Abstract
This study investigated the use of Bacillus subtilis protease powder (CTC E-ssentials™ MT-70N) as a carabeef tenderizer. The effect of the bacterial protease on the characteristics of carabeef was determined, and its effectiveness was compared to a commercial meat tenderizer containing papain. Only B. subtilis protease showed significant enzyme activity (80–190 U/g), while the commercial meat tenderizer had no activity (0 U/g). Results from the shear force device revealed that 0.35% B. subtilis protease was the optimal concentration required to induce significant tenderization in carabeef (282 g/cm2) and reduce carabeef toughness by 80%. Proximate analysis showed that carabeef treated with B. subtilis protease had significantly higher crude protein (37%) than the negative control (34%) and carabeef-treated commercial meat tenderizer (31%). Sensory evaluation revealed that carabeef treated with 0.35% B. subtilis protease is more tender than untreated carabeef and those treated with the commercial meat tenderizer. Moreover, the carabeef was not over-tenderized and is palatably acceptable. Hence, B. subtilis protease can be used as a meat tenderizer in place of available commercial tenderizers containing plant-derived proteases.
Keywords: Sensory evaluation, Carabao beef, Bacillus subtilis, Protease assay, Meat tenderizer
Introduction
Quality and overall acceptability of beef products are mostly judged by consumers based on tenderness (Neely et al. 1998). In these times when consumer acceptance for “enhanced”, processed, and “meal-ready” meat products are increasing, the tenderness of such products becomes even more significant (Ashie et al. 2002). Hence, it is important to develop a commercially applicable method that ensures a consistently tender beef product for consumer acceptance. And by improving beef tenderness, it will make the entire carcass more valuable, thus, allowing purveyors to sell consistently tender products, earn more profit, and meeting the demands of consumers (Pietrasik and Shand 2004). Carabeef is particularly in demand, especially in Islamic nations, because it is cheaper and its production meets halal standards. Thus, it is expanding in the markets of the Middle East, North Africa, and Southeast Asia (USDA 2012).
Of all the attributes of eating quality (i.e., appearance, juiciness, odor, taste, and tenderness/texture), tenderness is rated the most important factor affecting beef palatability and much research has been focused on improving tenderness (Vasanthi et al. 2007). Proteolytic enzymes derived from plants, such as papain, bromelain, and ficin, which have been widely used as meat tenderizers in America and Europe (Maqsood et al. 2018), are now being used in Asia, including the Philippines. In a recent study by Maqsood et al. (2018), these plant proteases can effectively tenderize camel meat, which is known as one of the toughest kinds of meat.
However, due to their broad substrate specificity, plant enzymes often over-tenderize meat. Over-tenderization leads to unpalatability and mushy texture. Hence, the ideal meat tenderizer would be a protease that specifically acts on connective tissue proteins (i.e., collagen and elastin) either at refrigeration or cooking temperatures (Gerelt et al. 2000). The tenderizer should also exhibit high activity at meat pH and room temperature but can be easily inactivated during cooking to avoid over-tenderization (Zhao et al. 2012). Such specificity is a characteristic of microbial proteases. Aside from acting specifically on collagen and elastin in meat, microbial proteases are milder meat tenderizers than their plant counterparts. Thus, the over-tenderization of meat can be avoided.
It is noted by Singh et al. (2009) that B. subtilis is generally recognized as safe (GRAS) organism by Food and Drug Administration (FDA) and is one of the most widely used bacteria for large-scale production of recombinant enzymes. Currently, there are no studies that have explored the use of bacterium-derived proteases for improving the tenderness of water buffalo (i.e., carabao) meat. It is the main objective of the study to evaluate the efficacy of manufactured protease powder obtained from Bacillus subtilis (Ehrenberg) Cohn as an alternative to plant proteases in tenderizing carabeef. Specifically, this study aims to (1) identify the most suitable concentration of B. subtilis protease needed to sufficiently tenderize carabeef; (2) compare the degree of meat tenderness treated with B. subtilis protease versus a commercial meat tenderizer containing papain; (3) determine the physicochemical and proximate composition of carabeef treated with the most effective B. subtilis protease concentration; and (4) evaluate the sensory properties and acceptability of oven-roasted carabeef tenderized by B. subtilis protease.
Materials and methods
Protein concentration and protease activity
Protein concentration was determined using the Lowry method. Enzyme activity of B. subtilis protease (CTC E-ssentials™ MT-70N) and commercial meat tenderizer (containing iodized salt, sugar, papain, calcium stearate, and soybean oil according to the product’s ingredients section) at different pH and temperature levels were tested using protease activity assay with casein as a substrate (Cupp-Enyard 2008). The protein samples were subjected to different pH levels (i.e., 6, 6.5, 7, 7.5, and 8) and temperature (i.e., 35 °C, 40 °C, 45 °C, 50 °C, and 55 °C) levels and their activity on casein were determined. Protease efficacy was evaluated by determining the amount of tyrosine liberated in the assay and comparing this with a tyrosine (Sigma-Aldrich, St. Louis, Missouri, United States) standard curve.
