Abstract
Mosquitoes that carry Wolbachia endosymbionts may help control the spread of arboviral diseases, such as dengue, Zika and chikungunya. Wolbachia frequencies systematically increase only when the frequency-dependent advantage due to cytoplasmic incompatibility exceeds frequency-independent costs, which may be intrinsic to the Wolbachia and/or can be associated with the genetic background into which Wolbachia are introduced. Costs depend on field conditions such as the environmental pesticide load. Introduced mosquitoes need adequate protection against insecticides to ensure survival after release. We model how insecticide resistance of transinfected mosquitoes determines the success of local Wolbachia introductions and link our theoretical results to field data. Two Ae. aegypti laboratory strains carrying Wolbachia were released in an isolated district of Rio de Janeiro, Brazil: wMelBr (susceptible to pyrethroids) and wMelRio (resistant to pyrethroids). Our models elucidate why releases of the susceptible strain failed to result in Wolbachia establishment, while releases of the resistant strain led to Wolbachia transforming the native Ae. aegypti population. The results highlight the importance of matching insecticide resistance levels in release stocks to those in the target natural populations during Wolbachia deployment.
Subject terms: Ecological modelling, Evolutionary ecology, Viral infection, Ecology
Introduction
The emergence and reemergence of arboviral diseases around the world is a significant concern for public health. High human mobility across countries, urban landscapes with poor sanitary conditions, and climate change all favor arthropod vector range expansion1–3. Among arboviruses with continental-wide distribution, dengue (DENV), chikungunya (CHIKV), Zika (ZIKV) and yellow fever (YFV) have caused recent outbreaks in multiple countries including Brazil4,5.
These four arboviruses are overwhelmingly transmitted by Aedes mosquitoes, with Ae. aegypti as the principal vector6–8. Aedes aegypti is closely associated with urban environments, such that females blood feed mainly on human hosts, lay eggs in domestic containers around human dwellings and rest inside houses9–11.
Since there are not effective vaccines or specific antiviral drugs available to low-income populations for DENV, CHIKV and ZIKV, control strategies target Ae. aegypti populations2,12. A relatively new strategy involves Wolbachia, intracellular maternally transmitted endosymbionts present in around 50% of insect species13,14. This bacterium, when transinfected into Ae. aegypti mosquitoes, reduces transmission of arboviruses such as DENV, CHIKV15 and ZIKV16. Thus, Wolbachia can be used for both population replacement and suppression. In replacement-oriented releases, an Ae. aegypti population highly competent for arbovirus is replaced by Wolbachia-carrying mosquitoes with significantly lower vector competence. Meanwhile, in suppression-oriented releases, the use of strains posing severe fitness costs could crash Ae. aegypti populations17, or combine incompatible and sterile insect techniques by releasing Ae. albopictus males18. Currently, Wolbachia has been deployed over 14 countries, including a variety of landscape, climate, demography and socioeconomic urban settings19–22.
Transinfected Wolbachia can be established in wild populations because they produce a frequency-dependent advantage for infected females by inducing cytoplasmic incompatibility (CI). The CI phenotype produces severe cell cycle defects in the male pronucleus, resulting in early embryonic lethality in crosses between Wolbachia-infected males and uninfected females23,24. Wolbachia frequencies tend to increase when the frequency-dependent CI advantage exceeds frequency-independent costs, which may be intrinsic to the Wolbachia, such as reduction in fecundity25, lower likelihood of surviving under starvation26, or associated with the genetic background into which Wolbachia are introduced, such as a genetic background susceptible to insecticides. Resistance to insecticides itself is likely to produce a fitness cost; overexpression of a resistance-conferring gene may result in a trade-off that involves resource reallocation at the expense of metabolic and developmental processes27,28 and mechanisms involving target-site modification may lead to a partial loss of function of a gene29–31.
Insecticidal based control is one of the most common approaches used to suppress Ae. aegypti populations in disease-endemic areas and can target both adult and larval stage of mosquito life cycle. Many studies have shown low insecticide efficiency due to development of resistance in wild Ae. aegypti populations32–36. Mutations in the voltage sodium channel gene produce a phenotype known as knockdown resistance (kdr). These mutations give rise to pyrethroids (PY) resistance, which has been related to fitness cost in many insects including Ae. aegypti28,30,37,38. Considering kdr mutations are globally spread in Ae. aegypti populations35, the genetics of released individuals must match those of native mosquitoes to foster invasion22,39. Insecticide resistance might be particularly useful for introducing Wolbachia infections with substantial fitness costs. Hoffmann and Turelli40 proposed an approach to facilitate Wolbachia invasion through insecticide-resistance selection, where insecticide-resistant mosquitoes infected with Wolbachia are deployed into an area in which insecticide usage suppresses wild population and thus enhances invasion. However, this strategy would require a susceptible native population, which may be rare around the globe35,41.
