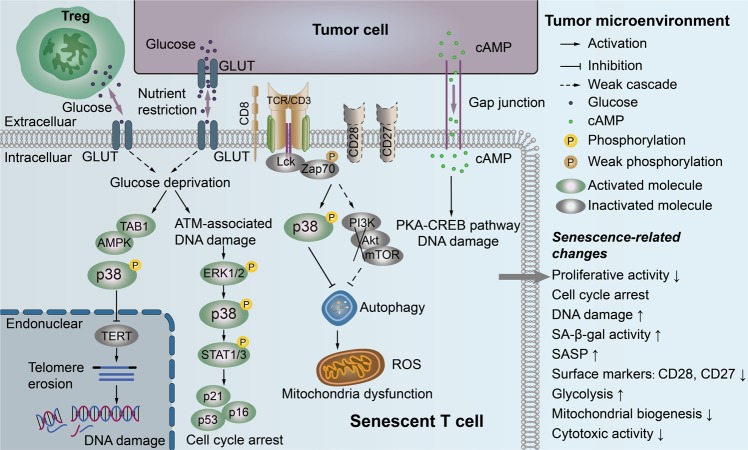
Fig. 2. Molecular pathways involved in T-cell senescence in the tumor microenvironment.
MAPK p38 plays a central role in the regulatory network of T-cell senescence. The glucose deprivation induced by tumor cells and/or Treg cells is attributed to the activation of AMPK-TAB1-dependent p38 activation, which induces TERT downregulation and/or telomere erosion, as well as the DNA damage response. Furthermore, the competition for glucose also triggers the ATM-associated DNA damage response and activates the MAPK ERK1/2 and p38 signaling pathways in T cells that functionally cooperate with the transcription factors STAT1 and STAT3 to induce cell cycle arrest and cell senescence in effector T cells. Tumor- and Treg cell-induced downregulation of CD28 and TCR signaling can activate MAPK p38 signaling or/and inhibit the PI3K-Akt-mTOR axis, subsequently inactivating autophagy and inducing mitochondrial dysfunction and elevated ROS levels in senescent T cells. Tumor-derived cAMP can be directly transferred into target T cells through gap junctions to activate PKA-CREB signaling, resulting in the activation of DNA damage response and induction of senescence in T cells. MAPK mitogen-activated protein kinase, TAB1 TAK1-binding protein 1, AMPK AMP-responsive protein kinase, ATM ataxia telangiectasia mutated, ERK extracellular signal-regulated protein kinase, STAT signal transducer and activator of transcription, cAMP endogenous cyclic adenosine monophosphate, Treg regulatory T cell, TERT telomerase reverse transcriptase, PKA protein kinase A, CREB cAMP response element-binding protein, SASP senescence-associated secretory phenotype, ROS reactive oxygen species.
Exhausted and senescent T cells coexist in the TME to favor tumor progression
It is well recognized that the suppressive tumor microenvironment can lead to an exhaustion profile in TILs and subsequent impairment of TIL effector functions in a variety of cancers, including lymphoma,75 melanoma,18 glioblastoma,76 and breast,77 ovarian,41 prostate,78 liver,79 and lung cancers.80 Accumulating evidence indicates that cancer cells can directly induce T-cell exhaustion during cross talk.81,82 Exhausted T cells in the TME highly express inhibitory receptors, strongly suppressing antitumor immune responses, and maintaining the suppressive microenvironment.38–41 Importantly, increased levels of exhausted T cells in patients are positively associated with a poor prognosis and poor outcomes in various cancers, indicating the important therapeutic implications for the restoration of TIL effector functions by targeting inhibitory receptors in the TME.75,77,80 Furthermore, current immune checkpoint blockade therapies targeting different inhibitory molecules have shown promising results in many types of cancers.22,23
Increased senescent CD8+ T-cell numbers have been found in patient TILs from various types of cancers, including lung cancers,26 colorectal cancer,83 head and neck cancer,27 endometrial cancer,84 ovarian cancer,85 lymphoma,86 hepatocellular carcinoma,65 and breast cancers,87,88 as well as in the metastatic satellite lymph nodes and peripheral blood of cancer patients.26,89 Furthermore, recent studies have demonstrated that both tumor-derived Treg cells and multiple types of tumor cells can also directly induce T-cell senescence.13–15,32,33,62 However, the role of these senescent T cells in tumor pathogenesis is still under investigation. The significant feature of these senescent T cells is the loss of their capacity for antitumor immunity, which is due to the downregulation of the expression of the costimulatory molecules CD27 and CD28, upregulation of the expression of some inhibitory molecules such as Tim-3,14,64–69 and decreased production of the effector molecules perforin and granzyme B.29,64,71 Furthermore, senescent T cells in the TME can directly suppress other immune cells, including T cells and dendritic cells (DCs), or function via bystander mechanisms mediated by the highly produced suppressive cytokines IL-10 and TGF-β1, resulting in amplified immune suppression in cancers.13–15,33 In addition, the unique SASP of senescent T cells may influence immune cells in the TME90,91 and promote cancer initiation and progression.92–94 These studies collectively suggest that exhausted and senescent T-cell populations show remarkable enrichment and coexistence in the circulation and/or tumor sites in cancer patients and that targeting both types of dysfunctional T cells is required for effective antitumor immunity and immunotherapy.