Determination of protease concentration for carabeef tenderization
Carabeef loin (longissimus dorsi muscles) on both sides of the carcasses were sampled from four male carabaos aging 7 years old. Samples were bought from a local market in Calauan, Laguna. These bought samples already underwent and passed rigor mortis. Then, samples were transported immediately to the lab. The carabeef loin was tempered overnight at 4 °C, and cut into equal pieces (5 × 4 × 4 cm3). Meat samples were rubbed with 0.15, 0.25, 0.35 or 0.45 g B. subtilis protease per 100 g of meat and were forked uniformly. Forking ensures that the enzyme will get through the inner portions of the meat. In this study, the efficacy of B. subtilis protease in tenderizing carabeef was compared with commercial meat tenderizer containing papain (1.1 g/100 g meat). Untreated meat samples served as the control. In both set-ups, meat samples were also forked. The application of 1.1 g commercial meat tenderizer per 100 g of meat was based on the manufacturer’s directions.
For three consecutive days (i.e., Tuesday, Wednesday, and Thursday), six meat samples were randomly assigned to each treatment and were held for 30 or 60 min at room temperature after forking. Each treatment had three replicates. An oven (WCG540XG, White-Westinghouse, USA) was preheated to 149 °C (DOT2 ProAccurate®, CDN, Portland, Oregon, USA) and the meat samples were roasted to an internal temperature of 71 °C (IRM200 ProAccurate®, CDN, Portland, Oregon, USA). Roasting ensures that the meat is cooked evenly on all sides. After cooking, the meat samples were allowed to rest for 10 min and were subjected to Warner–Bratzler shear force device (235 6 ×, Salter Brecknell, Fairmont, Minnesota, USA) to measure their tenderness. The results of the tenderness measurement were used to identify the most suitable concentration of B. subtilis protease and the holding time needed to achieve the best level of meat tenderness. This information was then used in the succeeding tests.
An alternative application of the enzymes to the meat was also done through injection. In this set-up, the enzymes were dissolved in distilled water, injected uniformly into the meat, and held at room temperature for 60 min. Distilled water and dissolved commercial meat tenderizer were used as controls. The volume of the enzyme injected was 10% of the total mass of the meat samples (Liu et al. 2011).
Physicochemical tests
Warner–Bratzler shear force device (235 6 ×, Salter Brecknell, Fairmont, Minnesota, USA) was used to measure cooked meat tenderness. A cork borer was used to get cylindrical samples of the cooked meat, parallel to the longitudinal axis of the muscle fibers. Three to four meat sample cores (1.27 cm in diameter) were taken from each cooked meat sample, wherein each core was perpendicularly sheared once (AMSA 2015). The shear force value will be computed using the value obtained from the shear force device and the diameter of the cork borer (Beattie et al. 2004). Carabeef color before and after roasting was assessed by the L* (lightness), a* (redness), b* (yellowness) system using a chroma meter (CR-410, Konica Minolta, Tokyo, Japan) to determine the colorimetric index of chromaticity. The standardization of the chroma meter was done using a white calibration plate.
Proximate analysis
Untreated carabeef and those treated with commercial meat tenderizer or B. subtilis protease providing the best degree of meat tenderness were analyzed for their proximate composition. The proximate composition including moisture, fat, protein (6.25 ×), and ash was measured following AOAC (2000) protocols.
Sensory evaluation
Ten trained sensory panelists were asked to evaluate the cooked meat samples in terms of color, aroma, flavor, tenderness, bitterness, juiciness, and general acceptability for three consecutive days. Each trait was scored on a 7-point scale from 1 to 7 (McGee et al. 2003). Range of scores are as follow: Color (1-very dark, 7-very brown); Aroma (1-very undesirable, 7-very desirable); Flavor (1-very weak meaty flavor, 7-very full meaty flavor); Tenderness (1-very tough, 7-very tender); Bitterness (1-no perceptible bitter flavor, 7-very strong bitter flavor); Juiciness (1-very dry, 7-very juicy); and General Acceptability (1-very unacceptable, 7-very acceptable).
The chosen panelists were veteran sensory analysts from the Philippine Carabao Center and Institute of Animal Science, College of Agriculture and Food Science, University of the Philippines Los Baños (UPLB), Laguna. The panel was comprised of animal science professors and researchers who had undergone systematic training that developed their ability to detect and express differences in cooked meat samples. Their training involved evaluating descriptors (e.g., tenderness) of carabeef at varying levels of doneness.
Before undergoing sensory evaluation, the panelists were briefed about the study so they can make informed decisions. When they agreed to participate, they signed a consent form but were free to withdraw anytime from the study. This is in line with the guidelines provided by IFST (2015). Guidelines published by the AMSA (2015) were also used.
Meat samples were frozen and thawed overnight. After cooking, the samples were kept warm (60 °C, ASTM E1871 2010) by wrapping them in aluminum foil, placing them in a plastic container, and putting them in a thermal bag before evaluation. The samples were then cut into small equal pieces (i.e., 5 cm × 2 cm × 1 cm) and placed in individual saucers with assigned three-digit random numbers. Selection of sliced meat samples from the steak was randomized to better accommodate variability in tenderness within a treatment (AMSA 2015). There were four saucers, and each contained one meat sample that represented one of each treatment (i.e., control, commercial meat tenderizer, 0.35% protease at 30 and 60 min cook time). The saucers were also arranged randomly on a tray before serving arbitrarily to the panelists. Samples were evaluated from left to right and all the quality attributes were scored sequentially before proceeding with the next treatment sample. Sensory evaluation was done in three sessions, and all four treatment samples were evaluated by each panelist in each session.
