Abstract
The aim of this study was to investigate the effect of atmospheric cold plasma (ACP) treatment on the microbial inactivation, physicochemical properties, and shelf-life of strawberry fruit with its extended in-package storage at room (25 °C) and refrigerated (4 °C) temperature. ACP treatment of 10, 15 and 30 min was studied on strawberry fruit using a dielectric barrier discharge (DBD) at 60 kV with an input voltage of 260 V at 50 Hz. The shelf-life of ACP treated strawberry was extended to 5 days at 25 °C and 9 days at 4 °C in sealed ACP package. However, non-treated packaged strawberry was degraded in 2 days. ACP treatment of 15 min resulted in 2 log reduction of microbial load and enhanced the concentration of chlorogenic acid, hyprin, phloretin, vanillin, gallic acid, 4-hydroxybenzaldehyde and rutin during in-package storage of 5 day (~ 120 h) at 25 °C with respect to control (p < 0.05). In addition, ACP treatment of 15 min at 60 kV was also found to increase the total phenolic content and antioxidant activity. However, total soluble solids, pH and moisture were not affected with ACP treatment (p > 0.05). Therefore, ACP treatment of 15 min with in-package storage of 5 days (~ 120 h) was found to be advantageous for increasing the shelf-life and functional quality of strawberry fruit.
Keywords: Atmospheric cold plasma (ACP), Strawberry, Microbial inactivation, Polyphenolic compounds, Antioxidant capacity, Shelf-life
Introduction
Strawberry (Fragaria × ananassa Duch.) is amongst the most popular berry fruit having the distinct flavor and taste (Herbst 2001). Strawberry is the profound source of ascorbic acid and bioactive compounds such as polyphenols (like caffeic acid, quinic acid, chlorogenic acid), flavonoids (like quercetin glycosides, kaempferol glycosides), anthocyanins (like pelargonidin and cyanidin glycosides) and tannins (like ellagic or gallic acid glycosides) (Giampieri et al. 2012; Silva Pinto et al. 2008).
Strawberry fruit is highly perishable and delicate in texture. Therefore, the regular allocation of fresh strawberries is difficult. Strawberry is susceptible to mechanical injury, physiological disorders, mould attacks, and texture loss (Romanazzi et al. 2013). Their short shelf-life is due to vulnerable to mould attacks and texture loss (Vardar et al. 2012). Though low temperature storage can effectively reduce the deterioration, but under controlled temperature conditions shelf-life of strawberry could rarely exceeds to 7–9 days (Erkan et al. 2008). Different methods such as physical methods (membrane filtration, drying), chemical and modified atmosphere packaging (MAP) have been employed in order to minimize the post-harvest losses by inactivating the microorganisms (Jouki and Khazaei 2014). Although these methods can effectively kill the microorganisms and increase the shelf-life of strawberry but they are laborious in nature, time consuming, costly and also render environmental hazards (Ito et al. 2012).
Furthermore, burgeoning awareness of consumers has led to an explosion of interest towards the consumption of healthy foods. Hence, there is a hike in the demand of minimally processed foods. Among the non-thermal technologies such as high-pressure processing (HPP), irradiation, power ultrasound, and ozone-assisted washing have showcased their strengths and benefitted the food industry with their potential of direct application. Similarly, atmospheric cold plasma (ACP) has been emerged as one of the prospective non-thermal technologies for rendering microbial safety to the whole fresh produce as well as preventing the degradation of polyphenols (Kovačević et al. 2016a). The excited charged species form uniform plasma discharge inside the sample holder and resulted in the inactivation of microbes (Ziuzina et al. 2014; Misra et al. 2014a, b).
The application of ACP-based microbial inactivation has been reported on whole fruits and their by-products such as kiwi (Ramazzina et al. 2015), grape (Moon et al. 2016), orange juice (Xu et al. 2017), white grape juice (Pankaj et al. 2017), tomato (Prasad et al. 2017) etc. In addition, the immediate effect of ACP has also been reported on microbial inactivation of strawberry inside sealed package with different gas mixtures (Misra et al. 2014a, b). However, the effect of ACP has not been explored on the shelf-life parameters (microbial, physicochemical and phenolics quality) during extended storage of packaged strawberry till 5 day at both room and refrigerated temperatures. In the present study, the potential effect of ACP treatment with its extended in-package storage duration (24 h–120 h) was investigated (as an essential post-harvest treatment) on microbial inactivation, physicochemical and shelf-life of strawberry fruit at room (25 °C) as well as refrigeration (4 °C) temperature. Previously, we have reported the effect of extended storage of 48 h of ACP treatment (15–30 min at 60 kV) on the inactivation of E. coli inoculated on tomato (Prasad et al. 2017). Therefore, the principal of extended storage was studied here on the microbial shelf-life and polyphenolics quality of strawberry.
Materials and methods
Procurement of strawberry sample
Freshly harvested matured strawberry fruit weighing 15–18 g each were procured from local market of Chandigarh. Samples were collected in three batches and each batch was analyzed in triplicate. Strawberries were exposed to blower in a laminar flow safety cabinet for 5 min at room temperature so as to remove the adhering dirt particles. There was no desiccation effect on fruits in 5 min laminar flow treatment.
Standards and reagents
Ascorbic acid, gallic acid, chlorogenic acid, rutin, hyprin, vanillin, 4-hydroxybenzaldehyde, phloretin, DPPH (2,2-diphenyl-1-picrylhydrazyl), and Folin–Ciocalteau reagent were procured from Sigma Aldrich (St. Louis, USA). Sodium carbonate, ortho-phosphoric acid, methanol (HPLC grade).