Molecular signaling responsible for T-cell exhaustion and senescence in the TME
Understanding the molecular signaling and pathways that control the generation and development of T-cell exhaustion and senescence in the TME will provide critical insights for the development of novel and effective combination therapies against cancer. Based on current studies, T-cell exhaustion is mainly mediated by inhibitory receptor-associated signaling, whereas T-cell senescence is regulated by mitogen-activated protein kinase (MAPK) signaling.13,14,55,95 Importantly, both of these signaling pathways are reversible processes that can be manipulated to enhance antitumor immunity.
Inhibitory receptor signaling mediates T-cell exhaustion
In the TME, there are multiple immunosuppressive components, such as malignant tumor cells, immunosuppressive cells, inhibitory cytokines, and receptors that can directly or indirectly trigger T-cell exhaustion.96 Among these components, multiple inhibitory receptor–ligand pairs can contribute to T-cell exhaustion by activating coinhibitory signals. The PD-1/PD-L1 pathway (PD-L1, PD ligand 1) is a well-studied inhibitory pathway that controls T-cell activation, and PD-1 is also the predominantly expressed inhibitory molecule in exhausted T cells.97 The regulation of T-cell activation by PD-1/PD-L1 may act through the following molecular processes: (1) tumor microenvironmental PD-L1 (expressed on tumor cells or tumor-derived suppressive immune cells) activates PD-1 on T cells, and SH2-domain containing tyrosine phosphatases (SHP-1 and/or SHP-2) are recruited to the phosphorylated immunoreceptor tyrosine-based switch motif (ITSM) in the cytoplasmic domain of PD-1 in T cells.98 The recruitment of SHP-1 and SHP-2 antagonizes TCR signaling by preventing the phosphorylation of proximal effector molecules such as ZAP70, which drives T-cell exhaustion.47 (2) PD-1 can inhibit the CD28-induced activation of the downstream PI3K/Akt/mTOR and Ras/MEK/ERK pathways, leading to the inhibition of T-cell metabolism and promotion of T-cell cycle arrest.99 (3) The PD-1-mediated pathway establishes an inhibitory loop in T cells to promote cell cycle arrest by increasing the expression of p27 and p15 and suppressing the expression of cyclin E-Cdk2 and Cdc25A.99 (4) With the regulation of activated PD-1 signaling, the transcription factor NFAT can promote the transcription of exhaustion-related genes (e.g., inhibitory receptors) without the cooperation of activator protein 1 (AP-1),50 while the upregulated expression of basic leucine transcription factor (BATF) induces negative transcriptional regulation of various effector genes (e.g., IFN-γ).100 In addition to PD1, CTLA-4 can also target the CD28 and PI3K/Akt/mTOR axis during T-cell activation, but the mechanisms are distinct from those of PD1 and involve activating protein phosphatase 2A (PP2A) to directly inhibit Akt101,102 (Fig. 1, Table 1). Modulating these dysregulated pathways in exhausted T cells by combined blockade of inhibitory receptors, such as coadministration of anti-PD-1/PD-L1 and anti-CTLA-4 antibodies, has been proven to dramatically reverse the exhausted T-cell state and enhance immune responses in patients with various cancers.24,38,97 Notably, recent studies have demonstrated that the PD-1-recruited SHP2 phosphatase strongly prefers the costimulatory receptor CD28 as a target for dephosphorylation, indicating that CD28 costimulation is required for exhausted CD8+ T cell rescue and for effective anti-PD-1 treatment of cancers.35,36 Interestingly, accumulated senescent T cells in the TME exhibit CD28 expression loss, which might be one reason for the unresponsiveness to immune checkpoint blockade therapy.35,36 These studies further highlight the necessity and importance of overcoming T-cell senescence simultaneously during immune checkpoint blockade therapy for cancers.