Statistical analyses
All tests were done in triplicates. Statistical analysis of data obtained was done using Statistical Tool for Agricultural Research (STAR). The normality of the data was determined first using the Shapiro–Wilk test. Normally distributed data undergone a parametric test, i.e., one-way analysis of variance (ANOVA) in a completely randomized design (CRD). Otherwise, data were subjected to a non-parametric test, i.e., the Mann–Whitney–Wilcoxon test. Tukey’s test or Least Significant Difference (LSD) test was used to determine significant differences among the various treatments. P values less than 5% were considered significant.
Results and discussion
Protein concentration of protease samples
The protein content of B. subtilis protease and commercial meat tenderizer were determined to establish if the enzymes contain potentially proteolytic protein. The B. subtilis protease had a protein content of 35.36 μg/g, which is far greater than the amount of protein found in the commercial meat tenderizer (1.42 μg/g) used in this study.
Sathiya (2013) was able to obtain a protein concentration of 3600 μg/mL B. subtilis crude protease and 4700 μg/mL from B. subtilis purified protease by cultivating the bacterium in dyes synthetic medium as its protease production medium at 37 °C for 48 h. Even so, B. subtilis protein content can still vary depending on the pH, temperature, and time of incubation (Younis et al. 2009), which may apply to other bacteria as well.
Protease assay at varying pH and temperature
pH
The pH at which the enzyme had the greatest activity was determined to identify the pH at which the enzyme is most active to effectively tenderize meat. As shown in Table 1, the enzyme activity of B. subtilis protease was greatest at 7.5 (136.71 U/g). In contrast, the commercial meat tenderizer did not exhibit enzyme activity (0 U/g) in all pH levels tested. Hence, the commercial meat tenderizer did not contain any proteolytic enzyme. Other ingredients of the commercial meat tenderizer used in this study include iodized salt, sugar, calcium stearate, and soybean oil. The 1.42 μg/g protein found in the commercial meat tenderizer can be attributed to the soybean oil present.
Table 1.
Enzyme activity of the commercial meat tenderizer and B. subtilis protease at different pH and temperature
| Enzyme activity (U/g) | ||
|---|---|---|
| Commercial meat tenderizer | B. subtilis protease | |
| pH | ||
| 6 | 0 | 81.62 |
| 6.5 | 0 | 101.5 |
| 7 | 0 | 126.09 |
| 7.5 | 0 | 136.71 |
| 8 | 0 | 115.34 |
| Temperature (°C) at pH 7.5 | ||
| 35 | 0 | 105.14 |
| 40 | 0 | 117.97 |
| 45 | 0 | 168.32 |
| 50 | 0 | 188.87 |
| 55 | 0 | 169.82 |
In this study, the optimum pH for the B. subtilis protease is 7.5. At pH 8, its enzyme activity started to decline. Yang et al. (2000) reported B. subtilis Y-108 protease activity of only 20.2 U/mL at pH 6 at 30 °C. Padmapriya and Williams (2012) isolated neutral B. subtilis protease that had an enzyme activity of 340 U/mL at pH 7 at 37 °C. Moreover, Sathiya (2013) reported that crude B. subtilis protease had a proteolytic activity of 77 U/mL at 37 °C, while purified B. subtilis protease had an activity of 533.55 U/mL also at 37 °C. These results suggest that B. subtilis protease activity, aside from pH and temperature, can also depend on the composition of the enzyme production medium, strain of the bacterium, and the purity of the enzyme.
Temperature
Enzyme activity of B. subtilis protease and the commercial meat tenderizer were also assessed at varying temperatures (Table 1). Since the B. subtilis protease is most active at pH 7.5, its activity was tested at various temperatures at pH 7.5. B. subtilis protease enzyme activity was highest at 50 °C (188.87 U/g), and the activity subsequently decreased at 55 °C (169.82 U/g). Still, there was no activity (0 U/g) from the commercial meat tenderizer at the temperatures tested.
Bacterial protease inactivation can be attributed to the cardinal temperature wherein the bacteria can grow and survive (Budde et al. 2006). It was noted by Budde et al. (2006) that B. subtilis’ growth range is between 11 and 52 °C. Beyond this, the bacterium’s proteins will lose its configuration and start to denature. Similarly, B. subtilis Y-108 protease was most active at 50 °C but was rapidly inactivated at higher temperatures. The protease activity was completely inactivated at 60 °C (Yang et al. 2000). Like any protein, enzymes have a heat threshold. At temperatures greater than 70 °C, most microbial alkaline proteases start to denature. This means that heat cleaves the bonds that hold the active conformation of the enzyme and unwinds it to a random configuration. Thus, the enzyme is unable to bind to the substrate and loses its activity (Ellaiah et al. 2002).