Atmospheric cold plasma (ACP) set up
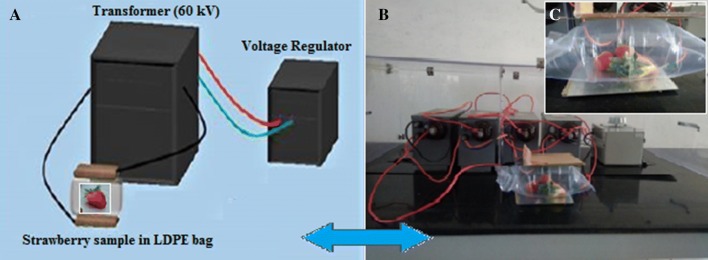
The dielectric barrier discharge (DBD) plasma set was assembled in lab as described in our previous study (Prasad et al. 2017). The DBD system consisted of two aluminum plate electrodes that were connected to a high voltage transformer (Flash Fabrication, S. No. 11110, 11221, 11222, 11223, India) with an input voltage of 260 V at 50 Hz and delivered a maximum high voltage output of 60 kV (Fig. 1). The cold plasma was generated in a low-density polypropylene (LDPE) packet that was filled with compressed air and acted for both as a sample holder and dielectric barrier (placed between the aluminum electrodes). The distance between the electrodes was 30 mm, which was equal to the LDPE packet filled with air and containing the strawberry sample.
Fig. 1.
Schematic presentation of ACP original device (a); Experimental set-up of assembled ACP (b); Strawberry was placed between the electrodes in LDPE packet that act as a dielectric barrier (c)
Enumeration of viable cells or microbial load examination
For the microbial count, the ACP treated and untreated samples of strawberry were analyzed during extended storage at refrigerated (4 ± 1 °C) and room (25 ± 1 °C) temperature. The ACP treated strawberry sealed packets were cut aseptically from the package and samples were taken out from the laminar flow. Strawberry samples were placed in sterile petri plate and rinsed with 0.9% saline water. The sample was rubbed for 2–3 min in the saline water. The resulting suspension was serially diluted using 0.9% saline water and plated on nutrient agar (NA) and potato dextrose agar (PDA) plates by spread plate method. The NA plates were incubated for 24 h at 37 °C and PDA plates were incubated for 48–72 h at 30 °C. Results obtained were evaluated in CFU/mL.
Extraction of phenolic compounds
Freeze dried sample (0.5 g) of treated and untreated strawberry was taken in a centrifuge tube. The sample was mixed with 20 mL of acidified methanol (HCl: water) in 50:50 (v/v) and was shaken at room temperature for about 1 h. The sample was then centrifuged for 10 min at 1575×g and the supernatant was recovered and filtered. Methanolic extract was used to quantify and identify the polyphenols using UPLC (Pérez-Jiménez et al. 2008).
Ultra-high-performance liquid chromatography (UPLC) analysis of extracted phenolic compounds
Separation of polyphenolic compounds of untreated and ACP treated strawberry samples were performed using Waters UPLC system in combination with PDA detector. Analysis of polyphenolic compounds was conducted by following the procedure described earlier (Bansal et al. 2014). The methanolic extract of ACP treated and untreated strawberry samples were centrifuged at 1575×g for 10 min and filtered through a 0.45 µm membrane filter (Pall Corporation, MI, USA). The sample volume of 20 µL was injected into the eclipse RP C-18 (4.6 × 100 mm × 5 µm) and the column temperature was set at 30 °C. All the chromatograms were extracted between 243 and 354 nm and results were expressed on dry weight basis.
Estimation of total phenolic content (TPC)
The concentration of phenolic compounds was determined by the Folin–Ciocalteu colorimetric method as described by Tezcan et al. (2009) with minor modifications. The methanolic extract of strawberry sample (0.1 mL) was mixed with 2.5 mL of the Folin–Ciocalteu reagent and 2 mL of 7.5% sodium carbonate. The mixture was then allowed to stand for 30 min at room temperature in the dark and the absorbance was measured at 760 nm using spectrophotometer (UV-3000, Navi Mumbai, India). Results were expressed as g per kg of gallic acid equivalents (GAE) of the sample on dry weight basis.
Estimation of antioxidant capacity
The free radical scavenging capacity was determined according to the method reported earlier (Rajan et al. 2011). Methanolic extract of strawberry sample (0.01 mL) was added to 3.9 mL of methanolic DPPH (0.025 g/L) and followed by addition of 0.09 mL of water. The mixture was then placed in dark for 30 min at room temperature. The absorbance of the samples was measured at 515 nm using spectrophotometer. The antioxidant capacity was determined as percent inhibition of DPPH of sample on dry weight basis and was calculated using the following formula:
Determination of physicochemical parameters
Total soluble solids (TSS) were determined by using a refractometer (ERMA, Japan) after standardizing it with distilled water. The pH of whole strawberry was measured through digital pH meter (Metler Toledo, Switzerland). The moisture content was measured by the moisture analyzer for 99 min at 105 °C (MA35 M, Sartorius, Germany). The results were expressed on fresh weight basis.
Statistical analysis
Statistical analyses were performed using SPSS version IBM SPSS Statistics (Version 22.0). The experiments were performed in triplicates. Mean and standard deviation were calculated.
Results and discussion
Effect of ACP treatment on microbial inactivation of in-packaged strawberry during its extended storage (at 25 and 4 °C)
Figure 2 presented the immediate microbial reduction in ACP treated strawberry samples. The total plate count and yeast/mould count of the control strawberries were 340 and 30 CFU/mL. Whereas, an immediate ACP treatment of 10 min was resulted in one log reduction of bacterial count (30 CFU/mL), whereas no growth of yeast was found. Likewise, no growth was found in 15 min and 30 min of ACP treated strawberry samples. Similarly, dielectric barrier discharge atmospheric cold plasma (DACP) treatment has been reported for significant and immediate decrease in the count of E. coli (1.1 log CFU/g) inoculated on the top of romaine lettuce at 42.6 kV for 10 min, when leaves were stacked in 7 layers (Min et al. 2017).
Fig. 2.