Direct evidence of the importance of matching the genetic background of native mosquitoes was provided when releasing Wolbachia-carrying mosquitoes in an isolated population in Rio de Janeiro, Brazil, with insecticide-resistant populations22. Releases failed to lead to stable establishment Wolbachia-transinfected when the released transinfected strain was susceptible to pyrethroids, whereas it was successful in a subsequent release with resistant wMel-infected Ae. aegypti22. Here, we perform an analysis of likely success/failure given insecticide-resistance in the field and varying intensities of insecticide use in the local human population. We model different scenarios of insecticide use and resistance. First, we evaluate the fitness cost of a colony of Ae. aegypti infected with the wMel Wolbachia strain maintained in laboratory for 18 generations (wMelBr), without insecticide pressure. Second, we study several different features on the likelihood of successful Wolbachia invasion. These are: (1) releasing Wolbachia in a mosquito with susceptible and resistant strains (the wMelBr and wMelRio strains from Garcia et al.22); (2) varying the insecticide use by local householders during the releases; (3) changing levels of insecticide resistance in Ae. aegypti wild populations; and (4) altering the fitness cost of Wolbachia and insecticide resistance. We identify scenarios in which insecticide resistance of wild Ae. aegypti populations challenge successful Wolbachia invasion.
Results
Quantifying the fitness cost due to insecticide resistance
We analyzed the frequencies of 1016Ile kdr mutations in the wMelBr colony without insecticide pressure across 18 generations. The frequency of the resistance gene decreased (Fig. 1), dropping from 0.75 to 0 after 18 generations. We estimate resistance fitness to be 0.75 (a fitness cost of 0.25). This value of parameter i was applied to the scenarios analyzed for Wolbachia invasion.
Figure 1.
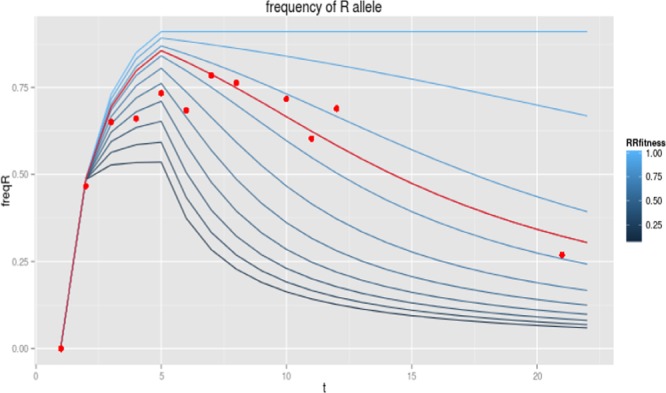
Observed and expected changes in the frequency of the resistance allele over time (laboratory generations). We assumed different fitness costs due to insecticide resistance based on the frequency of the kdr mutation, 1016Ile, along 18 generations when maintained under laboratory conditions, i.e., without insecticide pressure. Dots show the observed values and various curves constructed using the model show the expected frequencies when varying fitness of homozygous mosquitoes (factor i) from 0.1 to 1.0. The best fit using the lowest sum of residuals (curve in red color) has relative fitness i = 0.75.
Simulation scenarios
We considered Wolbachia releases and local wild Ae. aegypti mosquitoes resistant to the insecticides generally under two different sets of scenarios: deployment of Wolbachia infecting mosquitoes susceptible (wMelBr) or resistant (wMelRio) to insecticides. The intensity of insecticide application by local householders was also allowed to vary in these scenarios.
Scenario 1: Deployment of Wolbachia infecting a susceptible release strain (wMelBr) with wild resistant mosquitoes and insecticide pressures ranging from 0.0 to 0.9
Two outcomes were observed by releasing susceptible mosquitoes depending on whether there was no insecticide use (s = 0.0) or a low application intensity of s = 0.4 (Fig. 2A, blue and yellow line). As expected, in the absence of insecticide, Wolbachia invades rapidly. Additionally, the frequency of the R allele in the mosquitoes with Wolbachia increases due to introgression of the R allele in the first few generations. However, the frequency of R then decreases rapidly and is lost due to the continuous introduction of susceptible alleles through Wolbachia releases and due to fitness costs, resulting in a possible reversion of insecticide resistance status in the field after Wolbachia invasion (Fig. 2B, blue line). However, even an occasional insecticide application in the field selects R alleles in Wolbachia mosquitoes (Fig. 2B, yellow line).
Figure 2.
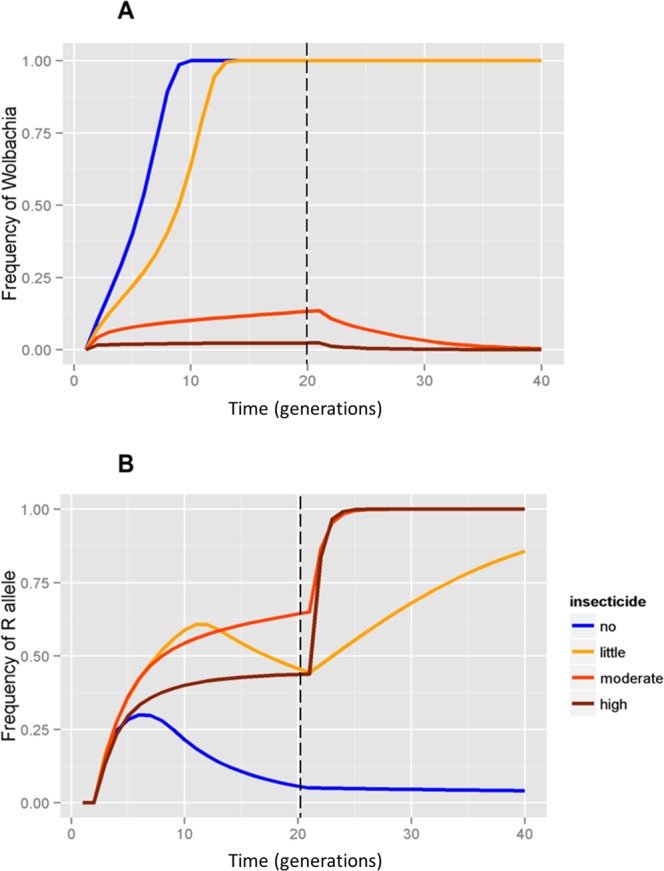
Releases of Wolbachia mosquitoes susceptible to insecticides. Frequency of (A) Wolbachia and (B) resistance alleles under different levels of insecticide use by local householders. Dashed line represents the end of releases.
