Abstract
The aim of this study was to compare different techniques in order to achieve a high extraction of phenolic compounds from propolis. For this purpose, it was investigated the use of double maceration (24 h at room temperature with continuous agitation at 250 rpm), double microwave treatments (1 min at 140 W) and double ultrasound-assisted extraction (15 min at 20 kHz) using 70% ethanol. The extraction efficiency was measured based on extraction yield, total phenolic content, flavones and flavonol content, and flavanone and dihydroflavonol content. The ultrasonic extraction had an extraction yield higher than microwave extraction and maceration. The yield of the propolis ranged between samples and between the years of propolis harvesting. Of the twelve quantified phenolic compounds, p-coumaric acid was the most abundant (271.65 mg/g propolis).
Keywords: Propolis, Maceration, Microwave, Ultrasound-assisted, Extraction
Introduction
Propolis is a sticky mixture formulated by honeybees (Apis melifera) from resins, exudate and beeswax (Nna et al. 2018). Propolis is used by bees for the construction, repair and protection of beehives due to its mechanical properties and biological activity (Chen et al. 2018). Propolis has a specific scent and its colour can range from yellow to dark brown (Meimandi-Parizi et al. 2018). It is composed of resins (50%), waxes (30%), essential oils (10%), pollen (5%) and other organic substances (5%). Propolis is composed of over 300 chemical compounds, of which flavonoids are considered the main components representing around of 25% of the total compounds (Marcucci 1995; Cornara et al. 2017; Veloz et al. 2015). According to the source plant materials, propolis is classified as: poplar, birch, green, red, Pacific or Canarian; this classification is based on the chemical constituents which present different bioactivities (Silva-Carvalho et al. 2015; Freitas et al. 2011).
In humans, propolis was widely used as a folk medicine worldwide since ancient times. Propolis is known to possess valuable biological properties (Sforcin 2016; Cornara et al. 2017) such as antibacterial (Oliveira et al. 2017; Roh and Kim 2018), antioxidant (Cruz et al. 2016), anti-inflammatory (Moreira et al. 2008), antineurodegenerative (Falcão et al. 2010), anti-tumor (Silva-Carvalho et al. 2014), antifungal (Roh and Kim 2018), anti-protozoan (Falcão et al. 2014), anti-viral (Yildirim et al. 2016), hepato-protective (Sheng et al. 2007), local-anesthetic (Moreira et al. 2008), and free-radical-scavenging (Al-Ani et al. 2018).
The extraction of bioactive compounds from propolis was made with different solvents but the common used is ethanol; the ethanol/water (70:30, v/v) mixture (Sforci and Bankova 2011) is widely preferred due to the fact that it is a non-toxic solvent and is an ideal solvent for the extraction of different compounds such as flavonoids or polyphenols from propolis (Escriche and Juan-Borrás 2018; Alm-Eldeen et al. 2017). The solvent type is not the only parameter which influences the extraction efficiency; other parameters known to impact the extraction process are temperature, time, and propolis particle dimensions (Sawaya et al. 2011). The propolis extracts are mainly prepared by maceration but it was shown that ultrasound-assisted extraction gave excellent results, spectacularly accelerating the process (Trusheva et al. 2007). Furthermore, supercritical extraction procedure was also applied on propolis, but resulted in lower extraction yields when compared to conventional techniques (Biscaia and Ferreira 2009; Jug et al. 2014).
In this study different extraction methods (maceration, ultrasonic and microwave) were analysed in order to establish the suitable method for the extraction of bioactive compounds from six different propolis samples using ethanol/water (70/30, v/v) mixture.
Materials and methods
Materials
The crude propolis was collected from local beekeepers from Suceava county (Romania) in 2017 (4 samples) and 2018 (2 samples). The samples from 2017 were collected in autumn, while the samples from 2018 were harvested in spring. Prior to analysis, the samples were kept at − 18 °C.
Reagents
The standards used: apigenin, caffeic acid, chrysin, p-coumaric acid, galangin, isorhamnetin, kaempferol, luteolin, myricetin, pinocembrin, rutin, and quercetin were purchased from PlantMetaChem (Gießen, Germany), and acetonitrile and ethanol were purchased from Sigma Aldrich (Germany). All reagents and standards used were of HPLC grade, and purified water from a Thermo system was used throughout the experiments.
Extraction procedure
The crude propolis (20 g) was grounded in frozen state. The extraction was conducted as: double maceration (M1, M2), double microwave treatment (Mi1, Mi2) and double ultrasonication (U1, U2). Each extraction was done in triplicate.
Maceration
1 g of grounded propolis was dissolved in 50 mL of 70% ethanol. The solution was stirred for 24 h at 250 rpm at room temperature using an orbital shaker. After that, the solution was centrifuged for 10 min at 4000 rpm and the supernatant was separated from the residue. The supernatant was filtered using filter paper Whatman 5. The residue was extracted one more time with 50 mL of 70% ethanol. The two supernatants were collected in volumetric flasks that were filled up to 50 mL using 70% ethanol.
Microwave-assisted extraction
1 g of grounded propolis was dissolved in 50 mL of 70% ethanol. The solution was placed in a microwave oven and heated for 1 min at 140 W (the temperature of the solution after 1 min was close to 60 °C). Following the microwave treatment, the solution was cooled to room temperature with cold water. After that, the solution was centrifuged for 10 min at 4000 rpm and the supernatant was separated from the residue. The supernatant was filtered using filter paper Whatman 5. The residue was extracted one more time with 50 mL of 70% ethanol for 1 min at 140 W. The two supernatants were collected in 50 mL volumetric flasks that were brought to volume with 70% ethanol.