Effect of ACP treatment with extended storage on the (i) total plate count of strawberry at (A) 25 °C and (B) 4 °C; (ii) yeast/mould count of strawberry at A 25 °C and B 4 °C. Vertical bars depict standard error
Figure 2 showed the effect of ACP treatment on the inactivation of bacterial and yeast/mould count of strawberry sample on 2, 5, 7 and 9 days at 4 and 25 °C. At 25 °C, there was visible fungal growth on 6th day and therefore considered for shelf life studies up to 5 days. While at 4 °C, there was no visible fungal growth up to 9th day and therefore considered for shelf life studies up to 9 days. Furthermore, it was found that during storage of ACP treated in-package strawberry, there was a significant microbial reduction in comparison to immediate ACP treatment. On the second day of in-package storage at 25 °C, there was 2 log reduction in the bacterial and yeast/mould count with ACP treatment of 10 min with respect to control sample of strawberry. Similarly, on increasing the treatment time to 15 and 30 min, no growth of bacteria and yeast/mould was found. Likewise, the reduction of 2 log in counts of bacteria and yeast/mould at 60 kV in ACP treatment has been reported in strawberry sample (Misra et al. 2014a). Moreover, 3-log reduction has also been reported in modified atmosphere packaged strawberries immediate after cold plasma treatment (Misra et al. 2014b). However, the shelf-life was not reported during in-package storage of strawberry for longer period i.e. up-to 5 days. Reduction of 6 log of E. coli has been reported in our previous study with the in-package storage of tomato to 48 h after ACP treatment at 60 kV (Prasad et al. 2017). The lesser reduction of microbes in strawberry might be due to wrinkled surface of the fruit in comparison to tomato. Hence, either longer duration at lower voltage or higher voltage would be required for more microbial reduction for smooth surface commodity. Similarly, ACP treatment of 90 min has been reported for 2.5–4.5 log reduction of Salmonella enteritidis at 15 kV on egg shell (Ragni et al. 2010). The control sample of strawberry was degraded on the second day with visible fungal growth. Similarly, no significant changes in bacterial growth were found on the 5 day in 10, 15, and 30 min of ACP treated strawberry. Likewise, no significant growth of yeast was found at 5 day (25 °C) in 15 and 30 min of ACP treated strawberry in sealed package. Increased microbial reduction with an increase in ACP treatment time could be a result of its reaction with the reactive species like atomic oxygen, singlet oxygen and hydroxyl radicals (Brandenburg et al. 2007). Maximum concentration of 3000 and 500 ppm of ozone and RNS (reactive oxygen and nitrogen species) has been achieved after 15 min of ACP treatment (Wan et al. 2017).
Furthermore, refrigerated storage had no significant effect on the inactivation of bacterial/yeast and mould count after 5 day of storage in comparison to 25 °C storage. This might be due to the agglomeration of highly charged active species. Therefore, ACP treatment was resulted in significant inactivation of microbes on 15 and 30 min ACP treatment (~ 60 kV) at 25 °C.
Effect of immediate ACP treatment on the concentration of polyphenolic compounds of strawberry analyzed by UPLC
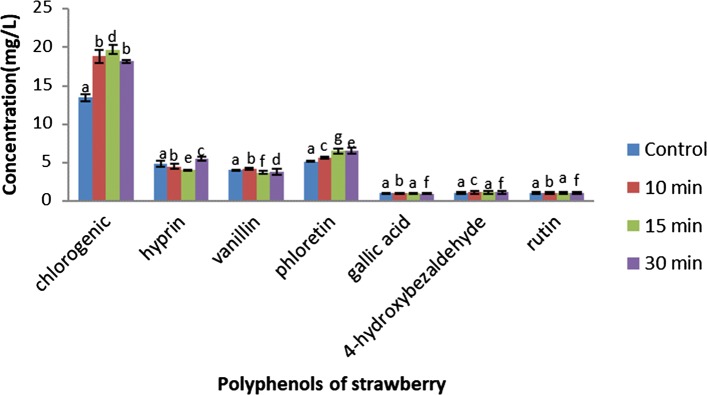
Polyphenolic content of control and ACP treated strawberry samples is shown in Fig. 3. UPLC analysis showed the presence of hydroxycinnamic acids (chlorogenic acid) as the major polyphenol in strawberry sample, which was followed by flavonoids (hyprin and rutin), hydroxy-benzoic acids (gallic acid), phenolic acid aldehydes (vanillin and 4-hydroxybenzaldehyde) and dihydrochalcones (phloretin).
Fig. 3.
Effect of immediate ACP treatment on the concentration of polyphenols of strawberry. Vertical bars depict standard error. Bars with different letters indicate significant difference (p < 0.05)
The amount of chlorogenic acid in untreated strawberry was found to be 13.45 ± 0.45 mg/L. However, the amount of chlorogenic acid was increased to 39.85% and 46.5% in ACP treated strawberry at 10 and 15 min of treatment time, respectively. Whereas, with increase in the treatment time to 30 min, the concentration was sustained to 35% in ACP treated strawberry sample. The similar effect of gas phase plasma has been observed on the stability of polyphenols in the chokeberry juice by employing the treatment of ACP jet at the power of 4 W for 3 and 5 min (Kovačević et al. 2016a). The concentration of hydroxycinnamic acids has been reported to increase in plasma treated juice compared to the untreated chokeberry juice (Kovačević et al. 2016a). Similarly, the plasma treated pomegranate juice contained higher content of hydroxycinnamic acids in comparison to control juices (Kovačević et al. 2016b). Significant increase in the content of phloretin content was observed in ACP treated samples (9.2% in 10 min, 25.4% in 15 min and 30 min, respectively) with respect to control sample (5.158 ± 0.05 mg/L). In addition, the concentration of 4-hydroxybenzaldehyde was further enhanced to 6.3%, 5.4%, and 5.9% at 10, 15, and 30 min, respectively. Moreover, the amount of rutin which is the major flavonoid (1.09 ± 0.09 mg/L in control sample) was found to be increased to 1.83% and 1.8% at 10 and 15 min of ACP treatment. In addition, the concentration of hyprin was significantly enhanced (13.9%) with an increase in time of ACP treatment to 30 min. These flavonoids might require more concentration of charged species in order to get released from their cellular membranes. The amount of vanillin and gallic acid were also increased to 3.6% and 0.4% in 10 min treatment time of ACP. With further increase in ACP treatment time (30 min), no significant increase in vanillin and gallic acid was found. Hence, immediate ACP treatment of 15 min was observed to be efficacious in enhancing the stimulation of phenolic compounds.