Wolbachia does not invade when insecticide susceptible mosquitoes are released and local householders undertake moderate or high insecticide applications (Fig. 2A, red and brown line). In these two scenarios, Wolbachia frequency did not increase above 25%. The R alleles are rapidly selected in Wolbachia mosquitoes, despite the release of Ae. aegypti on a timely basis (Fig. 2B, red and brown).
Scenario 2: Deployment of mosquitoes carrying Wolbachia on resistant strain (wMelRio) with wild mosquitoes resistant and insecticide pressures ranging from 0.0 to 0.9
When releasing mosquitoes carrying Wolbachia on a strain resistant to insecticides, invasion always succeeds (Fig. 3A), regardless of variation in insecticide application intensity from s = 0.0 to s = 0.9 (Fig. 3B, blue, yellow, red and brown line). Insecticide applications did not alter the Wolbachia invasion profile, except for a minor tendency for faster Wolbachia invasion when insecticide intensity is low. In the absence of the insecticide, the frequency of the R allele decreases in the field (Fig. 3B, line blue), as shown in scenario 1, but due to the fitness cost of resistance in the absence of the insecticide, rather than the introduction of susceptible alleles by Wolbachia mosquitoes as in scenario 1. However, with any level of insecticide applications, the R allele reaches fixation in Wolbachia mosquitoes in the field (Fig. 3B, yellow, red and brown line). These results are in agreement with the proposal by Turelli and Hoffman40 showing invasion of resistant mosquitoes in places with susceptible wild mosquitoes.
Figure 3.
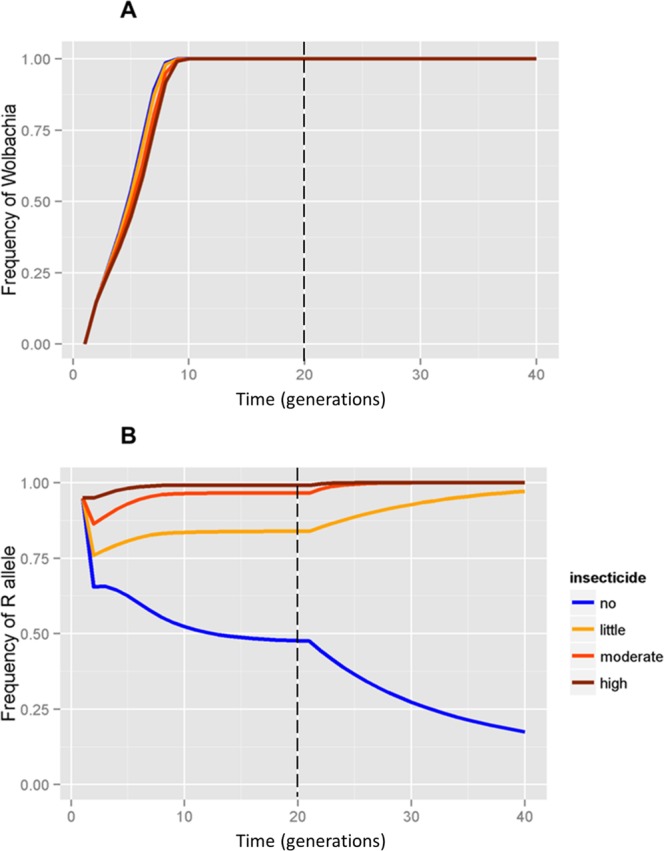
Releases of Wolbachia mosquitoes with comparable levels of insecticide resistance as those in the wild population. Frequency of (A) Wolbachia and (B) resistance alleles in field considering different levels of insecticide use by local householders. Dashed line represents the end of releases.
Discussion
We investigated how Wolbachia invasion success is influenced by the presence of insecticide resistance alleles in both the released and wild Aedes aegypti populations. Our model is based on the one by Hoffmann and Turelli40, but we take into account the fitness cost of pyrethroid resistance in order to analyze different scenarios of insecticide use and resistance. Considering Wolbachia deployment is undergoing expansion and the pyrethroid resistance in native Ae. aegypti populations is a worldwide phenomenon, Wolbachia success might be curtailed if the genetic background of released and local populations do not match, especially regarding insecticide resistance22. Therefore, results presented herein might inform release guidelines.