Ultrasound-assisted extraction
1 g of grounded propolis was dissolved in 50 mL of 70% ethanol. An ultrasonic probe was immersed into the solution and the extraction was made at 20 kHz (Sonoplus, Germany) for 15 min. After that, the solution was centrifuged for 10 min at 4000 rpm and the supernatant was separated from the residue. The supernatant was filtered using filter paper Whatman 5. The residue was extracted one more time with 50 mL of 70% ethanol for 15 min at 20 kHz. The two supernatants were collected in volumetric flasks and the solution was brought to a 50 mL volume with 70% ethanol.
Extraction yield
The extraction yield was expressed as balsam content (soluble ethanolic fraction) and determined according to Popova et al. (2007). 2 mL of ethanolic extract were evaporated to constant weight into an oven at 60 °C. The extraction yield was calculated as follows:
Total phenolic content (TPC) determination
For the TPC determination it was used the method described by Escriche and Juan-Borrás (2018). 100 µL of extract was mixed with 1900 µL of water and placed into a tube, and after that 100 µL of Folin–Ciocalteau reagent was added. After 2 min, 800 μL of 5% sodium carbonate (w/v) was added. This solution was maintained in a water bath at 40 °C for 20 min, and then the tube was rapidly cooled with crushed ice to stop the reaction. The determination was carried out at 760 nm. The results were expressed as mg gallic acid/g propolis and mg pinocembrin:galangin (1:1, w/w)/g propolis. The ethanolic solution was used as a blank and every assay was carried out in triplicate.
Flavone and flavonol content
The flavone and flavonol content was measured using the Popova et al. (2007) method. 2 mL of extract was mixed with 20 mL of methanol and 1 mL of AlCl3 (5%) in a 50 mL volumetric flask, and after a prior homogenization the flask was filled with methanol. The determination was carried out at 425 nm. The content was expressed as mg galangin/g propolis. Every assay was carried out in triplicate.
Flavanone and dihydroflavonol content
The flavanone and dihydroflavonol content was measured using the Popova et al. (2017) method. 1 mL of extract was mixed with 2 mL of DNP solution (1 g of dinitrophenylhydrazine (DNP) in 2 ml 96% H2SO4, diluted to 100 mL with methanol); the mix was heated to 50 °C for 50 min. After cooling, the mixture was diluted to 10 mL with 10% KOH in methanol. 1 mL of the solution was mixed with 20 mL of methanol in a 50 mL flask, and after homogenization the flask was filled with methanol. The determination was carried out at 486 nm. The content was expressed as mg pinocembrin/g propolis. Every assay was carried out in triplicate.
Chromatographic separation and determination of phenolic compounds
The phenolic compounds were separated and quantified using the method described by Coneac et al. (2008), Oroian and Ropciuc (2017). A High Performance Liquid Chromatography (HPLC) (Shimadzu, Kyoto, Japan) system equipped with a LC-20 AD liquid chromatograph, SIL-20A auto samples, CTO-20AC auto sampler and a SPD-M-20A diode array detector was used. The separation was carried out on a Zorbax SP-C18 column, with 150 mm length, 4.6 mm i.d., and 5 μm-diameter particle; the phenolics detection was set at 295 nm. The mobile phase was acetonitrile: water in a ratio of 48:52 (v/v), temperature was 30 °C, with a flow of 0.3 mL/min, and the injected sample volume was of 20 µL. The diluted standard solutions of quercetin, apigenin, myricetin, isorhamnetin, kaempherol, caffeic acid, chrysin, galangin, luteolin, p-coumaric acid, ferulic acid, and pinocembrin were analyzed under the same HPLC conditions and the calibration of the detector response was made. Data collection and subsequent processing were performed using the LC solution software 1.21 version (Shimadzu, Kyoto, Japan). The phenolics were identified by comparing the chromatographic retention times and UV spectra of each compound. The calibration curves were constructed via least-squares linear regression analyses of the ratio of the peak area of each representative compound versus the respective concentration. The regression analysis (n = 5) showed correlation coefficients (R2) higher than 0.99 for all the compounds. The quantitative results were expressed as mg of compound per g propolis.
Statistical analysis
The statistical analysis [ANOVA and Principal component analysis (PCA)] was performed using Unscrambler X 10.1 software system (Camo, Norway). The data corresponding to each variable were analysed by one-factor analysis of variance (ANOVA). Multiple comparisons were performed using the least significant difference test (LSD) and Fisher ratio (F), and statistical significance was set at α = 0.05.
Results and discussion
Extraction efficiency
The extraction of polyphenols from different products by liquid extraction using hot or cold solvents is common; the most used solvents for this purpose are aqueous mixtures with ethanol, methanol, acetone etc. (Antolovich et al. 2000). In “herbal medicine”, propolis is consumed as a macerated product in ethanol solutions, and for this reason in this paper the traditional method of propolis preparation was compared to two different extraction methods that are much faster.
In Table 1 is presented the extraction efficiency, expressed as g balsam on 100 g crude propolis, obtained using three different techniques: double maceration, double microwave treatment and double ultrasonic treatment. The microwave treatment, as mentioned in the “Materials and methods” section, was made for 1 min at 140 W because the temperature of the ethanolic solution after this time of exposure was around 60 °C, while the ultrasonic treatment was applied for 15 min at 20 kHz because the temperature of the ethanolic solution after this time was around 60 °C and the phenolic compounds extraction was decreasing (Mokrani and Madani 2016). In Table 1 is presented the extraction efficiency after the 1st, 2nd and 1st + 2nd extractions.