Effect of ACP treatment on the concentration of polyphenolic compounds of strawberry during its extended in-package storage (at 25 and 4 °C)
The effect of ACP treatment on the strawberry fruit with an extended in-package storage period at 25 °C is presented in Fig. 4. Chlorogenic acid was increased by 49.8%, 30.45% and 22.14% after the storage of 2 days at 10, 15 and 30 min of ACP treatment, respectively in comparison to immediate. Vanillin concentration was increased by 40%, 32% and 18.06% at 10, 15 and 30 min of ACP treatment, respectively. The concentration of phloretin was also increased in ACP treated strawberry to the extent of 20.9%, 8.58%, 3.96% in 10, 15, 30 min, respectively compared to the immediate ACP treated samples. 4-hydroxybenzaldehyde was also increased to 9.32% at 10 min and decreased to 1.7% at 15 min. These results imply that with increase in treatment time, the chlorogenic acid, vanillin, phloretin and 4-hydroxybenzaldehyde were decreased with respect to 10 min ACP treatment. The hyprin content was increased after 2 days in the 10 min (18%) and 15 min (33.9%) of ACP treatment. Therefore, extended storage was found to be significant with increased treatment for stimulation of polyphenols owing to the absolute performance of charged gas species (Pankaj et al. 2017). Moreover, concentration of hyprin was found stable (27.56%) in ACP treatment of 30 min. The polyphenol content was significantly decreased with higher plasma treatment time with an extended storage of 2 days (p < 0.05). This might be due to the oxidation of polyphenols in presence of elevated amount of reactive species (Makris and Rossiter 2002). Garofulić et al. (2015) have reported the reduction of phenolic acids like neochlorogenic acid and p-coumaric acid in sour cherry marasca juice. This was due to high intensity effect of plasma jet (5 min, 2.5 kV voltage at 25 kHz) of plasma treatment (Garofulić et al. 2015). The gallic acid which was measured after 2 days was found to be increased by only 2.0% and 1.193% for 15 min and 30 min of ACP treatment. This might be due to the ability of phenolic acids to scavenge free radicals generated by cold plasma. Therefore, gallic acid might have undergone the cleavage of its aromatic ring due to the formation of phenoxyl radical during electron/proton transfer and thereby, leading to the formation of small phenolic acids (Pankaj et al. 2017). ACP treatment showed 2.7% increase in rutin during ACP treatment of 10 min in comparison to the immediate ACP treatment. Further increase in ACP treatment was resulted in decrease of rutin. Overall, extended storage of 2 days was resulted in enhanced functional quality of strawberry in comparison to immediate ACP treatment.
Fig. 4.
Effect of ACP treatment with an extended storage on the concentration of polyphenols of strawberry stored at (i) 25 °C and (ii) 4 °C. Vertical bars depict standard error. Bars with different letters indicate significant difference (p < 0.05)
On further extending the sealed storage to 5 days, the chlorogenic acid was increased to 82.2%, 81.3% in 10, 15 min and fourfolds in the ACP treatment of 30 min at 25 °C with respect to 2 days storage at 25 °C. The vanillin was increased to about fourfolds in 10, 15 min and fivefold in 30 min of ACP treatment. The concentration of hyprin was enhanced to 126%, 274%, 314%, respectively in 10, 15, 30 min of ACP treatment in comparison to 2 days at 25 °C. Additionally, the 4-hydroxybenzaldehyde was increased to 89%, 152% and 121% in 10, 15 and 30 min of ACP treatment, respectively. Moreover, there was an increase of 119%, 112% and 124% in the concentration of phloretin in 10, 15 and 30 min of ACP treatment of strawberry. The amount of rutin was also increased by 36.8%, 41.6% and 32.7% in 10, 15 and 30 min of ACP treatment of strawberry, respectively. Similarly, the gallic acid was found to be increased by 12%, 13.8% and 11% in 10, 15 and 30 min of ACP treatment, respectively. Therefore, an extended storage of 120 h was found advantageous over 48 h for stimulating the bioactive compounds of fresh produce like strawberry.
The effect of ACP treatment on the strawberry fruit with extended storage period at 4 °C is presented in Fig. 4. After the storage of 2 days of ACP treatment at 4 °C, there was a significant decrease in the chlorogenic acid to the highest value of 27.4% in comparison to 2 days stored sample at 25 °C. However, after the extended storage of 5 days, there was an increase in the chlorogenic acid to 19.67% in 15 min treatment. While, the amount of vanillin and hyprin was decreased to 35.6% and 57% with extended storage of ACP treatment after 2 days. Our results were found in accordance with decrease in flavonols in Campineiro (a variety of strawberry) after 3 days at 6 and 16 °C compared to the room temperature (Cordenunsi et al. 2005). On contrary, hyprin was found to be increased to maximal amount of 28% till the storage of 5 d. In contrast, the phloretin was increased to 97.4% and 10.28% with ACP treatment of 10 and 15 min after the storage of 5 days in comparison to 2 d stored samples at 25 °C. However, the concentration of 4-hydroxybenzaldehyde was increased to 5.2% in 15 min ACP treatment after the storage of 2 days. No significant effect was found in the concentration of gallic acid and rutin in 10, 15, and 30 min of ACP treatment on the storage of 2 days. After the 5 days, a decrease of 10.5% was found in the gallic acid with ACP treatment of 15 min. Also, a decrease of 11.8% was found in the concentration of rutin with the ACP treatment.
Moreover with an extended storage up to 9 days, there was increase in the concentrations of chlorogenic acid, gallic acid, rutin, hyprin, vanillin and 4-hydroxybenzaldehyde under refrigerated conditions with respect to preceding storage day. An increase in chlorogenic acid could be attributed to the accumulation of sugars (glucose, fructose and sucrose) in potatoes during the cold storage (Hasegawa et al. 1966). An increase in phenolic acids has also been reported in the fruits and vegetables after 15 days storage at 4 °C (Galani et al. 2017).
