Abstract
In this study, the effect of high-pressure homogenization on the water-in-oil-in-water (W1/O/W2) double emulsions containing fish protein hydrolysate and fish oil encapsulated within a complex of whey protein concentrate and inulin were investigated in order to produce stable double emulsion. After adequacy of the positive influence of high-pressure homogenization at W1/O (one pass) and W1/O/W2 (three passes), the double emulsions were produced with (H) and without (HS) high-pressure homogenization. H samples were demonstrated lower CI of double emulsion and higher amounts of yield, total oil, encapsulated oil, EPA and DHA of microcapsules in comparison with HS samples. At subsequent step, response surface methodology were applied to optimize the high-pressure homogenization conditions (700–1500 Ba) of double emulsions in terms of minimum CI of emulsions and maximum microencapsulation efficiency and oxidation stability. Optimal conditions were obtained by using high-pressure homogenization at 1000 and 1100 Ba on W1/O and W1/O/W2, respectively.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04029-5) contains supplementary material, which is available to authorized users.
Keywords: Double emulsion, Fish protein hydrolysate, Fish oil, High-pressure homogenization, Response surface methodology
Introduction
The development of functional foods by using marine sources is a concerned issue. This is mainly due to the high content of omega-3 polyunsaturated fatty acids (ω-3 PUFA), especially eicosapentaenoic and docosahexaenoic acids (EPA and DHA, respectively), whose consumption has been related to some beneficial effects on human health, such as decreasing the risk of cardiovascular diseases or improving nervous system functions (Jiménez-Martín et al. 2016). Moreover, the use of fish protein hydrolysates (FPH) is an alternative method for using fish-by-products and some under-utilized fish species, i.e. pacific whiting (Merluccius productus), anchovy sprat (Clupeonella engrauliformis), and bigeye ilisha (Ilisha megaloptera) (Pacheco-Aguilar et al. 2008; Ovissipour et al. 2012). In addition, FPH is credited with antioxidant and anti-hypertension properties, and has high digestibility proteins with a balanced amino acid composition (Pacheco-Aguilar et al. 2008). Nevertheless, the addition of marine sources to food is challenging because of the high susceptibility to oxidation of ω-3 PUFA as well as the undesirable bitter taste, rancid and/or fishy flavours that they can impart.
The microencapsulation technique is a highly reported strategy to avoid the inconveniences of adding fish oil to food (Jiménez-Martín et al. 2016). It consists in retaining the oil droplets in an inner matrix by surrounding them with a protective coating. However, the encapsulation of ω-3 PUFA rich oil requires a previous treatment of oil-in-water emulsions. It is demonstrated that water-in oil-in water (W1/O/W2) double emulsions have potential application to be used as delivery systems for bioactive lipids as well as for trapping and protecting hydrophilic and lipophilic compounds (McClements et al. 2007).
Both the wall material and the emulsion preparation influence the emulsion stability (ability of system to endure changes in its physicochemical attributes during the time) as well as quality characteristics of the obtained microcapsules (Carneiro et al. 2013). Proteins and carbohydrates are the most used wall materials when applying spray-drying as microencapsulation technique. It is demonstrated that the use of complexes of proteins and carbohydrates improve the interfacial coverage and the stabilization of the double emulsions (Sapei et al. 2012). Carrillo-Navas et al. (2012) also observed higher stability in the double emulsion formulated with mesquite gum:maltodextrin:whey protein concentrate (WPC) (1:2:12) ratio in microcapsulated chia essential oil. WPC is a natural amphiphilic polymer containing surface-active parts with both hydrophobic and hydrophilic groups and functional properties. WPC with globular structure is placed in oil-in-water interface to surrounded oil droplets (Salimi et al. 2015). Inulin is a low price dietary fibre which consist of fructose units linking with β-(1,2) glucose at the end of the chain. In addition, inulin showed probiotic effects, improved calcium bioavailability, and decreases the risk of colon cancer (Robert et al. 2012).
Regarding to the preparation of emulsions, a two-stage method is normally used to produce double emulsion. It consists on producing the W1/O emulsion using hydrophobic surfactant, and then adding the hydrophilic surfactant to finally have the W1/O/W2 emulsion. High-shear conditions are indicated to prepare W1/O emulsion with small droplets, whereas mild shear conditions should be applied for the secondary emulsification step, to prevent the breakdown of internal particles (Van der Graaf et al. 2005). Due to the thermodynamic instability of double emulsion, extensive studies have been focused on the study of the different emulsification methods and devices at both W1/O and W1/OW2 emulsions. According to Van der Graaf et al. (2005), rotor stator and high-pressure homogenization are the most accurate emulsification systems. In the case of double emulsions, the use of rotor stator, alone or in combination with sonication or high-pressure homogenization, are normally applied to elaborate W1/O and/or W1/OW2 emulsions. Different combinations of these techniques have been reported. For example, (Sapei et al. 2012) have only used rotor stator, while (Kim et al. 2017) and (Fernández-Martín et al. 2017) have applied this technique in combination to sonication and high-pressure homogenization, respectively, on both W1/O and W1/O/W2 emulsions; (Li et al. 2017) have used rotor stator with high-pressure on W1/O and rotor stator alone on W1/O/W2; and (O’Regan and Mulvihill 2009) proposed the application of rotor stator on W1/O and rotor stator with high-pressure homogenization on W1/O/W2.
The optimization of techniques and procedures are normally performed by univariate strategies that do not take into account interactions between factors and only consider one response variable. In the case of the homogenization of double emulsions, conditions for both W1/O and W1/O/W2 emulsions should be established considering quality characteristics of the final emulsions and their corresponding microcapsules. In this sense, the response surface methodology (RSM) could be an appropriate tool since it analyzes the relationship between the independent variables and several dependent variables (responses) (Myers and Montgomery 2002). In fact, RSM has been broadly reported in the scientific literature to optimize the conditions of different procedures (Li et al. 2017).