MAPK signaling controls T-cell senescence
MAPK signaling is important for controlling cellular senescence.103,104 MAPK p38 is involved in central signaling by activating the cell cycle regulatory molecules p53, p21, and p16, which inhibits cell cycle progression to slow or completely arrest DNA replication.103–105 Recent studies suggest that MAPK signaling is specifically important in T-cell senescence.13,14 The metabolic master regulator AMP-responsive protein kinase (AMPK) is preferentially utilized by senescent CD4+ T cells to trigger p38 autophosphorylation by the scaffold protein TAK1-binding protein 1 (TAB1). This “intrasensory“ pathway activates p38 by sensing intracellular changes (e.g., glucose deprivation) and initiates the endogenous DNA damage response (DDR) to signal through the DDR checkpoint kinase ataxia telangiectasia mutated (ATM) under genotoxic stress. It inhibits T-cell proliferation, and downregulates the expression of telomerase reverse transcriptase (TERT) and key components of the TCR signalosome. Furthermore, the sestrin-dependent ERK-JNK-p38 MAPK activation complex controls distinct aspects of T-cell senescence.106 In addition, the senescent T-cell population can regain proliferation and telomerase activity via the inhibition of AMPK, TAB1 and/or p38 activation.72 Our recent work has identified that both naturally occurring Treg and tumor-derived Treg cells can compete with effector T cells for glucose, triggering AMPK activation and the ATM-associated DDR, and thereby induce senescence in T cells.13 Furthermore, ERK1/2 and p38 are selectively phosphorylated and activated during T-cell senescence mediated by Treg cells.13,14,33 In addition, the ATM-associated DDR, MAPK signaling, and STAT1 and STAT3 signaling cooperate together to control T-cell senescence during cross talk with Treg cells.13 These studies provide important molecular targets for the development of effective strategies to control T-cell senescence and restore T-cell effector functions for tumor immunotherapy (Fig. 2).
Metabolic control of T-cell exhaustion and senescence in the TME
Increasing evidence shows that malignant tumor cells and neighboring cells can establish nutrient-deprived conditions (e.g., glucose and amino acid restriction) to metabolically restrict and functionally impair T cells in the TME.107–109 Therefore, metabolic reprogramming is one of the critical tumor microenvironmental factors for controlling the development of both exhausted and senescent T cells in the TME.13,33,62,110
Metabolic regulation of T-cell exhaustion and dysfunction
Exhausted T cells display suppressed glycolysis and oxidative phosphorylation (OXPHOS) and dampened mitochondrial function during chronic infection.111 The metabolic and nutrient restrictions created by malignant tumors can also impair the glycolytic capacity and effector functions of TILs in the TME.107 PD-1/PD-L1 interactions can alter the metabolic program of effector T cells by inhibiting the PI3K/Akt/mTOR and Ras/MEK/ERK pathways and suppressing IFN-γ secretion in exhausted T cells.107,112 Recent transcriptomic analyses have demonstrated that PD-1 engagement in T cells significantly impairs the gene expression of molecules involved in major metabolic processes, including amino acid, nucleotide, carbohydrate metabolism, the Krebs cycle, and OXPHOS.113 Furthermore, PD-1 engagement suppresses glycolysis and OXPHOS and reduces the numbers of mitochondria and mitochondrial cristae.112,113 In contrast, PD-1 ligation promotes the fatty acid β-oxidation (FAO) of endogenous fatty acids and lipolysis to improve the efficiency of FAO usage via upregulation of the expression of the key enzymes carnitine palmitoyl transferase (CPT1A) and adiposite triglyceride lipase (ATGL).112,113 However, CTLA-4 signaling inhibits glycolysis without inducing FAO alterations in T cells.112 Therefore, PD-1 ligation impedes T-cell effector functions in response to glucose deprivation by limiting aerobic glycolysis and amino acid metabolism but promoting fatty acid metabolism.