Overall, with extended in-package refrigeration storage, the concentration of polyphenols was maximally retained till 9 days at 4 °C with ACP treatment in comparison to storage of 5 days at 25 °C.
Effect of ACP on total phenolic content (TPC) of strawberry during its extended storage (at 25 and 4 °C)
The effect of ACP on the total phenolic content was analyzed in terms of g/kg gallic acid equivalent at both the storage temperatures for 10 min, 15 min, and 30 min (Table 1). The average TPC of control sample was 37 ± 0.64 g/kg GAE. The TPC was found to be increased to 5.4% and 11.5% on 10 and 15 min, respectively on immediate basis. While, the concentration was decreased to 5.8% with 30 min of ACP treatment as sustained plasma treatment to longer time might have led to lower phenolic content. Likewise, significant increase was also reported in the TPC of blueberries on 1 min of ACP treatment but slight reduction was observed on 5 min treatment (Sarangapani et al. 2017).
Table 1.
Effect of the ACP treatment with extended storage on the antioxidant capacity (%) and total phenolic content (TPC) (g/kg GAE) of strawberry during storage at 4 and 25 °C
| Temperature (°C) | Antioxidant capacity (%) | Total phenolic content (g/kg GAE) | ||||
|---|---|---|---|---|---|---|
| 10 min | 15 min | 30 min | 10 min | 15 min | 30 min | |
| 25 | 52.25 ± 0.25a | 48.25 ± 0.28a | 42.375 ± 0.17a | 39 ± 0.99a | 41.27 ± 0.74a | 34.87 ± 0.20a |
| 25 | 48.875 ± 0.275b | 45.625 ± 0.98b | 43.25 ± 0.13a | 42.56 ± 0.24b | 40.116 ± 0.25b | 38.02 ± 0.29b |
| 4 | 45.125 ± 0.125c | 46.25 ± 0.03b | 50.875 ± 0.6b | 31.56 ± 0.65c | 36.23 ± 0.64c | 41.81 ± 0.03c |
| 25 | 49.125 ± 0.025b | 53.375 ± 0.3c | 46.75 ± 0.4c | 42.81 ± 0.96d | 49.6 ± 0.75d | 43.06 ± 0.12d |
| 4 | 50 ± 0.8d | 51.25 ± 0.25d | 41.5 ± 0.88d | 42.26 ± 0.33d | 44.014 ± 0.40e | 32.86 ± 0.94e |
| 4 | 45.75 ± 0.58c | 46.25 ± 0.1b | 42.5 ± 0.64a | 45.58 ± 0.28e | 45.4 ± 0.06f | 36.092 ± 0.80f |
| 4 | 45.875 ± 0.75c | 44.375 ± 0.28e | 42.125 ± 0.12a | 39.01 ± 0.29a | 38.9 ± 0.27g | 37.42 ± 0.70g |
Values are means ± standard deviations (SD) of triplicate repeated sets. Different superscripted letters in the same column are significantly different (p < 0.05)
After the storage of 2 day at 25 °C, ACP treatment was resulted in higher concentration of TPC at 10 min (9.13%) and that was sustained till 30 min (9.03%) (38 ± 0.29 g/kg GAE) with respect to immediate ACP treated sample. Furthermore, the TPC of samples was increased to 0.59%, 23% and 13.25% for 10, 15 and 30 min of ACP treatment, respectively on the storage of 5 day in comparison to storage samples of 2 day at 25 °C. The UV and shock waves increases the activity of phenylalanine ammonialyase, a primary enzyme for the synthesis of phenolic acids which in turn elevates the TPC (Wang et al. 2009).
In comparison with 25 °C stored samples of strawberry, a decrease was found in TPC of 26.4% and 9.69% with 10 and 15 min of ACP treatment. Whereas, an increase of 9.9% for 30 min of ACP treatment was recorded on storage of 2 day at 4 °C. After the storage of 5 day, the content was found to be decreased to 1.28% (10 min), 11.26% (15 min) and 23.68% (30 min) of ACP treatment. The content was increased to 7.87%, 3.15% and 9.8% for 10, 15 and 30 min, respectively, on 7 day in comparison to 5 day storage at 4 °C. On the 9 day, mere increase of 3.68% was seen on TPC of 30 min treated sample, but decrease of 14.4% and 14.3% was noticed on 10 and 15 min of ACP treated sample in comparison with sample stored on 7 day under refrigerated conditions. Results documented the highest concentration of TPC during the extended storage of 5 day post ACP treatment at 25 °C in comparison to 9 day at 4 °C.
Effect of ACP on antioxidant capacity of strawberry during its extended storage (at 25 and 4 °C)
The influence of ACP with its extended storage on the antioxidant capacity of strawberry fruit is shown in Table 1. In DPPH assay, the scavenging activity of control sample was 45.63%. Antioxidant capacity of ACP treated strawberry samples was increased to 14.5% and 5.75% in 10 and 15 min respectively, which was followed by decrease to 7.2% for 30 min on immediate treatment. Hence, longer duration of ACP treatment might led to lower antioxidant capacity. Similarly, the decrease in antioxidant capacity was observed with an increase in treatment time in the white grape juice treated with DBD at 80 kV (1–4 min) (Pankaj et al. 2017). An increase 2.06% on 2 day at 25 °C was observed in the antioxidant activity of sample treated with ACP for 30 min. While a decrease of 6% and 5.4% in antioxidant activity was observed during 10 and 15 min of ACP treatment. On the storage of 5 day, an increase was found in antioxidant capacity to the extent of 0.5%, 16.9% and 8.1% in 10, 15 and 30 min of ACP treatment, respectively. Therefore, the enhanced antioxidant capacity was in agreement with the increased TPC of ACP treated strawberry on the storage of 5 day at 25 °C.