Table 1.
Extraction efficiency (amount of extract expressed as g of extract per 100 g of crude propolis) of the three different methods applied on the propolis
| Sample | Maceration | Microwave | Ultrasonic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 1st + 2nd | 1st | 2nd | 1st + 2nd | 1st | 2nd | 1st + 2nd | |
| A | 61.2 (1.2) | 6.0 (0.2) | 67.2 (1.2) | 63.0 (1.0) | 9.0 (0.3) | 72.0 (1.2) | 68.0 (1.1) | 7.8 (0.3) | 75.8 (1.4) |
| B | 66.0 (1.3) | 6.4 (0.3) | 72.4 (1.4) | 68.6 (1.4) | 10.4 (0.5) | 79.0 (1.5) | 72.6 (1.6) | 9.2 (0.4) | 81.8 (1.7) |
| C | 32.3 (0.6) | 3.4 (0.2) | 35.6 (0.7) | 25.7 (0.5) | 10.4 (0.2) | 36.1 (0.6) | 31.9 (0.3) | 5.2 (0.1) | 37.1 (0.4) |
| D | 66.1 (1.2) | 25.0 (1.0) | 91.2 (1.4) | 88.8 (1.2) | 7.1 (0.2) | 95.9 (1.3) | 95.8 (0.8) | 0.9 (0.1) | 96.7 (0.8) |
| E | 40.4 (1.2) | 14.4 (0.1) | 54.8 (1.3) | 49.7 (0.7) | 10.0 (0.5) | 59.6 (0.9) | 53.0 (1.1) | 10.0 (0.1) | 63.0 (1.1) |
| F | 69.6 (1.1) | 4.1 (0.3) | 73.7 (1.2) | 59.2 (1.3) | 24.9 (0.7) | 84.1 (1.5) | 79.6 (0.2) | 6.6 (0.1) | 86.3 (0.3) |
A–B: samples from spring 2018, C–F: samples from autumn 2017
A great difference can be observed between the samples of propolis in terms of extraction yield. The highest extraction yield was three times higher than the lowest extraction yield, and these two samples were from 2017. According to Thamnopoulos et al. (2018), propolis is composed of resin, vegetable balsam, wax, essential and aromatic oils, pollen, and organic debris. However, the great differences observed in terms of propolis yield are because of the presence of different organic contaminants such as: insect remains or some adulterants that beekeepers mix in this product in order to increase the quantity of the finished product (Escriche and Juan-Borrás 2018). Stan et al. (2011) considered that the beekeeping practices have a great influence on the propolis yield due to the presence of different impurities in propolis. The propolis yield was used to characterize the active compounds present in the crude propolis, and can be considered a quality parameter.
The propolis yield was significantly different depending on the extraction. It can be observed that in all 6 samples the double ultrasonication reached the highest extraction yield (37.1–96.7 g balsam/100 g crude propolis), followed by double microwave treatment (36.1–95.9 g balsam/100 g crude propolis) and double maceration (35.6–91.2 g balsam/100 g crude propolis). When comparing the first extraction to the second extraction, it can be noted a high difference in terms of propolis yield, where normally the second extraction represents less than 30% of the first extraction. The increase of the extraction yield during the ultrasonication process is because of the mechanical effect; this effect causes a disruption of the material cell walls when the cavitation bubbles collapse at the surface of the solid matrix and therefore the mass transfer is enhanced and the contact surface area between solvent and material increases (Dranca and Oroian 2016).
The suitable method for reaching the highest yield is the double ultrasonication method, however, for preparing a propolis extract at home the microwave method is the accessible one because the microwave oven is normally present in all households. The results obtained in this study were in agreement with Liu et al. (2006) and Trusheva et al. (2007) which compared the extraction efficiency of ultrasonic- and microwave-assisted extraction and maceration for medicinal plants and propolis, and observed that the ultrasonic method reached the highest extraction yield for phenolic compounds. The yield reported in this study is similar to the Taiwanese propolis yield (Chen et al. 2018), Spanish and Honduras propolis yield (Escriche and Juan-Borrás 2018).
Total phenolic content: flavone and flavonol content and flavanone and dihydroflavonol content
In Table 2 are presented the average values and the standard deviation of total phenolic content, flavanone and flavonol content, flavanone and dihydroflavonol content in the propolis samples, and the analysis of variance (method of extraction and samples). The determination of these total compounds was made by using the three different methods (maceration, microwave and ultrasonication) in order to achieve the suitable process for extraction.
Table 2.