Several recent studies also facilitate a better understanding of the molecular and metabolic processes that occur during the development of T-cell exhaustion and dysfunction in the TME.114 It has been identified that the mitochondrial biogenesis regulator PPAR-gamma coactivator 1α (PGC1α) is a key checkpoint molecule regulating T-cell exhaustion.107,109,111,115 PD-1 can repress the expression of PGC1α and subsequently induce T-cell metabolic exhaustion via Akt/mTOR signaling, leading to a decreased mitochondrial mass and morphological and functional defects. Both anti-PD-1 antibody immunotherapy and PGC1α overexpression can rescue T-cell metabolism and improve the metabolic fitness of exhausted T cells in experimental tumor models.107,109,111,115 Furthermore, enhanced antitumor immunity mediated by 4-1BB costimulation is due to the promotion of the mitochondrial capacity and PGC1α expression in exhausted T cells via p38MAPK signaling.46
In addition, in the glucose-deficient TME, the accumulation of various cancer-generated metabolites, such as IDO, adenosine and lactate, is involved in the regulation of T-cell dysfunction to limit antitumor immunity.114,116–118 These hypoxia-derived metabolites are potent immune suppressors that can protect tumor cells from T cell-mediated antitumor immune responses.117,119 Lactate is the main metabolite of glycolysis utilized by malignant tumor cells.120,121 Intracellular lactate can trigger the phosphorylation/degradation of IκB-α and subsequently stimulate the NF-κB pathway for T cell suppression.122 In addition, lactate dehydrogenase A (LDHA)-associated lactic acid accumulation inhibits both T cells and NK cells, leading to tumor immune escape.123 The glycolytic metabolite phosphoenolpyruvate (PEP) has been shown to sustain tumor-specific CD4+ and CD8+ T-cell activation via regulation of Ca2+-NFAT signaling, and upregulating PEP production can enhance T-cell effector functions and promote overall animal survival in a melanoma tumor model.124 The glucose restriction in the TME of ovarian cancer inhibits the expression of the methyltransferase EZH2 in T cells, dampening T-cell polyfunctionality and survival mediated via miRNA-EZH2-Notch signaling.125 However, whether and how these glycolytic metabolites regulate T-cell exhaustion is still unclear. In addition to regulating glucose metabolism, tumor-derived cholesterol increases endoplasmic reticulum (ER) stress, resulting in the expression of PD-1 and 2B4 in T cells and causing T-cell exhaustion in the TME110 (Fig. 1). These studies clearly indicate that checkpoint blockade therapy can not only block the intrinsic negative signals between tumor cells and T cells but also rescue the metabolic processes of T cells to favor T-cell effector functions for antitumor immune responses.
Metabolic control of senescent T-cell development
Developed senescent CD8+ T cells are preferentially dependent on anaerobic glycolysis to produce energy rather than flexible mixed utilization of glycolysis and OXPHOS, resulting in mitochondrial dysfunction and increased production of reactive oxygen species (ROS).126 The mitochondrial disruption and ROS accumulation in senescent CD8+ T cells may molecularly result from the activation of p38 and inhibition of the PI3K/Akt/mTOR axis to regulate autophagy.95,126 However, limited information is known about the metabolic reprogramming that occurs during senescent T-cell development within the TME. Recent studies have demonstrated that both nTreg cells and tumor-derived Treg cells exhibit heightened glucose uptake and glycolysis capacities compared with other T-cell subsets (Th1, Th2, and Th17 cells).62 Furthermore, Treg cells exhibit accelerated glucose consumption and compete with responding effector T cells, initiating ATM-associated DNA damage and inducing senescence in the responder T cells.13,62 In addition to direct competition for glucose consumption, glucose metabolites may be involved in the regulation of T-cell senescence. It has been shown that cAMP is a critical component of the tumor-induced hypoxic microenvironment and a potent inhibitor of functional tumor-specific effector T cells117.[,127 Studies have identified that different types of tumor cells can also induce senescence in responder T cells.32,33 Tumor cells produce high amounts of cAMP, which can be transferred to responder T cells via gap junctions, resulting in the activation of the cAMP-induced PKA-CREB pathway and ATM-associated DNA damage response and the eventual induction of T-cell senescence.33,128,129 To identify strategies to prevent T-cell senescence for tumor immunotherapy, relatively recent studies have shown that the TLR8 signaling pathway can reverse the T-cell senescence induced by both human Treg cells and tumor cells.14,33,62 TLR8 signaling activation in Treg cells selectively inhibits glucose uptake, transport, and glycolysis in Treg cells, resulting in the reversal of Treg suppression and induction of T-cell senescence.62 Furthermore, TLR8 signaling can also inhibit tumor cell metabolism and downregulate cAMP levels produced by tumor cells.33 These studies collectively suggest that metabolic programming directs the molecular processes of senescence induction and development in T cells.