Physicochemical properties of roasted carabeef
Meat tenderness
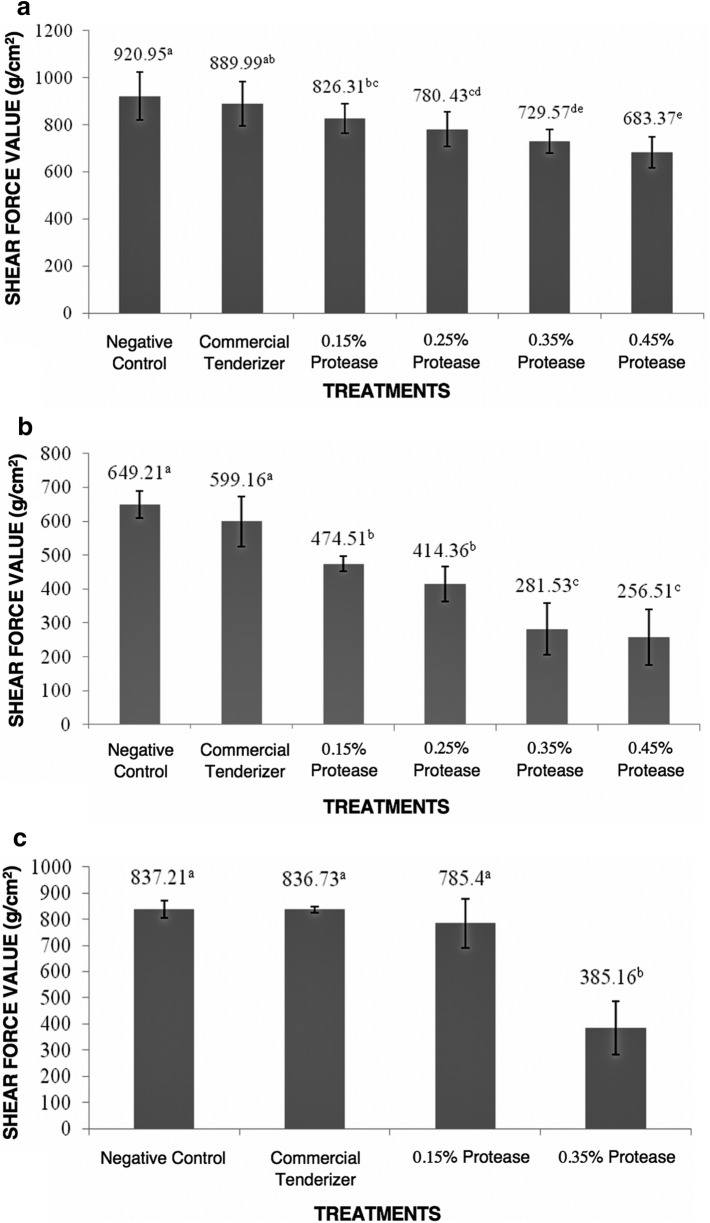
Shear force value (SFV) is the amount of force required to shear through roasted meat. Greater SFV means that meat is tougher and vice versa. Figure 1a shows the SFVs of carabeef treated with the commercial meat tenderizer and various concentrations of B. subtilis protease held at room temperature for 30 min. In this set-up, statistical analyses revealed that the SFV of all carabeef samples rubbed with B. subtilis protease was significantly lower than the negative control. Although at 0.15%, the SFV value was not significantly lower than the commercial meat tenderizer. Furthermore, no significant difference in SFV was observed in carabeef treated with commercial meat tenderizer and negative control. This means that the commercial meat tenderizer was not effective in tenderizing carabeef for 30 min.
Fig. 1.
a Mean shear force values of carabeef rubbed with the commercial meat tenderizer and B. subtilis protease held at room temperature for 30 min. b Mean shear force values of carabeef rubbed with the commercial meat tenderizer and B. subtilis protease held at room temperature for 60 min. c Mean shear force values of carabeef injected with the commercial meat tenderizer and B. subtilis protease held at room temperature for 60 min. Mean shear force value with the same superscript are not significantly different at P ≥ 0.05
Figure 1b shows the SFVs of carabeef treated with the commercial meat tenderizer and various concentrations of B. subtilis protease held at room temperature for 60 min. As shown in this figure, all carabeef samples rubbed with B. subtilis protease had significantly lower SFV than the negative control and the commercial meat tenderizer. B. subtilis protease at 0.15% and 0.25% rendered the carabeef significantly tender, while 0.35% and 0.45% rendered the carabeef very tender in terms of SFV. Meat is considered tender if SFV is between 346.5 and 462 g/cm2. Below these values, meat is considered very tender (Wulf et al. 1998). Still, the commercial meat tenderizer did not render carabeef significantly tender when compared to the negative control.
From the results shown in Fig. 1b, 0.15% and 0.35% B. subtilis protease concentrations were chosen for carabeef treatment through injection (Fig. 1c). Both the commercial meat tenderizer and 0.15% B. subtilis protease treatments did not have significantly lower SFV than the negative control. In contrast, carabeef injected with 0.35% B. subtilis protease solution was significantly more tender than that of the other treatments.