Analysis of variance of total phenolic content, flavone and flavonol content and flavanone and dihydroflavonol content from propolis using different methods of extraction
| Sample | Maceration | Microwave | Ultrasonic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 1st + 2nd | 1st | 2nd | 1st + 2nd | 1st | 2nd | 1st + 2nd | |||
| Total phenolic content (TPC) | |||||||||||
| A* | 319.3 (9.6) | 31.3 (0.9) | 350.6 (10.5) | 328.7 (9.9) | 46.7 (1.4) | 375.4 (11.3) | 354.5 (10.6) | 40.7 (1.2) | 395.2 (11.9) | ||
| A** | 338.4 (10.2) | 33.2 (1.0) | 371.6 (11.1) | 348.4 (10.5) | 49.5 (1.5) | 397.9 (11.9) | 375.8 (11.3) | 43.2 (1.3) | 418.9 (12.6) | ||
| B* | 344.4 (10.3) | 33.1 (1.0) | 377.5 (11.3) | 357.8 (10.7) | 54.0 (1.6) | 411.8 (12.4) | 378.6 (11.4) | 48.1 (1.4) | 426.7 (12.8) | ||
| B** | 365.0 (11.0) | 35.1 (1.1) | 400.1 (12.0) | 379.2 (11.4) | 57.3 (1.7) | 436.5 (13.1) | 401.03 (12.0) | 51.0 (1.5) | 452.3 (13.6) | ||
| C* | 168.3 (5.0) | 17.7 (0.5) | 185.9 (5.6) | 134.0 (4.0) | 54.1 (1.6) | 188.1 (5.6) | 166.4 (5.0) | 27.1 (0.8) | 193.6 (5.8) | ||
| C** | 178.3 (4.5) | 18.7 (0.5) | 197.1 (4.9) | 142.0 (3.6) | 57.3 (1.4) | 199.3 (5.0) | 176.4 (4.4) | 28.7 (0.7) | 205.2 (5.1) | ||
| D* | 463.3 (13.9) | 37.1 (1.1) | 500.4 (15.0) | 344.9 (10.3) | 130.6 (3.9) | 475.6 (14.3) | 499.4 (5.0) | 4.8 (0.1) | 504.2 (5.1) | ||
| D** | 491.0 (12.3) | 39.3 (1.0) | 530.4 (13.3) | 365.6 (9.1) | 138.5 (3.5) | 504.1 (12.6) | 529.4 (5.1) | 13.2 (0.1) | 534.4 (13.4) | ||
| E* | 211.0 (6.3) | 75.1 (2.3) | 286.0 (8.6) | 259.1 (7.8) | 52.0 (1.6) | 311.1 (9.3) | 276.3 (8.3) | 52.1 (1.6) | 328.4 (9.9) | ||
| E** | 223.6 (5.6) | 79.6 (2.0) | 303.2 (7.6) | 274.6 (6.9) | 55.1 (1.4) | 329.7 (8.2) | 292.9 (7.3) | 55.2 (1.4) | 348.1 (8.7) | ||
| F* | 362.8 (10.9) | 21.6 (0.6) | 384.4 (11.5) | 308.9 (9.3) | 129.7 (3.9) | 438.6 (13.2) | 415.4 (12.5) | 34.5 (1.0) | 449.9 (13.5) | ||
| F** | 384.6 (9.6) | 22.8 (0.6) | 407.4 (10.2) | 327.5 (8.2) | 137.5 (3.4) | 464.9 (11.6) | 440.3 (11.0) | 36.5 (0.9) | 476.8 (11.9) | ||
| Flavone and flavonol content (mg galangin/propolis) | |||||||||||
| A | 112.1 (3.4) | 10.5 (0.3) | 122.6 (3.7) | 115.4 (3.5) | 15.9 (0.5) | 124.5 (3.7) | 124.5 (3.7) | 13.8 (0.4) | 138.3 (4.1) | ||
| B | 121.0 (3.6) | 11.1 (0.3) | 132.1 (4.0) | 125.7 (3.8) | 18.5 (0.6) | 144.2 (4.3) | 133.0 (4.0) | 16.4 (0.5) | 149.4 (4.5) | ||
| C | 58.8 (1.8) | 5.6 (0.2) | 64.4 (1.9) | 46.7 (1.4) | 18.5 (0.6) | 65.2 (2.0) | 58.2 (1.7) | 9.0 (0.3) | 67.1 (2.0) | ||
| D | 162.9 (4.9) | 12.5 (0.4) | 175.4 (5.3) | 121.2 (3.6) | 45.5 (1.4) | 166.7 (5.0) | 175.7 (5.3) | 5.3 (0.1) | 176.5 (5.4) | ||
| E | 73.9 (2.2) | 25.9 (0.8) | 99.8 (3.0) | 90.9 (2.7) | 17.8 (0.5) | 108.6 (3.3) | 96.9 (2.9) | 17.8 (0.5) | 114.7 (3.4) | ||
| F | 127.5 (3.8) | 7.0 (0.2) | 134.5 (4.0) | 108.5 (3.3) | 45.2 (1.4) | 153.6 (4.6) | 146.0 (4.4) | 11.6 (0.3) | 157.6 (4.7) | ||
| Flavanone and dihydroflavonol content (mg pinocembrin/g propolis) | |||||||||||
| A | 63.2 (1.9) | 7.3 (0.2) | 70.5 (2.1) | 65.0 (2.0) | 10.3 (0.3) | 75.3 (2.3) | 70.0 (2.1) | 9.2 (0.3) | 79.2 (2.4) | ||
| B | 68.0 (2.0) | 7.7 (0.2) | 75.7 (2.3) | 70.7 (2.1) | 11.7 (0.4) | 82.4 (2.5) | 74.7 (2.2) | 10.6 (0.3) | 85.3 (2.6) | ||
| C | 33.9 (1.0) | 4.7 (0.1) | 38.6 (1.2) | 27.2 (0.8) | 11.7 (0.4) | 39.0 (1.2) | 33.5 (1.0) | 6.5 (0.2) | 40.0 (1.2) | ||
| D | 91.1 (2.7) | 8.5 (0.3) | 99.6 (3.0) | 68.2 (2.0) | 26.6 (0.8) | 94.8 (2.8) | 98.1 (2.9) | 2.2 (0.1) | 100.3 (3.0) | ||
| E | 42.2 (1.3) | 15.8 (0.5) | 58.0 (1.7) | 51.5 (1.5) | 11.3 (0.3) | 62.8 (1.9) | 54.9 (1.6) | 11.4 (0.6) | 66.2 (2.0) | ||
| F | 71.6 (2.1) | 5.4 (0.2) | 77.1 (2.3) | 61.2 (1.8) | 26.4 (0.8) | 87.6 (2.6) | 81.8 (2.5) | 7.9 (0.2) | 89.8 (2.7) | ||
| ANOVA results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample factor | Extraction factor | Sample × extraction | |||||||||
| Pino/Gala | Gal a | Gala | Pino | Pino/Gala | Gal a | Gala | Pino | Pino/Gala | Gal a | Gala | Pino |
| F value | |||||||||||
| 9246*** | 8564*** | 8406*** | 5600*** | 419*** | 319*** | 407*** | 193** | 85** | 81*** | 77*** | 41*** |
Pino pinocembrin, Gala galangin, Gal a gallic acid