We estimated the fitness cost due to insecticide resistance at 0.25 over a generation time, based on empirical observations of wMelBr pyrethroid resistance allele loss over 18 generations. This finding points to an expected performance decrease in an insecticide-free environment. Since several vector control programs historically rely on chemicals, this would likely lead to distinct levels of resistance between wild and released populations22. Therefore, simulations of Wolbachia invasion must consider the insecticide resistance status of both released and natural populations.
Our results indicate that invasion of a susceptible strain is only possible if local householders use insecticides at low levels. Therefore, we set the conditions to determine how the frequency of pyrethroids application by local householders can affect Wolbachia invasion in the field. If no insecticide is used, Wolbachia invades faster and insecticide susceptibility status in field mosquitoes may increase rapidly, mainly due to the introduction of S alleles by Wolbachia mosquitoes. With the chosen model, we observe that although Wolbachia invades, R alleles are still selected even if local householders engage in a low level of insecticide applications. For moderate or high frequencies of insecticide application, susceptible Wolbachia released mosquitoes would die quickly as wild-resistant mosquitoes are at an advantage to survive and reproduce. In these situations, Wolbachia frequency would not increase above 25% in the field, and its frequency would remain low as mass releases stop. This scenario provides a likely explanation for the unsuccessful invasion of wMel in Rio de Janeiro, since a susceptible strain (wMelBr) was released into a highly resistant field population. High insecticide pressure was likely based on information from local householders22. Field data showed that the wMelBr frequency reached 65% in the last week of release but sharply decreased afterwards when releases stopped. This partly fits scenario 1 (deployment of wMelBr susceptible mosquitoes into a native highly resistant population). In the field releases, between 12.5–24.2 mosquitoes were released per house weekly, for 20 consecutive weeks, and BG-Sentinel Traps were checked once a week, six days after release, and screened for Wolbachia22. Our model in scenario 1 suggests wMelBr frequency would not exceed 25% under moderate and high insecticide applications, but the higher frequency observed likely reflects weekly mass release of wMelBr-infected Ae. aegypti. The “real invasion” frequency expected from our model was probably reflected by the frequency of wMelBr a couple of weeks after releases stopped, which was around 20%. Garcia et al.22 hypothesized that given the high use of insecticides by households, only a small fraction of wMelBr-infected mosquitoes survived and reproduced, insufficient to overcome the threshold to promote invasion42, consistent with the modelled expectations presented here. Field releases were done on weekly basis, whereas our analytical results use generation time units, but these findings generally hold on different time scales.
The work by Hancock et al.43 evaluated how larval competition can modulate the invasion of Wolbachia at slower pace than often assumed. Also, a mathematical model structured by life stages analyzed the invasion of Wolbachia, also assuming diallelic locus model for insecticide resistance, among other traits evaluated44. Since overlapped generations are not observed, Hancock et al.44 rely on statistical models to obtain estimates. Such models exhibit the tradeoff between using overlapping and non-overlapped generations. Hancock et al., however, reported a number of 55 generations over a 4–5 month period. Since our model relies on generation time units, an initial intuition would require a long period of time if a generation takes multiple weeks. By contrast the overlapping generations may also signal invasion on time scales much shorter. Therefore, we believe that for this present study the most appropriate presentation is having a generation time unit, enabling elastic time scales, if necessary.
By contrast, when releasing a Wolbachia strain as resistant as the wild population (wMelRio), our results indicate that Wolbachia is able to invade irrespective of the intensity of insecticide application, and there is a decrease in the frequency of R alleles in the absence of insecticide which occurs more slowly than when a susceptible release strain introduces S alleles in field populations. The slow decrease in the frequency of R is consistent with studies that demonstrate slow insecticide resistance reversal when R alleles are at a high frequency45,46. For the other three intensities of insecticide application by local householders (0.4, 0.7, 0.9), selection maintains high frequencies of R alleles in the field. This matches what happened with a second round of releases in the same site in Rio de Janeiro which resulted in successful invasion by the wMelRio strain which had the same levels of insecticide resistance as the wild Ae. aegypti population22. It is worth noting that these results suggest that releasing resistant mosquitoes in places where susceptible ones dominate will also be successful even with little use of insecticide. This in fact is the proposal in the work by Turelli and Hoffman40 whose model demonstrated the success of Wolbachia invasion in this scenario. Ae. aegypti populations in various cities in Brazil already exhibit high levels of insecticide resistance28, but a strategy of releasing resistant Wolbachia mosquitoes in some areas that still have some susceptibility seems difficult to be adopted by authorities due to risk of raising resistance, if invasion does not happen successfully.
Insecticide resistance is frequently associated with a fitness cost on life-history traits such as larval development time and adult fecundity, longevity and locomotor activity28,31,47–49. The fitness cost due to insecticide resistance in our model was assessed by the rate of decrease of the kdr mutation in the strain wMelBr. This strain was backcrossed with Rio de Janeiro local populations50 and had a frequency of almost 70% of resistant genotypes. However, after eighteen generations with limited outcrossing (10% wild males every five generations) and no insecticide pressure, resistant genotypes dropped to 4%, resulting in a fitness loss estimate of 0.25. Brito et al.30 also observed 1016Ile kdr frequency decreasing to less than 30%, after 15 generations of Ae. aegypti without Wolbachia in laboratory cages, when starting from frequencies of 70% and 50% of kdr allelic frequency, consistent with the notion of a substantial fitness cost.