The main objective of this study was the use of RSM to set high-pressure homogenization conditions of double emulsions of WPC and inulin for microencapsulation of fish protein hydrolysate and fish oil. The effect of high-pressure homogenization on quality characteristics of emulsion and microcapsules was also evaluated.
Materials and methods
Materials
Fish protein hydrolysate (69.46% protein and 44.06% degree of hydrolysis) was extracted from Bigeye Ilisha (Ilisha megaloptera) by enzymatic process using Alcalase enzyme (Novazyme, Denmark) (Ovissipour et al. 2012). Fish oil (5.96% EPA, 25.83% DHA, 0.02% BHT) was kindly provided by Biomega Nutrition (Galicia, Spain), WPC and inulin were supplied by Arla Co. (Foods Ingredients, Denmark) and Sensus Co. (Netherlands), respectively. The oil-soluble emulsifier polyglycerol polyricinoleate (PGPR) was purchased from Palsgaard Co. (Denmark). Chemical materials (analytical grade) were purchased from Scharlau (Barcelona, Spain) and 2-thiobarbituric acid (TBA) as a reagent was supplied by Serva (Heidelberg, Germany).
Experimental design
Firstly, a trial was conducted to investigate the effect of (i) high-pressure homogenization on W1/O, W1/O/W2 or on both of them at 1000 bar (Ba), which can be considered intermediate homogenization conditions according to results on a previous experiment (unpublished data), and (ii) the number of homogenization passes (1 or 3) of each emulsion on emulsion stability using creaming index (CI) measurement. Results for this initial experiment indicated the adequacy or not of homogenizing at high pressure W1/O, W1/O/W2 or both emulsions.
Then, the influence of high-pressure homogenization of double emulsion on characteristics of emulsions (pH, CI) and microcapsules (moisture, yield, lipid content, encapsulation efficiency, oxidation and fatty acid composition) was analysed. This allowed selecting the most influenced parameters for carrying out the optimization assay of homogenization conditions.
Finally, experiments for optimising high-pressure homogenization conditions were conducted by using RSM. Table 1 shows the experimental design of Central Composite Design (CCD) followed in this study. It was carried out with two independent variables, high-pressure homogenization of W1/O (700–1500 bar) and W1/O/W2 (700–1500 bar), and three responses, CI of final emulsion and microencapsulation efficiency (MEE) and oxidation level of microcapsules. Optimized conditions pursued maximum MEE and minimum CI and oxidation levels. For that, it was applied a 23 factorial design with five central points and two axial points of each variable at distance of α = 1.682 from the design centre, having 13 combinations. The significant terms in the model were determined by analysis of variance (ANOVA) for each response. The adequacy of the models was determined using model analysis, lack-of-fit test, coefficient of determination (R2) and adjusted-R2 analysis (Joglekar and May 1987). The significance of the equation parameters for each response was evaluated by F-value at a probability of 0.05. Finally, expected and real values (obtained under the optimized conditions) of the response variables were compared. Design Expert trial-Version 7 (Stat-Ease, Inc., Minneapolis, MN) was used for data analysis, response surfaces and contour diagrams.
Table 1.
Uncoded and coded values of the independent variables used in the RSM design for optimization the homogenization conditions of double emulsions
| Symbol | Independent variable | Coded levels | ||||
|---|---|---|---|---|---|---|
| − 1.68 | − 1 | 0 | 1 | 1.68 | ||
| X1 | Pressure at W1/O (Ba) | 534.31 | 700 | 1100 | 1500 | 1665.69 |
| X2 | Pressure at W1/O/W2 (Ba) | 534.31 | 700 | 1100 | 1500 | 1665.69 |
W1/O/W2 double emulsion preparation
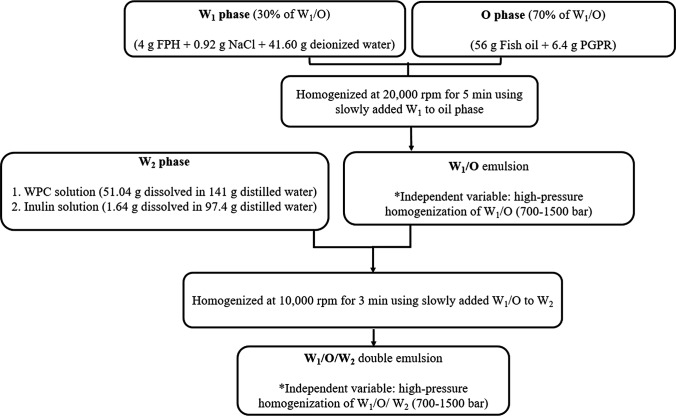
A two-step process was used to prepare W1/O/W2 double emulsions according to Sapei et al. (2012) (Fig. 1). The inner aqueous phase W1 was made with FPH (4 g) and NaCl (0.92 g) dissolved in distilled water (41.60 g) with gentle agitation at room temperature during 1 h and finally mixed at 50 °C during 5 min. The oil phase (O) contained fish oil (56 g) and 6.4 g PGPR, which were also mixed at 50 °C during 30 min. Then, the W1 phase (42.66% w/w) was added to the O phase (57.33% w/w) using Pasteur pipette dropwise in ice-water bath and homogenized at 20,000 rpm (Ultraturrax, T-25, Germany) for 5 min. Afterwards, the W1/O emulsions were passed through a high-pressure homogenizer (SPX, APV-200a, Silkeborg, Denmark) under the defined conditions in this work.
Fig. 1.
Schematic of water-in oil-in water (W1/O/W2) double emulsion
The outer aqueous phase (W2) contained complexes of whey protein (WPC) (51.04 g dissolved in 141 g distilled water) and inulin (1.64 g dissolved in 97.4 g distilled water). The polymers solutions were stored overnight at 4 °C to complete hydration. pH of both solutions was adjusting at 6 using 1 M HCl or 1 M NaOH. Afterward, the solutions were agitated at room temperature for 30 min. Finally, double emulsions were prepared by slowly adding W1/O to W2 phase and homogenizing at 10,000 rpm during 3 min (IKA, Germany). Then, the W1/O/W2 double emulsions were passed through high-pressure homogenization under the defined conditions in this work.