Conclusions and perspectives
It is now well recognized that the T-cell functional state in the TME is the key determining factor for successful antitumor immunity and immunotherapy.130,131 Exhaustion and senescence are two dominant dysfunctional states in T cells with unique phenotypic and functional features in cancer patients, which are obstacles to effective tumor immunotherapy. Understanding the molecular mechanisms and signaling pathways responsible for the generation and development of exhausted and senescent T cells in cancer patients is critical for the development of novel strategies for precision immunotherapy against cancer.
Given that success rates for current immune checkpoint blockade therapies are still limited in cancer patients,22,23 the exploration of other tolerogenic pathways utilized by malignant tumors and development of effective immunotherapies are urgently needed. Increasing evidence suggests that the development of T-cell senescence is also a general feature in the TME, which should be an emerging target for tumor immunotherapy.13–15,33,62 Many efforts have been made to explore T-cell exhaustion in different types of cancers in recent years, but limited information is known about the development and regulation of T-cell senescence in cancer patients. Future studies should focus on the identification of mechanisms responsible for the generation of senescent T cells in the TME and the investigation of the role of accumulated senescent T cells in the failure of current immunotherapies. Significant progress has been made in understanding the importance of metabolism in directing T-cell development, survival, and functions over the past several years.132–134 Furthermore, metabolic reprogramming is involved in the development of both exhausted T cells and senescent T cells in the TME.13,33,62,110 These studies facilitate rethinking the novel concept that metabolic reprogramming of T cells can be an important strategy to control T-cell exhaustion and senescence for tumor immunotherapy. Moreover, recent studies have already identified that TLR8 signaling selectively inhibits glucose metabolism in both Treg cells and tumor cells and reverses the suppressive and senescence-inducing effects on T cells.13,30,32,33,62 Therefore, TLR8 signaling-based metabolic reprogramming should be a potentially important strategy to prevent tumor-specific T-cell senescence and rejuvenate effector T-cell functions for successful cancer immunotherapy. In addition, future studies should explore how TLR8 signaling combined with checkpoint blockade strategies can reprogram T-cell metabolism and reverse T-cell senescence and exhaustion in the TME to enhance antitumor immunity and immunotherapy in different tumors.
Acknowledgements
Because of space limitations, we apologize to anyone whose excellent studies have been inadvertently omitted. This work was partially funded by grants from the American Cancer Society (RSG-10-160-01-LIB, to G.P.), Melanoma Research Alliance (to G.P.), and NIH (AI097852, AI094478, and CA184379 to G.P.).
Competing interests
The authors declare no competing interests.