We assume in the model that insecticide resistance is governed by a single diallelic locus, with alleles denoted R and S35,40. There are, however, various factors which impact insecticide resistance, for instance metabolic resistance. Further study on modeling these factors are important to advance knowledge on the insecticide resistance, but certainly will be helpful to better understand Wolbachia invasion possibilities. We also considered that wMel in Ae. aegypti has a small fitness cost, with minor alterations in larval competitive ability26,51, fecundity25 and fertility22. With these fitness costs and insecticide susceptibility in the release strain, invasion remains unlikely unless there is a sharp reduction in insecticide usage by local householders, which requires a significant effort from social scientists to change community behavior and vector control good practices. Successful releases will therefore likely require regular backcrossing of the release strain to maintain resistance in release material.
Methods
General model
The model is based on previous studies that have shown a fitness cost associated with PY target-site resistance, with a focus on two-allele representation of knockdown resistance based on 1016Ile kdr mutation30,47,48,52,53. Individuals can be classified by their resistance genotypes and Wolbachia infection state. Genotypes in a two-allele representation are given by RR, RS or SS for homozygous resistant, heterozygous and homozygous susceptible genotypes, respectively, as in Hancock et al.44 Insecticide susceptibility is typically a recessive trait48. The Wolbachia infection state is either uninfected (U) or infected (I). Without insecticides in the environment, homozygous-resistant mosquitoes have relative fitness given by a factor 1 – i compared to susceptible mosquitoes, hence a fitness cost given by i.
Turelli and Hoffmann40 developed a model in which a Wolbachia fitness cost Fc would apply over successive generations. We introduce in the present model a parameter to describe the fitness cost due to insecticide resistance. The model is designed from components that predict frequencies of resistance genotypes in successive generations and that consider varying intensities of insecticides application.
The first component evaluates frequencies f(XX,WS)t of XX newly entering individuals (zygotes) at generation t where XX = {RR, RS, SS} and WS is the Wolbachia infection state, WS = {U, I}. The frequency of Wolbachia over generation t is described by pt and the frequencies of R alleles in either Wolbachia mosquitoes or non-Wolbachia mosquitoes is given by rI,t and rU,t, respectively.
These frequencies can be modeled by recursive equations such as
where is given by:
The frequencies of Wolbachia and the R allele in adults will be impacted by the use of insecticides. We assume that a fraction 1-s survives to mate and generate offspring. Therefore, insecticide intensity is defined in indirect manner, such that its impact is measured by the fraction of adult mosquitoes surviving as a decreasing function. The most intense insecticide intensity usage will impact in less numbers of adult mosquitoes surviving to generate offspring. This follows Equations 2.4 given by Turelli and Hoffmann40. In the field s reflects intensity of insecticide use, whereas in the laboratory for rearing Wolbachia individuals no insecticide is used, hence s = 0. The model with s = 0 is used to estimate a best fit for parameter i, based on frequency changes of the R allele when laboratory Wolbachia mosquitoes are maintained as closed populations or crossed with field males.
Quantifying the fitness cost due to insecticide resistance (in laboratory conditions and without insecticide pressure)
Fitness costs due to Wolbachia presence and to insecticide resistance can be measured in the laboratory, where no insecticides are used during rearing of Wolbachia mosquito colonies. Estimates can be obtained from the model using a fixed Wolbachia fitness cost and varying costs due to insecticide resistance. We use the general model, with a particular approach that in the backcrossings we apply a frequency of resistance alleles equal to the one measured from field mosquitoes. Therefore, we expect an increase of frequency of resistance alleles during backcrossing generations. We vary the insecticide resistance cost Fc from 0.1 to 1 by increments of 0.01 and obtain for each cost value the sum of squared residuals considering the values predicted by the model and the frequencies observed in some of our lab generations (F5, F6, F7, F8, F9 and F18). The fitness cost due to the insecticide resistance is estimated as the cost producing the lowest sum of squared residuals.
Parameters used in the Wolbachia invasion model
We analyze different scenarios varying in the initial levels of insecticide resistance among wild mosquitoes, as well as in the insecticide application during releases. In order to define scenarios, we also need initial conditions for the presence of Wolbachia in the field and for levels of insecticide resistance in the release population. For all simulations we consider Wolbachia to be absent in the field prior to releases. We consider a frequency rU0 of the R allele in the local population prior to releasing Wolbachia mosquitoes. This parameter represents the level of insecticides resistance gene in Ae. aegypti wild population that receive Wolbachia releases. Based on published data, we use a value of 0.95 in our analyses reflecting the fact that most wild mosquitoes are homozygous for resistance (RR)22,48,52.