Microencapsulation by spray-drying
Double emulsions (400 g) were dried in a laboratory scale spray-dryer equipped with a 0.5 mm nozzle atomizer (Mini spray-dryer B-290, Buchi, Switzerland). The emulsions, kept at room temperature, were constantly and gently agitated in a magnetic stirrer during the spray-drying process. The aspirator rate was adjusted at 80%, feed rate was 1 L/h, inlet temperature was 180 °C and outlet temperature ranged 85–90 °C. The dried powders were collected and stored in plastic containers at − 80 °C for further analysis.
Emulsion analyses
Creaming index
The stability of the emulsions was measured by the CI. Freshly prepared double emulsions were placed in a 15 ml test tube to a height of 6 cm approximately and stored at room temperature during 7 days. Evaluation of the height of the distinctive clear layer upper emulsion was carried out (ht) and compared to total emulsion height (h0) to determine CI stability (Eq. 1). Analyses were done in triplicate.
| 1 |
pH measurement
pH was measured using a glass electrode pH meter (Eutech Instruments, Illkirch, France).
Microcapsule analyses
Microencapsulation yield
The amounts of microencapsulation yield (MY) were calculated based on the ratio between the quantity of microcapsules obtained and the solid content in the feed emulsions. MY (%) was expressed using Eq. (2) (Zhong et al. 2009), where M is the mass of collected product, and S is the non-solvent mass in the feed emulsions.
| 2 |
Microencapsulation efficiency measurement
Microencapsulation efficiency (MEE) was determined as a function of the encapsulated oil (within the core of the microcapsules) related to the total oil content of the microcapsules (no encapsulated oil plus oil within the core of the microcapsules). For that, the external (no encapsulated oil) and total oil of the microcapsules were quantified, and the MEE was calculated with the Eq. (3) provided by Velasco et al. (2006):
| 3 |
Extraction of surface oil
The amount of surface oil was determined according the method of Klinkesorn et al. (2006). Microcapsules (2.5 g) were mixed with 15 mL n-hexane in a falcon tube and vortex for 2 min. After centrifugation (2000 rpm, during 15 min), the supernatant was removed by filtration and the filter paper was washed twice with n-hexane. The solvent was evaporated in a rotary vacuum at 50 °C and the surface oil was determined gravimetrically.
Extraction of total oil
Sample (5 g) was placed in filtered Wathman No.42 and lipids were extracted by Soxhlet in a Büchi Universal Extraction System B-811 (Flawil, Switzerland) with hexane (100 ml), applying the parameters for extraction recommended by Büchi laboratories (BÜCHI Labortechnik AG 1998, Flawil, Switzerland). Extraction was performed for 2.30 h. The remaining solvent was evaporated at 70 °C in oven and total oil was quantified gravimetrically.
Moisture content
The moisture content of microcapsules was analysed according to the AOAC (2002) official method (reference 935.29).
Scanning electron microscopy
The scanning electron detector microscope FEI QUANTA 3D FEG (FEI Company, Hillsboro, EE.UU.) in high vacuum conditions mode state using EDT (Everhart–Thornley Detector) was employed to visualize the morphology of the microcapsules. Microcapsules samples were set on stubs and fastened by two-layer sticky coated carbon conductive adhesive sheet. Afterward, the fixed samples were exposed to a thin layer of conductive gold coating during 8 s to metallization (sputtering). This is done to reinforce the secondary electron signal. The metallized samples were imaged operating at 3 kV with focused electron beam of Ga+ (current of < 6e−4 Pa) and viewed with magnifications between 5000 and 15,000.
Oxidation measurement
Oxidation values of microcapsules were measured according to Hu and Zhong (2010). A ternary solvent mixture composed of 1-butanol:isopropanol:HCl 0.5 M (2:2:1, v/v/v) was used to completely dissolve the microcapsules matrix. TBA solution was achieved by mixing 4 g TBA in 500 mL distilled water. 0.01 g of samples were placed into a test tube, and 3160 µL of the ternary solvent and 6800 µL of TBA solvent were added and mixed using a vortex. The test tube was heated in a water bath at 95 °C for 2 h, and after cooling at room temperature the absorbance was measured at 532 nm by spectrophotometer (Hitachi U-2000, Giralt, Madrid, Spain) against the ternary solvent mixture used as a blank. At the same time, a standard curve was obtained using 1,1,3,3-tetramethoxypropane (TMP) solutions with concentration between 0.2 to 20.0 M, which was prepared as the samples. The amounts of malondialdehyde (MDA) were calculated based on the equation of standard curve, and oxidation level was expressed as mg MDA/kg oil.
Fatty acid composition
Fatty acid methyl esters (FAMEs) of microcapsules were achieved by applying direct transesterification method reported by Jiménez-Martín et al. (2016) and analysed by gas chromatography (GC) (Hewlett–Packard HP-5890A gas chromatograph). The GC was equipped with an on-column injector and a flame ionization detector, using a polyethylenglycol capillary column (Supelcowax-10, Supelco, Bellefonte, PA, USA) (60 m × 0.32 mm i.d. × 0.25 μm film thickness). The GC oven program temperature was as follows: initial temperature of 180 °C, 5 °C/min to 200 °C, 40 min at this temperature and thereafter 5 °C/min to 250 °C, and then kept for an additional 21 min. The temperatures of injector and detector were adjusted at 250 °C. Helium was employed as carrier gas at a flow rate of 0.8 ml/min. Individual FAME peaks were identified by comparison of their retention times with those of standards (Sigma, St. Louis, MO, USA). Peak areas were measured and FAMEs were quantified (mg FAME/g microcapsule) by external calibration curve method, using tridecanoic acid as internal standard.