At 4 °C, a subsequent increase was observed in the antioxidant capacity of ACP treated strawberry when treated for 15 min (1.37%), 30 min (17.63%). While it was decreased to 7.67% with ACP treatment of 10 min relative to storage of 2 day at room temperature. Moreover, an increase in the antioxidant capacity was found at 10 min (9.6%), 15 min (12.34%) and a decrease was observed at 30 min (9%) on the storage of 5 day. Previous studies have indicated an enhancing effect of indirect ACP treatment on the antioxidant activity of cashew apple juice when treated for 5 min at flow rate of 10 mL/min of nitrogen plasma. Furthermore, antioxidant capacity was reduced when treated for 10 and 15 min in cashew apple juice (Rodríguez et al. 2017). Antioxidant capacity was decreased to 8.5%, 9.76% and 2.40% in 10, 15 and 30 min of ACP treatment, respectively on storage of 7 day in comparison to 5 day at 4 °C. Till the storage of 9 d, the retention of antioxidant capacity was found sustained in 10 min of ACP treated strawberry sample with respect to 7 days. However, decrease of 4.05% and 3.82% was found in 15 min and 30 min of ACP treated samples with respect to 7 days stored strawberry samples at 4 °C.
The results indicated that ACP treatment for 15 min had significant effect on the antioxidant capacity of strawberry fruit with its extended storage at both temperatures. Whereas longer plasma treatment had led to decrease in antioxidant capacity owing to formation of large charged gas species.
Effect of ACP on physicochemical parameters of strawberry during its extended storage (at 25 and 4 °C)
The influence of ACP treatment on the total soluble solids (TSS) of strawberry is presented in Table 2. On an immediate ACP treatment, the TSS of strawberry was increased (5 ± 0.1% in untreated strawberries) with longer plasma exposure. Although, the maximum TSS value was found in the 30 min of ACP treated strawberry at both the temperatures. Similar results have been reported earlier, increase in treatment time has been reported to increase the TSS during the storage time of 1 and 4 day of cut-kiwi fruit (Ramazzina et al. 2015). However, ACP treatment for 10 min and 15 min had no significant effect on TSS of strawberry. Similarly, insignificant effect on TSS of orange juice was found in the DBD plasma treatment (Shi et al. 2011).
Table 2.
Effect of the ACP treatment with extended storage on the total soluble solids (%), pH and moisture (%) of strawberry during storage at 4 and 25 °C
| Days | Temp (°C) | TSS (%) | PH | Moisture (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 min | 15 min | 30 min | 10 min | 15 min | 30 min | 10 min | 15 min | 30 min | ||
| 0 | 25 | 5 ± 0.1a | 6 ± 0.2a | 6 ± 0.2a | 3.71 ± 0.0a | 3.72 ± 0.02a | 3.7 ± 0.01a | 91.5 ± 0.0a | 91.5 ± 0.16a | 91.6 ± 0.1a |
| 2 | 25 | 5 ± 0.1a | 5 ± 0.1b | 5.8 ± 0.2a | 3.87 ± 0.0a | 3.83 ± 0.02a | 3.7 ± 0.01a | 91.2 ± 0.11a | 91.13 ± 0.03a | 91.23 ± 0.2a |
| 2 | 4 | 5.2 ± 0.1a | 5 ± 0.1b | 5 ± 0.2b | 3.72 ± 0.01a | 3.96 ± 0.04b | 3.8 ± 0.05a | 91.3 ± 0.65a | 91.35 ± 0.35a | 91.44 ± 0.2a |
| 5 | 25 | 5 ± 0.1a | 5 ± 0.2b | 5.1 ± 0.1b | 3.46 ± 0.06b | 3.65 ± 0.05a | 3.6 ± 0.01a | 90.6 ± 0.95a | 90.34 ± 0.88a | 90.54 ± 0.14a |
| 5 | 4 | 5 ± 0.1a | 5 ± 0.2b | 5.2 ± 0.2b | 3.46 ± 0.04b | 3.54 ± 0.05c | 3.7 ± 0.1a | 91.3 ± 0.52a | 91.2 ± 0.29a | 91.43 ± 0.23a |
| 7 | 4 | 5 ± 0.1a | 5 ± 0.1b | 5.8 ± 0.2a | 3.83 ± 0.03a | 3.94 ± 0.03a | 4 ± 0.1b | 91.5 ± 0.29a | 91.3 ± 0.29a | 91.59 ± 0.2a |
| 9 | 4 | 5 ± 0.1a | 5 ± 0.2b | 5.1 ± 0.2b | 4.46 ± 0.05c | 4.52 ± 0.02d | 4.3 ± 0.05b | 91.5 ± 0.1a | 91.5 ± 0.01a | 91.52 ± 0.3a |
Values are means ± standard deviations (SD) of triplicate repeated sets. Different superscripted letters in the same column are significantly different (p < 0.05)
Table 2 showed the effect of ACP treatment on the pH of whole strawberry with its extended storage at 4 °C and 25 °C. A decrease was found in the pH of immediate ACP treated strawberry (3.71 ± 0.04, 3.72 ± 0.02, 3.75 ± 0.01) for the treatment time of 10, 15 and 30 min, respectively, with respect to control (3.83 ± 0.03). Decrease in the pH of DBD-ACP apple juice has been reported on treatment for 0 to 40 s with input power intensity of 30, 40 and 50 W due to the presence of active species with higher input power (Liao et al. 2018). The maximum pH increase was observed on storage of 9 day at 4 °C (16.4%, 18%, 14% for treatment time of 10, 15, 30 min, respectively). Nevertheless, there was no significant effect of ACP on the pH of treated and control strawberries. Similarly, an insignificant effect of microwave-powered cold plasma treatment on the pH of mandarin at storage temperature of 4 and 25 °C has been reported earlier (Won et al. 2017).
The effect of ACP on the moisture content of treated strawberry sample and untreated sample is shown in Table 2. The moisture content of untreated strawberry was 91.55 ± 0.05%. No significant effect of ACP treatment was found on the moisture content of stored strawberry. During storage at 25 °C, merely 1% decrease was observed due to faster dehydration/evaporation of moisture in all ACP treated samples. However, < 5% loss of moisture content does not have any major consequence on the tissue hardness (Wang et al. 2012).