Although not significant, a decrease in SFV can still be observed between the negative control and commercial meat tenderizer. This can be attributed to the salts present in the commercial meat tenderizer. Salt can contribute to the softening of the meat. It has been cited by Duranton et al. (2012) that salt tenderizes meat by protein denaturation. As they have stated, chloride ions unfold proteins and reveal its charged binding sites allowing further hydration to occur, which is linked to tenderization. In their study, salt significantly decreased shear force in their meat samples. Additionally, calcium from calcium stearate can activate calpains (Gerelt et al. 2005; Ji and Takahashi 2006). Calpains are endogenous enzymes found in meat that also have proteolytic activity causing meat tenderization (Koohmaraie and Geesink 2006).
Thus far, carabeef tenderization using microbial protease has not been reported. For comparison, Qihe et al. (2006) reported a marked decrease in toughness in beef treated with Bacillus sp. EL31410 protease. Sullivan and Calkins (2010) also reported that beef treated with B. subtilis protease showed improvement in tenderness. Collagen and elastin structures are significant factors that affect meat tenderness (Qihe et al. 2006). Meat tenderness is dependent on the amount, type, and extent of intermolecular cross-linking of these meat proteins (Ashie et al. 2002). According to Rawdkuen et al. (2013), Bacillus protease is capable of digesting muscle proteins (i.e., collagen and elastin) in meat. Rawdkuen and Benjakul (2012) also stated that Bacillus protease causes deformation and disruption of the intramuscular connective tissue structure in beef, which decreases the meat’s toughness.
Using the mean SFVs, the percentage differences of the commercial meat tenderizer, 0.15% and 0.35% of B. subtilis protease to the negative control were determined. B. subtilis protease at 0.25% and 0.45% were not tested for economic reasons and because these two concentrations did not have significantly lower SFV than 0.15% and 0.35% B. subtilis protease, respectively. For carabeef samples that were rubbed, forked, and held for 30 min, the meat toughness was decreased by 3.42% by the commercial tenderizer, 10.83% and 23.19% by the 0.15% and 0.35% B. subtilis protease, respectively. Carabeef that were rubbed, forked and held for 60 min, decreased the meat toughness by 8.02% by the commercial tenderizer, 31.09% and 79.01% by the 0.15% and 0.35% B. subtilis protease, respectively. On the other hand, carabeef treated through injection decreased meat toughness by 0.9% using the commercial meat tenderizer, 6.39% using 0.15% B. subtilis protease solution, and 73.96% using 0.35% B. subtilis protease solution. Based on these values, rubbing and forking was considered the most effective way to apply B. subtilis protease on meat samples in this study. Forking after rubbing ensures that the enzyme is also in the meat and not just on its surface. According to Singh et al. (2019), this is a more effective method of introducing an enzyme to meat because of its direct application. In contrast, application by injection will require dilution of the enzyme and may lead to seepage after injection. Nonetheless, an injection can still be used as an alternative way of applying the enzyme.
Both 0.35% and 0.45% B. subtilis protease concentrations sufficiently tenderized carabeef. Since the 0.45% B. subtilis protease concentration was not significantly lower than the 0.35% B. subtilis protease concentration, 0.35% was chosen in the succeeding tests for economic reasons. Sixty minutes holding time resulted in more tender carabeef than when held for only 30 min (P < 0.0001).
Meat color
Degree of lightness (L*), redness (a*), and yellowness (b*) of carabeef treated with the commercial meat tenderizer and 0.35% B. subtilis protease before and after roasting was determined. Statistical analyses showed that none of the L*, a*, and b* values across treatments were significantly different in both raw and roasted carabeef (P > 0.05). Hence, the carabeef samples had the same level of freshness and doneness, respectively, and application of B. subtilis protease to carabeef did not significantly alter the color measurements of the meat, whether raw or roasted.
Proximate composition of roasted carabeef
Table 2 shows the proximate composition of roasted carabeef treated with commercial meat tenderizer and 0.35% B. subtilis protease. The moisture and ash content of the samples were not significantly different among treatments. However, the crude fat in the negative control obtained significantly lower values with that of the commercial meat tenderizer and B. subtilis protease, while crude fat of the carabeef samples treated with commercial tenderizer was not significantly lower than B. subtilis protease. Moreover, crude protein in carabeef treated with B. subtilis protease had a significantly higher value than the two other treatments, but these two treatments were not significantly different from each other.
Table 2.
Proximate composition of roasted carabeef treated with commercial meat tenderizer and B. subtilis protease
| Parameters | Negative control | Commercial tenderizer | 0.35% B. subtilis protease |
|---|---|---|---|
| Moisture (%) | 63.49a | 63.94a | 63.08a |
| Crude fat (%) | 0.6b | 00.93a | 00.98a |
| Crude protein (%) | 33.52b | 31.31b | 36.83a |
| Ash (%) | 00.83a | 00.83a | 00.81a |
*Mean values with the same superscripts in the same row are not significantly different (P ≥ 0.05)
In a proximate analysis done by Macalood et al. (2013), samples containing protease do contain significantly higher amounts of crude protein. Moreover, proteases are capable of recovering significant quantities of protein hydrolysate (Abdulazeez et al. 2013). Hence, the B. subtilis protease had contributed to the higher crude protein of the sample. Additionally, Oliveros et al. (2007) mentioned that the level of meat trimming can plausibly explain the differences in proximate composition, especially crude fat. This is because the amount of lipid in meat cuts is dependent on the quantity of untrimmed deep muscle fat and superficial fat that persists after cutting and trimming (Aberle et al. 2001).