Results and discussion
Adequacy of homogenizing W1/O and/or W1/O/W2 emulsions at high-pressure
This study firstly evaluates the influence of high-pressure homogenization of W1/O, W1/O/W2 or of both emulsions on the stability of the double emulsions. The highest emulsion stability was found in double emulsion being homogenized in W1/O and W1/O/W2 (CI = 0%), while high-pressure homogenization at only W1/O or W1/O/W2 gave less stable double emulsions of FPH and fish oil (CI = 28.02 and 42.36%, respectively).
Following, the effect of the number of homogenization passes (1 or 3) at 1000 Ba of W1/O and W1/O/W2 was evaluated by means of CI (Table 2). Results on this trial showed higher stability in double emulsions applied one pass at W1/O and three passes at W1/O/W2 (CI = 0%), and three passes at both W1/O and W1/O/W2 emulsions (CI = 0%), whereas double emulsion homogenized with three passes at W1/O and one pass at W1/O/W2 (% CI) and one passes at both W1/O and W1/O/W2 were less stables (CI = 3.63 ± 1.32 and 16.36 ± 0.9%, respectively). These results could point out the great importance of applying high-pressure homogenization for preparing double emulsion of FPH and fish oil, especially in the second W1/O/W2 emulsion.
Table 2.
Effect of the number of high-pressure homogenization passes (1 or 3) of W1/O and W1/O/W2 on double emulsion stability (CI)*
| Sample | W1/O | W1/O/W2 | CI (%) | Result |
|---|---|---|---|---|
| 1 | 1 Passes | 1 Passes | 16.36 ± 0.9 | Unstable |
| 2 | 1 Passes | 3 Passes | 0.00 ± 00 | Stable |
| 3 | 3 Passes | 3 Passes | 0.00 ± 00 | Stable |
| 4 | 3 Passes | 1 Passes | 23.63 ± 1.32 | Unstable |
*Values expressed as average of triplicate (n = 3) analyses ± standard deviation
Taking into account these results, the use of one pass at W1/O and three passes at W1/O/W2 was considered in the present study for preparing double emulsion.
High-pressure homogenization effect on emulsion and microcapsules properties
Once the adequacy of high-pressure homogenization of double emulsions has been proved, the influence of this procedure (at 1000 Ba—one and three passes for W1/O and W1/O/W2, respectively) on quality parameters of emulsions and microcapsules was studied. Thus, the most determining parameters would be selected for being the analysed responses in the further RSM assay.
Table 3 shows CI and pH values of double emulsions prepared with (H) and without (HS) high-pressure homogenization. CI values were significantly higher in HS than in H double emulsions, indicating the major stability in high-pressure homogenized emulsion. This result is expected, since the main objective of high-pressure homogenization of emulsions is diminishing and standardizing the size of oil droplets, broke aggregates of droplets and spread them evenly, which improve the emulsion stability (McClements et al. 2007). Besides, CI of H double emulsions of the present study could be related to conformational changes in the protein structure of the wall material and its interaction with oil, because the high-pressure homogenization increases the droplet oil surface and also the number of hydrophobic groups present in the core of WPC, and consequently, may improve the oil-in water bindings (Shukat and Relkin 2011). San Martin-González et al. (2009) have also reported an increase in the stability of emulsions of corn oil in casein dispersion after applying high-pressure homogenization, which these authors related to the enhancement of the availability of emulsifying protein molecules.
Table 3.
Characteristics of double emulsions and microcapsules obtained from double emulsions prepared with (H) and without (HS) applying high pressure homogenization*
| HS | H | p | |
|---|---|---|---|
| Creaming index (%) | 25.88 ± 0.88 | 0.00 ± 00 | < 0.001 |
| pH | 6.22 ± 0.01 | 6.44 ± 0.00 | < 0.001 |
| Moisture (%) | 1.85 ± 0.71 | 1.75 ± 0.37 | 0.814 |
| Microencapsulation yield (%) | 17.38 ± 2.82 | 38.58 ± 4.69 | 0.001 |
| External oil (%) | 7.64 ± 0.35 | 16.77 ± 0.39 | < 0.001 |
| Total oil (%) | 12.82 ± 0.81 | 25.49 ± 0.98 | < 0.001 |
| Encapsulated oil (%) | 5.18 ± 0.45 | 8.72 ± 0.61 | < 0.001 |
| Microencapsulation efficiency (%) | 40.35 ± 1.00 | 34.17 ± 1.10 | 0.001 |
| EPA (mg/g microcapsule) | 2.56 ± 0.24 | 9.32 ± 1.25 | 0.001 |
| DHA (mg/g microcapsule) | 4.88 ± 0.53 | 19.99 ± 0.85 | < 0.001 |
| Lipid oxidation (mg MDA/kg oil) | 2.19 ± 0.02 | 2.14 ± 0.09 | 0.378 |
*Values expressed as average of triplicate (n = 3) analyses ± standard deviation
As for pH, H samples showed slightly higher values than HS ones, with values of 6.44 and 6.22, respectively, in agreement to the followed method that indicates adjusting pH at 6, which is needed to form the protein-carbohydrate complexes and produce stable emulsions. In fact, pH between 6 and 7 has been previously indicate to allow the stability of soybean oil-based emulsions with WPI (Hunt and Dalgleish 1994).
Table 3 shows results on quality characteristics of microcapsules from HS and H double emulsions. Moisture percentage did not show significant differences between microcapsules from HS and H double emulsions (1.85 and 1.75%, respectively). These values are within the range or even lower than moisture values found previously (Anwar and Kunz 2011; Do Carmo et al. 2017). It has been indicated a range values for moisture content of 3–10 g/100 g to assure an appropriate stability for dry foods (Klaypradit and Huang 2008). Anwar and Kunz (2011) found moisture percentages in the range of 2.23–3.01% for spray-dried microcapsules. And Do Carmo et al. (2017) reported moisture content of 4.24 ± 0.10% in microencapsulates of beetroot extract by spray-drying within WPI and inulin.