References
- 1.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 2.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 4.Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol. Immunother. 2006;55:1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li KK, Adams DH. Antitumor CD8+ T cells in hepatocellular carcinoma: present but exhausted. Hepatology. 2014;59:1232–1234. doi: 10.1002/hep.26779. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012;33:364–372. doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 9.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013;19:1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J. Immunol. Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat. Commun. 2018;9:249. doi: 10.1038/s41467-017-02689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye J, et al. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye J, et al. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J. Immunol. 2013;190:2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baitsch L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J. Clin. Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 26.Meloni F, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum. Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(-) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol. Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen WH, et al. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appay V, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montes CL, et al. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008;68:870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 31.Wolfram RM, et al. Defective antigen presentation resulting from impaired expression of costimulatory molecules in breast cancer. Int J. Cancer. 2000;88:239–244. [PubMed] [Google Scholar]
- 32.Ye J, Peng G. Controlling T cell senescence in the tumor microenvironment for tumor immunotherapy. Oncoimmunology. 2015;4:e994398. doi: 10.4161/2162402X.2014.994398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye J, et al. TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO Mol. Med. 2014;6:1294–1311. doi: 10.15252/emmm.201403918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu W, Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol. 2016;7:2111. doi: 10.3389/fmicb.2016.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamphorst AO, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hui E, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakuishi K, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuzaki J, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fourcade J, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chauvin JM, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J. Clin. Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canale FP, et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8(+) T cells. Cancer Res. 2018;78:115–128. doi: 10.1158/0008-5472.CAN-16-2684. [DOI] [PubMed] [Google Scholar]
- 45.Williams JB, et al. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J. Exp. Med. 2017;214:381–400. doi: 10.1084/jem.20160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menk AV, et al. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J. Exp. Med. 2018;215:1091–1100. doi: 10.1084/jem.20171068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheppard KA, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 48.Pereira RM, Hogan PG, Rao A, Martinez GJ. Transcriptional and epigenetic regulation of T cell hyporesponsiveness. J. Leukoc. Biol. 2017;102:601–615. doi: 10.1189/jlb.2RI0317-097R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mognol GP, et al. Exhaustion-associated regulatory regions in CD8(+) tumor-infiltrating T cells. Proc. Natl Acad. Sci. USA. 2017;114:E2776–E2785. doi: 10.1073/pnas.1620498114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez GJ, et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan O, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singer M, et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell. 2016;166:1500–1511. doi: 10.1016/j.cell.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia An-Liang, Wang Jin-Cheng, Yang Kun, Ji Dong, Huang Zheng-Ming, Xu Yong. Genomic and epigenomic perspectives of T-cell exhaustion in cancer. Briefings in Functional Genomics. 2018;18(2):113–118. doi: 10.1093/bfgp/ely005. [DOI] [PubMed] [Google Scholar]
- 54.Paley MA, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghoneim HE, et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell. 2017;170:142–157. doi: 10.1016/j.cell.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen DR, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Im SJ, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller BC, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campisi J, d’Adda, di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 62.Li L, et al. TLR8-mediated metabolic control of human treg function: a mechanistic target for cancer immunotherapy. Cell Metab. 2019;29:103–123. doi: 10.1016/j.cmet.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol. Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 65.Li H, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 66.Brenchley JM, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8(+) T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 67.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc. Natl Acad. Sci. USA. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8(+) T cell dysfunction in melanoma patients. J. Exp. Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang X, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J. Exp. Med. 2010;207:505–520. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang OO, et al. Decreased perforin and granzyme B expression in senescent HIV-1-specific cytotoxic T lymphocytes. Virology. 2005;332:16–19. doi: 10.1016/j.virol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 72.Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 2014;15:965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Childs BG, et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Disco. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodier F, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang ZZ, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J. Clin. Invest. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woroniecka K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin. Cancer Res. 2018;24:4175–4186. doi: 10.1158/1078-0432.CCR-17-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muenst S, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2013;139:667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Japp AS, et al. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol. Immunother. 2015;64:1487–1494. doi: 10.1007/s00262-015-1752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng C, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169:1342–1356. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 80.Thommen DS, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol. Res. 2015;3:1344–1355. doi: 10.1158/2326-6066.CIR-15-0097. [DOI] [PubMed] [Google Scholar]