Conclusion
The ACP treatment efficaciously extended the shelf-life of strawberry for 3 days at 25 °C. Significant reduction of 2-log was found in the microbial count of strawberry with ACP treatment at 60 kV in contrast to control. No significant effect was found in the microbial inactivation at refrigerated temperature during the extended storage. The concentration of bioactive compounds was significantly increased at 25 °C (p < 0.05) storage, particularly at ACP treatment for 15 min. ACP treatment has been found to significantly affect the total phenol content, antioxidant capacity with ACP treatment for 15 min, while overexposure of 30 min led to decrease in TPC at 25 °C. The total soluble solids, pH, moisture content of the strawberry fruit was not affected by the ACP treatment (p > 0.05). Therefore, ACP treatment (~ 60 kV for 15 min) was found to be an efficient post-harvest treatment for preserving strawberry at 25 °C.
Acknowledgements
We are thankful to the CEO, CIAB for his continuous support and encouragement. The authors are highly thankful to the Department of Biotechnology, Government of India for financial support.
Compliance with ethical standards
Conflict of interest
All authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sudha Rana and Deepak Mehta have contributed equally to this work.
Contributor Information
Vasudha Bansal, Email: vasu22bansal@gmail.com.
Sudesh Kumar Yadav, Email: sudesh@ciab.res.in.
References
- Bansal V, Sharma A, Ghanshyam C, Singla ML. Coupling of chromatographic analyses with pretreatment for the determination of bioactive compounds in Emblica officinalis juice. Anal Methods. 2014;6:410–418. doi: 10.1039/C3AY41375F. [DOI] [Google Scholar]
- Brandenburg R, Ehlbeck J, Stieber M, Woedtke Tv, Zeymer J, Schlüter O, Weltmann KD. Antimicrobial treatment of heat sensitive materials by means of atmospheric pressure Rf-driven plasma jet. Contrib Plasma Phys. 2007;47:72–79. doi: 10.1002/ctpp.200710011. [DOI] [Google Scholar]
- Cordenunsi BR, Genovese MI, do Nascimento JR, Hassimotto NM, dos Santos RJ, Lajolo FM. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 2005;91:113–121. doi: 10.1016/j.foodchem.2004.05.054. [DOI] [Google Scholar]
- da Silva Pinto M, Lajolo FM, Genovese MI. Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria x ananassa Duch.) Food Chem. 2008;107:1629–1635. doi: 10.1016/j.foodchem.2007.10.038. [DOI] [Google Scholar]
- Erkan M, Wang SY, Wang CY. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol Technol. 2008;48:163–171. doi: 10.1016/j.postharvbio.2007.09.028. [DOI] [Google Scholar]
- Galani JH, Patel JS, Patel NJ, Talati JG. Storage of fruits and vegetables in refrigerator increases their phenolic acids but decreases the total phenolics, anthocyanins and vitamin C with subsequent loss of their antioxidant capacity. Antioxidants. 2017;6(3):59. doi: 10.3390/antiox6030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofulić IE, Jambrak AR, Milošević S, Dragović-Uzelac V, Zorić Z, Herceg Z. The effect of gas phase plasma treatment on the anthocyanin and phenolic acid content of sour cherry Marasca (Prunus cerasus var. Marasca) juice. LWT Food Sci Technol. 2015;62:894–900. doi: 10.1016/j.lwt.2014.08.036. [DOI] [Google Scholar]
- Giampieri F, Tulipani S, Alvarez-Suarez JM, Quiles JL, Mezzetti B, Battino M. The strawberry: composition, nutritional quality, and impact on human health. Nutrition. 2012;28:9–19. doi: 10.1016/j.nut.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Johnson RM, Gould WA. Changes during storage, effect of cold storage on chlorogenic acid content of potatoes. J Agric Food Chem. 1966;14:165–169. doi: 10.1021/jf60144a020. [DOI] [Google Scholar]
- Herbst ST (2001) The new food lover’s companion: comprehensive definitions of nearly 6,000 food, drink, and culinary terms. Barron’s cooking guide. Hauppauge, NY: Barron’s Educational Series. ISBN 0764112589
- Ito M, Ohta T, Hori M. Plasma agriculture. J Korean Phys Soc. 2012;60:937–943. doi: 10.3938/jkps.60.937. [DOI] [Google Scholar]
- Jouki M, Khazaei N. Effect of low-dose gamma radiation and active equilibrium modified atmosphere packaging on shelf life extension of fresh strawberry fruits. Food Packag Shelf Life. 2014;1:49–55. doi: 10.1016/j.fpsl.2013.12.001. [DOI] [Google Scholar]
- Kovačević DB, Kljusurić JG, Putnik P, Vukušić T, Herceg Z, Dragović-Uzelac V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016;212:323–331. doi: 10.1016/j.foodchem.2016.05.192. [DOI] [PubMed] [Google Scholar]
- Kovačević DB, Putnik P, Dragović-Uzelac V, Pedisić S, Jambrak AR, Herceg Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016;190:317–323. doi: 10.1016/j.foodchem.2015.05.099. [DOI] [PubMed] [Google Scholar]
- Liao X, Li J, Muhammad AI, Suo Y, Chen S, Ye X, Liu D, Ding T. Application of a dielectric barrier discharge atmospheric cold plasma (Dbd-Acp) for Eshcerichia coli inactivation in apple juice. J Food Sci. 2018;83:401–408. doi: 10.1111/1750-3841.14045. [DOI] [PubMed] [Google Scholar]
- Makris DP, Rossiter JT. Hydroxyl free radical-mediated oxidative degradation of quercetin and morin: a preliminary investigation. J Food Compos Anal. 2002;15:103–113. doi: 10.1006/jfca.2001.1030. [DOI] [Google Scholar]