Accordingly, the application of high-pressure homogenization in the emulsions containing basil essential oil and arabic gum (Garcia et al. 2012) and, also, in the green coffee oil emulsions with different wall materials (modified starch or arabic gum with maltodextrin) (Silva et al. 2014) did not influence the moisture content of microparticles. In fact, the most influencing variables on moisture of the microcapsules are those of the drying process (such as the temperature of the inlet air), which are the same for both types of microcapsules of the present work.
Microcapsules from H emulsions showed significantly higher MY than HS microcapsules (38.85 and 17.38%, respectively) (Table 3), in agreement with Narayan et al. (2001), who have pointed out the atomization pressure as the main parameter affecting MY. Benchabane et al. (2007) have also noted the positive influence of pressure homogenization on MY of BSA-loaded microcapsules. These results are quite marked since the economic importance of MY.
Percentage of external, total and encapsulated oil of microcapsules from H double emulsions (16.77, 25.49 and 8.72%, respectively) were higher (p < 0.001) than of those from HS ones (7.64, 12.82 and 5.18%, respectively) (Table 3). Furthermore, MEE presented higher values in microcapsules from HS emulsions in comparison to H ones (40.35 ± 1.00 and 34.17 ± 1.10, respectively). These results are not aligned with findings of previous studies, which have shown a reduction in external oil and an increase in the internal oil in microencapsulates of D-Limonene (Soottitantawat et al. 2005) and basil essential oil (Garcia et al. 2012) due to pressure homogenization of emulsions. Nevertheless, Silva et al. (2014) obtained no effect of pressure homogenization on oil retention in microcapsules of green coffee oil and stated that this parameter is most affected by the composition of wall materials. High MEE and low oil content on particle surface of microcapsules have been related to high emulsion stability (Carneiro et al. 2013). In the present study, high-pressure homogenization improved emulsion stability and encapsulate oil but not MEE, as consequence of the higher surface oil. It is described that the high-pressure homogenization step diminishes and standardizes the size oil droplets (McClements 2004), which may result on a higher surface contact area of the oil droplets and explain the higher external oil of H microcapsules. This fact should not be a problem at first, since the decrease of surface oil does not confirm the preservation of the encapsulated oil (De Barros Fernandes et al. 2014). In addition, considering that denaturation and aggregation of WPC of double emulsion could take place during high-pressure homogenization (Desrumaux and Marcand 2002), the optimization of the high-pressure homogenization conditions should enhance MEE.
Table 3 also exposes EPA and DHA content, with higher content in microcapsules from H (9.32 and 19.99 mg/g microcapsule, respectively) than from HS emulsions (2.56 and 4.89 mg/g microcapsule, respectively). Considering that the same quantity of fish oil has been initially added to H and HS (56 g per 400 g of emulsion) (Fig. 1), the major content of EPA and DHA in H may be also ascribed to the reduction of the droplets size, as previously discussed for MEE, and it indicates the positive effect of high-pressure homogenization. Thus, it seems that, although high-pressure homogenization did not allow reducing the external oil of microcapsules, it may influence on protein structure that increase the absorption of protein at the oil–water interface and preserve fatty acids. This is corroborated by results on lipid oxidation, with no significant differences between microcapsules from H and HS emulsions (2.14 to 2.19 mg MDA/kg oil, respectively) (Table 3). Silva et al. (2014) have also reported that the application of high-pressure homogenization did not affect levels of lipid oxidation in microcapsules of green coffee oil. However, variations on high-pressure homogenization conditions could modify oxidation stability (Kuhn and Cunha 2012).
Thus, the obtained results clearly shown the notable influence of high-pressure homogenization on CI and MEE, which justified their inclusion as responses within the CDD study. Although, the influence on lipid oxidation has not been initially significant, this parameter has also been selected as response, due to high susceptibility to oxidation of fish oil.
Suitability of prediction models
High-pressure homogenization of W1/O (X1) and W1/O/W2 (X2) emulsions was tried to be optimized for obtaining the lowest CI values in double emulsions and the highest MEE and lipid oxidation stability in the corresponding microcapsules. The two full-factorial CCD involved 13 experiments, including five replicates of centre point for verifying any change in the estimation procedure and measuring the precision property. Experimental results on CI, MEE and oxidation levels are shown in Table 4. Results on analysis of the variance by means of Fisher’s F test for CI, MEE and oxidation values have also been exposed (supplementary material).
Table 4.
Experimental responses of the central composite design for optimisation the homogenization conditions of double emulsions
| Independent variable | Dependent variables (response) | ||||
|---|---|---|---|---|---|
| Run | Pressure at W1/O X1 |
Pressure at W1/O/W2 X2 |
Creaming index (%) | Microncapsulation efficiency (%) | Oxidation level (mg MDA/kg oil) |
| 1 | 700 | 700 | 0 | 0 | 9510.83 |
| 2 | 1100 | 1100 | 0 | 39.14 | 7212.13 |
| 3 | 1500 | 700 | 0 | 27.16 | 8743.67 |
| 4 | 534.31 | 1100 | 0 | 26.33 | 6831.46 |
| 5 | 1100 | 1100 | 0 | 32.88 | 7540.61 |
| 6 | 1100 | 534.31 | 0 | 20.63 | 6948.93 |
| 7 | 1500 | 1500 | 25.94 | 0 | 6955.77 |
| 8 | 1100 | 1100 | 0 | 37.85 | 6951.85 |
| 9 | 700 | 1500 | 43.95 | 50.77 | 12015.6 |
| 10 | 1100 | 1665.69 | 33.23 | 20.49 | 9368.97 |
| 11 | 1100 | 1100 | 0 | 38.36 | 6738.55 |
| 12 | 1665.69 | 1100 | 30.55 | 0 | 9041.97 |
| 13 | 1100 | 1100 | 0 | 36.26 | 7946.49 |
Values of the model F for CI, MEE and oxidation levels were 7.09, 33.65 and 1.28 respectively, which means the significance of the model for CI and MEE, with a 1.15 and 0.01% chance, respectively, that a so large model F value could occur due to noise. However, the model for oxidation levels did not show significance. Lower values than 0.05 were found for X2, X21 and X22 in CI, and X1, X1X2, X21 and X22 2 in MEE, which indicates that they are significant terms. Moreover, the obtained Rpred2 and Radj2 (0.835 and 0.714, and 0.960 and 0.931, for CI and MEE respectively) were in concordance, and the ratios of 7.316 and 18.334, respectively, for CI and MEE, were attained, which indicate an adequate signal. Thus, the response surface model for CI and MEE of microcapsules for double emulsions of FPH and fish oil are adequate and significant.