Our model considers that Wolbachia mosquitoes are released on a periodic units of time for nrel consecutive releases. In our analyses we considered nrel = 20 releases in all simulations based on Wolbachia releases carried out in Rio de Janeiro from Sept/2014 to Jan/201522. Each release of Wolbachia mosquitoes requires a release rate given by a ratio rrel representing the number of released individuals divided by the total number of mosquitoes present (released + local) per unit of time. This parameter covers the density of wild mosquitoes and the number of Wolbachia mosquitoes released per unit of time. The unit of time used here is the time for a mosquito generation since the model is based on non-overlapping generations. We use an rrel value of 0.10 based on releases in Brazil22 and for convenience we consider a timeframe of 40 mosquito generations (Table 1). Furthermore, our analysis indicates the frequency of the resistance allele within the total field population of Wolbachia mosquitoes, including the released mosquitoes (with releases lasting 20 units of time), plus field offspring, over the 40 generations period. Wild mosquitoes (without Wolbachia) were not taken into account due to a lack of initial gene flow from Wolbachia mosquitoes to the wild population, as a consequence of cytoplasmic incompatibility and complete maternal transmission40.
Table 1.
Fixed parameters in the model with respective descriptions and values used in simulations.
| Parameters | Description | Values | References |
|---|---|---|---|
| i | Fitness of homozygous resistant mosquitoes (0.0–1.0) | 0.75 | Brito et al. 201330 |
| h | Fitness factor for heterozygous mosquitoes (resistance nearly recessive) | 0.8 | Brito et al. 201848 |
| Fc | Fitness of Wolbachia-carrying mosquitoes | 0.8 | Turley et al. 201325; Hoffmann et. al 201454; Ross et al. 201626; Garcia et al. 201922 |
| rU0 | Local population frequency of R (95%) | 0.95 | Linss et al. 201452; Bellinato et al. 201655; Brito et al. 201848; Garcia et al. 201922 |
| nrel | Releases | 20 | Garcia et al. 201656 |
| rrel | Ratio of released individuals by the total number (released + local) per unit of time | 0.10 | Garcia et al. 201656 |
| Ttot | Total number of generations | 40 | — |
Construction of potential invasion scenarios
Our scenarios consider the intensity of insecticide used by the local human population and the resistance of Wolbachia mosquitoes (Table 2). We first consider that insecticide intensity s varies in the simulation scenario. We consider some scenarios with no application (s = 0.0), low use (s = 0.4), moderate use (s = 0.7), or high insecticide use (s = 0.9). We also define the frequencies frel of genotypes (RR, RS, SS) of released Wolbachia mosquitoes (Table 2). For the simulations done by releasing Wolbachia susceptible mosquitoes (wMelBr strain), the frequency profile was frel = (0.0, 0.0, 1.0). When releasing Wolbachia resistant mosquitoes (wMelRio strain), the frequency values frel = (0.95, 0.0, 0.05) are based on the status of wild resistant mosquitoes observed in previous studies22.
Table 2.
Variable parameters used in simulations.
| Insecticide intensity | Frequencies of genotypes (RR, RS, SS) | |
|---|---|---|
|
Scenario 1: Releasing susceptible Wolbachia mosquitoes (wMelBr strain) × wild resistant mosquitoes |
0.0 (none), 0.4 (low), 0.7 (moderate), 0.9 (high) |
(0, 0, 1) |
|
Scenario 2: Releasing resistant Wolbachia mosquitoes (wMelRio strain) × wild resistant mosquitoes |
0.0 (none), 0.4 (low), 0.7 (moderate), 0.9 (high) |
(0.95, 0, 0.05) |
Acknowledgements
We thank Dr. Michael Turelli for stimulating discussions about the formulation presented here and also about our results. We are grateful for support from CAPES, CNPq and Program Print-Fiocruz-CAPES.
Author contributions
Conception of the study (G.A.G., R.M.F. and D.A.M.V.), design of the work (G.A.G. and D.A.M.V.) acquisition and analysis (A.A.H. and D.A.M.V.), interpretation of data (G.A.G., A.A.H., R.M.F. and D.A.M.V.), write and revise the manuscript (G.A.G., A.A.H., R.M.F. and D.A.M.V.).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao-Lormeau V-M, Musso D. Emerging arboviruses in the Pacific. Lancet. 2014;384:1571–1572. doi: 10.1016/S0140-6736(14)61977-2. [DOI] [PubMed] [Google Scholar]
- 2.Liang G, Gao X, Gould EA. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg. Microbes Infect. 2015;4:1–5. doi: 10.1038/emi.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasconcelos PFC, Calisher CH. Emergence of Human Arboviral Diseases in the Americas, 2000–2016. Vector-Borne Zoonotic Dis. 2016;16:295–301. doi: 10.1089/vbz.2016.1952. [DOI] [PubMed] [Google Scholar]
- 4.Mota MT, et al. Mosquito-transmitted viruses – the great Brazilian challenge. Brazilian J. Microbiol. 2016;47:38–50. doi: 10.1016/j.bjm.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abreu, F. V. S. de et al. Combination of surveillance tools reveals that Yellow Fever virus can remain in the same Atlantic Forest area at least for three transmission seasons. Mem. Inst. Oswaldo Cruz114 (2019). [DOI] [PMC free article] [PubMed]