Sensory evaluation
Table 3 shows the median sensory scores by 10 experienced panelists for carabeef treated with commercial meat tenderizer and 0.35% B. subtilis protease for three consecutive days. Since the 7-point scale is an ordinal scale (Lim et al. 2009), median scores were used for analysis instead of the means because ordinal scale values cannot be added or divided.
Table 3.
Sensory characteristics of roasted carabeef rubbed with the commercial tenderizer and B. subtilis protease
| Parameters | Sensory scores | |||
|---|---|---|---|---|
| Negative control | Commercial tenderizer | 0.35% protease (30 min) | 0.35% protease (60 min) | |
| Color | 5a | 5a | 5a | 5a |
| Aroma | 6a | 6a | 5b | 5b |
| Flavor | 5a | 5a | 5a | 5a |
| Tenderness | 3b | 3b | 3b | 5a |
| Bitterness | 1a | 1a | 1a | 1a |
| Juiciness | 4a | 3a | 3a | 4a |
| General acceptability | 5a | 5a | 5a | 5a |
*Median scores with the same superscript in the same row are not significantly different (P ≥ 0.05)
The color was depicted slightly brown by the panelists for all treatments. Thus, none of the treatments were significantly different from each other in terms of color. The aroma was desirable for roasted carabeef treated with commercial meat tenderizer and slightly desirable for carabeef treated with B. subtilis protease. According to the product specification provided by CTCGroup Philippines, B. subtilis protease exudes an odor similar to fermentation. The significant difference in aroma can be attributed to the fermented odor exuded by the B. subtilis protease. The panelists evaluated the samples from all treatments to have slightly full meaty flavor and were not significantly different.
The panelists perceived carabeef samples treated with commercial meat tenderizer or B. subtilis protease for 30 min to be slightly tough, while carabeef samples treated with B. subtilis protease for 60 min was described as slightly tender. Statistical analysis of tenderness showed that carabeef treated with B. subtilis protease for 60 min was significantly tender than the other treatments. This coincides with the results of Qihe et al. (2006) and Sullivan and Calkins (2010) that described beef treated with Bacillus protease have greater sensory scores for tenderness than their control.
There was no perceptible bitter flavor among samples, and they were graded between slightly dry to neither juicy nor dry. Consequently, bitterness and juiciness among treatments were not significantly different. Overall, the general acceptability of the treatments was deemed slightly acceptable and was not significantly different across treatments.
It is noticeable that our analytical results (i.e., SFV) for tenderness did not coincide with the sensory evaluation done in this study. However, this is not uncommon, as several studies have similar results. According to Destefanis et al. (2008), the relationship between sensory evaluations and shear force measurements of beef tenderness can be highly variable. They concluded that SFV may not necessarily reflect consumer preference and product acceptability. In a study by Van Wezemael et al. (2014), the correlation between sensory analysis and SFV was even lower compared to what was reported by Destefanis et al. (2008). Their findings supported the results of Powell et al. (2011) who reported that consumers’ rating on beef tenderness is independent of SFV.
Van Wezemael et al. (2014) explained that the degree of juiciness can influence evaluations of tenderness, leading to a low correlation between sensory evaluation and shear force measurements. In our study, the juiciness of the B. subtilis protease treated carabeef for 30 min is rated a score of 3, which is slightly dry. This could explain why the 30 min B. subtilis protease-treated carabeef sample was also rated as slightly tough. Moreover, even though all of our meat samples in this study have the same level of acceptability, Verbeke et al. (2010) reported that qualitative research results are strongly subjective and that there is only a marginal correlation between sensory evaluations and instrumental measurements. Hence, consumer evaluations of tenderness and satisfaction may not necessarily agree with shear force measurements or analytical data (Van Wezemael et al. 2014).
Conclusion
This study investigated the efficacy of B. subtilis protease in tenderizing carabeef. Using 350 g/cm2 as standard for meat tenderness, results revealed that the optimal B. subtilis protease concentration that can sufficiently tenderize carabeef is 0.35% (280 g/cm2). At this concentration, the toughness of carabeef was reduced by 80%. Carabeef treated with 0.35% B. subtilis protease for an hour was significantly more tender than the untreated carabeef and carabeef treated with a commercial meat tenderizer. Measurement of meat tenderness and sensory evaluation of carabeef treated with the commercial meat tenderizer validated that commercial meat tenderizer used in this study is not an effective meat tenderizing agent as it lacked proteolytic enzymes. In conclusion, B. subtilis protease is an effective meat tenderizer that can be used in households and the food industry without causing over-tenderization.