- 81.Chen, J., Wu, X.-J. & Wang, G.-Q. Hepatoma cells up-regulate expression of programmed cell death-1 on T cells. World J. Gastroenterol.14, 6853 (2008). [DOI] [PMC free article] [PubMed]
- 82.Ozkazanc D, Yoyen-Ermis D, Tavukcuoglu E, Buyukasik Y, Esendagli G. Functional exhaustion of CD4(+) T cells induced by co-stimulatory signals from myeloid leukaemia cells. Immunology. 2016;149:460–471. doi: 10.1111/imm.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye SW, et al. Ex-vivo analysis of CD8+ T cells infiltrating colorectal tumors identifies a major effector-memory subset with low perforin content. J. Clin. Immunol. 2006;26:447–456. doi: 10.1007/s10875-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 84.Chang WC, et al. Clinical significance of regulatory T cells and CD8+ effector populations in patients with human endometrial carcinoma. Cancer. 2010;116:5777–5788. doi: 10.1002/cncr.25371. [DOI] [PubMed] [Google Scholar]
- 85.Webb JR, et al. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (alphaE/beta7 Integrin) in high-grade serous ovarian cancer. Gynecol. Oncol. 2010;118:228–236. doi: 10.1016/j.ygyno.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 86.Urbaniak-Kujda D, et al. Increased percentage of CD8+CD28- suppressor lymphocytes in peripheral blood and skin infiltrates correlates with advanced disease in patients with cutaneous T-cell lymphomas. Postepy Hig. Med. Dosw. 2009;63:355–359. [PubMed] [Google Scholar]
- 87.Gruber IV, et al. Down-regulation of CD28, TCR-zeta (zeta) and up-regulation of FAS in peripheral cytotoxic T-cells of primary breast cancer patients. Anticancer Res. 2008;28:779–784. [PubMed] [Google Scholar]
- 88.Sledge GW. Advances in HER2-positive breast cancer. Clin. Adv. Hematol. Oncol. 2008;6:98–100. [PubMed] [Google Scholar]
- 89.Filaci G, et al. CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J. Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 90.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 91.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 92.Wang T, et al. Senescent carcinoma-associated fibroblasts upregulate IL8 to enhance prometastatic phenotypes. Mol. Cancer Res. 2017;15:3–14. doi: 10.1158/1541-7786.MCR-16-0192. [DOI] [PubMed] [Google Scholar]
- 93.Capell BC, et al. MLL1 is essential for the senescence-associated secretory phenotype. Genes Dev. 2016;30:321–336. doi: 10.1101/gad.271882.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bavik C, et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 95.Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37:866–876. doi: 10.1016/j.it.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 96.Davoodzadeh Gholami M, et al. Exhaustion of T lymphocytes in the tumor microenvironment: significance and effective mechanisms. Cell Immunol. 2017;322:1–14. doi: 10.1016/j.cellimm.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 2016;375:1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 99.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front. Immunol. 2016;7:550. doi: 10.3389/fimmu.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Quigley M, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44:955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kwong J, et al. p38alpha and p38gamma mediate oncogenic ras-induced senescence through differential mechanisms. J. Biol. Chem. 2009;284:11237–11246. doi: 10.1074/jbc.M808327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang W, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lanna A, et al. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 2017;18:354–363. doi: 10.1038/ni.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang CH, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cascone T, et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018;27:977–987. doi: 10.1016/j.cmet.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McKinney EF, Smith KGC. Metabolic exhaustion in infection, cancer and autoimmunity. Nat. Immunol. 2018;19:213–221. doi: 10.1038/s41590-018-0045-y. [DOI] [PubMed] [Google Scholar]
- 110.Ma X, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bengsch B, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity. 2016;45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patsoukis N, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ogando J, et al. PD-1 signaling affects cristae morphology and leads to mitochondrial dysfunction in human CD8(+) T lymphocytes. J. Immunother. Cancer. 2019;7:151. doi: 10.1186/s40425-019-0628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang L, Romero P. Metabolic control of CD8(+) T cell fate decisions and antitumor immunity. Trends Mol. Med. 2018;24:30–48. doi: 10.1016/j.molmed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 115.Scharping NE, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45:374–388. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin. Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 118.Croci DO, et al. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol. Immunother. 2007;56:1687–1700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat. Rev. Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 120.Keshari KR, et al. Metabolic reprogramming and validation of hyperpolarized 13C lactate as a prostate cancer biomarker using a human prostate tissue slice culture bioreactor. Prostate. 2013;73:1171–1181. doi: 10.1002/pros.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodrigues TB, et al. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose. Nat. Med. 2014;20:93–97. doi: 10.1038/nm.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 123.Brand A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 124.Ho PC, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao E, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat. Immunol. 2016;17:95–103. doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Henson SM, et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J. Clin. Invest. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vang T, et al. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J. Exp. Med. 2001;193:497–507. doi: 10.1084/jem.193.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van Nguyen T, Puebla-Osorio N, Pang H, Dujka ME, Zhu C. DNA damage-induced cellular senescence is sufficient to suppress tumorigenesis: a mouse model. J. Exp. Med. 2007;204:1453–1461. doi: 10.1084/jem.20062453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pearce EL. Metabolism in T cell activation and differentiation. Curr. Opin. Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015;36:3–12. doi: 10.1016/j.it.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev. Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gupta PK, et al. CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog. 2015;11:e1005177. doi: 10.1371/journal.ppat.1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brenchley JM, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 137.Narita M, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]