- Min SC, Roh SH, Niemira BA, Boyd G, Sites JE, Uknalis J, Fan X. In-package inhibition of E. coli O157: H7 on bulk Romaine lettuce using cold plasma. Food Microbiol. 2017;65:1–6. doi: 10.1016/j.fm.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Misra NN, Moiseev T, Patil S, Pankaj SK, Bourke P, Mosnier JP, Keener KM, Cullen PJ. Cold plasma in modified atmospheres for post-harvest treatment of strawberries. Food Bioprocess Technol. 2014;7:3045–3054. doi: 10.1007/s11947-014-1356-0. [DOI] [Google Scholar]
- Misra NN, Patil S, Moiseev T, Bourke P, Mosnier JP, Keener KM, Cullen PJ. In-package atmospheric pressure cold plasma treatment of strawberries. J Food Eng. 2014;125:131–138. doi: 10.1016/j.jfoodeng.2013.10.023. [DOI] [Google Scholar]
- Moon AY, Noh S, Moon SY, You S. Feasibility study of atmospheric-pressure plasma treated air gas package for grape’s shelf-life improvement. Curr Appl Phys. 2016;16:440–445. doi: 10.1016/j.cap.2016.01.007. [DOI] [Google Scholar]
- Pankaj SK, Wan Z, Colonna W, Keener KM. Effect of high voltage atmospheric cold plasma on white grape juice quality. J Sci Food Agric. 2017;97:4016–4021. doi: 10.1002/jsfa.8268. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez J, Arranz S, Tabernero M, Díaz-Rubio ME, Serrano J, Goñi I, Saura-Calixto F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res Int. 2008;41:274–285. doi: 10.1016/j.foodres.2007.12.004. [DOI] [Google Scholar]
- Prasad P, Mehta D, Bansal V, Sangwan RS. Effect of atmospheric cold plasma (ACP) with its extended storage on the inactivation of Escherichia coli inoculated on tomato. Food Res Int. 2017;102:402–408. doi: 10.1016/j.foodres.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Ragni L, Berardinelli A, Vannini L, Montanari C, Sirri F, Guerzoni ME, Guarnieri A. Non-thermal atmospheric gas plasma device for surface decontamination of shell eggs. J Food Eng. 2010;100:125–132. doi: 10.1016/j.jfoodeng.2010.03.036. [DOI] [Google Scholar]
- Rajan S, Gokila M, Jency P, Brindha P, Sujatha RK. Antioxidant and phytochemical properties of Aegle marmelos fruit pulp. Int J Curr Pharm Res. 2011;3(2):65–70. [Google Scholar]
- Ramazzina I, Berardinelli A, Rizzi F, Tappi S, Ragni L, Sacchetti G, Rocculi P. Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biol Technol. 2015;107:55–65. doi: 10.1016/j.postharvbio.2015.04.008. [DOI] [Google Scholar]
- Rodríguez Ó, Gomes WF, Rodrigues S, Fernandes FA. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.) LWT Food Sci Technol. 2017;84:457–463. doi: 10.1016/j.lwt.2017.06.010. [DOI] [Google Scholar]
- Romanazzi G, Feliziani E, Santini M, Landi L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol Technol. 2013;75:24–27. doi: 10.1016/j.postharvbio.2012.07.007. [DOI] [Google Scholar]
- Sarangapani C, O’Toole G, Cullen PJ, Bourke P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov Food Sci Emerg Technol. 2017;44:235–241. doi: 10.1016/j.ifset.2017.02.012. [DOI] [Google Scholar]
- Shi XM, Zhang GJ, Wu XL, Li YX, Ma Y, Shao XJ. Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE Trans Plasma Sci. 2011;39:1591–1597. doi: 10.1109/TPS.2011.2142012. [DOI] [Google Scholar]
- Tezcan F, Gültekin-Özgüven M, Diken T, Özçelik B, Erim FB. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009;115:873–877. doi: 10.1016/j.foodchem.2008.12.103. [DOI] [Google Scholar]
- Vardar C, Ilhan K, Karabulut OA. The application of various disinfectants by fogging for decreasing postharvest diseases of strawberry. Postharvest Biol Technol. 2012;66:30–34. doi: 10.1016/j.postharvbio.2011.11.008. [DOI] [Google Scholar]
- Wan Z, Chen Y, Pankaj SK, Keener KM. High voltage atmospheric cold plasma treatment of refrigerated chicken eggs for control of Salmonella Enteritidis contamination on egg shell. LWT Food Sci Technol. 2017;76:124–130. doi: 10.1016/j.lwt.2016.10.051. [DOI] [Google Scholar]
- Wang CY, Chen CT, Wang SY. Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem. 2009;117:426–431. doi: 10.1016/j.foodchem.2009.04.037. [DOI] [Google Scholar]
- Wang RX, Nian WF, Wu HY, Feng HQ, Zhang K, Zhang J, Zhu WD, Becker KH, Fang J. Atmospheric-pressure cold plasma treatment of contaminated fresh fruit and vegetable slices: inactivation and physiochemical properties evaluation. Eur Phys J D. 2012;66:276. doi: 10.1140/epjd/e2012-30053-1. [DOI] [Google Scholar]
- Won MY, Lee SJ, Min SC. Mandarin preservation by microwave-powered cold plasma treatment. Innov Food Sci Emerg Technol. 2017;39:25–32. doi: 10.1016/j.ifset.2016.10.021. [DOI] [Google Scholar]
- Xu L, Garner AL, Tao B, Keener KM. Microbial inactivation and quality changes in orange juice treated by high voltage atmospheric cold plasma. Food Bioprocess Technol. 2017;10(10):1778–1791. doi: 10.1007/s11947-017-1947-7. [DOI] [Google Scholar]
- Ziuzina D, Patil S, Cullen PJ, Keener KM, Bourke P. Atmospheric cold plasma inactivation of Escherichia coli, Salmonella enterica serovar Typhimurium and Listeria monocytogenes inoculated on fresh produce. Food Micro. 2014;42:109–116. doi: 10.1016/j.fm.2014.02.007. [DOI] [PubMed] [Google Scholar]