*mg acid gallic/g propolis, **mg pinocembrin/galangin/g propolis, ***P < 0.001
The total phenolic content was expressed as mg gallic acid/g propolis (the most used reference substance for total phenolic content) (Oroian 2012) and mg pinocembrin/galangin/g propolis (the two substances are considered the suitable ones for propolis total phenolic content) (Cottica et al. 2015; Popova et al. 2007; Escriche and Juan-Borrás 2018). Some authors reported the total phenolic content as mg/g balsam (Cottica et al. 2015; Escriche and Juan-Borrás 2018), but we considered that it is better to express the total phenolic content as mg/g propolis because in many cases this product is a home-made “drug” and consumers do not have the possibility to measure the balsam content. In Table 2 it can be observed that the ultrasonication process is better than the microwave or maceration process for the extraction of total phenols, flavanone and dihydroflavonol content. In all the methods applied the second extraction represents an important percentage of the total content of phenolic compounds. The total phenolic content ranged between 185.9 and 500.4 mg gallic acid/g propolis; the difference between samples was significant (P < 0.05). The samples from 2018 (A and B) had similar content of phenolics, while in the case of the samples from 2017 (C to E) the difference was very high and may be caused by keeping the propolis under unsuitable conditions by the beekeepers. As in the case of extraction yield, it can be observed a significant difference between the methods used for the extraction (Table 2), and the interaction between the sample and extraction is a significant one for all the phenolic parameters determined.
In the scientific literature there are some studies which deal with the total phenolic content of propolis, but each group uses different calibration standards, as follows: pinocembrin and galangin (Popova et al. 2007), gallic acid, quercetin, 3,4-dihydroxybenzoic acid, caffeic acid and vanillin (González et al. 2003), gallic acid (Kumazawa et al. 2004; Cottica et al. 2015) or gallic acid and pinocembrin/galangin, pinocembrin and galangin (2:1) (Trusheva et al. 2007), and gallic acid (Di Capua et al. 2018). Our results are in agreement with the TPC reported in the case of Turkish propolis (Ozdal et al. 2018), Bulgarian propolis (Trusheva et al. 2007) and Canadian propolis (Cottica et al. 2015).
Phenolic profile
In Table 3 is presented the content of different phenolic compounds, expressed as mg of phenolic/g propolis, obtained using the three extraction methods proposed as: maceration (1st and 2nd), microwave (1st and 2nd) and ultrasonic (1st and 2nd). The table also presents the analysis of variance (ANOVA) for observing the influence of the method applied for extraction and whether the sample influences the content of individual phenolics. From the twelve phenolics separated, four of them were reported in concentrations higher than 50 mg/g propolis: chrysin, p-coumaric acid, myricetin, and rutin. Luteolin was the phenolic observed in the lowest concentration (between 2.47 and 6.70 mg/g propolis). All of the 12 quantified compounds showed significant differences between samples and all of them presented significant differences on the base of the method of extraction (Table 3). The most abundant phenolic was p-coumaric acid (271.65 mg/g propolis) presented in the sample from 2017. The sample which reached the highest concentration of p-coumaric acid also reached the highest concentration of all the phenolics determined. The results presented in this study were in agreement with Escriche and Juan-Borrás (2018). Other studies reported for European propolis from Ukraine and Bulgaria showed high concentration of pinobanksin (14.7 mg/g of ethanolic extract) and chrysin (120.4 of mg/g of ethanolic extract) (Kumazawa et al. 2004), for Chinese propolis caffeic acid (3.74 mg/g propolis) and pinobanksin-3-O-acetate (69.36 mg/g propolis), and for Honduras propolis trans-cinnamic acid (59 mg/g balsam) (Escriche and Juan-Borrás 2018).
Table 3.