It can be also observed (Eqs. 4 and 5) that CI response was affected by the quadratic term of X1 and X2, followed by interaction and linear terms of X2, while X1, X2 and the linear interaction expression of X1 influenced on MEE response.
| 4 |
| 5 |
Besides, the influence of the independent variables (pressure of homogenization at W1/O and W1/O/W2) on each response function (CI, MEE and oxidation values) was analyzed by means of the surface and contour plots of the models (Fig. 2).
Fig. 2.
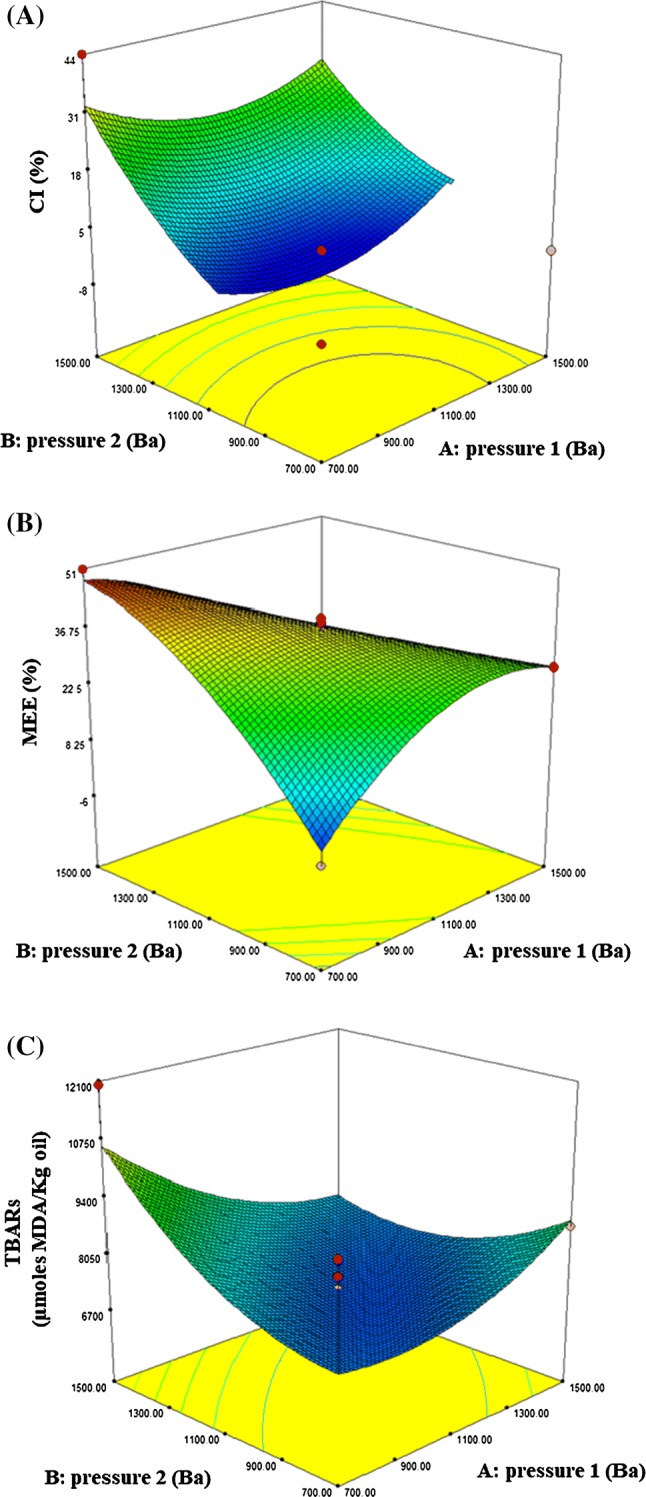
Response surface plots on the creaming index (CI) (a), microencapsulation efficiency (MEE) (b) and oxidation (TBARs) (c) of fish oil and FPH microcapsules as affected by homogenization conditions (pressure 1, at W1/O, and pressure 2, at W1/O/W2) of double emulsions
In the case of CI (Fig. 2a), it is observed that it significantly increases with increasing the X2 (from 1100 to 1500 Ba), and with high and low X1. Thus, the desired lowest values of CI of double emulsion were found at intermediate homogenization pressures (around 1100 Ba) at both X1 and X2. Regarding MEE (Fig. 2b), it increased as the X1 decreased and X2 increased, having the highest MEE percentage when using 700 Ba at X1 and 1500 Ba at X2. Although there is not significance for oxidation values, surface plots (Fig. 2c) shows that X1 and X2 between 700 and 1100 Ba achieved low oxidation values.
According to the over processing phenomenon, the stability of emulsion increases as the high-pressure homogenization increases until a certain range that allows to maintain the average of droplet size quite stable, above this certain range the stability of droplets decreases and also increase the collision and coalescence (Tornberg 1980). This fact can be observed in the case of X1, when the high-pressure homogenization of W1/O emulsion increases until 1100 Ba, the double emulsion stability increased. Above 1100 Ba the emulsion stability decreased
Selection and validation of the optimum conditions
Finally, optimization of X1 and X2 was developed to achieve minimum CI of emulsions and maximum MEE and oxidation stability, resulting on 1000 and 1100 Ba, respectively. (Table 3). Thus, whole procedure for preparing double emulsions of FPH and fish oil consists of applying 1000 Ba−1 pass and 1100 Ba−3 passes for homogenizing at W1/O and W1/O/W2, respectively.