Acknowledgements
We are thankful for CTCGroup Philippines (formerly CTC Far East Philippines, Inc.) for providing us with B. subtilis protease powder (CTC E-ssentials™ MT-70N) and a stock of commercially available meat tenderizer.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulazeez SS, Ramamoorthy B, Ponnusamy P. Proximate analysis and production of protein hydrolysate from king fish of Arabian Gulf coast—Saudi Arabia. Int J Pharma Bio Sci. 2013;3(1):138–144. [Google Scholar]
- Aberle ED, Forrest JC, Gerrard DE, Mills EW. Principles of meat science. 4. Dubuque, IA: Kendall/Hunt Publishing Company; 2001. p. 354. [Google Scholar]
- AMSA . Research guidelines for cookery, sensory evaluation, and instrumental tenderness measurements of meat. Champaign, IL: American Meat Science Association; 2015. [Google Scholar]
- AOAC . Official methods of analysis. 17. Gaithersburg, MD: The Association of Official Analytical Chemists; 2000. [Google Scholar]
- Ashie INA, Sorensen TL, Nielsen PM. Effects of papain and a microbial enzyme on meat proteins and beef tenderness. J Food Sci. 2002;67(6):2138–2142. doi: 10.1111/j.1365-2621.2002.tb09516.x. [DOI] [Google Scholar]
- ASTM E1871 . Standard guide for serving protocol for sensory evaluation of foods and beverages. West Conshohocken, PA: ASTM International; 2010. [Google Scholar]
- Beattie RJ, Bell SJ, Farmer LJ, Moss BW, Patterson D. Preliminary investigation of the application of Raman spectroscopy to the prediction of the sensory quality of beef silverside. Meat Sci. 2004;66(4):903–913. doi: 10.1016/j.meatsci.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Budde I, Steil L, Scharf C, Völker U, Bremer E. Adaptation of Bacillus subtilis to growth at low temperature: a combined transcriptomic and proteomic appraisal. Microbiology. 2006;152(3):831–853. doi: 10.1099/mic.0.28530-0. [DOI] [PubMed] [Google Scholar]
- Cupp-Enyard C. Sigma’s non-specific protease activity assay—casein as a substrate. J Vis Exp. 2008;19:899. doi: 10.3791/899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destefanis G, Brugiapaglia A, Barge MT, Dal Molin E. Relationship between beef consumer tenderness perception and Warner–Bratzler shear force. Meat Sci. 2008;78(3):153–156. doi: 10.1016/j.meatsci.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Duranton F, Simonin H, Chéret R, Guillou S, de Lamballerie M. Effect of high pressure and salt on pork meat quality and microstructure. J Food Sci. 2012;77(8):E188–E194. doi: 10.1111/j.1750-3841.2012.02816.x. [DOI] [PubMed] [Google Scholar]
- Ellaiah P, Srinivasulu B, Adinarayana K. A review on microbial alkaline proteases. JSIR. 2002;61(9):690–704. [Google Scholar]
- Gerelt B, Ikeuchi Y, Suzuki A. Meat tenderization by proteolytic enzymes after osmotic dehydration. Meat Sci. 2000;56:311–318. doi: 10.1016/S0309-1740(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Gerelt B, Rusman H, Nishiumi T, Suzuki A. Changes in calpain and calpastatin activities of osmotically dehydrated bovine muscle during storage after treatment with calcium. Meat Sci. 2005;70(1):55–61. doi: 10.1016/j.meatsci.2004.11.020. [DOI] [PubMed] [Google Scholar]
- IFST (2015) Guidelines for ethical and professional practices for the sensory analysis of foods. Institute of Food Science and Technology. https://www.ifst.org/our-resources/ifst-guidelines-ethical-and-professional-practices-sensory-analysis-foods. Accessed 1 July 2015
- Ji J-R, Takahashi K. Changes in concentration of sarcoplasmic free calcium during post-mortem ageing of meat. Meat Sci. 2006;73(3):395–403. doi: 10.1016/j.meatsci.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Koohmaraie M, Geesink GH. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci. 2006;74(1):34–43. doi: 10.1016/j.meatsci.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 2009;34:739–751. doi: 10.1093/chemse/bjp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Xiong YL, Rentfrow GK. Kiwifruit protease extract injection reduces toughness of pork loin muscle induced by freeze–thaw abuse. LWT. 2011;44(10):2026–2031. doi: 10.1016/j.lwt.2011.05.019. [DOI] [Google Scholar]
- Macalood JS, Vicente HJ, Boniao RD, Gorospe JG, Roa EC. Chemical analysis of Carica papaya L. crude latex. AJPS. 2013;4:1941–1948. doi: 10.4236/ajps.2013.410240. [DOI] [Google Scholar]
- Maqsood S, Manheem K, Gani A, Abushelaibi A. Degradation of myofibrillar, sarcoplasmic and connective tissue proteins by plant proteolytic enzymes and their impact on camel meat tenderness. J Food Sci Technol. 2018;55(9):3427–3438. doi: 10.1007/s13197-018-3251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MR, Henry KL, Brooks JC, Ray FK, Morgan JB. Injection of sodium chloride, sodium tripolyphosphate, and sodium lactate improves Warner–Bratzler shear and sensory characteristics of pre-cooked inside round roasts. Meat Sci. 2003;64(3):273–277. doi: 10.1016/S0309-1740(02)00189-4. [DOI] [PubMed] [Google Scholar]