Phenolic compounds profile (average values expressed as mg of compound/g propolis) presented in the six propolis sample extracted using maceration (M), microwave (Mi) and ultrasonic
| Parameter (mg/g propolis) | Extraction (1st + 2nd) | Sample | ANOVA sample | ANOVA extraction | Sample × ext | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |||||
| Apigenin | M | 9.31 | 10.03 | 4.94 | 13.29 | 8.26 | 10.21 | 250*** | 23*** | 7*** |
| Mi | 9.97 | 10.94 | 4.99 | 12.63 | 7.60 | 11.65 | ||||
| U | 10.50 | 11.33 | 5.14 | 13.39 | 8.17 | 11.95 | ||||
| Caffeic acid | M | 14.63 | 15.76 | 7.76 | 20.88 | 12.98 | 16.04 | 1001*** | 38*** | 9*** |
| Mi | 15.67 | 17.19 | 7.85 | 19.85 | 11.94 | 18.31 | ||||
| U | 16.50 | 17.81 | 8.08 | 21.04 | 12.84 | 18.78 | ||||
| Chrysin | M | 112.41 | 121.03 | 59.61 | 160.42 | 99.73 | 123.24 | 980*** | 36*** | 9*** |
| Mi | 120.35 | 132.03 | 60.29 | 152.48 | 91.70 | 140.62 | ||||
| U | 126.71 | 136.82 | 62.08 | 161.65 | 98.61 | 144.23 | ||||
| p-Coumaric acid | M | 188.90 | 203.39 | 100.17 | 269.59 | 167.59 | 207.10 | 871*** | 21*** | 3*** |
| Mi | 202.24 | 221.87 | 101.32 | 256.24 | 154.10 | 236.32 | ||||
| U | 212.94 | 229.91 | 104.32 | 271.65 | 165.71 | 242.37 | ||||
| Galangin | M | 15.96 | 17.19 | 8.46 | 22.78 | 14.16 | 17.50 | 276*** | 42*** | 10*** |
| Mi | 17.09 | 18.75 | 8.56 | 21.65 | 13.02 | 19.97 | ||||
| U | 17.99 | 19.43 | 8.82 | 22.96 | 14.00 | 20.48 | ||||
| Isorhamnetin | M | 15.96 | 17.19 | 8.46 | 22.78 | 14.16 | 17.50 | 324*** | 25*** | 8*** |
| Mi | 17.09 | 18.75 | 8.56 | 21.65 | 13.02 | 19.97 | ||||
| U | 17.99 | 19.43 | 8.82 | 22.96 | 14.00 | 20.48 | ||||
| Kaempferol | M | 9.31 | 10.03 | 4.94 | 13.29 | 8.26 | 10.21 | 267*** | 21*** | 6*** |
| Mi | 9.97 | 10.94 | 4.99 | 12.63 | 7.60 | 11.65 | ||||
| U | 10.50 | 11.33 | 5.14 | 13.39 | 8.17 | 11.95 | ||||
| Luteolin | M | 4.66 | 5.01 | 2.47 | 6.64 | 4.13 | 5.10 | 124*** | 12** | 3* |
| Mi | 4.98 | 5.47 | 2.50 | 6.32 | 3.80 | 5.82 | ||||
| U | 5.25 | 5.67 | 2.57 | 6.70 | 4.08 | 5.97 | ||||
| Myricetin | M | 172.94 | 186.20 | 91.70 | 246.80 | 153.43 | 189.59 | 794** | 19*** | 2** |
| Mi | 185.15 | 203.12 | 92.76 | 234.58 | 141.08 | 216.35 | ||||
| U | 194.94 | 210.49 | 95.51 | 248.69 | 151.71 | 221.89 | ||||
| Pinocembrin | M | 11.97 | 12.89 | 6.35 | 17.09 | 10.62 | 13.13 | 230*** | 37*** | 8*** |
| Mi | 12.82 | 14.06 | 6.42 | 16.24 | 9.77 | 14.98 | ||||
| U | 13.50 | 14.57 | 6.61 | 17.22 | 10.50 | 15.36 | ||||
| Rutin | M | 52.55 | 56.58 | 27.86 | 74.99 | 46.62 | 57.61 | 901*** | 35*** | 5** |
| Mi | 56.26 | 61.72 | 28.18 | 71.28 | 42.87 | 65.74 | ||||
| U | 59.23 | 63.96 | 29.02 | 75.56 | 46.10 | 67.42 | ||||
| Quercetin | M | 8.65 | 9.31 | 4.59 | 12.34 | 7.67 | 9.48 | 651*** | 10** | 1.5* |
| Mi | 9.26 | 10.16 | 4.64 | 11.73 | 7.05 | 10.82 | ||||
| U | 9.75 | 10.52 | 4.78 | 12.43 | 7.59 | 11.09 | ||||
The data were submitted to multifactorial analysis of variance with two factors: sample and extraction
Principal component analysis (PCA)
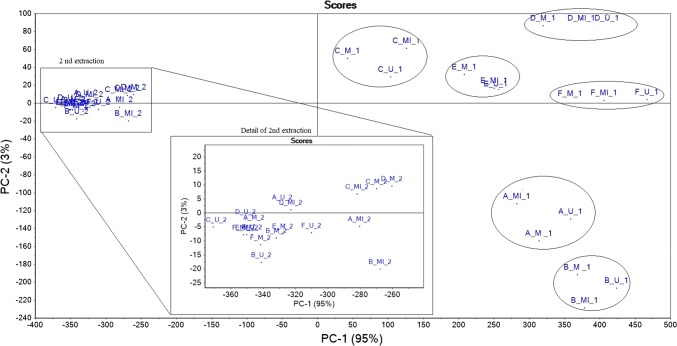
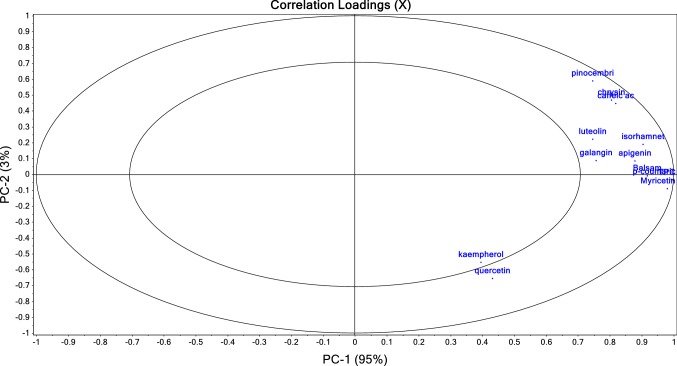
The principal component analysis was performed in order to evaluate the global effect of the extraction method applied and sample type on the phenolic profile and balsam content. In Figs. 1 and 2 are presented the scores and loadings of the propolis samples and phenolic profile/balsam content, respectively. The PC1 and PC2 explained 98% of the total variance. Figure 1 shows that the samples were divided two times, one time according to the year of harvest and the second time according to the extraction number. The PC1 separated the samples A and B (from 2018) from the samples C, D, E and F (from 2017), confirming that the year is strongly influencing the profile of phenolic compounds. When analysing the PCA plot in more detail, it can be observed that for the same sample there are some differences with respect to the extraction method applied. This indicates that the method of extraction has an effect on the analysed parameters as the score points are mainly grouped according to the type of propolis. The samples from the 2nd extraction are all found in the same place and no clear difference can be observed between them. The loading plot clearly shows that certain compounds are responsible for the differentiation between samples: kaempferol and quercetin are characteristic of the samples from 2018, and pinocembrin, chrysin, caffeic acid, luteolin, isorhamnetin, galangin, and apigenin are characteristic of the 2017 samples.