- 6.Lourenço-de-Oliveira R, Vazeille M, de Filippis AM, Failloux A. Aedes aegypti in Brazil: genetically differentiated populations with high susceptibility to dengue and yellow fever viruses. Trans. R. Soc. Trop. Med. Hyg. 2004;98:43–54. doi: 10.1016/S0035-9203(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 7.Vega-Rúa A, et al. Chikungunya Virus Transmission Potential by Local Aedes Mosquitoes in the Americas and Europe. PLoS Negl. Trop. Dis. 2015;9:e0003780. doi: 10.1371/journal.pntd.0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira-de-Brito A, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem. Inst. Oswaldo Cruz. 2016;111:655–658. doi: 10.1590/0074-02760160332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott TW, et al. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J. Med. Entomol. 1993;30:922–7. doi: 10.1093/jmedent/30.5.922. [DOI] [PubMed] [Google Scholar]
- 10.Braks MAH, Honório NA, Lourenço-De-Oliveira R, Juliano SA, Lounibos LP. Convergent Habitat Segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J. Med. Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- 11.Maciel-De-Freitas, R., Marques, W. A., Peres, R. C., Cunha, S. P. & Lourenço De Oliveira, R. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Mem Inst Oswaldo Cruz, Rio de Janeiro102 (2007). [DOI] [PubMed]
- 12.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining Challenges and Proposing Solutions for Control of the Virus Vector Aedes aegypti. PLoS Med. 2008;5:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B Biol. Sci. 2015;282:20150249–20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira CD, et al. Broader prevalence of Wolbachia in insects including potential human disease vectors. Bull. Entomol. Res. 2015;105:305–315. doi: 10.1017/S0007485315000085. [DOI] [PubMed] [Google Scholar]
- 15.Moreira LA, et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Caragata E, Dutra H, Moreira L. Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microb. Cell. 2016;3:293–295. doi: 10.15698/mic2016.07.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie SA, Townsend M, Paton CJ, Callahan AG, Hoffmann AA. Application of wMelPop Wolbachia Strain to Crash Local Populations of Aedes aegypti. PLoS Negl Trop Dis. 2015;9(7):e0003930. doi: 10.1371/journal.pntd.0003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X. et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 572(7767), 56–61 (2019). [DOI] [PubMed]
- 19.Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann AA, Ross PA, Rašić G. Wolbachia strains for disease control: ecological and evolutionary considerations. Evol. Appl. 2015;8:751–768. doi: 10.1111/eva.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TH, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit. Vectors. 2015;8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia G, et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl. Trop. Dis. 2019;13:e0007023. doi: 10.1371/journal.pntd.0007023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laven H. SPECIATION IN MOSQUITOES: Speciation by Cytoplasmic Isolation in the Culex Pipiens-Complex. Cold Spring Harb. Symp. Quant. Biol. 1959;24:166–173. doi: 10.1101/SQB.1959.024.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Landmann F, Orsi GA, Loppin B, Sullivan W. Wolbachia-Mediated Cytoplasmic Incompatibility Is Associated with Impaired Histone Deposition in the Male Pronucleus. PLoS Pathog. 2009;5:e1000343. doi: 10.1371/journal.ppat.1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turley AP, Zalucki MP, O’Neill SL, McGraw EA. Transinfected Wolbachia have minimal effects on male reproductive success in Aedes aegypti. Parasit. Vectors. 2013;6:36. doi: 10.1186/1756-3305-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross PA, Endersby NM, Hoffmann AA. Costs of Three Wolbachia Infections on the Survival of Aedes aegypti Larvae under Starvation Conditions. PLoS Negl. Trop. Dis. 2016;10:e0004320. doi: 10.1371/journal.pntd.0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevillon C. Population genetics of insecticide resistance in the mosquito Culex pipiens. Biol. J. Linn. Soc. 1999;68:147–157. doi: 10.1111/j.1095-8312.1999.tb01163.x. [DOI] [Google Scholar]
- 28.David MR, Garcia GA, Valle D, Maciel-de-Freitas R. Insecticide Resistance and Fitness: The Case of Four Aedes aegypti Populations from Different Brazilian Regions. Biomed Res. Int. 2018;2018:1–12. doi: 10.1155/2018/6257860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kliot A, Ghanim M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012;68:1431–1437. doi: 10.1002/ps.3395. [DOI] [PubMed] [Google Scholar]
- 30.Brito LP, et al. Assessing the Effects of Aedes aegypti kdr Mutations on Pyrethroid Resistance and Its Fitness Cost. PLoS One. 2013;8:e60878. doi: 10.1371/journal.pone.0060878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diniz DFA, et al. Fitness cost in field and laboratory Aedes aegypti populations associated with resistance to the insecticide temephos. Parasit. Vectors. 2015;8:662. doi: 10.1186/s13071-015-1276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcombe S, et al. Pyrethroid Resistance Reduces the Efficacy of Space Sprays for Dengue Control on the Island of Martinique (Caribbean) PLoS Negl. Trop. Dis. 2011;5:e1202. doi: 10.1371/journal.pntd.0001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maciel-de-Freitas R, et al. Undesirable Consequences of Insecticide Resistance following Aedes aegypti Control Activities Due to a Dengue Outbreak. PLoS One. 2014;9:e92424. doi: 10.1371/journal.pone.0092424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plernsub S, et al. Temporal frequency of knockdown resistance mutations, F1534C and V1016G, in Aedes aegypti in Chiang Mai city, Thailand and the impact of the mutations on the efficiency of thermal fogging spray with pyrethroids. Acta Trop. 2016;162:125–132. doi: 10.1016/j.actatropica.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Moyes CL, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017;11:e0005625. doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roiz D, et al. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl. Trop. Dis. 2018;12:e0006845. doi: 10.1371/journal.pntd.0006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster SP, et al. Analogous pleiotropic effects of insecticide resistance genotypes in peach–potato aphids and houseflies. Heredity (Edinb). 2003;91:98–106. doi: 10.1038/sj.hdy.6800285. [DOI] [PubMed] [Google Scholar]