The model predicted that the optimized independent variables would produce a stable double emulsion with CI = 0.10%, MEE = 37.87%, and lipid oxidation = 7352.76 mg MDA/kg oil. In order to confirm the appropriateness of the model equations, microcapsules were produced from double emulsions under the optimum conditions predicted by the model. This experiment was performed in triplicate, finding the following quality parameters: CI = 0%, MEE = 36.81% and lipid oxidation 6881.304 mg MDA/kg oil that were comparable with those predicted. This result confirms the validation of the high-pressure homogenization conditions for double emulsions of fish oil and FPH attained by RSM.
Conclusion
The preliminary experiments of this study approved the adequacy of high-pressure homogenization in one pass at W1/O and three passes at of W1/O/W2 for improving stability and encapsulation efficiency of double emulsion which incorporated FPH and fish oil. The use of high-pressure homogenization resulted in a higher creaming index of double emulsion and higher amounts of yield, total oil, encapsulated oil, EPA and DHA of microcapsules comparing with sample without high-pressure homogenization. The most important variable in the double emulsion procedure was the use of high-pressure homogenization at W1/O/W2. According to the optimal condition results, minimum creaming index and maximum microencapsulation efficiency and oxidation stability were achieved by using high-pressure homogenization at 1000 Ba−1 passes and 1100 Ba−3 passes on W1/O and W1/O/W2, respectively.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors, especially Trinidad Perez-Palacios, acknowledge to the Agencia Estatal de Investigación (AEI) and the Fondo Europeo de Desarrollo Regional (FEDER) the funding for this study, which was supported by the project AGL2016-73260-JIN (AEI/FEDER/UE).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anwar SH, Kunz B. The influence of drying methods on the stabilization of fish oil microcapsules: comparison of spray granulation, spray-drying, and freeze drying. J Food Eng. 2011;105:367–378. doi: 10.1016/j.jfoodeng.2011.02.047. [DOI] [Google Scholar]
- Association of Official Analytical Chemist (2002) Official methods of analysis of AOAC international. Vols. 1 and 2, 17th edn. AOAC International, Gaithersburg, Maryland
- Benchabane S, Subirade M, Vandenberg GW. Production of BSA-loaded alginate microcapsules: influence of spray dryer parameters on the microcapsule characteristics and BSA release. J Microencapsul. 2007;24:647–658. doi: 10.1080/02652040701500210. [DOI] [PubMed] [Google Scholar]
- Carneiro HC, Tonon RV, Grosso CR, Hubinger MD. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J Food Eng. 2013;115:443–451. doi: 10.1016/j.jfoodeng.2012.03.033. [DOI] [Google Scholar]
- Carrillo-Navas H, Cruz-Olivares J, Varela-Guerrero V, Alamilla-Beltrán L, Vernon-Carter EJ, Pérez-Alonso C. Rheological properties of a double emulsion nutraceutical system incorporating chia essential oil and ascorbic acid stabilized by carbohydrate polymer–protein blends. Carbohydr Polym. 2012;87:1231–1235. doi: 10.1016/j.carbpol.2011.09.005. [DOI] [Google Scholar]
- De Barros Fernandes RV, Borges SV, Botrel DA. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr Polym. 2014;101:524–532. doi: 10.1016/j.carbpol.2013.09.083. [DOI] [PubMed] [Google Scholar]
- Desrumaux A, Marcand J. Formation of sunflower oil emulsions stabilized by whey proteins with high-pressure homogenization (up to 350 MPa): effect of pressure on emulsion characteristics. Int J Food Sci Technol. 2002;37:263–269. doi: 10.1046/j.1365-2621.2002.00565.x. [DOI] [Google Scholar]
- Do Carmo EL, Teodoro RAR, Félix PHC, de Barros Fernandes RV, de Oliveira ÉR, Veiga TRLA, Botrel DA. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2017;249:51–59. doi: 10.1016/j.foodchem.2017.12.076. [DOI] [PubMed] [Google Scholar]
- Fernández-Martín F, Freire M, Bou R, Cofrades S, Jiménez-Colmenero F. Olive oil based edible W/O/W emulsions stability as affected by addition of some acylglycerides. J Food Eng. 2017;196:18–26. doi: 10.1016/j.jfoodeng.2016.10.011. [DOI] [Google Scholar]
- Garcia LC, Tonon RV, Hubinger MD. Effect of homogenization pressure and oil load on the emulsion properties and the oil retention of microencapsulated basil essential oil (Ocimum basilicum L.) Dry Technol. 2012;30:1413–1421. doi: 10.1080/07373937.2012.685998. [DOI] [Google Scholar]
- Hu ZX, Zhong QX. Determination of thiobarbituric acid reactive substances in microencapsulated products. Food Chem. 2010;123:794–799. doi: 10.1016/j.foodchem.2010.05.012. [DOI] [Google Scholar]
- Hunt JA, Dalgleish DG. Effect of pH on the stability and surface composition of emulsions made with whey protein isolate. J Agric Food Chem. 1994;42:2131–2135. doi: 10.1021/jf00046a011. [DOI] [Google Scholar]
- Jiménez-Martín E, Rojas TA, Gharsallaoui A, Carrascal JR, Pérez-Palacios T. Fatty acid composition in double and multilayered microcapsules of ω-3 as affected by storage conditions and type of emulsions. Food Chem. 2016;194:476–486. doi: 10.1016/j.foodchem.2015.08.046. [DOI] [PubMed] [Google Scholar]
- Joglekar AM, May AT. Product excellence through design of experiments. Cereal Foods World. 1987;32:857. [Google Scholar]