Fig. 1.
Principal component scores for the propolis samples (A, B—spring 2018, C, D, E and F—autumn 2017) extracted using different methods: maceration (M), ultrasonication (U) and microwave (Mi) for two times (1—first extraction, 2—second extraction)
Fig. 2.
Principal component loadings of the propolis extraction parameters determined
Conclusion
The article presents a comparative study on the extraction of bioactive compounds from propolis using three different methods (maceration, ultrasonication and microwave extraction). The selection of the most suitable method of extraction was based on the assessment of extraction yield, total phenolic content, flavones and flavonol content, and flavanone and dihydroflavonol content. The ultrasonication proved to be a better method for the extraction of bioactive compounds from propolis than microwave extraction and maceration. It was observed a great difference among the samples analysed that may be due to the impurities that are presented in the propolis samples. The PCA separated the samples according to the year and extraction time, and therefore it was concluded that the extraction year influences the propolis composition in terms of bioactive compounds.
Acknowledgements
This project is co-funded by the Ministry of Research and Innovation through Program 1—Development of the National R&D System, Subprogram 1.2—Institutional Performance—Projects for Excellence Financing in RDI, Contract No.18PFE/2018 and by the Romania National Council for Higher Education Funding, CNFIS, project number CNFIS-FDI-2019-0600.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Ani I, Zimmermann S, Reichling J, Wink M. Antimicrobial activities of european propolis collected from various geographic origins alone and in combination with antibiotics. Medicines. 2018;5(1):2. doi: 10.3390/medicines5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm-Eldeen AA, Basyony MA, Elfiky NK, Ghalwash MM. Effect of the Egyptian propolis on the hepatic antioxidant defense and pro-apoptotic p53 and antiapoptotic bcl2 expressions in aflatoxin B1 treated male mice. Biomed Pharmacother. 2017;87:247–255. doi: 10.1016/j.biopha.2016.12.084. [DOI] [PubMed] [Google Scholar]
- Antolovich M, Prenzler P, Robards K, Ryan D. Sample preparation in the determination of phenolic compounds in fruits. Analyst. 2000;125(5):989–1009. [Google Scholar]
- Biscaia D, Ferreira SR. Propolis extracts obtained by low pressure methods and supercritical fluid extraction. J Supercrit Fluids. 2009;51(1):17–23. [Google Scholar]
- Chen YW, Ye SR, Ting C, Yu YH. Antibacterial activity of propolins from Taiwanese green propolis. J Food Drug Anal. 2018;26(2):761–768. doi: 10.1016/j.jfda.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coneac G, Gafiţanu E, Hădărugă DI, et al. Flavonoid contents of propolis from the west side of Romania and correlation with the antioxidant activity. Chem Bull Politeh Uni Timis. 2008;53(67):56–60. [Google Scholar]
- Cornara L, Biagi M, Xiao J, Burlando B. Therapeutic properties of bioactive compounds from different honeybee products. Front Pharmacol. 2017;8:412. doi: 10.3389/fphar.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottica SM, Sabik H, Antoine C, et al. Characterization of Canadian propolis fractions obtained from two-step sequential extraction. LWT Food Sci Technol. 2015;60(1):609–614. [Google Scholar]
- Cruz M, Antunes P, Paulo L, et al. Antioxidant and dual dose-dependent antigenotoxic and genotoxic properties of an ethanol extract of propolis. RSC Adv. 2016;6(55):49806–49816. [Google Scholar]
- Di Capua A, Bejarano A, Adami R, Reverchon E. Preparation and characterization of Chilean propolis coprecipitates using supercritical assisted atomization. Chem Eng Res Des. 2018;136:776–785. [Google Scholar]
- Dranca F, Oroian M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason Sonochem. 2016;31:637–646. doi: 10.1016/j.ultsonch.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Escriche I, Juan-Borrás M. Standardizing the analysis of phenolic profile in propolis. Food Res Int. 2018;106:834–841. doi: 10.1016/j.foodres.2018.01.055. [DOI] [PubMed] [Google Scholar]
- Falcão SI, Vilas-Boas M, Estevinho LM, et al. Phenolic characterization of Northeast Portuguese propolis: usual and unusual compounds. Anal Bioanal Chem. 2010;396(2):887–897. doi: 10.1007/s00216-009-3232-8. [DOI] [PubMed] [Google Scholar]
- Falcão SI, Vale N, Cos P, et al. In vitro evaluation of Portuguese propolis and floral sources for antiprotozoal, antibacterial and antifungal activity. Phytother Res. 2014;28(3):437–443. doi: 10.1002/ptr.5013. [DOI] [PubMed] [Google Scholar]
- Freitas JA, Vanat N, Pinheiro JW, et al. The effects of propolis on antibody production by laying hens. Poult Sci. 2011;90(6):1227–1233. doi: 10.3382/ps.2010-01315. [DOI] [PubMed] [Google Scholar]