- Neely TR, Lorenzen CL, Miller RK, Tatum JD, Wise JW, Taylor JF, Buyck MJ, Reagan JO, Savell JW. Beef customer satisfaction: Role of cut, USDA quality grade, and city on in-home consumer ratings. J Anim Sci. 1998;76(4):1027–1033. doi: 10.2527/1998.7641027x. [DOI] [PubMed] [Google Scholar]
- Oliveros MCR, Manito CA, Del Barrio AN, Lapitan RM. Proximate composition and intramuscular fatty acid profile of meat from Brahman-grade cattle (Bos indicus) and crossbred carabao (Bubalus bubalis L.) Philipp J Vet Anim Sci. 2007;33(1):9–17. [Google Scholar]
- Padmapriya M, Williams BC. Purification and characterization of neutral protease enzyme from Bacillus subtilis. J Microbiol Biotechnol Res. 2012;2(4):612–618. [Google Scholar]
- Pietrasik Z, Shand PJ. Effect of blade tenderization and tumbling time on the processing characteristics and tenderness of injected cooked roast beef. Meat Sci. 2004;66(4):871–879. doi: 10.1016/j.meatsci.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Powell L, Nicholson KL, Huerta-Montauti D, Miller RK, Savell JW. Constraints on establishing threshold levels for Warner–Bratzler shear-force values based on consumer sensory ratings for seven beef muscles. Anim Prod Sci. 2011;51(10):959–966. doi: 10.1071/AN10267. [DOI] [Google Scholar]
- Qihe C, Guoqing H, Yingchun J, Hui N. Effects of elastase from a Bacillus strain on the tenderization of beef meat. Food Chem. 2006;98:624–629. doi: 10.1016/j.foodchem.2005.06.043. [DOI] [Google Scholar]
- Rawdkuen S, Benjakul S. Biochemical and microstructural characteristics of meat samples treated with different plant proteases. AJB. 2012;11(76):14088–14095. [Google Scholar]
- Rawdkuen S, Jaimakreu M, Benjakul S. Physicochemical properties and tenderness of meat samples using proteolytic extract from Calotropis procera latex. Food Chem. 2013;136(2):909–916. doi: 10.1016/j.foodchem.2012.08.077. [DOI] [PubMed] [Google Scholar]
- Sathiya G. Production of protease from Bacillus subtilis and its application in leather making process. Int J Res Biotechnol Biochem. 2013;3(1):7–10. [Google Scholar]
- Singh M, Patel SKS, Kalia VC. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb Cell Fact. 2009;8:38. doi: 10.1186/1475-2859-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Pradeep Kumar, Shrivastava Neeraj, Ojha B.K. Enzymes in Food Biotechnology. 2019. Enzymes in the Meat Industry; pp. 111–128. [Google Scholar]
- Sullivan GA, Calkins CR. Application of exogenous enzymes to beef muscle of high and low-connective tissue. Meat Sci. 2010;85(4):730–734. doi: 10.1016/j.meatsci.2010.03.033. [DOI] [PubMed] [Google Scholar]
- USDA . Livestock and Poultry: world markets and trade. Washington, DC: Foreign Agricultural Service; 2012. [Google Scholar]
- Van Wezemael L, De Smet S, Ueland Ø, Verbeke W. Relationships between sensory evaluations of beef tenderness, shear force measurements and consumer characteristics. Meat Sci. 2014;97(3):310–315. doi: 10.1016/j.meatsci.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Vasanthi C, Venkataramanujam V, Dushyanthan K. Effect of cooking temperature and time on the physicochemical, histological, and sensory properties of female carabeef (buffalo) meat. Meat Sci. 2007;76(2):274–280. doi: 10.1016/j.meatsci.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Verbeke W, Van Wezemael L, de Barcellos MD, Kugler JO, Hocquette JF, Ueland O, Grunert KG. European beef consumers’ interest in a beef eating-quality guarantee. Insights from a qualitative study in four EU countries. Appetite. 2010;54(2):289–296. doi: 10.1016/j.appet.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Wulf DM, Page JK, Schwotzer TR, Dunlap GR (1998) Final report to National Cattlemen’s Beef Association: using measurements of muscle color/pH/water-holding capacity to augment the current USDA beef carcass quality grading standards and improve the accuracy and precision of sorting carcasses into palatability groups. The Ohio State University, Columbus, OH
- Yang JK, Shih IL, Tzeng YM, Wang SL. Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microb Technol. 2000;26:406–413. doi: 10.1016/S0141-0229(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Younis MAM, Hezayen FF, Nour-Eldein MA, Shabeb MSA. Production of protease in low-cost medium by Bacillus subtilis KO strain. GJBB. 2009;4(2):132–137. [Google Scholar]
- Zhao G, Zhou M, Zhao H, Chen X, Xie B, Zhang X, He H, Zhou B, Zhang Y. Tenderization effect of cold-adapted collagenolytic protease MCP-01 on beef meat at low temperature and its mechanism. Food Chem. 2012;134(4):1738–1744. doi: 10.1016/j.foodchem.2012.03.118. [DOI] [PubMed] [Google Scholar]