- 38.Berticat C, et al. Costs and benefits of multiple resistance to insecticides for Culex quinquefasciatus mosquitoes. BMC Evol. Biol. 2008;8:104. doi: 10.1186/1471-2148-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wuliandari J, et al. Association between Three Mutations, F1565C, V1023G and S996P, in the Voltage-Sensitive Sodium Channel Gene and Knockdown Resistance in Aedes aegypti from Yogyakarta, Indonesia. Insects. 2015;6:658–685. doi: 10.3390/insects6030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann AA, Turelli M. Facilitating Wolbachia introductions into mosquito populations through insecticide-resistance selection. Proc. R. Soc. B Biol. Sci. 2013;280:20130371–20130371. doi: 10.1098/rspb.2013.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia G, et al. The impact of insecticide applications on the dynamics of resistance: The case of four Aedes aegypti populations from different Brazilian regions. PLoS Negl. Trop. Dis. 2018;12:e0006227. doi: 10.1371/journal.pntd.0006227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turelli M, Barton NH. Deploying dengue-suppressing Wolbachia: Robust models predict slow but effective spatial spread in Aedes aegypti. Theor. Popul. Biol. 2017;115:45–60. doi: 10.1016/j.tpb.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hancock, P. et al. Density-dependent population dynamics in Aedes aegypti slow the spread of wMel Wolbachia, Journal of Applied Ecology, Vol 53 (2016).
- 44.Hancock, P. A. et al. Predicting Wolbachia invasion dynamics in Aedes aegypti populations using models of density-dependent demographic traits, BMC Biology, (2016). [DOI] [PMC free article] [PubMed]
- 45.Melo-Santos MAV, et al. Resistance to the organophosphate temephos: Mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Trop. 2010;113:180–189. doi: 10.1016/j.actatropica.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Schechtman H, Souza MO. Costly Inheritance and the Persistence of Insecticide Resistance in Aedes aegypti Populations. PLoS One. 2015;10:e0123961. doi: 10.1371/journal.pone.0123961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martins AJ, et al. Effect of Insecticide Resistance on Development, Longevity and Reproduction of Field or Laboratory Selected Aedes aegypti Populations. PLoS One. 2012;7:e31889. doi: 10.1371/journal.pone.0031889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brito LP, et al. Levels of Resistance to Pyrethroid among Distinct kdr Alleles in Aedes aegypti Laboratory Lines and Frequency of kdr Alleles in 27 Natural Populations from Rio de Janeiro, Brazil. Biomed Res. Int. 2018;2018:1–10. doi: 10.1155/2018/2410819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaramillo-O. N, Fonseca-González I, Chaverra-Rodríguez D. Geometric Morphometrics of Nine Field Isolates of Aedes aegypti with Different Resistance Levels to Lambda-Cyhalothrin and Relative Fitness of One Artificially Selected for Resistance. PLoS One. 2014;9:e96379. doi: 10.1371/journal.pone.0096379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dutra HLC, et al. From Lab to Field: The Influence of Urban Landscapes on the Invasive Potential of Wolbachia in Brazilian Aedes aegypti Mosquitoes. PLoS Negl. Trop. Dis. 2015;9:e0003689. doi: 10.1371/journal.pntd.0003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Oliveira S, Villela DAM, Dias FBS, Moreira LA, Maciel de Freitas R. How does competition among wild type mosquitoes influence the performance of Aedes aegypti and dissemination of Wolbachia pipientis? PLoS Negl. Trop. Dis. 2017;11:e0005947. doi: 10.1371/journal.pntd.0005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linss JG, et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasit. Vectors. 2014;7:25. doi: 10.1186/1756-3305-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black WC., IV Coevolution of the Ile1,016 and Cys1,534 Mutations in the Voltage Gated Sodium Channel Gene of Aedes aegypti in Mexico. PLoS Negl. Trop. Dis. 2015;9:e0004263. doi: 10.1371/journal.pntd.0004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann, A. A. et al. of the wMel Wolbachia Infection following Invasion into Aedes aegypti Populations. PLoS Neglected Tropical Diseases8(9), e3115 (2014). [DOI] [PMC free article] [PubMed]
- 55.Bellinato DF. Resistance Status to the Insecticides Temephos, Deltamethrin, and Diflubenzuron in Brazilian Populations. BioMed Research International. 2016;2016:1–12. doi: 10.1155/2016/8603263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia, G. d. A., dos Santos, L. M. B., Villela, D. A. M. & Maciel-de-Freitas, R. Using Wolbachia Releases to Estimate Aedes aegypti (Diptera: Culicidae) Population Size and Survival. PLoS ONE11(8), e0160196 (2016). [DOI] [PMC free article] [PubMed]