- Kim YL, Mun S, Rho SJ, Do HV, Kim YR. Influence of physicochemical properties of enzymatically modified starch gel on the encapsulation efficiency of W/O/W emulsion containing NaCl. Food Bioprocess Technol. 2017;10:77–88. doi: 10.1007/s11947-016-1799-6. [DOI] [Google Scholar]
- Klaypradit W, Huang YW. Fish oil encapsulation with chitosan using ultrasonic atomizer. LWT-Food Sci Technol. 2008;41:1133–1139. doi: 10.1016/j.lwt.2007.06.014. [DOI] [Google Scholar]
- Klinkesorn U, Sophanodora P, Chinachoti P, Decker EA, McClements DJ. Characterization of spray-dried tuna oil emulsified in two-layered interfacial membranes prepared using electrostatic layer-by-layer deposition. Food Res Int. 2006;39:449–457. doi: 10.1016/j.foodres.2005.09.008. [DOI] [Google Scholar]
- Kuhn KR, Cunha RL. Flaxseed oil–whey protein isolate emulsions: effect of high pressure homogenization. J Food Eng. 2012;111:449–457. doi: 10.1016/j.jfoodeng.2012.01.016. [DOI] [Google Scholar]
- Li X, Wang L, Wang B. Optimization of encapsulation efficiency and average particle size of Hohenbuehelia serotina polysaccharides nanoemulsions using response surface methodology. Food Chem. 2017;229:479–486. doi: 10.1016/j.foodchem.2017.02.051. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Food emulsions: principles, particles, and techniques. 3. Boca Raton: CRC Press; 2004. [Google Scholar]
- McClements DJ, Decker EA, Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72:109–124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Myers RH, Montgomery DC. Response surface methodology: process and product optimization using designed experiments. 2. New York: Wiley; 2002. [Google Scholar]
- Narayan P, Marchant D, Wheatley MA. Optimization of spray drying by factorial design for production of hollow microspheres for ultrasound imaging. J Biomed Mater Res A. 2001;56:333–341. doi: 10.1002/1097-4636(20010905)56:3<333::AID-JBM1101>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- O’Regan J, Mulvihill DM. Water soluble inner aqueous phase markers as indicators of the encapsulation properties of water-in-oil-in-water emulsions stabilized with sodium caseinate. Food Hydrocoll. 2009;23:2339–2345. doi: 10.1016/j.foodhyd.2009.06.009. [DOI] [Google Scholar]
- Ovissipour M, Rasco B, Shiroodi SG, Modanlow M, Gholami S, Nemati M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. J Sci Food Agric. 2012;93:1718–1726. doi: 10.1002/jsfa.5957. [DOI] [PubMed] [Google Scholar]
- Pacheco-Aguilar R, Mazorra-Manzano MA, Ramírez-Suárez JC. Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Food Chem. 2008;109:782–789. doi: 10.1016/j.foodchem.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Robert P, García P, Reyes N, Chávez J, Santos J. Acetylated starch andinulin as encapsulating agents of gallic acid and their release behaviour in ahydrophilic system. Food Chem. 2012;134:1–8. doi: 10.1016/j.foodchem.2012.02.019. [DOI] [Google Scholar]
- Salimi A, Maghsoudlou Y, Jafari SM, Mahoonak AS, Kashaninejad M, Ziaiifar AM. Preparation of lycopene emulsions by whey protein concentrate and maltodextrin and optimization by response surface methodology. J Dispers Sci Technol. 2015;36:274–283. doi: 10.1080/01932691.2013.879833. [DOI] [Google Scholar]
- San Martin-González MF, Roach A, Harte F. Rheological properties of corn oil emulsions stabilized by commercial micellar casein and high pressure homogenization. LWT-Food Sci Technol. 2009;42:307–311. doi: 10.1016/j.lwt.2008.04.005. [DOI] [Google Scholar]
- Sapei L, Naqvi MA, Rousseau D. Stability and release properties of double emulsions for food applications. Food Hydro. 2012;27:316–323. doi: 10.1016/j.foodhyd.2011.10.008. [DOI] [Google Scholar]
- Shukat R, Relkin P. Lipid nanoparticles as vitamin matrix carriers in liquid food systems: on the role of high-pressure homogenisation, droplet size and adsorbed materials. Colloids Surf B: Biointerfaces. 2011;86:119–124. doi: 10.1016/j.colsurfb.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Silva VM, Vieira GS, Hubinger MD. Influence of different combinations of wall materials and homogenisation pressure on the microencapsulation of green coffee oil by spray drying. J Food Res Int. 2014;61:132–143. doi: 10.1016/j.foodres.2014.01.052. [DOI] [Google Scholar]
- Soottitantawat A, Bigeard F, Yoshii H, Furuta T, Ohkawara M, Linko P. Influence of emulsion and powder size on the stability of encapsulated d-limonene by spray drying. Innov Food Sci Emerg Technol. 2005;6:107–114. doi: 10.1016/j.ifset.2004.09.003. [DOI] [Google Scholar]
- Tornberg E. Functional characteristics of protein-stabilized emulsions: emulsifying behaviour of proteins in a sonifier. J Food Sci. 1980;45:1162–1168. doi: 10.1111/j.1365-2621.1980.tb07585.x. [DOI] [Google Scholar]
- Van der Graaf S, Schroën CGPH, Boom RM. Preparation of double emulsions by membrane emulsification: a review. J Membr Sci. 2005;251:7–15. doi: 10.1016/j.memsci.2004.12.013. [DOI] [Google Scholar]
- Velasco J, Marmesat S, Dobarganes C, Márquez-Ruiz G. Heterogeneous aspects of lipid oxidation in dried microencapsulated oils. J Agric Food Chem. 2006;54:1722–1729. doi: 10.1021/jf052313p. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Jin M, Davidson PM, Zivanovic S. Sustained release of lysozyme from zein microcapsules produced by a supercritical anti-solvent process. Food Chem. 2009;115:697–700. doi: 10.1016/j.foodchem.2008.12.063. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.