- González M, Guzmán B, Rudyk R, et al. Spectrophotometric determination of phenolic compounds in propolis. Acta Farm Bonaer. 2003;22(3):243–248. [Google Scholar]
- Jug M, Končić MZ, Kosalec I. Modulation of antioxidant, chelating and antimicrobial activity of poplar chemo-type propolis by extraction procures. LWT Food Sci Technol. 2014;57(2):530–537. [Google Scholar]
- Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84(3):329–339. [Google Scholar]
- Liu Z, Ding L, Zhang H, Hu X, Bu F. Comparison of the different extraction methods of flavonoids in Epimedium Koreamum Nakai by HPLC–DAD–ESI–MSn. J Liq Chromatogr Relat Technol. 2006;29(5):719–731. [Google Scholar]
- Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26(2):83–99. [Google Scholar]
- Meimandi-Parizi A, Oryan A, Sayahi E, Bigham-Sadegh A. Propolis extract a new reinforcement material in improving bone healing: an in vivo study. Int J Surg. 2018;56:94–101. doi: 10.1016/j.ijsu.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Mokrani A, Madani K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep Purif Technol. 2016;161:68–76. [Google Scholar]
- Moreira L, Dias LG, Pereira JA, Estevinho L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem Toxicol. 2008;46(11):3482–3485. doi: 10.1016/j.fct.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Nna VU, Bakar ABA, Mohamed M. Malaysian propolis, metformin and their combination, exert hepatoprotective effect in streptozotocin-induced diabetic rats. Life Sci. 2018;211:40–50. doi: 10.1016/j.lfs.2018.09.018. [DOI] [PubMed] [Google Scholar]
- Oliveira AV, Ferreira AL, Nunes S, et al. Antibacterial activity of propolis extracts from the south of Portugal. Pak J Pharm Sci. 2017;30(1):1–9. [PubMed] [Google Scholar]
- Oroian M. Physicochemical and rheological properties of Romanian honeys. Food Biophys. 2012;7(4):296–307. [Google Scholar]
- Oroian M, Ropciuc S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput Electron Agric. 2017;138:148–156. [Google Scholar]
- Ozdal T, Sari-Kaplan G, Mutlu-Altundag E, et al. Evaluation of Turkish propolis for its chemical composition, antioxidant capacity, anti-proliferative effect on several human breast cancer cell lines and proliferative effect on fibroblasts and mouse mesenchymal stem cell line. J Apic Res. 2018;57:1–12. [Google Scholar]
- Popova MP, Bankova VS, Bogdanov S, et al. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie. 2007;38(3):306–311. [Google Scholar]
- Popova M, Trusheva B, Bankova V. Content of biologically active compounds in Bulgarian propolis: a basis for its standardization. Bulg Chem Commun. 2017;49:115–120. [Google Scholar]
- Roh J, Kim KR. Antimicrobial activity of Korean propolis extracts on oral pathogenic microorganisms. J Dent Hyg Sci. 2018;18(1):18–23. [Google Scholar]
- Sawaya ACHF, da Silva Cunha IB, Marcucci MC. Analytical methods applied to diverse types of Brazilian propolis. Chem Cent J. 2011;5(1):27. doi: 10.1186/1752-153X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res. 2016;30(6):894–905. doi: 10.1002/ptr.5605. [DOI] [PubMed] [Google Scholar]
- Sforci JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133(2):253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Sheng J, Zhou J, Wang L, et al. Antioxidant activity of ethanol and petroleum ether extracts from Brazilian propolis. Eur Food Res Technol. 2007;225(2):249–253. [Google Scholar]
- Silva-Carvalho R, Miranda-Gonçalves V, Ferreira AM, et al. Antitumoural and antiangiogenic activity of Portuguese propolis in in vitro and in vivo models. J Funct Foods. 2014;11:160–171. [Google Scholar]
- Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Altern Med. 2015;2015:206439. doi: 10.1155/2015/206439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan L, Mărghitaş LA, Dezmirean D. Quality criteria for propolis standardization. Sci Pap Anim Sci Biotechnol. 2011;44(2):137–140. [Google Scholar]
- Thamnopoulos IAI, Michailidis GF, Fletouris DJ, et al. Inhibitory activity of propolis against Listeria monocytogenes in milk stored under refrigeration. Food Microbiol. 2018;73:168–176. doi: 10.1016/j.fm.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Trusheva B, Trunkova D, Bankova V. Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J. 2007;1(1):13. doi: 10.1186/1752-153X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloz JJ, Saavedra N, Lillo A et al (2015) Antibiofilm activity of Chilean propolis on Streptococcus mutans is influenced by the year of collection. BioMed Res Int, Article ID 291351 [DOI] [PMC free article] [PubMed]
- Yildirim A, Duran GG, Duran N, et al. Antiviral activity of hataypropolis against replication of herpes simplex virus type 1 and type 2. Med Sci Monit. 2016;22:422–430. doi: 10.12659/MSM.897282. [DOI] [PMC free article] [PubMed] [Google Scholar]